Abstract
Studies of regional hemispheric asymmetries point to relatively less activity in left frontal and right posterior regions in depression. Anxiety was associated with increased right posterior activity, which may be related to arousal and, in anxious-depressed individuals, offset the posterior asymmetry typically seen in depression. These asymmetries have been indexed by resting EEG or inferred through the use of lateralized auditory and visual tasks (e.g., dichotic listening and chimeric faces). However, associations between regional EEG activity and neurocognitive function in depression or anxiety remain unclear. The present study used matched verbal (Word Finding) and spatial (Dot Localization) tasks to compare task-related alpha asymmetries in depressed patients grouped according to level of trait anxiety. EEG and behavioral performance were recorded from depressed patients with high anxiety (n=14) or low anxiety (n=14) and 21 age- and education-matched healthy adults during the two tasks, and alpha power was averaged within each task. As predicted, the two patient groups exhibited opposite patterns of regional hemispheric alpha asymmetry. Greater right than left central-parietal activation was seen in the high-anxiety depressed group during the spatial task, whereas greater left than right frontal-central activation was found in the low-anxiety depressed group during the verbal task. Group differences in task performance were in the expected direction but did not reach statistical significance. These results are consistent with Heller’s two-dimensional model of depression and anxiety and highlight the sensitivity of task-related EEG alpha in discriminating among subgroups of depressed patients differing in trait anxiety.
Keywords: Alpha Asymmetry, Anxiety, Depression, Electroencephalography, Verbal/Spatial Processing
INTRODUCTION
Major depressive disorder is among the most common of psychiatric illnesses, with a lifetime prevalence of 13 percent.1 Approximately one third of individuals with depression also have an anxiety disorder.1,2 Individuals with this comorbidity are often more severely depressed3 and less likely to respond to antidepressant treatment.4 Linking particular neurocognitive abnormalities with symptom profiles should lead to an increased understanding of the pathways to clinical impairment and could highlight the importance of comorbidity in treatment planning and research design.
Comparisons of EEG activity within the alpha frequency band over homologous right and left hemisphere regions have been used to study regional brain asymmetry in depression. Alpha is generally presumed to indicate relative deactivation of brain regions over which it is recorded,5 and regional alpha power at rest has been reported to be inversely correlated with cerebral blood flow as measured by PET6 and fMRI.7 There is evidence that depressed patients display a frontal asymmetry characterized by relatively less alpha power (therefore, greater activity) over right than left frontal regions,8,9 but there have also been inconsistent findings.10–12 Posterior alpha in depression has been shown to exhibit an asymmetry opposite to that of frontal alpha, indicative of greater left than right activity. This asymmetry has been found in both currently and previously depressed individuals8,13 and in offspring of two depressed parents.14 Posterior asymmetries were also found to be unchanged by antidepressant treatment.15 However, not all studies have found this pattern of posterior asymmetry.16
Heller’s two-dimensional model proposes that in both anxiety and depression, there is an asymmetry consisting of right greater than left frontal activity, thought to be associated with increased negative affect in both disorders. In contrast, greater right parietotemporal activity is thought to reflect increased arousal, which is characteristic of some anxiety disorders. Less activity in this region reflects decreased arousal, seen in depression as symptoms of fatigue and hypersomnia.17 When listening to sad or fearful stories, individuals high in trait anxiety symptoms had a rightward shift in parietal alpha asymmetry, indicative of greater right parietal activity.18 In a study of patients with post-traumatic stress disorder (PTSD), level of arousal appeared to predict posterior alpha asymmetry more than did severity of depression.19 However, this asymmetry is not necessarily a feature of all anxiety disorders. Whereas an alpha asymmetry indicating greater right activity was found in individuals high in anxious arousal, individuals with anxious apprehension, who are characterized by worry and a tendency to ruminate, did not show an asymmetry.20 It is possible that conflicting research findings for posterior asymmetries in depression may be due to the presence of comorbid anxiety, in which posterior asymmetry may be opposite to that seen in depression alone.13,21
Functional asymmetries have been assessed with tasks involving lateralized presentation of stimuli. Dichotic listening studies using verbal and nonverbal stimuli have supported opposite asymmetries in depression with and without anxiety, with an increased right hemisphere advantage for nonverbal and/or a decreased left hemisphere advantage for verbal stimuli in those with comorbid anxiety and depression relative to those with depression alone.22–24 Opposite hemispheric biases in depression with and without anxiety have also been found in studies employing chimeric faces25,26 and comparing reaction times to right- and left-lateralized stimuli.27
Electrophysiological asymmetries pointing to right posterior hypoactivation, along with behavioral asymmetries on lateralized tasks, suggest that there may also be abnormalities in cognitive functions controlled by right posterior brain regions in some depressed individuals. In others, in particular those with comorbid anxiety, there may be relative hyperactivation of these regions, which might be expected to result in better performance on cognitive tasks mediated by right posterior regions. A study measuring event-related potentials during phonetic and tonal target detection (oddball) tasks in patients with either a depressive disorder, anxiety disorder, or comorbidity of these disorders showed task-dependent hemispheric asymmetries in N2-P3 amplitude. The greater left than right temporoparietal asymmetry for phonetic as opposed to tonal stimuli was largest in patients with depression alone, smallest in patients with anxiety disorders alone, and intermediate for the comorbid patients.28 However, there have been few other electrophysiological studies of functional asymmetries comparing patients having depression with and without anxiety.
