Abstract
Greater left than right reductions of P3 amplitude in schizophrenia during auditory oddball tasks have been interpreted as evidence of left-lateralized dysfunction. However, the contributions of methodological factors (response mode, stimulus properties, recording reference), which affect event-related potential (ERP) topographies, remain unclear. We recorded 31-channel ERPs from 23 schizophrenic patients and 23 age- and gender-matched healthy controls (all right-handed) during tonal and phonetic oddball tasks, varying response mode (left press, right press, silent count) within subjects. Performance accuracy was high in both groups but patients were slower. ERP generator patterns were summarized by temporal Principal Components Analysis (PCA; unrestricted Varimax) from reference-free current source density (CSD; spherical spline Laplacians) waveforms, which sharpen scalp topographies. CSD represents the magnitude of the radial current flow entering (source) and leaving (sink) the scalp. Both patients and controls showed asymmetric frontolateral and parietotemporal N2 sinks peaking at 240 ms and asymmetric parietal P3 sources (355 ms) for targets (tonal R > L, phonetic L > R), but frontocentral N2 sinks and parietal P3 sources were bilaterally reduced in patients. A response-related midfrontal sink and accompanying centroparietal source (560 ms) were highly comparable across groups. However, a superimposed left temporal source was larger for silent count compared to button press, and this difference was smaller in patients. In both groups, left or right press produced opposite, region-specific asymmetries originating from central sites, modulating the N2/P3 complex. The results suggest bilaterally reduced neural generators of N2 and P3 in schizophrenia during auditory oddball tasks, but both groups showed comparable topographic effects of task and response mode. However, additional working memory demands during silent count may partially overlap in time the generation of the N2/P3 complex and differentially affect the asymmetry of P3 subcomponents, particularly when employing conventional ERP measures.
Keywords: Button Press, Current Source Density, Event-Related Potentials, N2/P3 Complex, P300 Asymmetry, Principal Components Analysis, Schizophrenia, Silent Count, Tonal/Phonetic Oddball Tasks
INTRODUCTION
The amplitudes of auditory event-related potentials (ERPs) during simple tone discrimination or “oddball” tasks have been found to be reduced in patients having schizophrenia (e.g.,1–11). This includes early ERPs, such as N1, that are related to sensory or attentional processing, and also the later cognitive N2 and P3 potentials. It has been hypothesized that the reductions of N2 and P3 are related to pathophysiology of temporal lobe structures involved in the generation or modulation of these auditory ERPs.3,12 Additional support for this hypothesis has come from findings of greater reduction in N2 or P3 amplitude to tones in schizophrenia over left than right temporal lobe sites,3,12–16 and from evidence that this is related to asymmetric abnormalities of temporal lobe structures.17–19 Some studies have, however, found bilateral, rather than asymmetric, P3 reduction in schizophrenia20,21 and neuroimaging data showing comparable volume reductions of both left and right temporal lobes.1,22 Further complicating the interpretation of these conflicting findings is evidence showing that reduced P3 may be associated with reversed asymmetries in schizophrenia when handedness is considered, with right-handed patients showing greater reductions of the left temporal regions, and left-handed patients show the opposite pattern.23 In a meta-analysis of P300 asymmetry in schizophrenia, Jeon and Polich24 found no consistent asymmetrical P3 reductions; however, effect sizes for group comparisons were nevertheless larger over left than right temporal sites. Although clinical or neuroanatomical differences between patients may account for the difference in P3 findings across these studies, another important contributor could be the different response modes used in the oddball tasks.13 Most studies in which ERPs were recorded during silent counting of oddball tones found greater reduction of P3 amplitude over left (T7) than right (T8) temporal lobe sites in schizophrenic patients (cf. Pivik et al.25 for modifications to the 10–20 system electrode nomenclature), whereas only a few studies using a button press response found this P3 asymmetry.
The difference in findings for silent-counting and button-press responses could stem from movement-related potentials (MRPs) in the latter task. In oddball tasks where a right button press was the predominant mode of response (e.g.,20,21), lateralized MRPs, which are negative in polarity and larger over frontocentral sites contralateral to the hand movement, might have interfered with demonstration of the P3 asymmetry difference between schizophrenic patients and controls. However, studies comparing P3 findings for silent-counting and button-pressing responses have yielded conflicting findings.26,27 Ford et al.26 measured ERPs of 17 men with schizophrenia and 11 healthy controls in three tasks in which subjects counted oddball tones or pressed a button with either the right or left hand. While button presses significantly affected P3 amplitude and asymmetry over left and right motor cortices (C3/4), schizophrenic patients did not show smaller P3 over left than right temporal lobe sites (T7/8) in either silent-counting or button-pressing tasks. In contrast, Salisbury et al.27 found that overall P3 amplitude was smaller and P3 asymmetry was reduced when healthy controls (n = 46) responded with the right finger as compared to silent counting of oddball tones. Using a reaction-time procedure to correct for contamination by MRPs restored the P3 asymmetry in the button-pressing task to the normal level, and the MRP-corrected data for 36 healthy men in this study differed significantly in P3 asymmetry when compared to schizophrenic patients in their prior study. Moreover, while a frontal P3 asymmetry was observed for right button presses to targets (Go-P3) in a follow-up study,28 this asymmetry was absent in healthy adults (n = 34) when response requirements were reversed (i.e., button press to nontargets; NoGo-P3). The authors also observed a frontally reduced P3 amplitude in the Go condition when compared to NoGo and silent-counting conditions, resulting in an anterior shift of the P3 topography (i.e., NoGo-anteriorization), which can be interpreted as a superposition of an anterior negativity associated with a motor response. However, no comparison was made of ERPs for right versus left hand responses in healthy adults, or for silent-counting versus button-pressing responses in schizophrenic patients.
It is also possible that the added cognitive demands of the silent-counting oddball task may enhance any P3 asymmetry differences between schizophrenic patients and controls. Although the silent counting task has the advantage of avoiding the problem of motor potentials, it adds an additional working memory load because the subject must keep a running count of the number of oddballs. Neuroimaging studies suggest that maintenance of verbal information in working memory would be expected to increase activation in left frontal, temporal and parietal regions in healthy adults, but not in schizophrenic patients.29,30 In this regard, Salisbury et al.27 reported that their healthy adults had significant left-larger-than-right P3 asymmetry over lateral temporal sites in the silent-counting task, but not in the button-pressing task. Healthy adults tested in tasks with button-presses to oddball tones have actually shown greater P3 amplitude over right than left frontocentral sites,31–33 which is opposite the left-lateralized P3 seen in healthy adults by Salisbury et al.27 for the silent-counting task. Tenke et al.34 found evidence that the right-lateralized P3 in the button-pressing task was related to two different processes. First, it is due in part to response-related asymmetries associated with right-hand button presses. Second, it is related to the process of pitch discrimination, presumably reflecting the superiority of right hemisphere regions for pitch perception. The presence of a right-lateralized P3 to standard and novel stimuli for which no motor response is given also indicates that this P3 asymmetry is not due solely to overlapping MRPs.31,32 Its relation to right hemispheric dominance for processing tonal stimuli is supported by its association with left ear (right hemisphere) advantage for dichotic pitch discrimination.34
Studies measuring ERPs during phonetic and tonal oddball tasks have provided direct evidence of the contribution of lateralized neurocognitive processes to N2 and P3 asymmetries.10,33,35,36 Both healthy adults and schizophrenic patients showed task-related asymmetries of N2 and P3 in the expected direction, with larger amplitudes over right hemisphere sites for the tonal task, but over left hemisphere sites for the phonetic task. If abnormal N2 and P3 asymmetries in schizophrenia reflect a left-sided deficit in temporal lobe regions,17,18 one might expect such abnormalities to be particularly evident in the phonetic oddball task. Contrary to expectations, Kayser et al.10 did not find evidence of a specific left-sided reduction of N2 or P3 in schizophrenic patients in either the tonal or phonetic task. However, it should be noted that a silent-counting condition was not included in this study and the schizophrenic patients did not show an overall reduction of P3 amplitude, which may have contributed to the absence of a difference in P3 asymmetry between patients and controls.37 Although task-related asymmetries of N2 and P3 were modulated by response hand, evaluation of response hand effects was limited because right versus left button press was only compared across subjects.
Another important methodological difference between these studies is the choice of EEG recording reference. For instance, whereas Salisbury et al.14,27,28 employed a commonly-used linked-earlobes reference, Ford et al.20,26 recorded EEG with sternovertebral reference, and our lab10,33,34 and others8,11 have used nose-referenced recordings. The dependency of surface potentials on a recording reference (e.g., linked-mastoids, nose, average),38–40 and the way by which ERP components are quantified,41–44 crucially affect the identification and statistical analysis of N2 and P3. While the relative ERP topography is not affected by the reference choice (cf. discussion in Kayser et al.10), different references can substantially affect ERP amplitudes, peak latencies and their local maxima, depending on the direction of the underlying neuronal generator, which, of course, may differ between groups. For example, relatively preserved P3 amplitudes in schizophrenia may be a direct consequence of using a nose reference; at the same time, this recording reference is likely to amplify differences in N2 amplitude between schizophrenic patients and healthy controls.8,10,11,45 The critical point is that the choice of the recording reference may affect what aspect (e.g., time interval, scalp location) of the ERP signal is ultimately analyzed.
