Abstract
Medial temporal lobe and temporoparietal brain regions are among the earliest neocortical sites to undergo pathophysiologic alterations in Alzheimer’s disease (AD), although the underlying white matter changes in these regions is less well known. We employed diffusion tensor imaging to evaluate early alterations in regional white matter integrity in participants diagnosed with mild cognitive impairment (MCI). The following regions of interests (ROIs) were examined: 1) anterior cingulum (AC); 2) posterior cingulum (PC); 3) genu of the corpus callosum; 4) splenium of the corpus callosum; and 5) as a control site for comparison, posterior limb of the internal capsule. Forty nondemented participants were divided into demographically-similar groups based on cognitive status (MCI: n = 20; normal control: n = 20), and fractional anisotropy (FA) estimates of each ROI were obtained. MCI participants showed greater posterior white matter (i.e., PC, splenium) but not anterior white matter (i.e., AC, genu) changes, after adjusting for age, stroke risk, and whole brain volume. FA differences of the posterior white matter were best accounted for by changes in radial but not axial diffusivity. PC FA was also significantly positively correlated with hippocampal volume as well as with performance on tests of verbal memory, whereas stroke risk was significantly correlated with genu FA and was unrelated to PC FA. When investigating subtypes of our MCI population, amnestic MCI participants showed lower PC white matter integrity relative to those with non-amnestic MCI. Findings implicate involvement of posterior microstructural white matter degeneration in the development of MCI-related cognitive changes and suggest that reduced FA of the PC may be a candidate neuroimaging marker of AD risk.
Keywords: Aging, diffusion tensor imaging, memory, mild cognitive impairment, posterior cingulum, white matter
INTRODUCTION
Several structural neuroimaging studies of Alzheimer’s disease (AD) and mild cognitive impairment (MCI), a transitional state validated as qualitatively distinct from both normal aging and dementia [1, 2], have shown striking gray matter changes in medial temporal lobe (MTL) structures. The MTL is well known to be especially important for memory formation, and studies have shown that the entorhinal cortex and hippocampal formation represent vulnerable brain areas that are pathologically involved early in the AD process [3–7]. However, functional neuroimaging studies using PET and SPECT techniques have found metabolic and perfusion reductions primarily in the posterior cingulate cortex [8–10], well before the severe neurodegeneration is demonstrated in this region in moderate to severe AD [11]. Recently, Zhang et al. [12] suggested that this regional dissociation observed between structural and functional alterations may be due to decreased “neuronal traffic” between MTL structures and the posterior cingulate. Within this framework, cingulum fibers connecting the MTL and the posterior cingulate may be negatively impacted early in the AD process and, as AD pathology advances, commissural connections (e.g., the corpus callosum) may also undergo changes. Indeed, histopathological studies have shown that, in addition to degenerative changes in the MTL system, there is increased gray matter atrophy and volume losses in posterior brain regions relative to anterior regions in early AD [13, 14]. While these gray matter alterations have been well described in the literature, the exact mechanisms underlying these brain changes have not yet been fully characterized.
Recently, there has been a growing body of research to suggest that white matter pathology may be related to age-related cognitive impairment and possibly represent one of the earliest changes in the development of dementia [15, 16]. Disruption of white matter in AD has been observed in neuroimaging [17, 18] and postmortem studies [19] as well as in animal models [20]. However, although there have been increasing numbers of studies focusing on diffusion tensor imaging (DTI), there have been several conflicting findings, and thus the associations of white matter changes with various clinical states (e.g., MCI, dementia) remain unclear [21]. Moreover, elucidating the role of white matter changes in MCI has been particularly challenging given that little attention has been paid to differences across MCI subtypes, the pattern of changes observed, and the role of important risk factors (e.g., cerebrovascular disease risk) that may be related to white matter degeneration. Thus, although several imaging studies have shown that white matter alterations occur across the aging spectrum, it remains to be seen whether the pattern of these changes are central to MCI. Moreover, it is unclear how important vascular risk variables may be associated with microstructural white matter changes as well as concomitant neuropsychological decline.
DTI has been shown to be a useful technique for characterizing the structure of white matter in vivo, and this enhanced visualization may allow early microstructural changes within the white matter to be more fully captured (see [22] for review). Interestingly, studies using DTI have shown a dissociation in terms of pattern of white matter changes in aging and AD. Specifically, in the context of normal aging, white matter changes appear to occur first in anterior regions (e.g., genu of the corpus callosum; [23, 24]), whereas AD-related changes appear to be associated with posterior alterations in the white matter (e.g., splenium of the corpus callosum; [25–27]). Collectively, these findings could be a consequence of Wallerian degeneration which describes microstructural changes to white matter fibers as reflecting atrophy of the corresponding cerebral cortex. Alternatively, regional white matter change may be explained by demyelination. In terms of the latter, there has been some interest in the retrogenesis hypothesis [18, 28, 29] which posits that—in pathological states such as MCI or AD—progression of white matter degradation occurs inversely to normal developmental myelination patterns (see [30]). This developmental heterogeneity may underlie the increased susceptibility of later-myelinating fiber systems such as association fibers to myelin breakdown [17, 31, 32]. Indeed, it has recently been suggested that myelin breakdown is at the core of the earliest changes involved in both normal and pathological aging [33, 34]. Another possibility is that both processes, Wallerian degeneration secondary to neuronal death within the MTL and primary demyelination, simultaneously occur in the early stages of AD.
DTI studies examining brain and cognitive changes in MCI are needed in order to examine early white matter changes in important predilection sites (e.g., posterior cingulum, subregions of the corpus callosum). Some recent studies of AD have found differences in parahippocampal white matter [35–37], and one study has found lower parahippocampal white matter integrity in MCI [35]. In line with these findings, Teipel et al. [38] showed decreased functional connectivity in posterior regions in amnestic MCI using combined EEG and DTI. However, although some studies have shown greater posterior callosal white matter degradation (i.e., splenium) in MCI [26, 39–41], other studies have not found white matter changes in this region in this population [12, 42, 43]. For example, a recent combined voxel-based morphometry and DTI study investigating both white matter density and diffusion indices showed anterior involvement of the anterior callosal subregion in MCI. However, interestingly, this change was related to white matter density but not fractional anisotropy (FA). Although there is generally some agreement that temporoparietal connections are more impacted than frontal connections in the context of AD [12, 27, 44], the findings in MCI are more disparate and thus there is currently great interest in better understanding the pattern of degradation as it occurs in MCI.
Few studies have investigated changes in the tissue characteristics of callosal subregions in MCI and its subtypes and how any observed changes may be associated with the presence and severity of vascular risk as well as neuropsychological functioning. Thus, the present study used DTI to examine white matter changes in older adults with MCI and demographically-matched normal control (NC) participants in order to understand how regional white matter changes may be related to hippocampal volumes, stroke risk, and cognition. In addition to the cingulum (anterior and posterior portions), subregions of the corpus callosum (i.e., the genu and splenium) were selected. Integrity of the internal capsule was also measured as a control site for comparison given that little change is expected in this brain region in the context of MCI (site of sensorimotor fiber convergence) and with respect to the retrogenesis hypothesis. We predicted that, consistent with studies of AD, white matter changes in MCI would be most pronounced in posterior regions versus anterior regions. In addition, we expected that DTI indices of lower white matter integrity would be associated with poorer neuropsychological functioning, reduced hippocampal volumes, and increased stroke risk. Finally, we examined for differences by MCI subtype (i.e., non-amnestic versus amnestic MCI) in white matter integrity as well as whether any observed white matter changes may be best accounted for changes indexed by axial diffusivity (DA) or by radial diffusivity (DR). Taken together, we examined white matter integrity in MCI and its subtypes as well as its associations with a sophisticated comprehensive assessment of cognition; we further examined for important associations with hippocampal volume, APOE genotype, and stroke risk.