One way to test the hypothesis that depression and anxiety exhibit opposite functional asymmetries in posterior regions is to compare performance and EEG recorded during cognitive tasks which selectively engage the left or the right posterior regions. EEG alpha in healthy adults recorded during a set of matched verbal (Word Finding) and nonverbal (Dot Localization) tasks confirmed greater activation over the left and right hemispheres, respectively.29 As would be expected given a right posterior deficit, depressed patients show a selective weakness on the Dot Localization task.30,31 Further, depressed patients failed to demonstrate the task-specific posterior asymmetries found in controls, instead showing relative left-sided activation during both tasks.31 Relative right hemisphere hypoactivation during the spatial task was also associated with higher levels of depression in college students, both before and after partialling out level of anxiety.32 However, a study of men having a depressive disorder using the Word Finding and Dot Localization tasks reported finding left frontotemporal hypoactivation.33 Conflicting findings for depressed patients could arise from the failure to take into account comorbid anxiety disorders, or methodological differences across studies, e.g., in the reference electrode.34
The present study measured EEG and behavioral performance during the Dot Localization and Word Finding tasks in depressed patients having either high or low trait anxiety and in healthy controls. Depressed patients with low levels of anxiety were predicted to show an increase in the normal EEG asymmetry of greater left than right activation during the verbal task and a reduction in the normal asymmetry of greater right than left activation during the spatial task. In contrast, in depressed patients with high levels of anxiety, the right temporoparietal region was expected to show increased activation during both tasks, with an opposite effect of decreasing the normal asymmetry in the verbal task and increasing the normal asymmetry in the spatial task. While it was expected that depressed patients with low anxiety would show a relative performance deficit on Dot Localization consistent with right hemisphere dysfunction, patients with high anxiety were instead expected to show similar performance on the two tasks or better Dot Localization than Word Finding performance due to increased activation in right posterior regions.
METHODS
Participants
Currently depressed patients were recruited from inpatient and outpatient clinics at the New York State Psychiatric Institute. DSM-IV Diagnoses were made by research psychiatrists based on a structured interview. Age-matched healthy comparison participants were recruited from postings around the medical center and from online advertisements. Healthy controls were free of past or present DSM-IV diagnosis. All participants were fluent in English and had learned English prior to age 7. Handedness was determined via the score on the Edinburgh Handedness Inventory,35 and left-handed participants (laterality quotient < 0) were excluded.
Self-ratings were obtained from the Beck Depression Inventory (BDI)36 and the State-Trait Anxiety Inventory-Form Y (STAI-Y).37 The patients were classified as high-anxiety depressed or low-anxiety depressed via the median split procedure based on level of self-rated trait anxiety. Patients with trait anxiety scores = 82 were classified as high-anxiety depressed (range = 82 – 97) and those with scores < 82 were classified as low-anxiety depressed (range = 60 – 80). For descriptive purposes, these groups are referred to as high- and low-anxiety, although patients in the low-anxiety depressed group may still have some level of anxiety. Trait anxiety scores from healthy controls did not overlap with patient scores.
The final sample included 14 participants in the high-anxiety depressed group, 14 participants in the low-anxiety depressed group, and 21 participants in the control group. Of the 28 patients, 22 had a diagnosis of major depressive disorder, 7 had a diagnosis of dysthymia (3 of whom also met criteria for major depressive disorder), and 2 had a diagnosis of bipolar II disorder. Nine of the patients also met criteria for an anxiety disorder (1 social phobia, 4 simple phobia, 1 panic disorder, and 3 obsessive-compulsive disorder). These 9 included 8 of 14 in the high-anxiety depressed group and 1 of 14 in the low-anxiety depressed group. Demographic information for the final sample is reported in Table 1. There were no significant group differences in age, years of education, handedness score, or in the proportion of men to women.
Table 1.
Participant characteristics
| High-anxiety depressed | Low-anxiety depressed | Control | |
|---|---|---|---|
| Gender | |||
| M/F | 8/6 | 4/10 | 9/12 |
| Age (yrs.) | |||
| Mean | 39.9 | 31.4 | 34.0 |
| SD | 11.7 | 12.8 | 11.8 |
| Education (yrs.) | |||
| Mean | 15.1 | 15.9 | 15.8 |
| SD | 2.8 | 1.8 | 3.1 |
| Handedness (Laterality Quotient) | |||
| Mean | 79.6 | 79.1 | 82.5 |
| SD | 18.7 | 29.0 | 26.3 |
| BDIa | |||
| Mean | 31.1 | 22.9 | 1.8 |
| SD | 9.6 | 5.9 | 2.3 |
| STAI-Y Trait Anxietyb | |||
| Mean | 86.7 | 71.7 | 44.1 |
| SD | 4.6 | 7.4 | 7.8 |
Significant difference among groups in BDI score (F (2,46) = 106.25, p < .001); High-anxiety depressed > Low-anxiety depressed > Control.
