Abstract
Modern spiders spin high-performance silk fibers with a broad range of biological functions, including locomotion, prey capture and protection of developing offspring 1,2. Spiders accomplish these tasks by spinning several distinct fiber types that have diverse mechanical properties. Such specialization of fiber types has occurred through the evolution of different silk-producing glands, which function as small biofactories. These biofactories manufacture and store large quantities of silk proteins for fiber production. Through a complex series of biochemical events, these silk proteins are converted from a liquid into a solid material upon extrusion.
Mechanical studies have demonstrated that spider silks are stronger than high-tensile steel 3. Analyses to understand the relationship between the structure and function of spider silk threads have revealed that spider silk consists largely of proteins, or fibroins, that have block repeats within their protein sequences 4. Common molecular signatures that contribute to the incredible tensile strength and extensibility of spider silks are being unraveled through the analyses of translated silk cDNAs. Given the extraordinary material properties of spider silks, research labs across the globe are racing to understand and mimic the spinning process to produce synthetic silk fibers for commercial, military and industrial applications. One of the main challenges to spinning artificial spider silk in the research lab involves a complete understanding of the biochemical processes that occur during extrusion of the fibers from the silk-producing glands.
Here we present a method for the isolation of the seven different silk-producing glands from the cobweaving black widow spider, which includes the major and minor ampullate glands [manufactures dragline and scaffolding silk] 5,6, tubuliform [synthesizes egg case silk] 7,8, flagelliform [unknown function in cob-weavers], aggregate [makes glue silk], aciniform [synthesizes prey wrapping and egg case threads] 9 and pyriform [produces attachment disc silk] 10. This approach is based upon anesthetizing the spider with carbon dioxide gas, subsequent separation of the cephalothorax from the abdomen, and microdissection of the abdomen to obtain the silk-producing glands. Following the separation of the different silk-producing glands, these tissues can be used to retrieve different macromolecules for distinct biochemical analyses, including quantitative real-time PCR, northern- and western blotting, mass spectrometry (MS or MS/MS) analyses to identify new silk protein sequences, search for proteins that participate in the silk assembly pathway, or use the intact tissue for cell culture or histological experiments.
Keywords: Cellular Biology, Issue 47, Spider silk, silk-producing glands, fibroins, structural proteins, spidroins
Protocol
1. Anesthetizing the spider and isolation of the abdomen
Transfer the spider from a glass jar into a cardboard box lined with plastic (Figure 1A). We typically collect spiders from woodpiles, garages or bushes and house them in the lab in glass jars. Because the spider can not climb up the plastic lining, it provides an efficient method to transfer the spider from a glass jar or coffee can into a smaller vial for anesthetizing purposes. While handling the spider at this point, you should be wearing two pairs of latex gloves or gardening gloves for safety purposes.
While the spider is in the box lined with plastic, approach the spider from its backside and let the spider slide into the plastic culture vial. After the spider enters the vial, immediately place the plug over the top (Figure 1B). We recommend using a vial that is approximately 101.6 mm x 31.75 mm (L x D). This minimizes the amount of carbon dioxide gas needed for the anesthetizing step.
Anesthetize the spider by introducing carbon dioxide gas at 5-10 pounds per square inch for 10 min (Figure 1C). This amount of gas will only knock the spider out for around 5 min, so after anesthetization you must immediately proceed to steps 1.4-1.5.
Place the anesthetized spider into a small dissection dish and use forceps to position the spider dorsal side up (red hourglass facing down).
Clip the pedicel (narrow stalk connecting the cephalothorax and the abdomen) to release the abdomen from the rest of the spider (Figure 1D). Store the cephalothorax in the freezer overnight in a petri dish and discard after frozen the next day. Be careful when moving the cephalothorax as the fangs of the spider are present on this segment.
Fasten the abdomen to the Sylgard material in the small dissection dish using a single insect pin. Insert the insect pin through the hole made from the pedicel clipping (Figure 1E). This can easily be identified as the site where the fluid is emerging from the clipping of the pedicel. Make sure the spinneret (opposite end of the pedicel) is oriented toward the bottom (closest to you).