The importance of using reference-independent descriptors of topographic P3 abnormalities in schizophrenia has been described by Strik et al.4,5 who also reported left-lateralized P3 reductions in patients using a silent count oddball paradigm with pure tones. We have proposed a two-step, generic analytic strategy to overcome these limitations.48,49 First, reference-dependent surface potentials are transformed into reference-free current source density (CSD; surface Laplacian) waveforms, which are an estimate of the local current flow normal to the scalp.39,40 While this transform not only eliminates redundant, volume-conducted contributions, yielding sharper topographies than ERPs,34,50 another core advantage is that, for a given EEG montage, any EEG reference will render the same, unique CSD waveforms, which completely removes the interpretational ambiguities stemming from the choice of recording reference. Second, to further disentangle temporally and spatially overlapping components, unique and orthogonal variance patterns are identified in these reference-free data by unrestricted Varimax-PCA using the covariance matrix,42,43,48,51 yielding neuronal generator patterns that may be directly related to known neuroanatomical circuits responsible for cognitive processing.52,53,55,56
The present study was conducted to take full advantage of the combined CSD-PCA technique for distinguishing and quantifying the separate contributions of cognitive and response-related factors to ERP asymmetries in schizophrenic patients and healthy controls during auditory target detection tasks. The study design provides a direct “within-subjects” comparison of N2/P3 amplitude and asymmetry during left button press, right button press, and silent count target detection tasks, and provides information on whether response mode effects modulate patient-control differences in N2 and P3 asymmetry. Moreover, the use of both phonetic and tonal oddball tasks is of particular value for further testing hypotheses of left temporal lobe dysfunction in schizophrenia (e.g.,17,46,47).
Our initial reports using this design in healthy adults focused on the methodological comparison of PCA solutions derived from ERP or CSD waveforms (n = 66),48 and the suitability of using these methods for both high- and low-density EEG montages (n = 17).49 The task- and response-related findings of these prior reports provide the groundwork for the current study. Three CSD components represented the target-related N2/P3 complex: 1) an N2 sink, which showed task-dependent topographic maxima with opposite hemispheric asymmetries (tones: frontotemporal R > L; syllables: parietotemporal L > R); 2) a mid-parietal P3 source, which also showed task-dependent, asymmetric enhancements; and 3) a mid-frontal sink accompanied by centroparietal sources occurring around the time of the subjects’ response to targets. The CSD component structure therefore clearly separated a classical P3b positivity from an overlapping anterior negativity.27 While response mode modulated the earlier task-dependent effects, or substantially altered their overall amplitude, an intriguing finding was a selective enhancement of source activity over left lateral temporal sites only for silent count (cf. Figure 8 in Kayser et al.48). This effect was associated with the mid-frontal sink/centroparietal source factor, and was therefore observed for a time period when participants updated their memory in the silent count condition. If this effect is indeed due to the added cognitive demand when silently counting targets, it would be of particular interest to investigate whether it may be reduced in patients and may account for asymmetric P3 reductions in schizophrenia, which have been more frequently reported for silent-counting oddball tasks.
MATERIALS AND METHODS
Participants
Thirty-three inpatients (22 male, 11 female) at New York State Psychiatric Institute were recruited for the study, excluding left-handed individuals and those with a history of neurological illness or substance abuse. Data from 10 patients (7 male, 3 female) were excluded from the study due to an insufficient number of correct, artifact-free trials (at least 14 for any target condition) or a low signal-to-noise ratio, which prevented a recognizable ERP component structure in the individual waveforms. The 23 patients included in the final patient sample met DSM-IV58 criteria for schizophrenia (paranoid, n = 11; undifferentiated, n = 6; disorganized, n = 1) or schizoaffective disorder (depressed type, n = 3; bipolar type, n = 2). Diagnoses were based on clinical interviews by psychiatrists and a semistructured interview59 including items from commonly-used instruments (e.g., SCID-P; SANS, SAPS).60–62 Symptom ratings were obtained using the Positive and Negative Syndrome Scale (PANSS).63 The total brief psychiatric rating scale (BPRS) score indicated that patients were mildly-to-moderately disturbed (Table 1). Most patients (n = 13) did not receive antipsychotic medications for at least 14 days before testing. Ten patients were treated with olanzapine (n = 5), clozapine (n = 2), risperidone (n = 1), haloperidol (n = 1), or fluphenazine (n = 1), with chlorpromazine equivalents ranging from 150 to 1506 mg/day.65
Table 1.
Means, standard deviations (SD), and ranges for demographic and clinical variables
| Variable | Patients (n = 23, 15 male) | Healthy Controls (n = 23, 15 male) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age (years) | 31.1 | 10.4 | 18–53 | 30.5 | 8.2 | 21–51 |
| Handedness (LQ)a | 70.7 | 34.7 | 0–100 | 70.3 | 23.0 | 5–100 |
| Education (years)b | 13.6 | 2.3 | 10–18 | 15.2 | 1.5 | 12–18 |
| Onset age (years) | 22.8c | 7.7 | 8–43 | |||
| Illness duration (years) | 9.3c | 10.5 | 0–33 | |||
| Total BPRS | 39.4c | 9.6 | 24–64 | |||
| PANSS general | 33.9c | 8.9 | 17–58 | |||
| PANSS positive | 18.2c | 5.7 | 10–29 | |||
| PANSS negative | 14.4c | 4.7 | 7–23 | |||
BPRS: brief psychiatric rating scale; PANSS: positive and negative syndrome scale
Laterality quotient64 can vary between −100.0 (completely left-handed) and +100.0 (completely right-handed).
The available data for 20 patients (13 male) and 21 healthy controls (14 male) yielded a marginally significant group main effect, F[1,40] = 3.48, p = .07.
n = 21.
Patients were compared to 23 healthy volunteers (15 male) selected from a larger sample (N = 66) included in our previous report.48 Control participants, who had been recruited from the New York metropolitan area and paid US$15/hr, were without a history of neurological illness or substance abuse and without current or past psychopathology based on a standard screening interview (SCID-NP).66 Importantly, patient and control participants had been tested under the same protocol and during the same time period. Without knowledge about their behavioral performance or ERP data, healthy adults were carefully matched to individual patients with regard to gender, age, and handedness (Table 1). While patients tended to have less education than control participants, a typical characteristic of schizophrenia samples, the absolute difference was rather small (i.e., about 1.5 years).
Hearing acuity was assessed using standard audiometric procedures, which required all participants to have a difference of less than 10 dB between ears at threshold and a hearing loss no greater than 25 dB at 500, 1,000, or 2,000 Hz. The ethnic composition in both groups was representative for the New York region, including several racial categories in each group (patients vs. controls: White 9/15, Black 6/3, Hispanic 4/3, Asian 2/1, more than one race or other, 2/1). The experimental protocol had been approved by the institutional review board and was undertaken with the understanding and written consent of each participant.
Stimuli and procedure
The study protocol was a modification of our earlier auditory target detection (oddball) paradigms using tonal and phonetic stimuli,10,33 as already described in detail.48 Briefly, stimuli were either two complex tones, consisting of 250-ms square waves (25 ms rise and decay time) with fundamental frequencies of 444 and 485 Hz corresponding to the major notes A4 and B4, or two consonant-vowel syllables (/da/, /ta/), spoken by a male voice and approximately matched to the complex tones by discriminability, duration, and root mean squared amplitude (cf. Figure 1 in Kayser and Tenke48). These tonal (T) and phonetic (P) stimuli have been shown to produce opposite perceptual asymmetries in dichotic listening studies (e.g.,67,68), and therefore provide a probe of cognitive processes predominantly performed by the right or left hemisphere. All stimuli were presented binaurally via a matched pair of TDH-49 earphones at a comfortable hearing level (72 dB SPL), with earphone orientation counterbalanced across participants.
During twelve 80-trial blocks (960 trials total), participants listened to a series of either tones or syllables (6 blocks each) consisting of 20% target and 80% nontarget stimuli (fixed 2,000 ms SOA). The assignment of target and nontarget stimulus was systematically alternated in two consecutive blocks (A4 vs. B4, /da/ vs. /ta/). Participants were instructed to respond to infrequent target stimuli as quickly and accurately as possible using one of three response modes: 1) a right-hand button press (R); 2) a left-hand button press (L); or 3) by silently counting (S) the targets (participants reported their target count at the end of the block). These response modes were systematically crossed with task type (T, P), and assignment order was counterbalanced across participants (e.g., PL-TL-PR-TR-PS-TS or TL-PL-TS-PS-TR-PR). To reduce ocular artifacts, participants were instructed to fixate a cross on a monitor while listening to the stimuli.
Data acquisition, recording, and artifact procedures
Nose-referenced scalp EEG (AFz ground) was continuously recorded at 200 samples/s from 30 extended 10–20-system locations (4 midline and 13 lateral pairs of tin electrodes embedded in a Lycra stretch cap) within .1–30 Hz (−6dB/octave), along with bipolar recordings of vertical and horizontal eye movements (for complete montage and recording details, see 48). Volume-conducted blink artifacts were effectively removed from the raw EEG by means of spatial PCA generated from identified blinks and artifact-free EEG periods.69
Recording epochs of 2,000 ms (including a 200 ms pre-stimulus baseline) were extracted off-line from the blink-corrected continuous data, tagged for A/D saturation, and low-pass filtered at 20 Hz (−24 dB/octave). To maximize the number of artifact-free epochs, volume-conducted horizontal eye movements were reduced by computing the linear regressions between the horizontal EOG and the EEG differences of homologous lateral recording sites (i.e., Fp2 - Fp1, F8 - F7, etc.) for each epoch, and the correlated eye activity was then removed by applying ±beta weight/2 to each lateral EEG signal (cf. 52). Residual artifacts due to amplifier drift, muscle or movement-related activity, or residual eye activity were identified on a channel-by-channel and trial-by-trial basis by employing a reference-free electrical distance measure.70 Artifactual surface potentials were replaced by spherical spline interpolation50 using the data from artifact-free channels if eight or less channels were affected; otherwise, a trial was rejected. Artifact detection and electrode replacement was verified by visual inspection.