Significant difference among groups in STAI-Y Trait Anxiety, F (2,46) = 170.13, p < .001; High-anxiety depressed > Low-anxiety depressed > Control.
Self-ratings of depression (BDI) and trait anxiety (STAI-Y) were compared among groups and are summarized in Table 1. As expected, trait anxiety differed significantly between groups (F (2,46) = 170.13, p < .001). Pairwise testing indicated that all groups differed from each other (all p-values < .001), with highest anxiety in the high-anxiety depressed group and lowest anxiety in the control group. A significant group difference in BDI depression scores was also found (F (2,46) = 106.25, p < .001). Pairwise testing revealed that both patient groups were significantly more depressed than controls (p-values < .001). Additionally, the high-anxiety depressed group had significantly higher BDI scores than the low-anxiety depressed group (p = .003). These results are unsurprising in light of evidence that self-report measures of depression and anxiety are highly correlated.38 Overall, depressed men and women did not differ on level of depression (t (26) = .54, p = .59) or anxiety (t (26) = −0.42, p = .68).
Tasks
A pair of psychometrically matched verbal (Word Finding) and spatial (Dot Localization) tasks39–41 were used to examine EEG asymmetries related to verbal and nonverbal task performance in depression. In the Word Finding task, individuals are presented with a written definition and must determine the word being defined (e.g., “A person who works for a skilled worker to learn a trade” Answer: “apprentice”). The Dot Localization task is a measure of visual localization which presents individuals with two open rectangles, one above and slightly to the left of the other. The top rectangle contains two dots, and the bottom rectangle contains a matrix of numbers. Individuals are required to indicate the two numbers in the matrix that would be covered by dots if the rectangle containing dots were placed over the rectangle containing numbers. Number matrices ranged from 13 to 50 numbers.
The Word Finding and Dot Localization tasks were adapted from their original booklet form to be administered on a PC. Thirty-item forms of each task were created, and approximately equal difficulty of the forms was verified by calculating the mean and standard deviation percent correct and response latency for each item based on a separate, preliminary sample of nine healthy adults. The tasks were administered in a fixed order (Word Finding first, Dot Localization second) consistent with the normative procedure and prior studies.39–41 In both tasks, participants pressed a button to indicate they were ready to respond. Responses were given verbally and recorded by an examiner. The Word Finding score was the number of definitions correctly identified. The Dot Localization score was the number of trials on which both numbers were correctly identified.
EEG Recording and Analysis
Continuous EEG was recorded from 12 sites (F3/4; C3/4; P3/4; F7/8; T7/8; P7/8) using a 72-channel Biosemi ActiveTwo system (256 samples/s; DC-128 Hz; 24-bit resolution; active reference composed of common mode sense [PO1] and driven right leg [PO2]). Additional channels included nose (offline reference) and bipolar eye channels (left and right outer canthi; above and below right eye) to monitor lateral eye movements and blinks. Data were converted to the 16-bit continuous data format (CNT) used by NeuroScan and re-referenced to the nose using PolyRex software.42 A high pass filter of 0.3 Hz (−24 dB/octave) was applied. Volume-conducted blink artifacts were removed from the raw EEG using a spatial singular value decomposition filter generated from identified blinks and artifact-free EEG periods.43 One-second EEG epochs (50% overlap) were extracted from the continuous recording during the two task conditions, beginning at the onset of each task stimulus and ending with the participant’s button press, and screened for electrolyte bridges.44 Horizontal eye artifacts were removed from EEG epochs using a linear regression of lateral EEG differences to remove correlated activity (+/− beta weight/2) of each lateral channel.45 To maximize the number of artifact-free epochs, a reference-free approach was used to identify artifactual EEG epochs for any given trial,46 which is based on the electrical distance measure introduced by Tenke and Kayser.44 Using this measure, channels with extreme values outside a median-based range47 were flagged, and these artifactual surface potentials were replaced by spherical spline interpolation48 from artifact-free channels of the complete montage. Removal of artifactual epochs was then verified interactively.
Power spectra were computed by Fast Fourier Transform (1Hz resolution) from artifact-free EEG epochs after data were baseline corrected and cosine-tapered over the entire epoch. Power spectra were averaged across all available epochs for each task at each electrode. Power density was averaged across 8–13 Hz (alpha) and transformed using natural logarithms to normalize the data.
Because different reference schemes have been shown to affect EEG asymmetry,34 for comparative purposes the EEG data were also mathematically re-referenced to the averaged mastoids, and additionally these averages were analyzed.
Statistical Analysis
Gender was not included as a factor in the ANOVA models due to some small cell sizes. However, exploratory analyses including gender did not yield main effects or interactions involving gender for task accuracy or task-related alpha asymmetries.
Performance data
To test the hypothesis that the groups would differ with regard to accuracy on the verbal and spatial tasks, the data were analyzed using repeated-measures ANOVA, with a between-subjects factor of group (high-anxiety depressed, low-anxiety depressed, and control) and a within-subjects factor of task (verbal and spatial).