MATERIALS AND METHODS
Participants
This study included 40 age- and education-matched nondemented participants (MCI: n = 20, NC = 20; see Table 1 for demographic comparisons) who were drawn from a larger cohort of individuals enrolled in an ongoing, longitudinal study of normal aging and MCI (PI: Mark Bondi, PhD), as well as through the community via word-of-mouth and flyers. Participants were consecutively enrolled and selected because they had undergone both neuroimaging (DTI) and comprehensive neuropsychological testing. Cognitive status of each participant was initially based upon clinical examination by a staff neurologist as well as an extensive medical, laboratory, and cognitive evaluation. Exclusion criteria included: 1) past history of head injury with loss of consciousness or serious neurologic disorder (e.g., multiple sclerosis, Parkinson’s disease, epilepsy); 2) significant history of alcohol or drug abuse; 3) history of learning disability; 4) history of stroke; 5) current/past history of severe psychiatric illness; or 6) MRI contraindications (e.g., claustrophobia, pacemakers, or metal implants). The appropriate institutional review boards approved this study and all participants provided written informed consent.
Table 1.
Demographic, clinical, global cognitive, and brain MRI morphometric characteristics of normal control (NC) participants and patients with mild cognitive impairment (MCI)
| Groups
|
p | ||
|---|---|---|---|
| MCI (n = 20) | NC (n = 20) | ||
| Mean SD | Mean SD | ||
| Age (years) | 77.70 (6.61) | 78.30 (6.32) | 0.77 |
| Gender (women/men)○ | 8/20 | 12/20 | 0.21 |
| Education (years) | 15.90 (2.69) | 16.70 (2.06) | 0.30 |
| APOE genotype (ε4/non-ε4) | 4/16 | 7/13 | 0.29 |
| Stroke risk (FSRP) | 15.5 (7.10) | 16.3 (11.20) | 0.79 |
| Depression (GDS) | 6.28 (4.70) | 6.16 (5.08) | 0.94 |
| Total DRS score | 137.65 (5.22) | 142.55 (1.76) | <0.001* |
| Activities of daily living† | 0.09 (0.68) | −0.08 (1.05) | 0.63 |
| Hippocampal volume (mm3) | 6056.21 (113.66) | 6072.40 (80.03) | 0.96 |
| Whole brain volume (cm3) | 1314.54 (145.8) | 1279.09 (110.44) | 0.39 |
| Gray matter volume (cm3) | 515.68 (74.3) | 515.95 (53.9) | 0.97 |
| White matter volume (cm3) | 464.18 (78.5) | 448.99 (70.6) | 0.52 |
| CSF volume (cm3) | 334.68 (40.0) | 315.14 (42.5) | 0.14 |
| Gray matter proportion (%) | 39.18 (2.95) | 40.38 (3.94) | 0.28 |
| White matter proportion (%) | 35.22 (3.80) | 34.93 (2.85) | 0.79 |
| CSF volume proportion (%) | 25.60 (2.96) | 24.69 (3.11) | 0.35 |
APOE = apolipoprotein E gene; FSRP = Framingham Stroke Risk Profile [55]; GDS = Geriatric Depression Scale; DRS = Dementia Rating Scale; CSF = Cerebrospinal fluid;
Chi-square test with continuity correction;
Activities of Daily Living = composite score representing Money Management and Health and Safety subscales of the Independent Living Scales (ILS).
Diagnosis of Mild Cognitive Impairment
Participants were determined to have a diagnosis of MCI based on consensus diagnosis using all available neurological, cognitive, and functional data. MCI criteria used for diagnosis were based on an adaptation of those described by Petersen and Morris [45] and discussed in detail in Delano-Wood et al. [46]. Specific criteria include: 1) normal activities of daily living; 2) absence of dementia; and 3) mild quantifiable cognitive impairment within one or more domains (i.e., attention, language, memory, executive function, visuospatial function). Given that the Mini-Mental Status Examination (MMSE) [47] has shown limited utility in terms of distinguishing normal control participants from MCI [48–50], we chose to use the Mattis Dementia Rating Scale (DRS), a more comprehensive, commonly used general test of overall cognitive ability. A cutoff of 127 was employed to ensure that no participant scored more than 1.5 standard deviation (SD) below the published normative mean values for this test. Table 1 shows that all participants exhibited normal global cognitive functioning. In addition, the entire sample was free of functional impairment (i.e., ability to effectively and independently execute important activities of daily living [ADLs] as assessed by the Independent Living Scales (ILS) [51], an ecologically valid performance-based measure. A composite score representing Money Management and Health and Safety subscales of the ILS was used in all analyses.
To determine mild deficits on individual cognitive tests, we used a cut-off of 1.2 SDs (after applying norms adjusted for age, education, and gender), signifying a level of performance worse than 88.5% of the population and indicative of mild to moderate impairment [46, 52]. Although there is currently no standard cut-off criterion for defining cognitive impairment in MCI, Petersen et al. [53] and Petersen and Morris [45] have suggested employing a 1.5 SD cut-off, although Busse et al. [54] demonstrated that a more liberal cut-off (i.e., 1 SD below the mean) provides higher sensitivity (while maintaining reasonable specificity) than the traditional cut-off of 1.5 SD. Thus, we struck a balance between the recommendations of Petersen and colleagues [45, 53] and Busse et al. [54] by using a cut-off of 1.2 SD. All participants were categorized into one of four subgroups [single-domain amnestic MCI (n = 6); single-domain non-amnestic MCI (n = 5); multiple-domain amnestic-MCI (n = 5); and multiple-domain non-amnestic MCI (n = 4)] based on neuropsychological test scores [45]. Although examination of all four MCI subtypes (e.g., single-versus multi-domain, amnestic versus non-amnestic) could not adequately be carried out due to the relatively small number of individuals within each cell, we collapsed MCI subtypes to reflect both an amnestic MCI subgroup (single- and multiple-domain amnestic MCI participants) as well as a non-amnestic MCI subgroup (single- and multiple-domain non-amnestic MCI participants).
Stroke risk assessment
The Framingham Stroke Risk Profile (FSRP) [55], the most widely used scale of cerebrovascular risk burden, was administered to each participant at the time of cognitive testing. The FSRP was developed to predict a 10-year probability for risk of stroke and is based on several risk factors identified from roughly 40 years of longitudinal study within the Framingham Heart Study, and it has been shown to predict stroke in aging populations [56]. The FSRP provides gender-corrected scores based on the following risk factors: age, systolic blood pressure, diabetes mellitus, cigarette smoking, history of cardiovascular disease, atrial fibrillation, left ventricular hypertrophy as identified by electrocardiogram, and use of antihypertensive medications.
Apolipoprotein E genotyping
APOE genotyping was conducted using a polymerase chain reaction method discussed in detail elsewhere [57]. APOE genotyping showed that 11 of the 40 individuals (27.5% of the sample) included in the current study possessed at least one copy of the APOE-ε4 allele. As can be seen in Table 1, the breakdown of APOE-ε4 allele distribution is as follows: (MCI APOE-ε4 positive: 4/20 [20%]; NC APOE-ε4 positive: 7/20 [35%]; χ2 = 1.13, p = 0.29). The following represents the breakdown of APOE-ε4 allele distribution across MCI subtype: (amnestic MCI APOE-ε4 positive: 3/11 [27%]; NC APOE-ε4 positive: 1/9 [11%]; p = 0.37) (Table 2).
Table 2.