Stimulus-locked ERP waveforms were averaged from artifact-free trials using the entire 2-s epoch (correct responses only for button press). The mean number of trials used to compute target ERP averages across response mode (M ± SD, minimum in any condition, controls vs. patients) were 29.2 ± 2.2 (14) vs. 29.3 ± 1.8 (21) for the tonal task, and 29.7 ± 1.8 (22) vs. 29.0 ± 2.0 (19) for the phonetic task. There were no significant differences between patients and controls, response mode, or task, as virtually identical mean number of trials were observed for each response mode and task in each group. Furthermore, a satisfactory signal-to-noise ratio for each condition was confirmed by visual inspections of the individual ERP waveforms of each participant. ERP waveforms were screened for electrolyte bridges,71 low-pass filtered at 12.5 Hz (−24 dB/octave), and baseline-corrected using the 100 ms preceding stimulus onset.
Current Source Density (CSD) and Principal Components Analysis (PCA)
Averaged ERP waveforms were transformed into current source density (CSD) estimates (μV/cm2 units) using a spherical spline surface Laplacian50 as detailed elsewhere (e.g.,48,53; for documented Matlab code, see 54). To determine common sources of variance in these reference-free transformations of the original ERP data, CSD waveforms were submitted to temporal principal components analysis (PCA) derived from the covariance matrix, followed by unrestricted Varimax rotation of the covariance loadings. However, only a limited number of meaningful, high-variance CSD factors were retained for further statistical analysis (for complete rationale, see 42,43,48,51). By virtue of the reference-independent Laplacian transform, CSD factors have an unambiguous component polarity and topography.
Stimulus-locked CSD waveforms (220 sample points spanning the time interval from −100 to 995 ms around stimulus-onset) were submitted to temporal PCA (MatLab emulation of BMDP-4M algorithms),42 with an input data matrix consisting of 220 variables and 17,112 observations stemming from 46 participants, 12 conditions (target/nontarget stimuli, tonal/phonetic task, left press/right press/silent count response mode) and 31 electrode sites, including the nose.
Statistical analysis
For PCA factors of interest (i.e., those covering variance associated with target detection ERP effects), factor scores for target stimuli were submitted to repeated measures ANOVA with task (tonal, phonetic) and response mode (left press, right press, silent count) as a within-subjects factors, and group (controls, patients) as a between-subjects factor. Guided by our previous findings,10,33,48 repeated measures ANOVA were conducted for subsets of recording sites at which PCA factor scores were largest and most representative of the associated CSD components. These subsets consisted of either midline or lateral, homologous recording sites over both hemispheres, thus adding either site, or site and hemisphere as within-subjects factors to the design. The rationale for selecting a “subset” of recording sites as a “representative” measure for a factor is given below with the factor descriptions. For this reason, and to avoid needless complexity, site effects are only reported if they are of critical relevance to the study objectives.
For the behavioral data, only button press responses were analyzed. Response latency (mean response time of correct responses) and percentages of correct responses were submitted to repeated measures ANOVA with task (tonal, phonetic) and response hand (left, right) as within-subjects factors, and group (controls, patients) as the between-subjects factor.
To increase statistical power, gender was not included as a design factor given that the small number of women resulted in uneven cell sizes, and also because the investigation of sex differences was not the primary objective of the study; importantly, however, the gender ratio (15 male, 8 female) was equal for patients and controls.
Greenhouse-Geisser epsilon (ε) correction was used to compensate for violations of sphericity when appropriate.72 Simple effects and contrasts (BMDP-4V)73 provided means to systematically examine interaction sources, or to further explore group effects even in the absence of superordinate interactions. A conventional significance level (p < .05) was applied for all effects.
RESULTS
Behavioral data
The mean response latency for correct button press responses was about 115 ms slower in patients compared to controls for both tones (M ±SD, controls vs. patients; 422.8 ±116.5 vs. 541.6 ±123.8 ms) and syllables (448.7 ±117.6 vs. 557.6 ±138.1 ms; group main effect, F[1,44] = 11.1, p = .002), and across groups about 20 ms faster for tones than syllables (task main effect, F[1,44] = 5.55, p = .02), which is in accordance with our previous findings.10,33,48 Performance accuracy was high for both groups, and likely subject to ceiling effects, although the mean hit rate was marginally lower for patients compared to controls (95.9 ±7.1 vs. 98.2 ±5.2%; group main effect, F[1,44] = 2.97 p = .09). However, there was no task difference in performance accuracy (tonal vs. phonetic, 97.1 ±6.8 vs. 97.0 ±6.0%), nor were there any interactions with group. There were also no significant behavioral effects involving response hand.
Electrophysiologic data
Grand mean CSD waveforms
Figures 1 and 2 show the grand mean, reference-free CSD waveforms of patients and controls at all 31 recording sites for tonal and phonetic targets (pooled across response mode) and nontargets (pooled across task and response mode), and for targets for each response mode (pooled across task). The original, nose-referenced ERP waveforms were highly comparable to those of our prior studies10,33,48 (corresponding ERP figures and animated topographies of ERP and CSD waveforms can be obtained at URL http://psychophysiology.cpmc.columbia.edu/SczOddball2010.html).
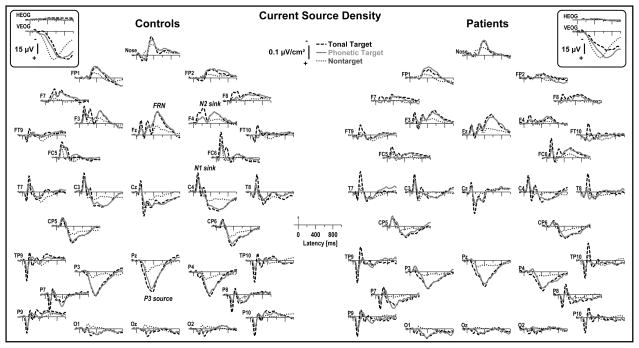
Figure 1.
Grand mean reference-free current source density (CSD) [μV/cm2] waveforms (−100 to 995 ms) for 23 healthy adults and 23 schizophrenic patients comparing tonal and phonetic target (averaged across response mode) and nontarget stimuli (averaged across task and response mode) at all 31 recording sites. Horizontal and vertical electrooculograms (EOG) [μV] are shown before artifact correction (insets). Distinct CSD components are labeled for controls (italics) and included frontocentral N1 and N2 sinks (approximate peak latencies were 105 ms at C4 and 230 ms at F4), a mid-parietal P3 source (375 ms at Pz), and a midfrontal sink (FRN; 480 ms at Fz).
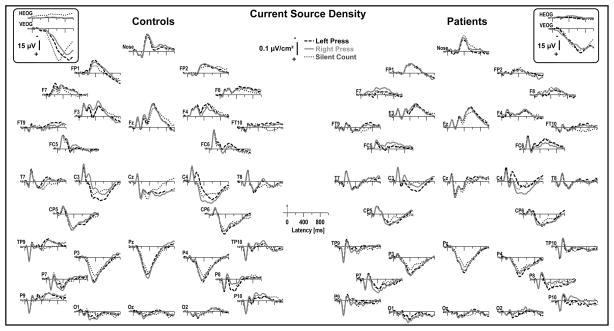
Figure 2.
CSD waveforms as in Figure 1 for targets comparing response mode (averaged across task). For both groups, response-related negativities associated with a button press were clearly evident over medial-central sites (C3/4), revealing prominent sinks contralateral to the responding hand superimposed on the task-related component structure.
The expected target/nontarget condition effects were clearly evident, particularly for the tonal task in healthy adults (Figure 1, left), revealing frontocentral N2 sinks (approximate peak latency 230 ms; e.g., site F4) and mid-parietal P3 sources (375 ms; Pz) for target stimuli only. However, target-related N2 sinks and P3 sources were smaller in patients (Figure 1, right). In contrast, a late mid-frontal sink for targets (480 ms; Fz), which we have repeatedly observed in various paradigms48,53,55,56 and linked to a frontal response negativity (FRN),53 was equally robust in controls and patients. Across tasks, a prominent frontocentral N1 sink corresponded to a lateral temporoparietal source (105 ms; e.g., see sites Cz, C3/4, FC5/6 and TP9/10, P9/10), although this was smaller for syllables compared to tones. This early frontocentral N1 sink was followed by lateral temporal N1 sinks for tones (160 ms; T7/8) but not for syllables.
This CSD component structure was modulated in both groups by the different response requirements (Figure 2). Most notably, CSD waveforms revealed marked button press effects over medial-central sites (C3/4), shifting the contralateral CSD waveforms in a negative direction for most of the recording epoch. By comparison, silent count responses had arelative smaller P3source over mid-parietal sites (e.g., Pz). Thus, overall CSD waveforms were highly comparable in controls and patients across task and response mode, despite notable reductions of prominent CSD components (N2 sink, P3 source) in patients. Importantly, eye movements were also comparable across group, task, and response mode (see bipolar eye activity traces included in Figures 1 and 2), and evidently did not affect the corresponding CSDs derived from blink-corrected continuous EEG data.
PCA component waveforms and topographies
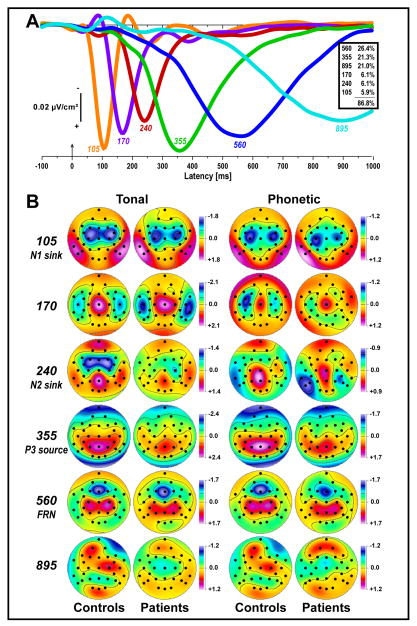
Figure 3 shows the time courses of factor loadings for the first six CSD factors extracted (86.8% explained variance after rotation) and the corresponding topographies of factor scores, separately plotted for tasks and groups. Labels were chosen to indicate the peak latency of the factor loadings relative to stimulus onset, and are supplemented by a brief functional interpretation if the factor had a signature topography. The mere purpose of these identifying labels is to ease referring to these CSD factors, which nevertheless consist of characteristic time courses and entire topographies.