Task-related EEG data
Log power measures were evaluated using repeated-measures ANOVA. The between-subjects factor was group (high-anxiety depressed, low-anxiety depressed, and control), and the within-subjects factors were task (verbal and spatial), hemisphere (left and right), and region (frontal, central, and parietal). Frontal electrodes were F3, F4, F7, and F8. Central electrodes were C3, C4, T7, and T8. Parietal electrodes were P3, P4, P7, and P8. Within each region, power was averaged across medial and lateral sites after preliminary analyses indicated no significant effects of interest for this variable.
F-ratios were evaluated using degrees of freedom computed with the Greenhouse-Geisser epsilon correction on tests involving more than 1 degree of freedom on within-subjects factors. Significant omnibus F-tests involving factors with more than two levels were followed-up with Bonferroni-adjusted pairwise comparisons (GLM procedure, SPSS Statistics 17.0).
RESULTS
Performance
Overall accuracy on the tasks did not differ significantly by group (F (2,46) <1; ns). Contrary to expectation, there was no significant Group x Task interaction (F (2,46) < 1, ns). Post-hoc analyses were used to determine whether there were more subtle group differences in Word Finding versus Dot Localization task performance. To control for between-subject variability in overall level of performance due to general cognitive factors such as attention and motivation, which can be assumed to influence the tasks equally, task-asymmetry scores were calculated as the accuracy difference between verbal and spatial tasks.49 A positive score, indicating better performance on the spatial than the verbal task, was achieved in 36 percent of high-anxiety depressed participants, 18 percent of low-anxiety depressed participants, and 24 percent of controls. Although these proportions are in the expected direction based on the hypothesis of increased right hemisphere activation in anxiety states, Fisher’s exact test revealed that the groups did not differ significantly (Fisher’s exact test, p = .37). Despite the lack of between-group differences, evaluation of whether scores on verbal and spatial tasks differed within each group was made using two-tailed paired t-tests.30 Better performance on the verbal than the spatial task was seen in the low-anxiety depressed (t (13) = 2.64, p = .02) and control (t (20) = 2.29, p = .03) groups, but the high-anxiety depressed group had essentially equal performance on the two tasks (t (13) = 0.28, p = .79). Percent correct for each task and asymmetry scores for the three groups are shown in Table 2.
Table 2.
Mean (SD) percent correct on Word Finding and Dot Localization tasks
| Word Finding | Dot Localization | Difference | |
|---|---|---|---|
| High-anxiety depressed | 75.0 (17.3) | 72.9 (23.2) | 2.1 (29.0) |
| Low-anxiety depressed | 83.6 (9.1) | 71.2 (16.2) | 12.4 (17.6)a |
| Control | 81.1 (13.4) | 72.9 (20.4) | 8.3 (16.5)a |
Significant difference in accuracy for Word Finding and Dot Localization tasks (p<.05)
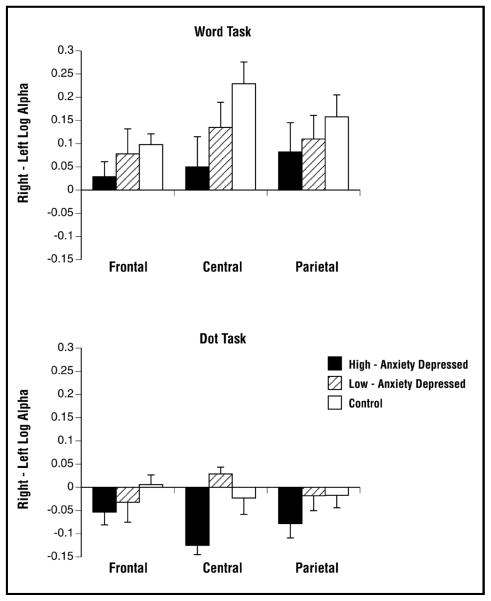
Task-Related Alpha
Alpha asymmetries (right – left log alpha power) during the Word Finding and Dot Localization tasks for each group at frontal, central, and parietal regions are displayed in Figure 1. For the nose-referenced data, the predicted 4-way Group x Task x Hemisphere x Region interaction approached significance (F (4,92) = 2.29, epsilon = .825, p = .08). This interaction reflects group differences in patterns of regional hemispheric asymmetry for each task. Significant hemispheric asymmetries were in the expected direction for the spatial and verbal tasks, but each group showed an asymmetry predominantly in only one or the other of the tasks. Specifically, paired t-tests of hemispheric effects with group, task, and region held constant indicated that the high-anxiety depressed group showed less alpha (greater activation) over right than left central (p = .003) and parietal (p = .03) regions in the spatial task, but had no significant asymmetries in the verbal task. In contrast, the low-anxiety depressed group showed less alpha (greater activation) over the left than right hemisphere in frontal (p = .01) and central (p = .03) regions in the verbal task, while displaying no significant asymmetry in the spatial task. The control group had a pattern similar to that seen in the low-anxiety depressed group, with significantly less alpha (greater activation) over the left than right hemisphere in all regions for the verbal task (frontal p = .002, central p < .001, parietal p < .001) and no significant asymmetry for the spatial task. The 4-way interaction was not significant with a linked-mastoids reference (F (4, 86) = 1.43, p = .24).