2. Removal of the exoskeleton
Make the first incision through the exoskeleton using a pair of microscissors, starting at the hole from the pedicel clipping. Cut laterally until the dorsal side is reached on both sides (Figure 1F). Using a pair of forceps, peel back the anterior exoskeleton segment and insert a second pin at this location. Following the insertion of the second pin, turn the dissecting tray caudally (90° angle) from the initial lateral cuts and continue cutting until the spinnerets are reached (Figure 1G). This incision will be perpendicular or vertical to the initial lateral cut. Do not cut through the spinnerets when the vertical incision is made (posterior relative to the hourglass and it has a circular appearance). Failure to insert the second pin will result in the abdomen spinning when the vertical incisions are performed.
Submerge the abdomen in dissecting buffer solution (0.1 M Sodium Chloride, 0.015 M Sodium Citrate, 0.1% Diethyl Pyrocarbonate).
Peel the remaining exoskeleton back starting from the initial, lateral incision. When this is completed, the fatty tissue should be clearly visible (Figure 1H).
3. Isolation of the silk-producing glands
Using forceps begin teasing away the fat layer (Figure 1H). The fat will have a whitish to yellowish appearance. Continue until most of the fat is removed and the silk glands are clearly visible. The tubuliform gland consists of three pairs of very long, cylindrical tubes that are abundant in the abdominal cavity. The major ampullate, which is found in pairs, is a large, crescent shaped ampulla with a long convoluted distal tail and a proximal duct that decreases in width as it approaches the spider's spinnerets. The minor ampullate is very similar in morphology to the major ampullate, but it is significantly smaller. The flagelliform gland, also occurring in pairs, is round and multilobular with a small cylindrical zig-zag duct that extends down toward the spinnerets. The aggregate gland (found in pairs) is a very large, multilobular gland that has a very large excretory duct which has large, irregular lobes on it. The aciniform glands occur in large numbers and resemble short, small fingerlike projections. The pyriform gland is the smallest gland in the abdomen and has many compacted, cylindrical ducts that resemble a fan with many small excretory ducts that extend down to the spinnerets.
We recommend removing the glands in the following order: tubuliform, major ampullate, flagelliform, minor ampullate, aggregate, aciniform, and then the pyriform (Figure 2A-B).
Beginning with the tubuliform, tease these glands off of the rest of the glands - they should be sitting on top of the other glands (Figure 2A). There are three pairs of tubuliform glands in a single spider. Allow them to float free but remain attached to their spinnerets by their ducts.
Next, tease away the major ampullate glands. There are two major ampullate glands present in one spider. During the removal procedure, make sure the ducts remain attached to the glands (Figure 2A).
Continue to tease the rest of the glands away from each other, working carefully to avoid puncturing any of the glands, especially the aggregate and flagelliform glands. Each gland should be free floating all the way to its exit point in the exoskeleton to the spinnerets. These glands are also found in pairs. Remove each gland by pinching the duct with forceps and carefully pulling until it breaks away from the exit point. The major ampullate, flagelliform, minor ampullate, and aggregate glands are found in pairs. The aciniform and pyriform glands occur in many more pairs.
Remove each gland by pinching the duct with forceps and carefully pulling until it breaks away from the exit point down by the spinneret. We typically store the glands wet; however, we do not add additional buffer.
As the glands are removed, place them into sterile pre-labeled 1.5 ml microcentrifuge tubes. Individual glands are shown in Figure 3A-G. Keep these glands on ice and then flash freeze them in liquid nitrogen. Following flash freezing in liquid nitrogen, place them at -80°C for storage.