Figure 3.
Unrestricted temporal PCA solution. A: Time courses of Varimax-rotated covariance loadings for the first six CSD factors extracted (86.8% total variance explained; see inset). Labels indicate the peak latency of the factor loadings relative to stimulus onset. B: Corresponding factor score topographies of tonal and phonetic targets for 23 healthy adults and 23 schizophrenic patients (pooled across response mode). Dots indicate the spherical positions of the 31-channel EEG montage (nose at top). All maps are 2D-representations of spherical spline surface interpolations (m = 4; λ = 10−5)50 derived from the mean factors scores available for each recording site.
CSD factors corresponded to N1 sink (peak latency 105 ms; off-midline, medial-central sinks associated with lateral temporoparietal sources; 5.9% explained variance), temporal N1 sink (170 ms, lateral-temporal sinks paired with a vertex source; 6.1%), N2 sink (240 ms, frontocentral sinks for tones and left lateral-parietal sinks for syllables paired with a mid-parietal source; 6.1%), and P3 source (355 ms; broad medial-parietal maximum; 21.3%). Two additional high-variance factors corresponded to late activity around the time subjects responded (560 ms; mid-frontal sink paired with off-midline, centroparietal sources [FRN]; 26.4%) and beyond (895 ms; unsystematic topography; 21.0%). For both groups, these factors closely matched those reported previously for these tasks in larger samples of healthy adults34,48,49; however, patients appeared to have reduced amplitudes of the tonal N2 sink and P3 source across tasks. The use of a common extraction was validated by separate PCA solutions derived from the CSD data for patients only (n = 23) or controls only (n = 23), which revealed highly comparable factor structures (correlations between corresponding factor loadings were .95 ≤ r ≤ .99). Because factors 240, 355, and 560 showed robust target/nontarget condition effects across tasks, including those typically attributed to the N2/P3 complex,10,74 the remainder of this report focuses on these three CSD-PCA components.
Repeated measures ANOVA
Table 2 summarizes the primary statistics obtained for the three CSD-PCA factors for target stimuli at representative sites.
Table 2.
Summary of F ratios (and ε corrections) from repeated measures ANOVA performed on CSD-PCA factors scores for targets at selected sites
| Factor (Sites)
|
||||||
|---|---|---|---|---|---|---|
| 240 N2 sink |
355 P3 source |
560 FRN |
||||
| (F3/4, FC5/6, C3/4) | (P7/8, P9/10, TP9/10) | (P3/4, P7/8, CP5/6) | (Pz) | (Fz) | (C3/4, CP5/6, P3/4) | |
| G | 4.96* | 6.13* | 4.78* | |||
| T | 42.2**** | 48.8**** | 4.22* | |||
| T x G | 10.6** | |||||
| R | 3.14 (0.90) | 3.31* (0.98) | 4.57* (0.93) | 12.1**** (0.93) | 2.60 (0.72) | 21.8**** (0.85) |
| R x G | 2.81 (0.93) | |||||
| R x T | 2.69 (0.96) | |||||
| H | 6.11* | — | — | |||
| T x H | 4.06* | 12.2*** | — | — | ||
| R x H | 15.6**** (0.97) | 11.0**** (0.99) | — | — | ||
| R x H x G | 2.89 (0.97) | — | — | |||
| R x T x H | 2.46 (0.98) | — | — | |||
G = Group (patients, controls); T = task (tonal, phonetic); R = response mode (right press, left press, silent count); H = hemisphere (left, right) Only F ratios with p < .10 are reported for effects pooled over site (subsets as indicated; for effects involving response mode, df = 2, 88; for all other tabled effects, df = 1, 44)
—Effect not applicable
p ≤ .05;
p ≤ .01;
p ≤ .001;
p ≤ .0001
N2 sink
Factor 240 corresponded to N2 sink amplitude for targets, which showed a marked task-dependent topography of this component: it was most prominent over frontocentral sites for tones, with a modest shift toward the right hemisphere, whereas it was greatest over left lateral temporoparietal sites for syllables (Figure 3B, row 3). To adequately represent the task-dependent topographic specificity of this component, two separate ANOVA were computed using three homologous pairs over frontocentral (F3/4, FC5/6, C3/4) or temporo-parietal (P7/8, P9/10, TP9/10) regions.
The analysis at anterior sites revealed significant effects of group and task x group, which originated from reduced N2 sink amplitude for patients, particularly for the tonal task (M ±SD, controls vs. patients, −0.96 ±1.37 vs. −0.27 ±1.27) but not for syllables (−0.01 ±1.06 vs. 0.04 ±1.01). Apart from a highly significant task main effect that simply confirmed the presence of N2 sink for tones but not syllables at these sites, there was also a highly significant response mode x hemisphere interaction (Figure 4). Simple hemisphere main effects for each response mode revealed right-greater-than-left N2 sinks for left press (LH vs. RH, −0.18 ±1.14 vs. −0.54 ±1.46; F[1,44] = 10.6, p = .002) and silent count (−0.14 ±0.95 vs. −0.30 ±1.25; F[1,44] = 3.73, p = .06), but left-greater-than-right N2 sinks for right press (−0.41 ±1.25 vs. −0.23 ±1.36; F[1,44] = 3.89, p = .05). Although there was no significant overall task x hemisphere interaction at these anterior sites, a simple interaction effect at FC5/6 (F[1,44] = 6.34, p = .02) stemmed from a right-greater-than-left N2 sink for tones (LH vs. RH, −0.44 ±1.33 vs. −0.48 ±1.34), and vice versa for syllables (−0.01 ±0.95 vs. 0.31 ±1.15).
Figure 4.
N2 sink topographies (mean scores of factor 240) for 23 schizophrenic patients and 23 healthy controls. Topographies are shown for tonal and phonetic target stimuli and each response mode.
In contrast, the analysis of N2 sink at posterior sites did not reveal any significant effects involving group. Apart from another highly significant task main effect, confirming the presence of N2 sink for syllables but not tones, significant effects of hemisphere and task x hemisphere originated from a left-greater-than-right N2 asymmetry for the phonetic task (LH vs. RH; for syllables, −0.63 ±1.03 vs. −0.25 ±0.93) but not for tones (0.26 ±1.17 vs. 0.41 ±1.10). However, a highly significant task x hemisphere x site x group interaction, F[2,88] = 5.96, p = .005, ε = 0.91, could be traced to a greater phonetic N2 sink for patients compared to controls at site P7 (−0.89 ±0.92 vs. −0.27 ±1.09; simple group main effect, F[1,44] = 6.13, p = .02; Figure 4). A response mode main effect resulted from a greater N2 sink for silent count compared to left press (−0.14 ±1.10 vs. 0.03 ±1.18; F[1,44] = 5.94, p = .02), while the contrasts of these response modes to right press (−0.05 ±1.12) were not significantly different.
P3 source
Factor 355 corresponded to P3 source amplitude for targets, spanning parietal sites with a midline maximum in both tasks (Figure 3B, row 4). Two separate ANOVA were computed using three homologous pairs over medial and lateral centroparietal sites (P3/4, CP5/6, P7/8) or at midline (Pz).