Figure 1.
Regional alpha asymmetries recorded during the Word Finding and Dot Localization tasks.
Figure 1 also illustrates the group differences in overall hemispheric asymmetries, which are most prominent in the central region. The low-anxiety depressed patients and controls had a tendency toward greater left hemisphere activation (greater right alpha), whereas high-anxiety depressed patients had a tendency toward greater right hemisphere activation (greater left alpha). In order to further examine this difference in overall hemispheric asymmetry, a combined asymmetry score was computed for each region by averaging alpha asymmetries (right – left log alpha power) across the two tasks. This is similar to a combined index of perceptual asymmetry used in a dichotic listening study of depressed patients with or without a comorbid anxiety disorder.23 Combined asymmetry scores for the three groups are presented in Table 3. The results confirmed that group differences were strongest in the central region, where the groups differed significantly in combined asymmetry (F (2,46) = 3.71, p = .03). The control group differed from the high-anxiety depressed group (p = .04) in showing relatively stronger left hemisphere activation across tasks, whereas the high anxiety-depressed group showed relatively greater right hemisphere activation across tasks. The low-anxiety depressed group did not differ significantly from either the high-anxiety depressed (p = .14) or control (p = 1.0) group. There were no group differences in combined asymmetry scores for either the frontal (F (2,46) = 1.32, p = .28) or parietal regions (F (2,46) < 1, ns).
Table 3.
Mean (SD) combined asymmetry (right – left log alpha power) across tasks
| Frontal | Centrala | Parietal | |
|---|---|---|---|
| High-anxiety depressed | −.012 (.100) | −.038 (.141) | .002 (.153) |
| Low-anxiety depressed | .023 (.161) | .082 (.153) | .046 (.122) |
| Control | .052 (.085) | .101 (.165) | .070 (.149) |
Significant difference among groups in combined asymmetry scores, F(2,46)=3.71, p=.03.
BDI and STAI-Y scores were significantly correlated in the combined sample of depressed patients (Pearson r = .57, p = .002). Correlations between each of these scores and the combined asymmetry score were examined to determine whether depression or trait anxiety were related to hemispheric asymmetry. The association of combined central asymmetry with trait anxiety approached significance (r = −.309, p = .06) in the expected direction, i.e., greater right-hemisphere activation (less alpha) associated with higher trait anxiety, whereas the association of combined central asymmetry with depression was not significant (r = −.10, p = .31). This suggests that trait anxiety was a better predictor of alpha asymmetry during cognitive processing than depression. Nonsignificant correlations with BDI and STAI-Y scores were obtained for the combined frontal asymmetry and combined parietal asymmetry scores and for the individual task asymmetry scores in each of the three regions.
DISCUSSION
Electrophysiological Data
The results were in accordance with predictions made on the basis of the two-dimensional model of depression and anxiety.25 Group differences in task-related regional hemispheric alpha asymmetries were found, with different asymmetry patterns for depressed patients with high and low trait anxiety. Significant task-related hemispheric asymmetries were all in the expected direction, but the groups differed in terms of which task produced an asymmetry. In the high-anxiety depressed group, the Dot Localization (spatial) task was accompanied by greater activation over right than left central and parietal regions, but no hemispheric asymmetries were present during the Word Finding (verbal) task. In contrast, the low-anxiety depressed group had no significant regional asymmetries in the spatial task, but showed greater activation over left than right frontal and central regions during the verbal task.
These results were not found for the linked-mastoids reference, suggesting that the nose reference scheme may be more sensitive in detecting these task-related asymmetries, owing to the topographies of signal and noise across the recording montage (including the reference). Similarly, in an earlier study using these tasks, correlations between depression and alpha asymmetry in the spatial task were greater for a nose than a linked-ears reference.32
It has been previously demonstrated that these tasks asymmetrically activate brain regions in non-depressed adults, with greater right-sided activation during the spatial task and greater left-sided activation during the verbal task in both the central and parietal regions (using both averaged-ears and vertex reference montages)29,31 with no task-related alpha asymmetry present in a depressed sample.31 Results for the healthy participants in the present study suggested a pattern consisting of the expected asymmetry only in the verbal task, with a nonsignificant opposite asymmetry in the spatial task. Upon closer inspection, this lack of asymmetry in the spatial task in healthy adults is consistent with previous findings in which hemispheric differences appeared to be primarily the result of asymmetries in the verbal task.31 Similarly, in the dichotic listening literature, left hemisphere advantages for verbal tasks are typically more robust than right-hemisphere advantages for nonverbal tasks.23,50 Although a recent study reported that subclinical depression was associated with reduced right hemisphere activation during the spatial task,32 undiagnosed depression is unlikely to account for our negative finding because the present sample of healthy adults was screened for psychopathology (also see their low BDI score in Table 1). Finally, right-handed button press and verbal responding may have increased the left-hemisphere demand of both tasks, reducing the right greater than left asymmetry in the spatial task.