MCI subgroup differences (amnestic versus nonamnestic MCI) on demographic, clinical, DTI, and brain MRI morphometric characteristics
| Amnestic MCI (n = 11) | Nonamnestic MCI (n = 9) | p | |
|---|---|---|---|
| Age | 77.9 (7.3) | 78.8 (5.3) | 0.77 |
| Gender (male/female) | 7/11 (64%) | 5/9 (56%) | 0.71 |
| Stroke Risk (FSRP) | 17.3 (7.0) | 13.3 (7.0) | 0.23 |
| APOE-ε4 Status (ε4/non-ε4) | 3/11 (27%) | 1/9 (11%) | 0.37 |
| DRS Total Score | 135.8 (4.3) | 139.9 (5.6) | 0.08 |
| Depression (GDS) | 7.8 (5.1) | 4.8 (3.9) | 0.18 |
| Posterior Cingulum (PC) FA | 0.38 (0.04) | 0.41 (0.02) | 0.03* |
| Right PC FA | 0.40 (0.05) | 0.43 (0.02) | 0.11 |
| Left PC FA | 0.37 (0.05) | 0.41 (0.03) | 0.04* |
| Splenium | 0.63 (0.04) | 0.63 (0.05) | 0.72 |
| Genu | 0.51 (0.08) | 0.53 (0.04) | 0.41 |
| Anterior Cingulum (AC) FA | 0.28 (0.05) | 0.28 (0.03) | 0.84 |
| Right AC FA | 0.27 (0.07) | 0.27 (0.04) | 0.97 |
| Left AC FA | 0.31 (0.04) | 0.29 (0.03) | 0.39 |
| Internal Capsule (IC) FA | 0.50 (0.08) | 0.54 (0.04) | 0.23 |
| Hippocampal volume (mm3) | 5772.9 (1327.7) | 6402.4 (787.1) | 0.23 |
| Right hippocampus | 3012.8 (666.5) | 3206.3 (451.3) | 0.47 |
| Left hippocampus | 2760.1 (698.6) | 3196.2 (399.7) | 0.12 |
| Whole brain volume (cm3) | 1298.29 (118.45) | 1334.41 (179.34) | 0.60 |
| Gray volume | 501.42 (69.62) | 533.10 (180.27) | 0.36 |
| White volume | 469.32 (84.00) | 457.90 (75.72) | 0.76 |
| CSF | 327.55 (33.73) | 343.41 (47.04) | 0.39 |
FSRP = Framingham Stroke Risk Profile; APOE = Apolipoprotein-E; DRS = Dementia Rating Scale; GDS = Geriatric Depression Scale.
Neuropsychological assessment
All participants were administered a comprehensive cognitive battery that was created to maintain consistency with that used in our UCSD Alzheimer’s Disease Research Center. The battery, which is described in extensive detail elsewhere [58] includes tests of global cognitive function (Mattis Dementia Rating Scale), attention [Wechsler Adult Intelligence Scale-Revised (WAIS-R) Digit Span subtest], language (WAIS-R Vocabulary Subtest, American National Adult Reading Test, Boston Naming Test, Verbal Fluency), memory (California Verbal Learning Test-Version 1; Wechsler Memory Scale – Revised Logical Memory), visuospatial functioning (WISC-R Block Design and Digit Symbol subtests; Clock Drawing test, simple motor functioning (Grooved Pegboard), and executive functioning (Tower, Color-Word Interference, Sorting, and Trail Making Number-Letter Switching subtests from the Delis-Kaplan Executive Function System (D-KEFS) [59, 60]).
Data reduction of neuropsychological measures
Composite scores were created to represent the following cognitive domains: recall memory, recognition memory, executive functioning, attention, and visuospatial skills. Significant correlations between the tests that comprise each composite score supported this approach. Raw scores for each of the measures listed below were z-transformed using the means and standard deviations of the control group, and the z-scores for the individual tests were averaged to create a summary score for each cognitive domain. The specific measures that comprise each domain include: 1) Recall Memory was measured with the Wechsler Memory Scale – Revised [61] Logical Memory sub-tests (WMS-R; Immediate and Delayed Free Recall) and the California Verbal Learning Test (CVLT; Trials 1–5 Total Recall and Long Delay Free Recall; [62]); 2) Recognition Memory was assessed with WMS-R Logical Memory Total Recognition Correct subtest and CVLT Total Recognition Correct (Hits) subtest; 3) Attention was measured with the Attention sub-scale of the DRS and the Digit Span subtest of the WAIS-R [61]; 4) Visuospatial Skills were assessed with the Block Design subtest of the Wechsler Intelligence Scale for Children – Revised (WISC-R; [63]) and the Construction subscale of the DRS; 5) Executive Functioning was measured with the following DKEFS subtests: Number-Letter Sequencing (Total Correct), and Letter and Category Verbal Fluency (Total Words Correct) [59, 60]. Tests within each cognitive domain were submitted to a principal components analysis (PCA) to verify that all tests within a domain loaded on only one factor, which was the case for each domain. Ranges for the individual factor loadings were the following for each cognitive domain: Recall Memory (0.85–0.94); Recognition Memory (0.79–0.83); Attention (0.75–0.77); Visuospatial Skills (0.76–0.79); and Executive Functioning (0.73–0.83).
MRI acquisition and processing
All images were acquired on a GE Signa LX 1.5 Tesla scanner located at the VA San Diego Health-care System. High-resolution T1-weighted anatomic images were collected with an SPGR sequence (124 slices acquired in the sagittal plane; 1.2 mm slice thickness; 256 × 256 matrix; field of view [FOV] = 250 mm; resulting in a 1 mm2 in-plane resolution). For the DTI sequence, 12 axial images through the corpus callosum, cingulum, and posterior limb of the internal capsule were acquired, and diffusion was encoded with a single-shot stimulated-echo sequence with spiral acquisition along 42 diffusion directions with b = 1990 s/mm2. A non-diffusion weighted image (b = 0) was also acquired. The image matrix was chosen to allow for rapid acquisition of 7 scans (NEX = 7), which were averaged to improve SNR. DTI quantification was preceded by eddy current correction based on the inversion recovery images, using a six-parameter affine correction on a slice-by-slice basis [64]. Image parameters for the DTI sequence were: TE = 100 ms, 64 × 64 image matrix, slice thickness = 3.8 mm, FOV = 24 cm2, and TR = 2.5 s. The images were reconstructed onto a 128 × 128 × 12 grid with a voxel size of 1.875 × 1.875 × 3.8 mm3.
Image processing prior to region of interest (ROI) analyses included co-registered structural and diffusion-weighted data resampled to 1 mm3 voxels [65]. Solving the six independent equations with respect to MDxx, MDxy, etc., yielded the elements of the diffusion tensor. The general diffusion tensor was then diagonalized, yielding eigenvalues λ1, λ2, λ3, as well as eigenvectors that defined the predominant diffusion direction. The transformation was then applied to the DTI images before calculation of FA, a measure which reflects white matter integrity (i.e., orientation coherence). DA was defined as the amount of diffusion corresponding to the principal diffusion direction (DA = λ1) and DR was defined as the average of the two eigenvalues orthogonal to the principal diffusion direction (DR = (λ2 + λ3)/2) [66].
Derivation of DTI regions of interest
In general, DTI data analysis can be conducted in two ways. In a whole-brain, voxel-based analysis the whole white matter is investigated simultaneously. Voxel-based approaches have the advantage of being automated (i.e., no need to manually identify regions of interest) and allows for the detection of findings in regions beyond any a priori regions of interest. However, voxel-based approaches are subject to stringent multiple comparison corrections, which may obscure the detection of subtle changes in small regions. ROI analysis, on the other hand is not subject to the same stringent multiple comparison correction, and may thereby be more sensitive to subtle changes. The disadvantages of an ROI approach are that there may be placement errors across subjects which may lead to erroneous findings (assessable via intra-rater reliability analysis), and, unlike voxel-based approaches, the analysis is restricted to only those identified ROIs. In the present study, we employed an ROI approach across multiple white matter regions in an effort to increase our power of detecting a significant effect within those regions the literature has previously identified as being sensitive to the early effects of AD.
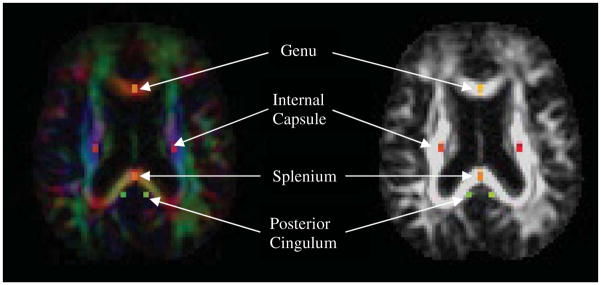
All analyses were conducted with Analysis of Functional NeuroImages (AFNI) software [67]. Individualized, manually outlined versus Talairach daemon-defined ROIs were chosen, in part, to minimize partial volume effects and more accurately reflect individual variation within white matter tracts (e.g., corpus callosum). Consistent with Zhang et al. [12], the ROIs were standard (fixed) across all participants. Color-coded directionality maps of diffusion were created and simultaneously displayed for easy visualization of the white matter fiber tracts (red: left-to-right direction, green: anterior-to-posterior direction, blue: superior-to-interior direction). Rectangular ROIs were drawn based on the identification of white matter tracts on the color-coded maps [68]. Similar to the methodology of Zhang et al. [12], three pairs of individually, manually-outlined ROIs (fixed across participants) were placed on axial slices to maximally encompass each of the following white matter regions: 1) bilateral posterior cingulate regions, at the middle level of the dorsal curve of the cingulum fibers; 2) genu and splenium ROIs (center of anterior and posterior CC); and 3) bilateral regions at the posterior limb of internal capsule, where sensorimotor fiber converge. FA values within each ROI were averaged and, to account for possible differences in fiber thickness with head size, group analyses of DTI data were adjusted for total whole brain volume. An experienced operator (LDW) who was blinded to group conducted the ROI drawings. Intrarater reliability was computed for 10 randomly chosen subjects, which yielded intraclass correlations averaging 0.96.