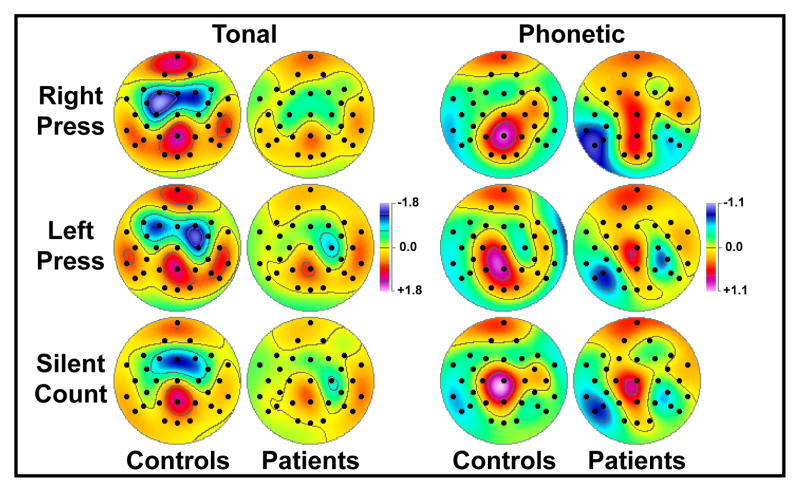
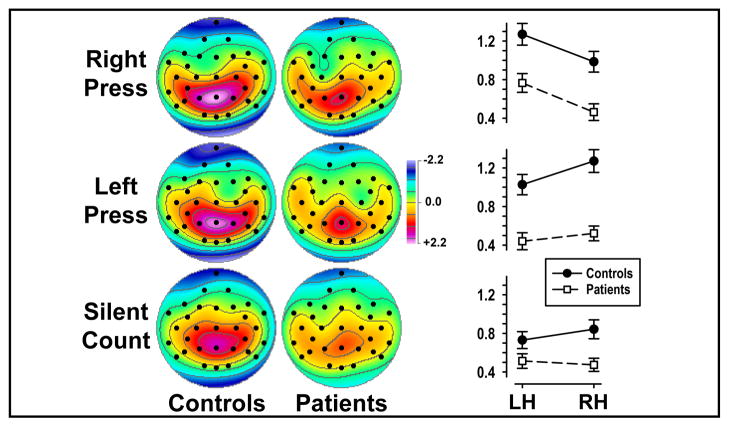
Both analyses yielded significant group main effects, with patients having reduced P3 source (at Pz, controls vs. patients, 1.95 ±1.31 vs. 1.22 ±1.31). Across groups, tones resulted in a greater P3 source than syllables at Pz (1.69 ±1.38 vs. 1.48 ±1.34). Also across groups, significant response mode main effects in both analyses stemmed from greater P3 source to button press (at Pz; for right press, 1.76 ±1.29; for left press, 1.72 ±1.44) than silent count (1.27 ±1.30; simple contrasts, left/right press vs. silent count, both F[1,44] ≥ 18.2, both p ≤ .0001; left vs. right press, F[1,44] < 1.0, n.s.), and this interacted with hemisphere at medial and lateral centroparietal sites (Figure 5). Whereas left-greater-than-right P3 source was associated with right press (LH vs. RH, 1.02 ±1.26 vs. 0.72 ±1.17), the opposite asymmetry was seen for left press (0.73 ±1.17 vs. 0.90 ±1.22), with no hemisphere difference for silent count (0.62 ±0.97 vs. 0.66 ±1.00). These response-related P3 source asymmetries were indirectly caused by superimposed anterior sinks contralaterally to the response hand, which are evident for both groups over left or right frontocentral sites for right or left button presses (Figure 5, rows 1 and 2) but not for silent count (Figure 5, row 3). Difference topographies between right or left press and silent count clarified that button press was associated with a superimposed, dipole-like sink-source generator pattern spanning the left or right motor cortex. While these lateralized, response-related dipoles were not completely symmetric for right and left button presses, they were highly comparable across groups. Finally, a significant task x hemisphere interaction resulted from opposite P3 source asymmetries, favoring the right hemisphere for tones (LH vs. RH, 0.76 ±1.18 vs. 0.85 ±1.16) and the left hemisphere for syllables (0.83 ±1.13 vs. 0.67 ±1.11), and these task-dependent asymmetries were present in both patients and controls (Figure 3, row 4).
Figure 5.
P3 source topographies (mean scores of factor 355) for 23 schizophrenic patients and 23 healthy controls for each response mode (pooled across task), and pooled means (±SEM) at medial and lateral centroparietal sites (P3/4, CP5/6, P7/8).
Frontal Response Negativity (FRN)
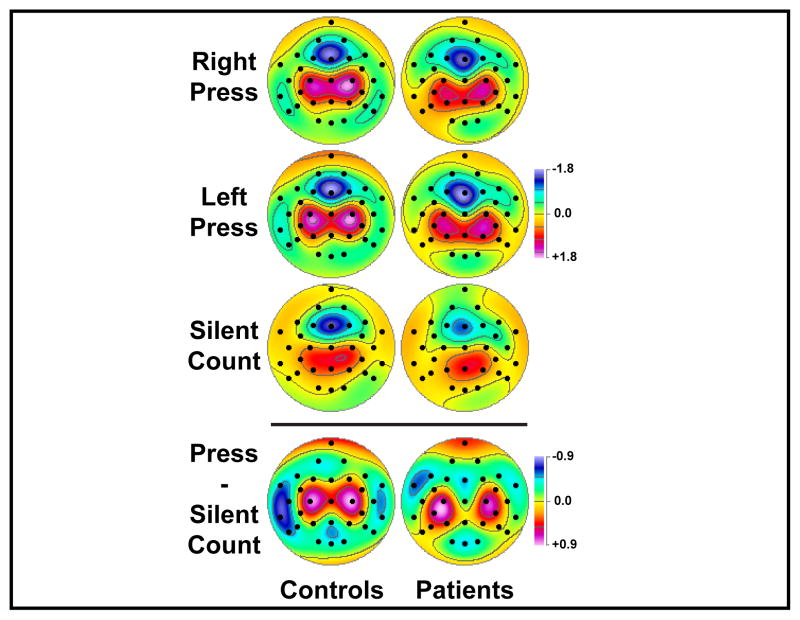
For targets, factor 560 corresponded to a large, focal mid-frontal sink accompanied by off-midline centroparietal sources (Figure 5, row 6), which were analyzed by two separate ANOVA employing only site Fz or three homologous pairs over the centroparietal region (C3/4, CP5/6, P3/4). The only significant effect that emerged from these analyses was a response mode main effect for the centroparietal source, which was due to greater amplitudes for button press (for right press, 0.88 ±1.22; for left press, 1.02 ±1.25) compared with silent count (0.44 ±0.81; simple contrasts, left/right press vs. silent count, both F[1,44] ≥ 12.2, both p ≤ .0001), and also greater amplitude for left than right button press (F[1,44] = 4.23, p = .05; Figure 6).
Figure 6.
FRN topographies (mean scores of factor 560) for 23 schizophrenic patients and 23 healthy controls for each response mode (pooled across task), and the topographic difference of button press (average of right and left press) minus silent count.
As in our prior study,48 the net effect of detecting targets by either pressing a response button or by silently counting was computed by subtracting the topography of factor 560 for silent count from the mean topography for left and right press (Figure 6, bottom row). As previously seen for the larger sample of healthy adults, this net difference revealed a relative source for the silent count condition over lateral regions of the left hemisphere, particularly for controls (cf. dark blue region at left-lateral sites in the difference topography). Another post-hoc ANOVA was therefore computed for factor 560 using two homologous pairs of lateral-temporal sites (T7/8, TP9/10). This analysis confirmed across groups the greater 560 source at temporal sites for silent count (0.04 ±0.93) versus right (−0.30 ±1.17) and left (−0.30 ±1.07) button presses (response mode main effect, F[2,88] = 9.72, p = .0004, ε = .83; simple contrasts, right/left press vs. silent count, F[1,44] ≥ 11.5, both p = .001; right vs. left press, F[1,44] < 1.0, n.s.). However, these response-related effects were substantially reduced in patients (right press, −0.13 ±1.17; left press, −0.10 ±1.10; silent count, 0.00 ±0.92) compared to controls (right press, −0.48 ±1.14; left press, −0.51 ±0.99; silent count, 0.08 ±0.94), resulting in a significant response mode x group interaction (F[2,88] = 4.48, p = .02, ε = .83; group interaction contrasts, right/left press vs. silent count, F[1,44] ≥ 4.44, both p < .05; right vs. left press, F[1,44] < 1.0, n.s.).
DISCUSSION
The present study did not provide support for the hypothesis that different response requirements during simple auditory target detection tasks (i.e., using a button press or silent count) differentially affect amplitude or asymmetry of N2 and P3 components in schizophrenia patients and healthy controls,3,12–16 and is thereby consistent with the P3 findings reported by Ford et al.26 This study systematically compared the effects of left or right button press versus silent count on all subcomponents of the N2/P3 complex in a fully-crossed, within-subjects design in schizophrenia. While response mode impacted on all subcomponents of the N2/P3 complex, with most of the modulations originating from medial-central sites and attributable to motor activities associated with a button press, the response-related effects on N2 and P3 were highly comparable among patients and controls, which is in close agreement with previous findings.10,26 Consistent with numerous prior ERP studies in schizophrenia,1,2 schizophrenic patients showed bilateral reduction in amplitudes of sink and source activity underlying the N2 and P3 components, that is, the defining electrophysiologic correlates of tone discrimination tasks. Notably, patients had marked reductions of N2 sink amplitude over frontocentral sites during the tonal task, and of P3 source amplitude over lateral- and mid-parietal sites during both tasks. It would therefore appear that deficits in stimulus categorization, which are commonly associated with N2, depend on the specific cortical substrate primarily involved in N2 generation – the tonal N2 sink is consistent with possible contributions of anterior cingulate cortex (ACC).95,96 Of critical importance for the present study, however, is that these reductions of N2 sink or P3 source were not greater over the left than right hemisphere. Although the interpretation of the lack of asymmetric P3 reductions is generally problematic, several unique paradigmatic features of the present study strengthen this conclusion.
While the basic paradigm closely matched standard auditory oddball tasks, it employed tonal and phonetic stimuli suitable to specifically probe left or right hemispheric functions.33,48,75 Most importantly, patients and controls showed the expected task-related asymmetries of N2 sinks and P3 sources favoring the right hemisphere for tones but the left hemisphere for syllables, thereby replicating our previous findings using tonal and phonetic oddball tasks in a large sample (n = 66) of schizophrenic patients.10 Moreover, in the present study, the left-lateralized parietal N2 sink for syllables was even more robust in patients than healthy controls, suggesting preserved categorization of phonemes (i.e., early encoding of linguistic information) in schizophrenia. The preserved task-related N2/P3 asymmetries in schizophrenia challenges the implicit notion that greater reductions of N2/P3 amplitude over left than right temporal sites are per se indicative of a left-lateralized dysfunction. This assumption would have predicted left-lateralized reductions of N2/P3 components regardless of task, or particularly for phonetic stimuli known to engage left-hemispheric processing.33,35,36 Instead, right-greater-than-left N2 and/or P3 amplitudes for tones,31,33,34 which are commonly used as stimuli in standard oddball tasks, represent the normal asymmetry pattern that is consistent with functional hemispheric differences for pitch processing in healthy adults.85,86
The present study employed a combined CSD-PCA approach that is not subject to the interpretational ambiguities stemming from the recording reference or ERP component quantification, which may have contributed to prior conflicting reports concerning P3 asymmetry. Apart from this methodological advantage, the capability of these combined methods to separate task- and response-related effects for temporally and spatially overlapping components, which are highly blurred in volume-conducted surface potentials,28,34,76 allowed the identification of a late, left-lateralized source. This source was unique to silent-counting of targets and approximated the timing of the subjects’ manual response, suggesting an increased, left-lateralized memory load associated with updating the internal target count. Most importantly, this left-lateralized source was markedly reduced in patients, which is consistent with impairments of working memory in schizophrenia.77,78 The left temporoparietal topography of this silent-counting effect resembles topographic abnormalities observed in schizophrenia for the late parietal old/new effect, which is widely considered an electrophysiological correlate of conscious recollection.93 In three independent studies, we found largely preserved late positive old/new effects in patients over mid-parietal sites, but markedly reduced old/new effects over left lateral parietal regions.81,82,84 While the current finding of a reduced left-lateralized source in patients supports the idea that the added cognitive demand in silent-counting oddball tasks may increase the likelihood of observing lateralized ERP reductions in schizophrenia, particularly when employing a conventional analysis of volume-conducted surface potentials using a bilateral recording reference (linked-ears or -mastoids), it also provides evidence that this silent count effect is not directly linked to classic N2 or P3 but rather to a distinct component likely associated with working memory or other cognitive processes.