The lack of significant task-related asymmetry in a depressed sample in a prior study31 may have been related to failure to distinguish between depressed patients with high and low trait anxiety levels. While partialling out the effects of anxiety from depression in a healthy sample did not affect the task-related alpha asymmetry,32 depressed patients having high anxiety in the current study showed different task-related asymmetries when compared to patients with low anxiety and healthy adults. From the current findings, it may be inferred that depressed individuals with low trait anxiety have a tendency toward greater left-hemisphere activity, which in effect maintained the expected task-related asymmetry in the verbal task but reduced it in the spatial task. In contrast, depressed individuals with high trait anxiety have an opposite tendency, which heightened the right hemisphere advantage on the spatial task and decreased the left hemisphere advantage on the verbal task. These results are in line with previous EEG evidence of greater left than right posterior activity in both currently and previously depressed individuals8,13 and a reduction in the normal right hemisphere superiority for face processing in depressed patients.51 The opposite pattern observed in the high-anxiety depressed patients in the present study is consistent with evidence of greater right that left hemisphere activity measured by dichotic listening studies in patients with comorbid major depressive and anxiety disorders,22–24 opposite patterns of asymmetry in reaction time to lateralized stimuli in depressed versus anxious patients,27 chimeric face perception studies in individuals grouped according to levels of trait anxiety and depression,25,26 and resting alpha asymmetry at posterior sites in patients with comorbid major depressive and anxiety disorders.13,21 While few studies have examined electro-physiological asymmetries associated with cognitive functions, results from this study are also consistent with an ERP study suggesting opposite effects of anxiety and depression in functional asymmetry.28
Performance Data
The prediction of differential task performance between the groups was only partially supported. Low-anxiety depressed patients and controls performed better on the verbal than spatial task, whereas high-anxiety depressed patients performed approximately equally on the two tasks; however, the interaction of group and task did not reach statistical significance, suggesting that the group differences in task performance were not sufficiently robust to yield statistical significance.
Future studies would benefit from the use of more sensitive scales to discriminate between depression and anxiety. Scales used to measure depression and anxiety have been found to be correlated.38 It is likely that the overlap of symptoms measured by the BDI and STAI-Y scales, which was clearly reflected in higher levels of depression, as well as anxiety, in the high-anxiety than the low-anxiety depressed group, resulted in non-optimal classification of depressed patients as high- or low-anxiety. It remains possible that greater depression severity also contributed to greater relative right-sided activation in the high-anxiety depressed group, but this appears unlikely in light of evidence that depression severity is unrelated to posterior asymmetry.19,50 Examination of the effectiveness of EEG measures during verbal and spatial tasks in differentiating patients with anxiety disorders alone from those having either depression alone or comorbidity of anxiety and depressive disorders would further clarify relationships between psychiatric symptoms and cognitive functions, and could potentially aid clinicians in diagnosis. Future research might also explore whether task-related EEG asymmetries are associated with treatment response in depressed patients.50
In conclusion, the presence of electrophysiological and perceptual findings indicative of opposite posterior asymmetries in depressed individuals with low and high anxiety has been fairly well supported. The present investigation extends previous work by revealing these same patterns during the performance of verbal and spatial cognitive tasks. While in their current form, the tasks do not reliably discriminate on the basis of performance between depressed patients with high and low anxiety, the ability of task-related alpha asymmetry to distinguish between these groups indicates the sensitivity of EEG spectral analysis in detecting subtle neurocognitive differences.
Acknowledgments
This research was supported in part by National Institute of Mental Health grant MH36295 (G.E.B.). We are grateful to E.N. Miller for providing us with the paper-and-pencil versions of the cognitive tasks and to Chris Kroppmann, Daniel Alschuler, Shiva Fekri, and Jennifer Schaller for assistance with data collection.
Footnotes
DISCLOSURE AND CONFLICT OF INTEREST
C.B.G. Manna, C.E. Tenke, N.A. Gates, J. Kayser, J.C. Borod, and G.E. Bruder have no conflict of interest in relation to this article. For work unrelated to the present study, Dr. Stewart has been on the speakers bureau of, been a consultant to, or received research support from Eli Lilly, Forest, GlaxoSmithKline, Organon, Shire, Pfizer, BioVail, and Somerset Pharmaceuticals. Dr. McGrath has provided scientific consultation or served on advisory boards for GlaxoSmithKline and Somerset Pharmaceuticals. He has received research grant support from Eli Lilly, GlaxoSmithKline, Lipha, and Organon Pharmaceuticals.