Derivation of hippocampal volumes
Hippocampal volumes were obtained bilaterally via inspection and manual outlining performed in the coronal plane. Images were realigned perpendicular to the anterior-posterior commissure line; however, they were not transformed into standard space coordinates. Regions of interest were defined using AFNI software and completed by an experienced operator (AJJ), who was blind to participant identity and group membership. High levels of intra- and inter-rater reliability for the procedure have been previously established (intraclass correlation coefficients >0.90; [69]). Hippocampal ROIs were delineated using a stereotactic approach adapted from methods published previously [69, 70]. Briefly, the anterior bound of the hippocampus was chosen as the coronal slice through the fullest portion of the mammillary bodies, and the posterior boundary was traced on the last coronal slice on which the superior colliculi could be visualized.
Segmentation of structural images
Whole brain images were skull-stripped and segmented into gray matter, white matter, and cerebrospinal fluid (CSF) compartments. Following N3 bias correction of field inhomogeneities [71], each scan was processed with one or both of the following automated methods to most fully remove all non-brain material: FreeSurfer’s Hybrid Watershed Algorithm and/or Brain Surface Extractor (Version 3.3; [72]), which have been demonstrated to be effective for processing images from older adults [73]. When necessary, scans were manually edited to remove any residual non-brain material (e.g., skull). Tissue segmentation was performed using FSL’s FAST (FMRIB’s Automated Segmentation Tool) [74], whole brain volume was derived, and total gray matter, white matter, and CSF volumes were normalized [75]. Specifically, each compartment was normalized by dividing the respective structure volume by the whole brain volume and multiplying by 100 to correct for intersubject differences in overall brain size.
Statistical analyses
Group comparisons (i.e., MCI versus NC; amnestic versus non-amnestic MCI) were performed with ANCOVA, independent samples t-tests, or Chi-Square tests, as appropriate. Multivariate hierarchical regression was used to determine the relationship between DTI indices and diagnosis, adjusting for important covariates (e.g., age, stroke risk, hippocampal and whole brain volumes). In addition, Pearson product-moment correlations examined associations between DTI indices and cognitive and stroke risk variables of interest. Moreover, t-tests and analysis of covariance was used to test group differences on DR and DA, adjusting for age and gray matter volume. All statistical tests were two-tailed and a significance level of p < 0.05 was used for all analyses. Multiple comparisons were addressed using the Bonferonni correction for all analyses. All analyses were conducted in SPSS (Version 14.0).
RESULTS
Demographic, clinical, and morphometric characteristics by group
There were no significant differences between MCI and NC participants on any demographic (i.e., age, education, gender), clinical (i.e., FSRP [stroke risk], ILS [activities of daily living], and GDS [depression]), structural MR whole-brain segmentation volumetric measurements, or on total hippocampal volume (all p-values ≥ 0.16; see Table 1).
DTI difference between MCI and control groups
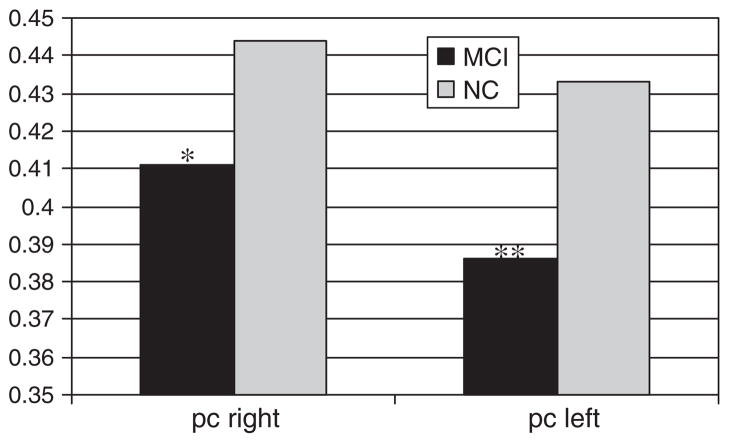
Independent samples t-tests demonstrated that FA of both posterior white matter indices were significantly lower in the MCI group than in the NC group (posterior cingulum [PC] FA: t = 3.40(38), d = 1.10, p = 0.002; splenium FA: t = 2.13(38), d = 0.69, p = 0.04). When adjusting for multiple comparisons, only PC FA is statistically significantly lower in the MCI group. Figure 2 shows that, when directly investigating PC FA by brain hemisphere, FA was significantly lower in the MCI group bilaterally (left PC FA: t = 2.85(38), d = 0.92, p = 0.007; right PC FA: t = 2.37(38), d = 0.77, p = 0.02). The MCI and NC groups did not significantly differ on FA of the anterior white matter regions (anterior cingulum; p = 0.22; genu: p = 0.52). Moreover, as expected, the groups did not significantly differ on FA of the posterior limb of the internal capsule (p = 0.57).
Fig. 2.
Posterior cingulum FA hemispheric differences by group. pc = posterior cingulum. *p = 0.023, **p = 0.007.
Prediction of MCI status from posterior white matter DTI indices
A series of multivariate hierarchical regression analyses were modeled to assess prediction of posterior white matter ROIs (PC and splenium) by diagnostic group (MCI and NC), adjusting for age, total stroke risk, and whole brain volume. Results showed that, above and beyond the effects of the covariates entered into the model, lower PC FA predicted MCI status (β = −0.51, ΔR2 = 0.25, p = 0.001); however, only a trend existed for the prediction of splenium FA by MCI status after adjusting for age, total stroke risk, and whole brain volume (β = −0.30, ΔR2 = 0.065, p = 0.07). In order to examine the differential contribution of hippocampal volume, another multivariate stepwise regression analysis was run. After adding hippocampal volume to the regression, results showed that the prediction of PC FA by MCI status remained significant (diagnosis: β = −0.48, ΔR2 = 0.22, p = 0.001; hippocampal volume, β = 0.32, ΔR2 = 0.11, p = 0.045). There were no associations between MCI status and frontal white matter indices (all p > 0.40).
DTI differences by MCI subtype
Exploratory analyses were conducted to test for differences between amnestic and non-amnestic MCI groups. As shown in Table 2, independent samples t-tests showed that the two MCI subgroups did not differ on any demographic, clinical (e.g., stroke risk, depressive symptomatology), or morphometric variable (e.g., whole brain volumes). One-way ANCOVA (comparing amnestic MCI, non-amnestic MCI, and NC groups) adjusting for age and whole brain volume demonstrated that the subgroups differed only on PC FA (F(39) = 7.88, ηp2 = 0.32, p = 0.001). Tukey post hoc testing showed that the significant ANCOVA was largely driven by a group difference between NC and amnestic MCI groups (p = 0.001). NC participants did not differ significantly from those with non-amnestic MCI (p = 0.15), nor did the MCI subgroups differ significantly from each other (p = 0.91).