The silent count effect was observed during a late CSD factor (560 ms peak latency) interval and was maximum over left temporal sites. While the underlying component is substantially masked in surface potentials, CSD-PCA methods have repeatedly revealed a mid-frontal, response-related negativity (FRN) accompanied by off-midline centroparietal sources during this time interval in a wide range of ERP paradigms – including tonal,34 phonetic,48,49 novelty,56 and dichotic55 oddball tasks, as well as continuous recognition memory tasks using spoken or read words and faces.53,82,84 Thus, the FRN is a characteristic and replicable CSD component. Although this component is observed around the time subjects respond48 or approximately 50 ms after the recorded response onset,53,82 and its topography is highly similar to the error-related negativity (ERN; Ne) for error but also correct responses,87–90 the fact that it is also present during the silent count condition strongly suggests that is it not contingent on a motor response. Still, FRN peak latencies during button presses, as identified in the grand mean CSD waveforms, were about 75 ms longer for patients compared to controls, thereby approximating but not fully matching the 115-ms slower response latency of patients. While it is possible that the larger FRN amplitude during button press than silent count (cf. trend in Table 2), which was supported for the larger sample of healthy adults,48 may reflect an incomplete motor response or response inhibition, as suggested by Go/NoGo response tasks,28,97–99 the blocked assignment of response mode should have eliminated the need to inhibit motor responses during silent count; however, given the lack of NoGo trials in the present paradigm, these issues are beyond the scope of this report. As we have previously interpreted this stimulus-locked CSD component as an index of processes linked to response selection, evaluation, or monitoring,48 it is of interest to note that there were no group differences in the overall FRN amplitude, that is, neither in its midfrontal sink nor in its centroparietal source. This suggests that patients did not show evidence of any response monitoring deficit91,92 during these tonal and phonetic oddball tasks, in which their accuracy was on par with that of controls. This is in striking contrast to our recent finding of a markedly reduced response-locked FRN in schizophrenia during auditory and visual word recognition memory tasks.82
Typical limitations of ERP studies in schizophrenia involve ongoing treatment with antipsychotic medication, small sample size, sample heterogeneity with respect to clinical features, and differences in task performance. Differential medication effects on auditory ERPs have been reported, for example, with clozapine treatment being associated with P3 enhancement,8 or risperidone treatment associated with reduced P3 latency.79 In a previous report,80 we found reduced N2 amplitudes in schizophrenia during these tonal and phonetic oddball tasks primarily for unmedicated patients. This is consistent with the current study, which included more unmedicated than medicated patients; however, it is not clear to what extent drug treatment had a moderating influence on the current findings. While the number of patients included in the present study (n = 23) was substantially smaller than the sample size of our previous study (n = 66),10 which likely caused a reduction in statistical power for detecting less robust effects, the current patient sample was reasonably large compared to sample sizes of many other studies. Also, the current study included a particularly well-matched sample of healthy controls. Although patients responded more slowly than healthy controls, their performance accuracy during these less-demanding target detection tasks was more than adequate and on par with those of healthy adults. The well-preserved, highly-comparable CSD component structure in patients also bolstered the assumption that task disengagement was not a concern.
In summary, we found bilateral reductions of N2 sink and P3 source amplitudes during tonal and phonetic oddball tasks in schizophrenic patients. The present study also found evidence that silently counting targets during these oddball tasks imposes a response requirement that differs substantially from a button press condition and is associated with a late, relative positivity over left temporal sites, which is reduced in patients. This positivity may influence the laterality of overlapping ERP components (e.g., P3) observed for different tasks (e.g., tonal vs. phonetic) or study groups (i.e., heathy controls vs. schizophrenic patients), particularly with conventional ERP measures that are influenced by the EEG recording reference. The employed CSD-PCA approach clearly separated this superimposed silent-counting effect from N2 sink and P3 source activity, that is, neuronal current generator patterns underlying these two prototypical ERP components. However, it is not likely that this methodological insight can easily account for reports of asymmetric P3 reductions in schizophrenia during typical oddball tasks. Rather, it seems more parsimonious to hypothesize that differences in neuroanatomy between patient samples involving asymmetric impairments of temporal lobe structures (as previously argued by O’Donnell et al.19 and Ford et al.26) are a major contributor to inconsistent findings of asymmetric N2/P3 reductions in schizophrenia across different laboratories. Patients without asymmetric structural impairments may not show asymmetric neurophysiologic deficits during simple tonal oddball tasks, which thereby appear unsuitable to probe a left-lateralized, language-related dysfunction in schizophrenia.46,47,94 In contrast, more demanding tasks that specifically probe linguistic and/or mnemonic processes are better suited to study specific impairments of cognitive function, and these have less ambiguously pointed to a left-lateralized dysfunction of phonological processing in schizophrenia.52,81–84
Acknowledgments
This research was supported by grants MH050715 and MH066597 from the National Institute of Mental Health (NIMH). We thank Dan Alschuler, Nil Bhattacharya, Nathan Gates, Carlye Griggs, Christopher Kroppmann, Paul Leite, Mia Sage, Jennifer Schaller, Stewart Shankman, and Barbara Stuart with data collection, storage, and preprocessing. Preliminary analyses of these data were presented at the 3rd Joint Meeting of the EEG and Clinical Neuroscience Society (ECNS) and International Society for Neuroimaging in Psychiatry (ISNIP) in Boston, Massachusetts, September 13-17, 2006, and at the 46th Annual Meeting of the Society for Psychophysiological Research (SPR) in Vancouver, British Columbia, Canada, October 25-29, 2006. The authors greatly appreciate access to waveform plotting software developed and provided by Charles L. Brown, III.