4. Representative Results
When removing the different glands, extreme care should be taken while handling the flagelliform and aggregate glands, as these two structures can be easily punctured and damaged with the forceps. Furthermore, it is worth noting that morphologically the flagelliform and aggregate glands look very similar prior to their removal and they are often intertwined with each other. To prevent cross-contamination of these tissues upon removal, the dissector should carefully tease these structures apart. Additionally, upon the initial exposure of the silk-producing glands, the aciniform and pyriform glands will be the most difficult to immediately visualize due to their smaller size and anatomical location. Often there is the presence of many eggs. Moreover, additional care needs to be taken when removing the pyriform gland, as this tissue is extremely sticky and will often adhere to your forceps. When this procedure is done properly, it is possible to obtain all seven silk-producing glands from a single spider in a highly purified manner. Typically, one can recover microgram quantities of total RNA and protein from a single spider dissection. An example of using the total RNA for qPCR to examine the mRNA levels of a tubuliform restricted fibroin gene, TuSp1, is shown (Figure 4). Representative experiments that involve protein lysates collected from the glands e.g. in-solution tryptic digestion followed by MS analysis (could also be used for MS/MS analysis) or an amino acid composition analysis is shown (Figure 5-6, respectively).
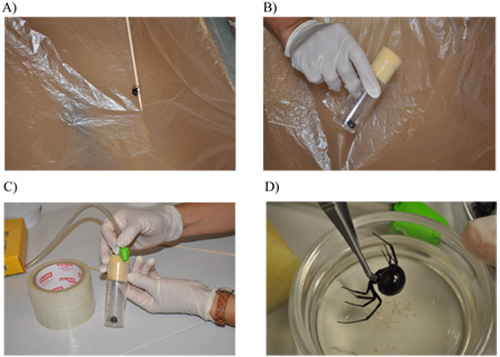
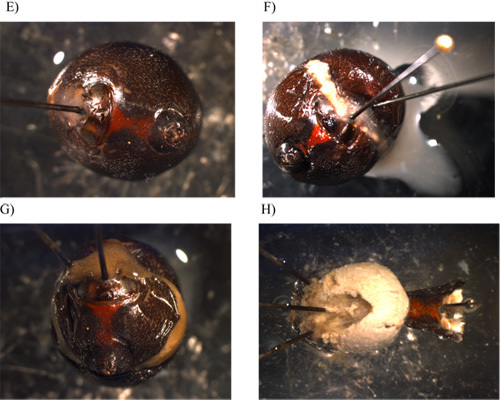 Figure 1. Handling, anesthetizing, and removal of the exoskeleton from the abdomen of a female black widow spider. A) Transfer of the spider into a cardboard box lined with plastic to facilitate moving the spider into a smaller plastic vial. B) Spider placed into a plastic vial with plug. C) Anesthetizing the spider with carbon dioxide gas. D) Separation of the cephalothorax from the abdomen using microscissors. E) Immobilization of the abdomen in the dissecting tray using insect pins. F) View after a lateral incision is made with the scissors and a portion of the exoskeleton is pealed back with forceps. G) Incisions being made perpendicular to the initial lateral cut. H) Removal of the exoskeleton and exposure of the fat layer.
Figure 1. Handling, anesthetizing, and removal of the exoskeleton from the abdomen of a female black widow spider. A) Transfer of the spider into a cardboard box lined with plastic to facilitate moving the spider into a smaller plastic vial. B) Spider placed into a plastic vial with plug. C) Anesthetizing the spider with carbon dioxide gas. D) Separation of the cephalothorax from the abdomen using microscissors. E) Immobilization of the abdomen in the dissecting tray using insect pins. F) View after a lateral incision is made with the scissors and a portion of the exoskeleton is pealed back with forceps. G) Incisions being made perpendicular to the initial lateral cut. H) Removal of the exoskeleton and exposure of the fat layer.
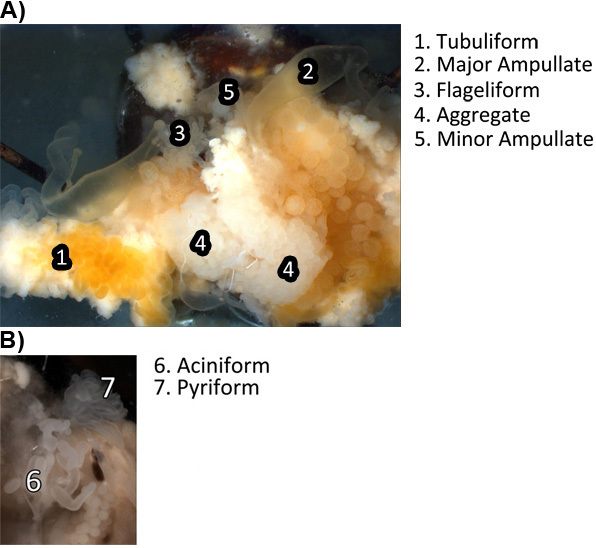 Figure 2. Visualization of the seven different silk-producing glands while intact in the abdomen of the spider as well as surrounding tissues. A) Images of the tubuliform, major ampullate, flagelliform, aggregate, minor ampullate tissues at 12X magnification. B) Image of the aciniform and pyriform glands at 12X magnification.