Further analyses explored PC FA hemispheric differences by MCI diagnosis. One-way ANCOVA (adjusting for age and whole brain volume) revealed that, collapsed across group, MCI status was associated with lower FA in both the left PC (F(39) = 6.19, ηp2 = 0.21, p = 0.005) as well as the right PC (F(39) = 4.15, ηp2 = 0.14, p = 0.02). Tukey post hoc testing showed that for both the left PC and right PC, the NC group differed significantly from the amnestic MCI subgroup (p = 0.003 and p = 0.018, respectively). NC participants did not differ significantly from those with non-amnestic MCI (left PC: p = 0.49; right PC; p = 0.62), and the MCI subgroups did not differ significantly from each other (left PC: p = 0.15; right PC: p = 0.27). No other white matter ROI significantly differed between MCI subgroups. Interestingly, MCI subgroups did not differ by hippocampal volume (p = 0.23).
Associations between regional white matter indices, cognition, and stroke risk
FA of the PC was significantly and positively related to hippocampal volume (r = 0.33, p = 0.04) as well as to both memory composite scores (Recall: r = 0.50, p = 0.001; Recognition: r = 0.49, p = 0.009). Importantly, however, the association of PC FA with hippocampal volume does not reach significance after adjusting for multiple comparisons. Stepwise multiple hierarchical regression showed that PC FA significantly predicted Recall Memory, even after adjusting for hippocampal volume and stroke risk (β = 0.43, ΔR2 = 0.16, p = 0.007). Collapsed across group, elevated stroke risk was significantly associated with advancing age (r = 0.40, p = 0.01), decreased FA in the genu (r = −0.34, p = 0.03), and total hippocampal volume (r = −0.32, p = 0.04). A trend was revealed between elevated total stroke risk and lower percent gray matter volume (r = −0.31, p = 0.055).
Examination of diffusion components of posterior DTI indices
We next examined group differences in DA and DR for each ROI that were significantly different between groups in the prior analyses (i.e., PC and splenium). Compared to the NC group, the MCI group showed significantly higher DR in the PC (t38 = −4.88, d = −1.58, p < 0.001) as well as the splenium (t38 = −3.16, d = −1.03, p = 0.003). There was no significant difference in DA across groups (PC: t38 = −0.70, p = 0.49; t38 = −1.18, p = 0.24). The group differences in PC and splenium DR remained significant when controlling for age and GM volume (F1,36 = 23.85, p < 0.001, ηp2 = 0.40 and F1,36 = 9.98, p = 0.003, ηp2 = 0.22, respectively).
DISCUSSION
Using DTI to investigate regional differences in white matter integrity, we found that, in comparison to normally aging control participants, those with MCI showed lower FA of posterior—but not anterior—white matter regions examined. Specifically, independent of several important variables (e.g., age and whole brain volumes), older adults with MCI demonstrated considerably diminished white matter integrity in the posterior cingulum and, to a lesser extent, the splenium relative to cognitively normal older adults. Additionally, results showed that, above and beyond the effects of age, stroke risk, hippocampal volume, and whole brain volume, diminished white matter integrity of the PC was strongly predictive of MCI status as well as neuropsychological performance on recall and recognition memory tasks. Finally, exploratory analyses showed that those with amnestic MCI showed decreased white matter integrity of the PC, although this effect was only significant for the left PC.
Results of the current study are consistent with Chua et al. [76] who also found that DTI indices of the posterior cingulate region discriminated MCI from cognitively normal participants. In this study, both nonamnestic- and amnestic-MCI subgroups showed degradation of the PC, although those with amnestic-MCI showed more profound changes in this region coupled with additional changes to the splenium and parahippocampal white matter. Zhang et al. [12] also showed decreased PC white matter integrity in MCI as well as a gradient of PC white matter damage across MCI and AD (lower integrity in AD versus MCI). Their results also demonstrated FA reductions predominantly in the left PC, although white matter degeneration of the splenium was not seen in MCI but was evident in AD. Furthermore, in line with our findings, Zhang et al. [12] showed that, compared to classifications of MCI and AD using hippocampal volume loss alone, DTI white matter indices of the PC increased sensitivity and group classification rates. Our results complement these findings by suggesting that neuropathologic changes associated with MCI may first affect the PC before spreading to other white matter structures important for cognition (e.g., splenium). Our results extend the findings of Chua et al. [76] and Zhang et al. [12] by relating regional white matter integrity to stroke risk burden and neuropsychological functioning.
Given that white matter changes in the PC were most salient in our MCI group, it may be that this region—an important outflow tract of the MTL that connects the posterior cingulate gyrus to the hippocampus [77]—is especially vulnerable and compromised early in the course of MCI. Indeed, other studies have found greater posterior versus anterior involvement in dementia [25–27, 78], while an anterior-posterior gradient of atrophy and degeneration have been found in the context of normal aging [79–83]. Within MCI, there are a few studies showing a posterior-anterior gradient of change [24, 26, 42], and some studies have found especially large reductions in FA in parahippocampal regions in this population [12, 27, 69]. However, other studies have shown conflicting results with the results of the current study. For example, although Stenset et al. [84] found lower FA in the PC region in participants with MCI, their results showed that FA of the genu but not the splenium was lower in their MCI group. Differences across DTI studies of MCI likely reflect a number of study methodological differences, including definition of and tools used to diagnose MCI, specific scanning parameters employed, and DTI processing and analysis approach.
Our findings of early PC involvement in MCI are in line with studies that have shown reduced gray matter density in this population [85, 86], and with neuropathologic data demonstrating neuronal degeneration in the PC in early AD [87, 88]. Moreover, AD-related neuropathologic changes in the PC cortex and in MTL structures have been recently found using in vivo molecular imaging [89]. The findings of our study speak to the potential specific vulnerability of cingulum fibers—particularly in the left hemisphere—in MCI, and they raise the question of selective involvement of this brain region in the context of progressive cognitive impairment. Indeed, it may be that AD-related cognitive deficits are secondary to a disruption of critical cortical memory networks involving the MTL and posterior cingulate-retrosplenial cortices. Additionally, although PC FA was associated with hippocampal volume and memory performance across the sample, there were no group (MCI versus NCs) differences by hippocampal volume. These findings suggest that macrostructural volume losses to the hippocampus likely occur later than white matter microstructural changes in the progression of cognitive impairment. Accordingly, our results suggest that DTI-derived indices of white matter integrity may offer increased utility over and above hippocampal volume differences in the prediction of risk of MCI and conversion to dementia such as AD.
Consistent with other studies in the literature, stroke risk was related to anterior but not posterior white matter integrity (see [90] and [15] for review). This finding was not surprising given that histopathological and imaging studies have shown preferential vulnerability of the frontal-subcortical circuitry due to vascular compromise [92, 93]. A potential hypothesis that may reconcile the finding that FA of the PC is related to hippocampal volume and memory performance but not related to stroke risk is the amyloid “cortical hub” theory put forth by Reisa Sperling and colleagues [94, 95]. This theory suggests that amyloid-β deposition in “cortical hubs” (brain areas that are highly connected to multiple other regions) may disrupt cortical networks prior to clinically-diagnosed AD. Specifically, this theory posits that the ‘default mode network’ important for memory function (i.e., posterior cingulate cortex and medial prefrontal, hippocampal, and inferior parietal cortical regions) is preferentially negatively affected by synaptic pathologies prior to neuronal death and atrophy [94, 96–98]. Indeed, several studies have shown that roughly one- to two-thirds of participants with MCI have higher [11C] Pittsburgh compound B (PiB) uptake indicative of significant amyloid-β burden, particularly in the posterior cingulate region [99, 100]. As recently suggested by Sperling et al. [101], white matter changes may be initiated through the accumulation of amyloid-β which may subsequently trigger the release and activation of various pro-inflammatory neurotoxic substances and microglia [102, 103].