Footnotes
DISCLOSURE AND CONFLICT OF INTEREST
J. Kayser, C.E. Tenke, R. Gil and G.E. Bruder have no conflict of interest in relation to this article.
References
- 1.Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36(6):667–682. [PubMed] [Google Scholar]
- 2.Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40(5):684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- 3.McCarley RW, Faux SF, Shenton ME, Nestor PG, Adams J. Event-related potentials in schizophrenia: their biological and clinical correlates and a new model of schizophrenic pathophysiology. Schizophr Res. 1991;4(2):209–231. doi: 10.1016/0920-9964(91)90034-o. [DOI] [PubMed] [Google Scholar]
- 4.Strik WK, Dierks T, Franzek E, Stober G, Maurer K. P300 asymmetries in schizophrenia revisited with reference-independent methods. Psychiatry Res. 1994;55(3):153–166. doi: 10.1016/0925-4927(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 5.Strik WK, Dierks T, Franzek E, Stober G, Maurer K. P300 in schizophrenia: interactions between amplitudes and topography. Biol Psychiatry. 1994;35(11):850–856. doi: 10.1016/0006-3223(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 6.Salisbury DF, O’Donnell BF, McCarley RW, Shenton ME, Benavage A. The N2 event-related potential reflects attention deficit in schizophrenia. Biol Psychol. 1994;39(1):1–13. doi: 10.1016/0301-0511(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 7.Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry. 1995;52(7):550–558. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- 8.Umbricht D, Javitt D, Novak G, Bates J, Pollack S, Lieberman J, Kane J. Effects of clozapine on auditory event-related potentials in schizophrenia. Biol Psychiatry. 1998;44(8):716–725. doi: 10.1016/s0006-3223(97)00524-6. [DOI] [PubMed] [Google Scholar]
- 9.Mathalon DH, Ford JM, Pfefferbaum A. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biol Psychiatry. 2000;47(5):434–449. doi: 10.1016/s0006-3223(99)00277-2. [DOI] [PubMed] [Google Scholar]
- 10.Kayser J, Bruder GE, Tenke CE, Stuart BK, Amador XF, Gorman JM. Event-related brain potentials (ERPs) in schizophrenia for tonal and phonetic oddball tasks. Biol Psychiatry. 2001;49(10):832–847. doi: 10.1016/s0006-3223(00)01090-8. [DOI] [PubMed] [Google Scholar]
- 11.Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006;59(8):762–772. doi: 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Salisbury DF, O’Donnell BF, McCarley RW, Nestor PG, Faux SF, Smith RS. Parametric manipulations of auditory stimuli differentially affect P3 amplitude in schizophrenics and controls. Psychophysiology. 1994;31(1):29–36. doi: 10.1111/j.1469-8986.1994.tb01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruder GE, Tenke CE, Rabinowicz E, Towey JP, Malaspina D, Amador X, Kaufmann CA, Gorman JM. Electrophysiologic studies of brain activity in schizophrenia. In: Kaufmann CA, Gorman JM, editors. Schizophrenia: New directions for clinical research and treatment. Larchmont, NY: Mary Ann Liebert, Inc; 1996. pp. 17–33. [Google Scholar]
- 14.Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW. First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiatry. 1998;55(2):173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Souza VB, Muir WJ, Walker MT, Glabus MF, Roxborough HM, Sharp CW, Dunan JR, Blackwood DH. Auditory P300 event-related potentials and neuropsychological performance in schizophrenia and bipolar affective disorder. Biol Psychiatry. 1995;37(5):300–310. doi: 10.1016/0006-3223(94)00131-L. [DOI] [PubMed] [Google Scholar]
- 16.Turetsky BI, Colbath EA, Gur RE. P300 subcomponent abnormalities in schizophrenia: I. Physiological evidence for gender and subtype specific differences in regional pathology. Biol Psychiatry. 1998;43(2):84–96. doi: 10.1016/S0006-3223(97)00258-8. [DOI] [PubMed] [Google Scholar]
- 17.McCarley RW, Shenton ME, O’Donnell BF, Faux SF, Kikinis R, Nestor PG, Jolesz FA. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry. 1993;50(3):190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell BF, Shenton ME, McCarley RW, Faux SF, Smith RS, Salisbury DF, Nestor PG, Pollak SD, Kikinis R, Jolesz FA. The auditory N2 component in schizophrenia: relationship to MRI temporal lobe gray matter and to other ERP abnormalities. Biol Psychiatry. 1993;34(1–2):26–40. doi: 10.1016/0006-3223(93)90253-a. [DOI] [PubMed] [Google Scholar]
- 19.O’Donnell BF, McCarley RW, Potts GF, Salisbury DF, Nestor PG, Hirayasu Y, Niznikiewicz MA, Barnard J, Shen ZJ, Weinstein DM, Bookstein FL, Shenton ME. Identification of neural circuits underlying P300 abnormalities in schizophrenia. Psychophysiology. 1999;36(3):388–398. doi: 10.1017/s0048577299971688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford JM, White PM, Csernansky JG, Faustman WO, Roth WT, Pfefferbaum A. ERPs in schizophrenia: effects of antipsychotic medication. Biol Psychiatry. 1994;36(3):153–170. doi: 10.1016/0006-3223(94)91221-1. [DOI] [PubMed] [Google Scholar]
- 21.Pfefferbaum A, Ford JM, White PM, Roth WT. P3 in schizophrenia is affected by stimulus modality, response requirements, medication status, and negative symptoms. Arch Gen Psychiatry. 1989;46(11):1035–1044. doi: 10.1001/archpsyc.1989.01810110077011. [DOI] [PubMed] [Google Scholar]
- 22.Zipursky RB, Marsh L, Lim KO, DeMent S, Shear PK, Sullivan EV, Murphy GM, Csernansky JG, Pfefferbaum A. Volumetric MRI assessment of temporal lobe structures in schizophrenia. Biol Psychiatry. 1994;35(8):501–516. doi: 10.1016/0006-3223(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 23.Holinger DP, Faux SF, Shenton ME, Sokol NS, Seidman LJ, Green AI, McCarley RW. Reversed temporal region asymmetries of P300 topography in left- and right-handed schizophrenic subjects. Electroenceph Clin Neurophysiol. 1992;84:532–537. doi: 10.1016/0168-5597(92)90042-a. [DOI] [PubMed] [Google Scholar]
- 24.Jeon YW, Polich J. P300 asymmetry in schizophrenia: a meta-analysis. Psychiatry Res. 2001;104(1):61–74. doi: 10.1016/s0165-1781(01)00297-9. [DOI] [PubMed] [Google Scholar]
- 25.Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30(6):547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- 26.Ford JM, Mathalon DH, White PM, Pfefferbaum A. Left temporal deficit of P300 in patients with schizophrenia: effects of task. Int J Psychophysiol. 2000;38(1):71–79. doi: 10.1016/s0167-8760(00)00131-8. [DOI] [PubMed] [Google Scholar]
- 27.Salisbury DF, Rutherford B, Shenton ME, McCarley RW. Button-pressing affects P300 amplitude and scalp topography. Clin Neurophysiol. 2001;112(9):1676–1684. doi: 10.1016/s1388-2457(01)00607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salisbury DF, Griggs CB, Shenton ME, McCarley RW. The NoGo P300 ‘anteriorization’ effect and response inhibition. Clin Neurophysiol. 2004;115(7):1550–1558. doi: 10.1016/j.clinph.2004.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barch DM, Csernansky JG, Conturo T, Snyder AZ. Working and long-term memory deficits in schizophrenia: is there a common prefrontal mechanism? J Abnorm Psychol. 2002;111(3):478–494. doi: 10.1037//0021-843x.111.3.478. [DOI] [PubMed] [Google Scholar]
- 30.Stevens AA, Goldman Rakic PS, Gore JC, Fulbright RK, Wexler BE. Cortical dysfunction in schizophrenia during auditory word and tone working memory demonstrated by functional magnetic resonance imaging. Arch Gen Psychiatry. 1998;55(12):1097–1103. doi: 10.1001/archpsyc.55.12.1097. [DOI] [PubMed] [Google Scholar]
- 31.Alexander JE, Bauer LO, Kuperman S, Morzorati S, O’Connor SJ, Rohrbaugh J, Porjesz B, Begleiter H, Polich J. Hemispheric differences for P300 amplitude from an auditory oddball task. Int J Psychophysiol. 1996;21(2–3):189–196. doi: 10.1016/0167-8760(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 32.Bruder GE, Tenke CE, Towey JP, Leite P, Fong R, Stewart JE, McGrath PJ, Quitkin FM. Brain ERPs of depressed patients to complex tones in an odd-ball task: relation of reduced P3 asymmetry to physical anhedonia. Psychophysiology. 1998;35(1):54–63. [PubMed] [Google Scholar]
- 33.Kayser J, Tenke CE, Bruder GE. Dissociation of brain ERP topographies for tonal and phonetic oddball tasks. Psychophysiology. 1998;35(5):576–590. doi: 10.1017/s0048577298970214. [DOI] [PubMed] [Google Scholar]
- 34.Tenke CE, Kayser J, Fong R, Leite P, Towey JP, Bruder GE. Response-and stimulus-related ERP asymmetries in a tonal oddball task: a Laplacian analysis. Brain Topogr. 1998;10(3):201–210. doi: 10.1023/a:1022261226370. [DOI] [PubMed] [Google Scholar]
- 35.Celsis P, Doyon B, Boulanouar K, Pastor J, Demonet JF, Nespoulous JL. ERP correlates of phoneme perception in speech and sound contexts. Neuroreport. 1999;10(7):1523–1527. doi: 10.1097/00001756-199905140-00024. [DOI] [PubMed] [Google Scholar]
- 36.Henkin Y, Kishon-Rabin L, Gadoth N, Pratt H. Auditory event-related potentials during phonetic and semantic processing in children. Audiol Neurootol. 2002;7(4):228–239. doi: 10.1159/000063739. [DOI] [PubMed] [Google Scholar]
- 37.Hill H, Weisbrod M. The relation between asymmetry and amplitude of the P300 field in schizophrenia. Clin Neurophysiol. 1999;110(9):1611–1617. doi: 10.1016/s1388-2457(99)00112-1. [DOI] [PubMed] [Google Scholar]
- 38.Nunez PL, Westdorp AF. The surface Laplacian, high resolution EEG and controversies. Brain Topogr. 1994;6(3):221–226. doi: 10.1007/BF01187712. [DOI] [PubMed] [Google Scholar]
- 39.Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. New York: Oxford University Press; 2006. [Google Scholar]
- 40.Tenke CE, Kayser J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA) Clin Neurophysiol. 2005;116(12):2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Dien J. Addressing misallocation of variance in principal components analysis of event-related potentials. Brain Topogr. 1998;11(1):43–55. doi: 10.1023/a:1022218503558. [DOI] [PubMed] [Google Scholar]
- 42.Kayser J, Tenke CE. Optimizing PCA methodology for ERP component identification and measurement: theoretical rationale and empirical evaluation. Clin Neurophysiol. 2003;114(12):2307–2325. doi: 10.1016/s1388-2457(03)00241-4. [DOI] [PubMed] [Google Scholar]
- 43.Kayser J, Tenke CE. Trusting in or breaking with convention: towards a renaissance of principal components analysis in electrophysiology. Clin Neurophysiol. 2005;116(8):1747–1753. doi: 10.1016/j.clinph.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Beauducel A, Debener S. Misallocation of variance in event-related potentials: simulation studies on the effects of test power, topography, and baseline-to-peak versus principal component quantifications. J Neurosci Methods. 2003;124(1):103–112. doi: 10.1016/s0165-0270(02)00381-3. [DOI] [PubMed] [Google Scholar]
- 45.Bruder G, Kayser J, Tenke C, Rabinowicz E, Friedman M, Amador X, Sharif Z, Gorman J. The time course of visuospatial processing deficits in schizophrenia: an event-related brain potential study. J Abnorm Psychol. 1998;107(3):399–411. doi: 10.1037//0021-843x.107.3.399. [DOI] [PubMed] [Google Scholar]