Figure 2. Visualization of the seven different silk-producing glands while intact in the abdomen of the spider as well as surrounding tissues. A) Images of the tubuliform, major ampullate, flagelliform, aggregate, minor ampullate tissues at 12X magnification. B) Image of the aciniform and pyriform glands at 12X magnification.
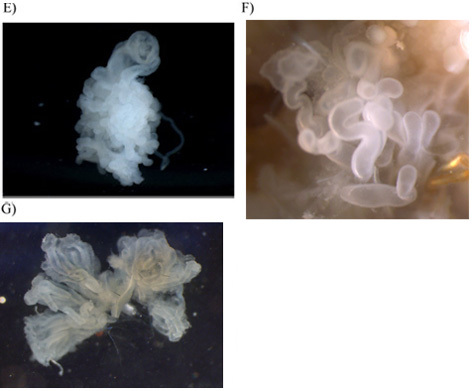 Figure 3. Photographs of the seven silk-producing glands after their removal from the abdomen. All images were captured at 20X magnification with the exception of the aciniform and pyriform glands, which were done at 40X. A) major ampullate gland; B) major and minor ampullate (right side) for size comparison; C) tubuliform; D) flagelliform; E) aggregate; F) aciniform; G) pyriform.
Figure 3. Photographs of the seven silk-producing glands after their removal from the abdomen. All images were captured at 20X magnification with the exception of the aciniform and pyriform glands, which were done at 40X. A) major ampullate gland; B) major and minor ampullate (right side) for size comparison; C) tubuliform; D) flagelliform; E) aggregate; F) aciniform; G) pyriform.
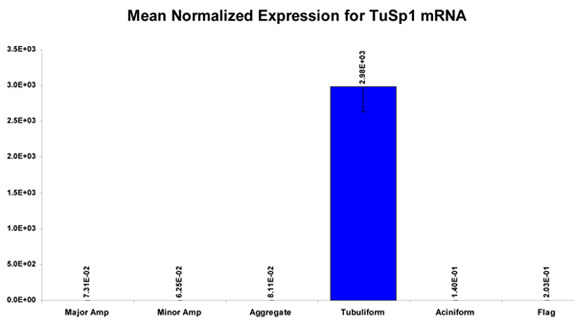 Figure 4. Representative results of the expression pattern of the silk gene, TuSp1, after surveying the TuSp1 mRNA levels in the different silk-producing glands (excluding the pyriform gland) using quantitative real-time PCR (qPCR) following isolation of total RNA from the glands.
Figure 4. Representative results of the expression pattern of the silk gene, TuSp1, after surveying the TuSp1 mRNA levels in the different silk-producing glands (excluding the pyriform gland) using quantitative real-time PCR (qPCR) following isolation of total RNA from the glands.
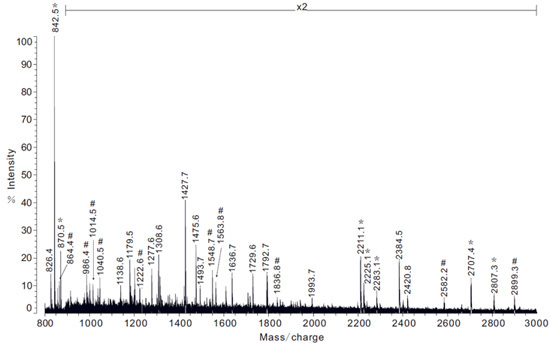 Figure 5. Example of MS analysis of protein extracts obtained from the pyriform gland following in-solution tryptic digestion. Note: x2 represents 2-fold magnification of spectrum intensity. Spectrum peptide ion masses that correspond to regions of the PySp1 fibroin are shown with the symbol #.