Other possibilities for our finding of reduced white matter integrity in posterior regions in MCI include changes associated with Wallerian degeneration, demyelination, and/or vascular territory compromise. First, changes in FA may arise from white matter fiber change reflecting degeneration/atrophy of the corresponding cerebral cortex. Alternatively, our findings could be explained by the retrogenesis hypothesis [29] which posits that later-myelinating fibers (e.g., limbic pathways such as the cingulum; see [104, 105]), are more susceptible to myelin breakdown than earlier-myelinating fibers (e.g., posterior limb of the internal capsule). However, we did not observe changes in the genu of the corpus callosum which is known to myelinate much later in development when compared to other white matter regions [104, 105]. Taken together, our results speak to partial support for both Wallerian degeneration and demyelination. First, although whole brain volumes did not differ between MCI and NC groups, lowered FA of the PC predicted decreased hippocampal volumes across all subjects. Thus, early degeneration of the hippocampus may have lead to subsequent damage of its associated white matter tracts (i.e., PC). Examination of the intrinsic differences in the posterior DTI indices provided support for demyelination as well. Specifically, we observed a change in DR that was not accompanied by a similar change in DA, suggesting changes specific to demyelination. These findings are generally consistent with recently reported studies in MCI [68] and AD [27, 30]. However, it is important to note that there has been recent debate in the literature regarding what these diffusion parameters actually measure. For example, Madden et al. [106] recently argued that, although myelin may contribute to DTI-based radial diffusivity, factors other than demyelination (i.e., fiber arrangement and packing, decreased axonal diameter, and reduction in axons or axonal density) may affect this index making it more a composite index of white matter microstructure than any specific myelin marker.
Although additional prospective studies are needed in order to better identify the mechanism of white matter degeneration seen in MCI, there is evidence from several studies suggesting that disruption of white matter may occur early in the course of AD [92]. Indeed, myelin degradation may be central to the earliest changes involved in brain aging and, recently, Bartzokis [34] suggested that changes in the myelin may represent a primary disease process for dementia such as AD, even preceding the formation of plaques and tangles. However, in the context of neurodegenerative dementias such as AD, neuronal loss is earliest and most severe in the MTL and, thus, consistent with the Wallerian degeneration, it might be expected that the most significant alterations in white matter would be found in the tracts connecting this region to important neocortical areas [3]. It may be that as hippocampal damages progress, the functioning of connected areas such as the cingulate cortex is disrupted. It remains to be seen whether Wallerian degeneration, retrogenesis, or some combination lead to degradations in critical white matter tracts in the transition of normal aging to MCI to AD. Moreover, such transitions may be further influenced by cerebrovascular risk given our observed association of increased stroke risk with anterior FA and decreased gray matter (percent of whole brain volume). Taken together, our results suggest that these three different mechanisms (i.e., Wallerian degeneration, demyelination, and vascular risk) may act differentially on specific white matter tracts and would be predicted by each of the hypotheses based on areas of AD-related histopathologic damage, developmental myelination, and vascular territory.
Despite these novel findings, results presented here need to be replicated in larger samples, particularly since data gleaned from MCI subtypes is critically important in order to elucidate possible differing etiologies. Additionally, our DTI observations could be related to vascular factors such as deterioration of cerebral hemodynamics and white matter lesion pathology due to underlying cerebral vascular disease. Although we had no measure of white matter lesion in the current study, we expect generally low burden given that vascular burden for this sample was fairly low as indexed by the FSRP, and our MCI and NC groups did not differ on this measure. Moreover, our DTI sequence does not afford us the ability to capture differences with respect to crossing fibers, and partial voluming effects (i.e., inclusion of CSF)—particularly with respect to smaller structures such as the cingulum bundle—may have biased our DTI findings in MCI toward lower FA. However, any FA attenuation would have been expected to be constant across groups, there were no gross differences in overall brain volume between groups, and our methodology was chosen, in part, to minimize partial voluming effects by selecting central voxels within each ROI. Despite these limitations, our results show that white matter changes are evident in at-risk older adults and further validate the use of DTI to capture subtle, early white matter changes before significant atrophy (e.g., whole brain, hippocampus) is present.
In summary, our study is among the very few that has (1) examined MCI subtypes, (2) provided a sophisticated comprehensive assessment of cognition as opposed to rudimentary use cognitive screening measures or reliance on very few measures, and (3) examined for the importance of APOE genotyping and stroke risk assessments—all within a single study. In short, we found that a loss of microstructural white matter integrity is evident in early MCI. Importantly, this loss appears to be selective to the posterior white matter, disproportionately affecting the PC. Additionally, results showed a positive association between white matter integrity of the PC and verbal memory performance on neuropsychological tests, suggesting that white matter integrity of PC fibers may be a supplementary marker and early predictor of future cognitive decline and development of dementia such as AD. In order to assess whether white matter changes represent a primary or secondary even in the etiology of progressive cognitive impairment as well as to determine if DTI indices predict cognitive decline and progression to AD and other dementia types, prospective, longitudinal follow-up of our samples will be essential. Altogether, results further suggest that selective posterior microstructural white matter degeneration may play a particular role in MCI-related cognitive changes, and they suggest that reduced FA of the PC may be a candidate biomarker of progression to AD.
Fig. 1.
White matter regions of interest (ROIs) on color-coded directional maps (left) and fractional anisotropy maps (right). PC region (green) is represented bilaterally at the middle of the dorsal curve of cingulum fibers; Genu (yellow) and splenium (orange) ROIs traced at center of anterior and posterior corpus callosum; IC (pink) is represented bilaterally at posterior limb.
Table 3.
Group comparisons of posterior diffusion measures and brain volume indices in participants with mild cognitive impairment (MCI) and healthy normal controls (NC)a
| NC (n = 20) | MCI (n = 20) | p | |
|---|---|---|---|
| Posterior cingulum (PC) FA | 0.438 (0.049) | 0.392 (0.037) | 0.002* |
| PC DA | 1.776 (0.044) | 1.784 (0.035) | 0.49 |
| PC DR | 0.770 (0.032) | 0.817 (0.029) | <0.001* |
| Splenium FA | 0.653 (0.030) | 0.629 (0.041) | 0.04 |
| Splenium DA | 1.829 (0.097) | 1.861 (0.072) | 0.81 |
| Splenium DR | 0.793 (0.035) | 0.831 (0.042) | 0.003* |
| Whole brain volume (cm3) | 1279.09 (110.44) | 1314.54 (145.83) | 0.39 |
| Gray matter volume (%) | 40.38 (3.94) | 39.18 (2.95) | 0.28 |
| White matter volume (%) | 34.93 (2.85) | 35.22 (3.80) | 0.79 |
| CSF Volume (%) | 24.69 (3.11) | 25.60 (2.96) | 0.35 |
FA = fractional anisotropy; PC = posterior cingulum; DA = Axial diffusivity; DR = Radial diffusivity.
Mean (SD).
Acknowledgments
This work was supported by grants from the National Institutes of Health (K24 AG026431, R01 AG12674, R01 MH64729, R01 MH75870, and P50 AG05131), by a Career Development Award and Merit Review Research Program from the Department of Veterans Affairs, and by Investigator Initiated (IIRG 07-59343) and New Investigator (NIRG 09-134067) Research Grants from the Alzheimer’s Association. The authors gratefully acknowledge the assistance of staff, patients, and volunteers of the UCSD Alzheimer’s Disease Research Center, and the UCSD Laboratory of Cognitive Imaging.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1110).