References
- 1.Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- 2.Schatzberg AF, Samson JA, Rothschild AJ, Bond TC, Regier DA. McLean Hospital depression research facility: early-onset phobic disorders and adult-onset major depression. Br J Psychiatry (Supplement) 1998;34:29–34. [PubMed] [Google Scholar]
- 3.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fava M, Uebelacker LA, Alpert JE, Nierenberg AA, Pava JA, Rosenbaum JF. Major depressive subtypes and treatment response. Biol Psychiatry. 1997;42:568–576. doi: 10.1016/S0006-3223(96)00440-4. [DOI] [PubMed] [Google Scholar]
- 5.Shagass C. Electrical activity of the brain. In: Greenfield NS, Sternbach RA, editors. Handbook of Psychophysiology. New York: Rinehart & Winston; 1972. pp. 263–328. [Google Scholar]
- 6.Cook IA, O’Hara R, Uijtdehaage SHJ, Mandelkern M, Leuchter AF. Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalogr Clin Neurophysiology. 1998;107:408–414. doi: 10.1016/s0013-4694(98)00092-3. [DOI] [PubMed] [Google Scholar]
- 7.Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, Krakow K. EEG-correlated fMRI of human alpha activity. Neuroimage. 2003;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- 8.Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. J Abnorm Psychol. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- 9.Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- 10.Debener S, Beauducel A, Nessler D, Brocke B, Heilemann H, Kayser J. Is resting anterior EEG alpha asymmetry a trait marker for depression? Neuropsychobiology. 2000;41:31–37. doi: 10.1159/000026630. [DOI] [PubMed] [Google Scholar]
- 11.Harmon-Jones E, Abramson LY, Sigelman J, Bohlig A, Hogan ME, Harmon-Jones C. Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. J Pers Soc Psychology. 2002;82:610–618. [PubMed] [Google Scholar]
- 12.Reid SA, Duke LM, Allen JJ. Resting frontal electroencephalographic asymmetry in depression: inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389–404. [PubMed] [Google Scholar]
- 13.Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, et al. Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biol Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- 14.Bruder GE, Tenke CE, Warner V, Nomura Y, Grillon C, Hille J, et al. Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biol Psychiatry. 2005;57:328–335. doi: 10.1016/j.biopsych.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Bruder GE, Sedoruk JP, Stewart JW, McGrath PJ, Quitkin FM, Tenke CE. Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: pre- and post-treatment findings. Biol Psychiatry. 2007;63:1171–1177. doi: 10.1016/j.biopsych.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaffer CE, Davidson RJ, Saron C. Frontal and parietal electroencephalogram asymmetry in depressed and nondepressed subjects. Biol Psychiatry. 1983;18:753–762. [PubMed] [Google Scholar]
- 17.Heller W, Nitschke JB. The puzzle of regional brain activity in depression and anxiety: the importance of subtypes and comorbidity. Cogn Emot. 1998;12:421–447. [Google Scholar]
- 18.Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. J Abnormal Psychol. 1997;106:376–385. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- 19.Metzger LJ, Paige SR, Carson MA, Lasko NB, Paulus LA, Pitman RK, Orr SP. PTSD arousal and depression symptoms associated with increased right-sided parietal EEG asymmetry. J Abnormal Psychol. 2004;113:324–329. doi: 10.1037/0021-843X.113.2.324. [DOI] [PubMed] [Google Scholar]
- 20.Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–637. [PubMed] [Google Scholar]
- 21.Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, Bruder GE. Electroencephalographic asymmetries in adolescents with major depression: influence of comorbidity with anxiety disorders. J Abnormal Psychol. 2000;109:797–802. doi: 10.1037//0021-843x.109.4.797. [DOI] [PubMed] [Google Scholar]
- 22.Bruder GE, Schneier FR, Stewart JW, McGrath PJ, Quitkin F. Left hemisphere dysfunction during verbal dichotic listening tests in patients who have social phobia with or without comorbid depressive disorder. Am J Psychiatry. 2004;161:72–78. doi: 10.1176/appi.ajp.161.1.72. [DOI] [PubMed] [Google Scholar]
- 23.Bruder GE, Wexler BE, Stewart JW, Price LH, Quitkin FM. Perceptual asymmetry differences between major depression with or without a comorbid anxiety disorder: a dichotic listening study. J Abnorm Psychol. 1999;108:233–239. doi: 10.1037//0021-843x.108.2.233. [DOI] [PubMed] [Google Scholar]
- 24.Pine DS, Kentgen LM, Bruder GE, Leite P, Bearman K, Ma Y, Klein RG. Cerebral laterality in adolescent major depression. Psychiatry Res. 2000;93:135–144. doi: 10.1016/s0165-1781(00)00101-3. [DOI] [PubMed] [Google Scholar]
- 25.Heller W, Etienne MA, Miller GA. Patterns of perceptual asymmetry in depression and anxiety: implications for neuropsychological models of emotion and psychopathology. J Abnorm Psychol. 1995;104:327–333. doi: 10.1037//0021-843x.104.2.327. [DOI] [PubMed] [Google Scholar]
- 26.Keller J, Nitschke JB, Bhargava T, Deldin PJ, Gergen JA, Miller GA, Heller W. Neuropsychological differentiation of depression and anxiety. J Abnormal Psychol. 2000;109:3–10. [PubMed] [Google Scholar]