Figure 5. Example of MS analysis of protein extracts obtained from the pyriform gland following in-solution tryptic digestion. Note: x2 represents 2-fold magnification of spectrum intensity. Spectrum peptide ion masses that correspond to regions of the PySp1 fibroin are shown with the symbol #.
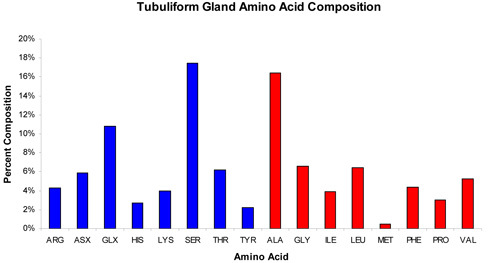 Figure 6. Typical result of the amino acid composition profile of the proteins extracted from the tubuliform gland. Note: ASX = Asp and Asn; GLX = Glu and Gln. Blue coloration reflects amino acids with polar side chain groups whereas red coloration represents amino acid residues with non-polar side chain groups.
Figure 6. Typical result of the amino acid composition profile of the proteins extracted from the tubuliform gland. Note: ASX = Asp and Asn; GLX = Glu and Gln. Blue coloration reflects amino acids with polar side chain groups whereas red coloration represents amino acid residues with non-polar side chain groups.
Discussion
Our methodology for the microdissection of the silk-producing glands from the black widow spider offers an effective means to obtain highly purified silk-producing glands. Dissections can be completed in 1.5 to 3 hrs, yielding a complete set of the seven distinct silk-producing glands from cobweavers. Obtaining highly purified silk-gland samples allows investigators the ability to perform a broad range of biochemical studies, including identification of new silk or chaperone proteins stored within the glandular luminal contents by using mass spectrometry, analysis of mRNA levels for genes selectively expressed in different glands, and culturing of specific glands in vitro to study the mechanism of silk production.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by a NSF RUI Grant MCB-0950372 entitled Molecular Characterization of Black Widow Spider Silks.
References
- Vollrath F, Knight DP. Int. J. Biol. Macromol. 1999;24:243–243. doi: 10.1016/s0141-8130(98)00095-6. [DOI] [PubMed] [Google Scholar]
- Gosline JM, Guerette PA, Ortlepp CS. J. Exp. Biol. 1999;202:3295–3295. doi: 10.1242/jeb.202.23.3295. [DOI] [PubMed] [Google Scholar]
- Gosline JM, DeMont ME, Denny MW. Endeavour. 1986;10:31–31. [Google Scholar]
- Hinman MB, Jones JA, Lewis RV. Trends Biotechnol. 2000;18(9):374–374. doi: 10.1016/s0167-7799(00)01481-5. [DOI] [PubMed] [Google Scholar]
- Lewis RV, Xu M. Proc. Natl. Acad. Sci. 1990;87:7120–7120. doi: 10.1073/pnas.87.18.7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin MA, Lewis RV. Protein Sci. 1998;7(3):667–667. doi: 10.1002/pro.5560070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Lewis RV. Appl. Phys. A-Mater. 2006;82:265–265. [Google Scholar]
- Hu X, Lawrence B, Kohler K. Biochemistry. 2005;44(30):10020–10020. doi: 10.1021/bi050494i. [DOI] [PubMed] [Google Scholar]
- Vasanthavada K, Hu X, Falick AM. J Biol Chem. 2007;282(48):35088–35088. doi: 10.1074/jbc.M705791200. [DOI] [PubMed] [Google Scholar]
- Hayashi CY, Blackledge TA, Lewis RV. Mol. Biol. Evol. 2004;21(10):1950–1950. doi: 10.1093/molbev/msh204. [DOI] [PubMed] [Google Scholar]
- Blasingame E, Tuton-Blasingame T, Larkin L. J Biol Chem. 2009;284(42):29097–29097. doi: 10.1074/jbc.M109.021378. [DOI] [PMC free article] [PubMed] [Google Scholar]