References
- 1.Petersen RC. Mild cognitive impairment as a diagnostic entity (key symposium) J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith GE, Ivnik RJ. Normative neuropsychology. In: Petersen RC, editor. Mild Cognitive Impairment: Aging to Alzheimer’s Disease. Oxford University Press Inc; New York: 2003. pp. 63–88. [Google Scholar]
- 3.Callen DJ, Black SE, Gao F, Caldwell CB, Szalai JP. Beyond the hippocampus: MRI volumetry confirms widespread limbic atrophy in AD. Neurology. 2001;57:1669–1674. doi: 10.1212/wnl.57.9.1669. [DOI] [PubMed] [Google Scholar]
- 4.deToledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer’s disease: In vivo detection of entorhinal cortex atrophy. Ann NY Acad Sci. 2000;11:240–753. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- 5.deToledo-Morrell L, Stoub TR, Bulgakova M, Wilson RS, Bennett DA, Leurgans S, Wu J, Turner DA. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging. 2004;25:1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, Beckett LA, deToledo-Morrell L. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 7.Du Z, Ma S, Lin RC, Dodel F, Gao KR, Bales LC, Triarhou E, Chernet KW, Perry DL, Nelson S, Luecke LA, Phebus FP, Bymaster S, Paul M. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 9.Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 10.Petrella JR, Coleman RE, Doraiswamy PM. Neuroimaging and early diagnosis of Alzheimer disease: A look to the future. Radiology. 2003;226:315–336. doi: 10.1148/radiol.2262011600. [DOI] [PubMed] [Google Scholar]
- 11.Brun A, Gustafson L. Distribution of cerebral degeneration in Alzheimer’s disease. A clinico-pathological study. Arch Psychiatr Nervenkr. 1976;223:15–33. doi: 10.1007/BF00367450. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Schuff N, Hahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe MD, Chui H, Jagust WJ, Miller BL, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 15.Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Bartzokis G, Cummings J, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in aging and Alzheimer’s disease: A magnetic resonance imaging study. Arch Neurol. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- 18.Bartzokis G. Age-relatedmyelin breakdown: A developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Englund E. Neuropathology of white matter changes in Alzheimer’s disease and vascular dementia. Dement Geriatr Cogn Disord. 1998;9:6–12. doi: 10.1159/000051183. [DOI] [PubMed] [Google Scholar]
- 20.Wirths O, Weis J, Szczygielski J, Multhaup G, Bayer TA. Axonopathy in an APP/PS1 transgenic mouse model of Alzheimer’s disease. Acta Neuropathol. 2006;111:312–319. doi: 10.1007/s00401-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 21.Wozniak JR, Lim KO. Advances in white matter imaging: A review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci Biobehav Rev. 2006;30:762–774. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minati L, Grisoli M, Bruzzone MG. MR spectroscopy, functional MRI, and diffusion-tensor imaging in the aging brain: A conceptual review. J Geriatr Psychaitry Neurol. 2007;20:3–21. doi: 10.1177/0891988706297089. [DOI] [PubMed] [Google Scholar]
- 23.Head D, Snyder AZ, Girton AZ, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer’s disease. Cereb Cortex. 2005;15:732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- 24.Delano-Wood L, Bondi MW, Jak AJ, Horne NR, Schweinsburg BC, Frank LR, Wierenga CE, Delis DC, Theilmann RJ, Salmon DP. Stroke risk modifies regional white matter differences in mild cognitive impairment. Neurobiol Aging. 2010;31:1721–1731. doi: 10.1016/j.neurobiolaging.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo E, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer’s type: Evidence form diffusion tensor imaging. Cerebral Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- 26.Medina D, deToledo-Morrell L, Urresta F, Gabrieli JDE, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi S, Yonezawa H, Takahashi J, Kudo M, Inoue T, Tohgi H. Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neurosci Lett. 2002;332:45–48. doi: 10.1016/s0304-3940(02)00914-x. [DOI] [PubMed] [Google Scholar]
- 28.Reisberg B, Franssen EH, Hasan SM, Monteiro I, Boksay I, Souren LE, Kenowsky S, Auer SR, Elahi S, Kluger A. Retrogenesis: Clinical, physiologic, and pathologic mechanisms in brain aging, Alzheimer’s and other dementing processes. Eur Arch Psychiatry Clin Neurosci. 1999;249(Suppl 3):28–36. doi: 10.1007/pl00014170. [DOI] [PubMed] [Google Scholar]
- 29.Ringman JM, O’Neill J, Geschwind D, Medina L, Apostolova LG, Rodriguez Y, Schaffer B, Varpetian A, Tseng B, Ortiz F, Fitten J, Cummings JL, Bartzokis G. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer’s disease mutations. Brain. 2007;130:1767–1776. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]
- 30.Stricker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, Frank LR, Salmon DP, Bondi MW. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer’s disease supports retrogenesis. Neuroimage. 2009;45:10–16. doi: 10.1016/j.neuroimage.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- 32.Kemper T. Neuroanatomical and neuropathological changes during aging and dementia. 2. Oxford University Press; New York, NY: 1994. [Google Scholar]
- 33.Bartzokis G, Lu PH, Geschwind DH, Tingus K, Huang D, Mendez MF, Edwards N, Mintz J. Apolipoprotein E affects both myelin breakdown and cognition: Implications for age-related trajectories of decline into dementia. Biol Psychiatry. 2007;62:1380–1387. doi: 10.1016/j.biopsych.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Bartzokis G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging. 2011;32:1341–1371. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Spulber G, Lehtimaki KK, Kononen M, Hallikainen I, Grohn H, Kivipelto M, Hallikainen M, Vanninen R, Soininen H. Diffusion tensor imaging and Tract-Based Spatial Statistics in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2009;32:1558–1571. doi: 10.1016/j.neurobiolaging.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Rose SE, McMahon KL, Janke AL, O’Dowd B, de Zubicaray G, Strudwick MW, Chalk JB. Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2006;77:1122–1128. doi: 10.1136/jnnp.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salat DH, Tuch DS, van der Kouwe AJ, Greve DN, Pappu V, Lee SY, Hevelone ND, Zaleta AK, Growdon JH, Corkin S, Fischl B, Rosas HD. White matter pathology isolates the hippocampal formation in Alzheimer’s disease. Neurobiol Aging. 2010;31:244–256. doi: 10.1016/j.neurobiolaging.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teipel SJ, Pogarell O, Meindl T, Dietrich O, Sydykova D, Hunklinger U, Georgii B, Mulert C, Reiser MF, Möller HJ, Hampel H. Regional networks underlying interhemispheric connectivity: An EEG and DTI study in healthy ageing and amnestic mild cognitive impairment. Hum Brain Mapp. 2009;30:2098–2119. doi: 10.1002/hbm.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naggara O, Oppenheim C, Rieu D, Raoux N, Rodrigo S, Dalla Barba G, Meder JF. Diffusion tensor imaging in early Alzheimer’s disease. Psychiatry Res. 2006;146:243–249. doi: 10.1016/j.pscychresns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Cho H, Yang DW, Shon YM, Kim BS, Kim YI, Choi YB, Lee KS, Shim YS, Yoon B, Kim W, Ahn KJ. Abnormal integrity of corticocortical tracts in mild cognitive impairment: A diffusion tensor imaging study. J Korean Med Sci. 2008;23:477–483. doi: 10.3346/jkms.2008.23.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ukmar M, Makuc E, Onor ML, Garbin G, Trevisiol M, Cova MA. Evaluation of white matter damage in patients with Alzheimer’s disease and in patients with mild cognitive impairment by using diffusion tensor imaging. Neuroradiology. 2007;14:201–208. doi: 10.1007/s11547-008-0286-1. [DOI] [PubMed] [Google Scholar]
- 42.Fellgiebel A, Wille P, Muller MJ, Winterer G, Scheurich A, Vucurevic G, Schmidt LG, Stoeter P. Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: A diffusion tensor imaging study. Demen Geriatr Cog Dis. 2004;18:101–108. doi: 10.1159/000077817. [DOI] [PubMed] [Google Scholar]
- 43.Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg ST. White matter damage in Alzheimer disease and mild cognitive impairment: Assessment with diffusion-tensor MR imaging and parallel imaging techniques. Radiology. 2007;243:483–492. doi: 10.1148/radiol.2432051714. [DOI] [PubMed] [Google Scholar]
- 44.Rose SE, Chen F, Chalk JB, Zelaya FO, Strugnell WE, Benson M, Semple J, Doddrell DM. Loss of connectivity in Alzheimer’s disease: An evaluation of white matter tract integrity with colour coded MR diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2000;69:528–530. doi: 10.1136/jnnp.69.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62:1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- 46.Delano-Wood L, Bondi MW, Sacco J, Abeles N, Jak AJ, Libon DJ, Bozoki A. Heterogeneity in Mild Cognitive Impairment: Differences in neuropsychological profile and assorted white matter lesion pathology. J Intern Neuropsych Soc. 2009;15:906–914. doi: 10.1017/S1355617709990257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 48.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 49.Trenkle DL, Shankle WR, Azen SP. Detecting cognitive impairment in primary care: Performance assessment of three screening instruments. J Alzheimers Dis. 2007;11:323–335. doi: 10.3233/jad-2007-11309. [DOI] [PubMed] [Google Scholar]