- 27.Liotti M, Sava D, Rizzolatti G, Caffarra P. Differential hemispheric asymmetries in depression and anxiety: a reaction-time study. Biol Psychiatry. 1991;29:887–899. doi: 10.1016/0006-3223(91)90055-q. [DOI] [PubMed] [Google Scholar]
- 28.Bruder GE, Kayser J, Tenke CE, Leite P, Schneier FR, Stewart JW, Quitkin FM. Cognitive ERP’s in depressive and anxiety disorders during tonal and phonetic oddball tasks. Clin Electroencephalogr. 2002;33:119–124. doi: 10.1177/155005940203300308. [DOI] [PubMed] [Google Scholar]
- 29.Davidson RJ, Chapman JP, Chapman LJ, Henriques JB. Asymmetrical brain electrical activity discriminates between psychometrically-matched verbal and spatial cognitive tasks. Psychophysiology. 1990;27:528–543. doi: 10.1111/j.1469-8986.1990.tb01970.x. [DOI] [PubMed] [Google Scholar]
- 30.Miller EN, Fujioka TA, Chapman LJ, Chapman JP. Hemispheric asymmetries of function in patients with major affective disorders. J Psychiatry Res. 1995;29:173–183. doi: 10.1016/0022-3956(95)00011-s. [DOI] [PubMed] [Google Scholar]
- 31.Henriques JB, Davidson RJ. Brain electrical asymmetries during cognitive task performance in depressed and nondepressed subjects. Biol Psychiatry. 1997;42:1039–1050. doi: 10.1016/s0006-3223(97)00156-x. [DOI] [PubMed] [Google Scholar]
- 32.Rabe S, Debener S, Brocke B, Beauducel A. Depression and its relation to posterior cortical activity during performance of neuropsychological verbal and spatial tasks. Pers Individ Dif. 39:601–611. [Google Scholar]
- 33.Flor-Henry P, Lind JC, Koles ZJ. A source-imaging (low-resolution electromagnetic tomography) study of the EEGs from unmedicated males with depression. Psychiatry Res. 2004;130:191–207. doi: 10.1016/j.pscychresns.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Tenke CE, Kayser J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA) Clin Neurophysiol. 2005;116:2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 37.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual of the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 38.Tanaka-Matsumi J, Kameoka VA. Reliabilities and concurrent validities of popular self-report measures of depression, anxiety, and social desirability. J Consult Clin Psychol. 1986;54:328–333. doi: 10.1037//0022-006x.54.3.328. [DOI] [PubMed] [Google Scholar]
- 39.Fujioka TAT. Unpublished doctoral dissertation. University of Wisconsin; Madison: 1986. Left-hemisphere deficit in schizophrenia: a test of the hypothesis using psychometrically matched tasks. [Google Scholar]
- 40.Miller EN. Unpublished doctoral dissertation. Department of Psychology, University of Wisconsin; Madison: 1986. Hemispheric asymmetries of function in patients with major affective disorders. [Google Scholar]
- 41.Miller EN, Fujioka TA, Chapman LJ, Chapman JP. Psychometrically matched tasks for assessment of hemispheric asymmetries of function. Brain Cog. 1995;28:1–13. doi: 10.1006/brcg.1995.1030. [DOI] [PubMed] [Google Scholar]
- 42.Kayser J. Polygraphic recording data exchange – PolyRex Version 1.2. New York State Psychiatric Institute: Division of Cognitive Neuroscience; 2006. ( http://psychophysiology.cpmc.columbia.edu/software/PolyRex/) [Google Scholar]
- 43.NeuroScan, Inc. EDIT 4.3 – Offline analysis of acquired data (Document number 2203, Revision D) II. El Paso, TX: Compumedics Neuroscan; 2003. SCAN 4.3. [Google Scholar]
- 44.Tenke CE, Kayser J. A convenient method for detecting electrolyte bridges in multichannel electroencephalogram and event-related potential recordings. Clin Neurophysiol. 2001;112:545–550. doi: 10.1016/s1388-2457(00)00553-8. [DOI] [PubMed] [Google Scholar]
- 45.Kayser J, Tenke CE, Gates NA, Kroppmann CJ, Gil RB, Bruder GE. ERP/CSD indices of impaired verbal working memory subprocesses in schizophrenia. Psychophysiology. 2006;43:237–252. doi: 10.1111/j.1469-8986.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 46.Kayser J, Tenke CE. Electrical distance as a reference-free measure for identifying artifacts in multichannel electroencephalogram (EEG) recordings. The 46th annual meeting of the Society for Psychophysiological Research (SPR); Vancouver, BC. October 25–29, 2006. [Google Scholar]
- 47.Junghofer M, Elbert T, Tucker DM, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37:523–532. [PubMed] [Google Scholar]
- 48.Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [Corrigenda EEG 02274. Electroencephalogr Clin Neurophysiol 1990; 76, 565] [DOI] [PubMed] [Google Scholar]
- 49.Deptula D, Manevitz A, Yozawitz A. Asymmetry of recall in depression. J Clin Exp Neuropsychol. 1991;13:854–870. doi: 10.1080/01688639108405103. [DOI] [PubMed] [Google Scholar]
- 50.Bruder GE, Stewart JW, McGrath PJ, Deliyannides D, Quitkin FM. Dichotic listening tests of functional brain asymmetry predict response to fluoxetine in depressed women and men. Neuropsychopharmacology. 2004;29:1752–1761. doi: 10.1038/sj.npp.1300519. [DOI] [PubMed] [Google Scholar]
- 51.Jaeger J, Borod JC, Peselow E. Depressed patients have atypical hemispace biases in the perception of emotional chimeric faces. J Abnorm Psychol. 1987;6:321–324. doi: 10.1037//0021-843x.96.4.321. [DOI] [PubMed] [Google Scholar]