- 46.Crow TJ. Temporal lobe asymmetries as the key to the etiology of schizophrenia. Schizophr Bull. 1990;16(3):433–443. doi: 10.1093/schbul/16.3.433. [DOI] [PubMed] [Google Scholar]
- 47.Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997;20(8):339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- 48.Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin Neurophysiol. 2006;117(2):348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 49.Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clin Neurophysiol. 2006;117(2):369–380. doi: 10.1016/j.clinph.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 50.Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping [Corrigenda EEG 02274, EEG Clin. Neurophysiol, 1990, 76, 565] Electroenceph Clin Neurophysiol. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- 51.Kayser J, Tenke CE. Consensus on PCA for ERP data, and sensibility of unrestricted solutions. Clin Neurophysiol. 2006;117(3):703–707. [Google Scholar]
- 52.Kayser J, Tenke CE, Gates NA, Kroppmann CJ, Gil RB, Bruder GE. ERP/CSD indices of impaired verbal working memory subprocesses in schizophrenia. Psychophysiology. 2006;43(3):237–252. doi: 10.1111/j.1469-8986.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 53.Kayser J, Tenke CE, Gates NA, Bruder GE. Reference-independent ERP generator patterns of auditory and visual word recognition memory. Psychophysiology. 2007;44(6):949–967. doi: 10.1111/j.1469-8986.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 54.Kayser J. Current Source Density (CSD) Interpolation using Spherical Splines - CSD Toolbox. Division of Cognitive Neuroscience, New York State Psychiatric Institute; 2009. ( http://psychophysiology.cpmc.columbia.edu/CSDtoolbox.html) [Google Scholar]
- 55.Tenke CE, Kayser J, Shankman SA, Griggs CB, Leite P, Stewart JW, et al. Hemispatial PCA dissociates temporal from parietal ERP generator patterns: CSD components in healthy adults and depressed patients during a dichotic oddball task. Int J Psychophysiol. 2008;67(1):1–16. doi: 10.1016/j.ijpsycho.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tenke CE, Kayser J, Stewart JW, Bruder GE. Novelty P3 reductions in depression: characterization using principal components analysis (PCA) of current source density (CSD) waveforms. Psychophysiology. 2010;47(1):133–146. doi: 10.1111/j.1469-8986.2009.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nunez PL, Pilgreen KL, Westdorp AF, Law SK, Nelson AV. A visual study of surface potentials and Laplacians due to distributed neocortical sources: computer simulations and evoked potentials. Brain Topogr. 1991;4(2):151–168. doi: 10.1007/BF01132772. [DOI] [PubMed] [Google Scholar]
- 58.Diagnostic and Statistical Manual of the Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. (DSM-IV) [Google Scholar]
- 59.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 60.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R: Patient Edition. Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- 61.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) The University of Iowa; Iowa City, IA: 1983. [Google Scholar]
- 62.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) The University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- 63.Kay SR, Opler LA, Fishbein A. Positive and negative syndrome scale (PANSS) rating manual. Multihealth System Inc; Toronto, Canada: 1992. [Google Scholar]
- 64.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 65.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 66.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis-I Disorders - Non-patient Edition (SCID-NP) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 67.Berlin CI, Hughes LF, Lowe Bell SS, Berlin HL. Dichotic right ear advantage in children 5 to 13. Cortex. 1973;9(4):394–402. doi: 10.1016/s0010-9452(73)80038-3. [DOI] [PubMed] [Google Scholar]
- 68.Sidtis JJ. The complex tone test: implications for the assessment of auditory laterality effects. Neuropsychologia. 1981;19(1):103–111. doi: 10.1016/0028-3932(81)90050-6. [DOI] [PubMed] [Google Scholar]
- 69.NeuroScan, Inc. SCAN 4.3 - Vol. II. EDIT 4.3 - Offline analysis of acquired data (Document number 2203, Revision D) El Paso, TX: Compumedics Neuroscan; 2003. [Google Scholar]
- 70.Kayser J, Tenke CE. Electrical distance as a reference-free measure for identifying artifacts in multichannel electroencephalogram (EEG) recordings. Psychophysiology. 2006;43:S51. [Google Scholar]
- 71.Tenke CE, Kayser J. A convenient method for detecting electrolyte bridges in multichannel electroencephalogram and event-related potential recordings. Clin Neurophysiol. 2001;112(3):545–550. doi: 10.1016/s1388-2457(00)00553-8. [DOI] [PubMed] [Google Scholar]
- 72.Keselman HJ. Testing treatment effects in repeated measures designs: an update for psychophysiological researchers. Psychophysiology. 1998;35(4):470–478. [PubMed] [Google Scholar]
- 73.Dixon WJ, editor. BMDP Statistical Software Manual: To Accompany the 7.0 Software Release. University of California Press; Berkeley, CA: 1992. [Google Scholar]
- 74.Maiste AC, Wiens AS, Hunt MJ, Scherg M, Picton TW. Event-related potentials and the categorical perception of speech sounds. Ear Hear. 1995;16(1):68–90. doi: 10.1097/00003446-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 75.Bruder G, Kayser J, Tenke C, Amador X, Friedman M, Sharif Z, Gorman J. Left temporal lobe dysfunction in schizophrenia: event-related potential and behavioral evidence from phonetic and tonal dichotic listening tasks. Arch Gen Psychiatry. 1999;56(3):267–276. doi: 10.1001/archpsyc.56.3.267. [DOI] [PubMed] [Google Scholar]
- 76.Tandonnet C, Burle B, Hasbroucq T, Vidal F. Spatial enhancement of EEG traces by surface Laplacian estimation: comparison between local and global methods. Clin Neurophysiol. 2005;116(1):18–24. doi: 10.1016/j.clinph.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 77.Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- 78.Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39(6):889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- 79.Umbricht D, Javitt D, Novak G, Bates J, Pollack S, Lieberman J, Kane J. Effects of risperidone on auditory event-related potentials in schizophrenia. Int J Neuropsychopharmcol. 1999;2(4):299–304. doi: 10.1017/S1461145799001595. [DOI] [PubMed] [Google Scholar]
- 80.Bruder GE, Kayser J, Tenke CE, Friedman M, Malaspina D, Gorman JM. Event-related potentials in schizophrenia during tonal and phonetic oddball tasks: relations to diagnostic subtype, symptom features and verbal memory. Biol Psychiatry. 2001;50(6):447–452. doi: 10.1016/s0006-3223(01)01168-4. [DOI] [PubMed] [Google Scholar]
- 81.Kayser J, Bruder GE, Friedman D, Tenke CE, Amador XF, Clark SC, et al. Brain event-related potentials (ERPs) in schizophrenia during a word recognition memory task. Int J Psychophysiol. 1999;34(3):249–265. doi: 10.1016/s0167-8760(99)00082-3. [DOI] [PubMed] [Google Scholar]
- 82.Kayser J, Tenke CE, Gil RB, Bruder GE. Stimulus- and response-locked neuronal generator patterns of auditory and visual word recognition memory in schizophrenia. Int J Psychophysiol. 2009;73(3):186–206. doi: 10.1016/j.ijpsycho.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Angrilli A, Spironelli C, Elbert T, Crow TJ, Marano G, Stegagno L. Schizophrenia as failure of left hemispheric dominance for the phonological component of language. PLoS One. 2009;4(2):4507. doi: 10.1371/journal.pone.0004507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kayser J, Tenke CE, Kroppmann CJ, Fekri S, Alschuler DM, Gates NA, et al. Current source density (CSD) old/new effects during recognition memory for words and faces in schizophrenia and in healthy adults. Int J Psychophysiol. 2010;75(2):194–210. doi: 10.1016/j.ijpsycho.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zatorre RJ. Neural specializations for tonal processing. Ann N Y Acad Sci. 2001;930:193–210. doi: 10.1111/j.1749-6632.2001.tb05734.x. [DOI] [PubMed] [Google Scholar]
- 86.Zatorre RJ, Evans AC, Meyer E, Gjedde A. Lateralization of phonetic and pitch discrimination in speech processing. Science. 1992;256:846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]
- 87.Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000;51(2–3):87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 88.Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3(5):516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- 89.Vidal F, Hasbroucq T, Grapperon J, Bonnet M. Is the ‘error negativity’ specific to errors? Biol Psychol. 2000;51(2–3):109–128. doi: 10.1016/s0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- 90.Roger C, Benar CG, Vidal F, Hasbroucq T, Burle B. Rostral Cingulate Zone and correct response monitoring: ICA and source localization evidences for the unicity of correct- and error-negativities. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.02.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ullsperger M. Performance monitoring in neurological and psychiatric patients. Int J Psychophysiol. 2006;59(1):59–69. doi: 10.1016/j.ijpsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 92.Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J Abnorm Psychol. 2002;111(1):22–41. [PubMed] [Google Scholar]
- 93.Paller KA, Kutas M. Brain potentials during memory retrieval provide neurophysiological support for the distinction between conscious recollection and priming. J Cogn Neurosci. 1992;4(4):375–391. doi: 10.1162/jocn.1992.4.4.375. [DOI] [PubMed] [Google Scholar]
- 94.Flor-Henry P. Lateralized temporal-limbic dysfunction and psychopathology. Ann N Y Acad Sci. 1976;280:777–797. doi: 10.1111/j.1749-6632.1976.tb25541.x. [DOI] [PubMed] [Google Scholar]
- 95.van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77(4–5):477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- 96.van Veen V, Carter CS. Error detection, correction, and prevention in the brain: a brief review of data and theories. Clin EEG Neurosci. 2006;37(4):330–335. doi: 10.1177/155005940603700411. [DOI] [PubMed] [Google Scholar]
- 97.Burle B, Vidal F, Bonnet M. Electroencephalographic nogo potentials in a no-movement context: the case of motor imagery in humans. Neurosci Lett. 2004;360(1–2):77–80. doi: 10.1016/j.neulet.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 98.Smith JL, Johnstone SJ, Barry RJ. Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clin Neurophysiol. 2008;119(3):704–714. doi: 10.1016/j.clinph.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 99.Weisbrod M, Kiefer M, Marzinzik F, Spitzer M. Executive control is disturbed in schizophrenia: evidence from event-related potentials in a Go/NoGo task. Biol Psychiatry. 2000;47(1):51–60. doi: 10.1016/s0006-3223(99)00218-8. [DOI] [PubMed] [Google Scholar]