- 50.Diniz BS, Yassuda MS, Nunes PV, Radanovic M, Forlenza OV. Mini-mental state examination performance in mild cognitive impairment subtypes. Int Psychogeriatr. 2007;19:647–656. doi: 10.1017/S104161020700542X. [DOI] [PubMed] [Google Scholar]
- 51.Loeb PA. Independent Living Scales Manual. The Psychological Corporation; San Antonio, Texas: 1996. [Google Scholar]
- 52.Delano-Wood L, Abeles N, Sacco J, Wierenga CE, Horne NR, Bozoki A. Regional white matter pathology in mild cognitive impairment: Differential influence of lesion type on neuropsychological functioning. Stroke. 2008;39:794–800. doi: 10.1161/STROKEAHA.107.502534. [DOI] [PubMed] [Google Scholar]
- 53.Petersen RC, Doody RS, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor MN. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 54.Busse A, Hensel A, Guhne U, Angemeyer MC, Riedel-Heller SG. Mild cognitive impairment: Long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 55.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: Adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 56.Truelsen T, Lindenstrom E, Boyesen G. Comparison of probability of stroke between the Copenhagen city heart study and the Framingham study. Stroke. 1994;25:802–807. doi: 10.1161/01.str.25.4.802. [DOI] [PubMed] [Google Scholar]
- 57.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 58.Salmon DP, Butters N. Neuropsychological assessment of dementia in the elderly. In: Katzman R, Rowe JW, editors. Principles of Geriatric Neurology. FA Davis; Philadelphia: 1992. pp. 144–163. [Google Scholar]
- 59.Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System (D-KEFS) The Psychological Corporation; San Antonio, Texas: 2001. [Google Scholar]
- 60.Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System- technical manual. The Psychological Corporation; New York: 2001. [Google Scholar]
- 61.Wechsler D. Wechsler Adult Intelligence Scale –Revised Manual. San Antonio: The Psychological Corporation; 1981. [Google Scholar]
- 62.Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. Psychological Corporation; New York: 1987. [Google Scholar]
- 63.Wechsler D. Wechsler Intelligence Scale for Children – Revised. Psychological Corporation; New York: 1974. [Google Scholar]
- 64.Frank LR. Anisotropy in high angular resolution diffusion-weighted MRI. Magnetic Resonance Med. 2001;45:935–939. doi: 10.1002/mrm.1125. [DOI] [PubMed] [Google Scholar]
- 65.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- 66.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 68.Fellgiebel A, Muller MJ, Wille P, Dellani PR, Scheurich A, Schmidt LD, Stoeter P. Color-coded diffusion-tensor imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol Aging. 2005;26:1193–1198. doi: 10.1016/j.neurobiolaging.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Jak AJ, Houston WS, Corey-Bloom J, Nagel BJ, Bondi MW. Differential cross-sectional and longitudianal impact of APOE genotype on hippocampal volume in nondemented older adults. Dem Geriat Cog Dis. 2007;23:282–289. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nagel BJ, Palmer SL, Reddick WE, Glass JO, Helton KJ, Wu S, Xiong X, Kun LE, Gajjar A, Mulhern RK. Abnormal hippocampal development in children with medulloblastoma treated with resk-adapted irradiation. Am J Neuroradiol. 2004;25:1575–1582. [PMC free article] [PubMed] [Google Scholar]
- 71.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 72.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. NeuroImage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 73.Fennema-Notestine C, Ozyurt IB, Brown GG, Clark CP, Morris S, Bischoff-Grethe A, Bondi MW, Jernigan TL, Fischl B, Segonne F, Shattuck DW, Leahy RM, Rex DE, Toga AW, Smith SM. The Human Brain Morphometry BIRN. Quantitative evaluation of automated skull-stripping methods applied to contemporary and legacy images: Effects of diagnosis, bias correction, and slice location. Hum Brain Map. 2006;27:99–113. doi: 10.1002/hbm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov randomfield model and the expectation-maximization algorithm. IEEE Transactions on Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 75.Bigler ED, Tate DF. Brain volume, intracranial volume, and dementia. Invest Radiol. 2001;36:539–546. doi: 10.1097/00004424-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 76.Chua TC, Wen W, Chen X, Kochan N, Slavin MJ, Trollor JN, Brodaty H, Sachdev PS. Diffusion tensor imaging of the posterior cingulate is a useful biomarker of mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17:602–613. doi: 10.1097/JGP.0b013e3181a76e0b. [DOI] [PubMed] [Google Scholar]
- 77.Schmahmann JD, Pandya DN. Cerebral white matter—Historical evolution of facts and notions concerning the organization of the fiber pathways of the brain. J Hist Neurosci. 2007;16:237–267. doi: 10.1080/09647040500495896. [DOI] [PubMed] [Google Scholar]
- 78.Catheline G, Periot O, Amirault M, Braun M, Dartigues J-F, Auriacombe S, Allard M. Distinctive alterations of the cingulum bundle during aging and Alzheimer’s disease. Neurobiol Aging. 2008;31:1582–1592. doi: 10.1016/j.neurobiolaging.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 79.Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 80.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, Buckner RL. Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2007;30:432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in pre-frontal white matter measured by diffusion tensor imaging. Ann NY Acad Sci. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- 83.Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 84.Stenset V, Bjornerud A, Fjell AM, Walhovd KB, Hofoss D, Due-Tonnessen P, Gjerstad L, Fladby T. Cingulum fiber diffusivity and CSF T-tau in patients with subjective and mild cognitive impairment. Neurobiol Aging. 2011;32:581–589. doi: 10.1016/j.neurobiolaging.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 85.Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 86.Frisoni GB, Testa C, Zorzan A, Sabattoli F, Beltramello A, Soininen H, Laakso MP. Detection of grey matter loss in mild Alzheimer’s disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2002;73:657–664. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vogt BA, Van Hosesen GW, Vogt LJ. Laminar distribution of neuron degeneration in posterior cingulate cortex in Alzheimer’s disease. Acta Neuropathol. 1990;80:581–589. doi: 10.1007/BF00307624. [DOI] [PubMed] [Google Scholar]
- 88.Vogt BA, Vogt LJ, Vrana KE, Gioia L, Meadows RS, Challa VR, Hof PR, Van Hoesen GW. Multivariate analysis of laminar patterns of neurodegeneration in posterior cingulate cortex in Alzheimer’s disease. Exp Neurol. 1998;153:8–22. doi: 10.1006/exnr.1998.6852. [DOI] [PubMed] [Google Scholar]
- 89.Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Hurggeren AC, Cole GM, Vinters HV, Thompson PM, Huang SC, Satyamurthy N, Phelps ME, Barrio JR. PET of brain amyloid and tau in mild cognitive impairment. N Engl Med. 2006;355:2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 90.Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging. 2002;23:421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 91.Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 2009;1297:41–56. doi: 10.1016/j.brainres.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee DY, Fletcher E, Martinez O, Ortega M, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, DeCarli C. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology. 2009;73:1722–1728. doi: 10.1212/WNL.0b013e3181c33afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee DY, Fletcher E, Martinez O, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, DeCarli C. Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and Alzheimer’s disease. Stroke. 2010;41:1791–1797. doi: 10.1161/STROKEAHA.110.582163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Drzezga A, Becker A, Van Dijk KRA, Sreenivasan A, Talukdar T, Sullivan C, Schultz AP, Sepulcre J, Putcha D, Greve D, Johnson KA, Sperling RA. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain. 2011;134:1635–1646. doi: 10.1093/brain/awr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sperling RA, LaViolette PS, O’Keefe KO, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker A, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons with dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 97.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P. Beta-amyloid imaging and memory in non-demented individuals: Evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 100.Forsberg A, Engler H, Almkvist O, Blomquist G, Hagman G, Wall A, Ringheim A, Långström B, Nordberg A. PET imaging of amyloid deposits in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 101.Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen K, Iribarren P, Hu J, Chen J, Gong W, Cho EH, Lockett S, Dunlop NM, Wang JM. Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. J Biol Chem. 2006;281:3651–3659. doi: 10.1074/jbc.M508125200. [DOI] [PubMed] [Google Scholar]
- 103.Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 105.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. FA Davis Company; Philadelphia: 1967. pp. 3–70. [Google Scholar]
- 106.Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]