Abstract
Purpose
Aromatase inhibitors (AIs) are effective for treatment of hormone receptor–positive breast cancer, but adherence and persistence with therapy are poor. Predictors of treatment discontinuation are not clearly defined. It is unknown whether patients with intolerable toxicity from one AI are able to tolerate another.
Patients and Methods
Women with early-stage breast cancer initiating AI therapy were enrolled onto a multicenter, prospective, open-label randomized trial of exemestane versus letrozole. Patients completed symptom questionnaires at baseline and serially during therapy. Patients who developed AI-associated intolerable symptoms and discontinued treatment were given the option to switch to the other study AI after a 2- to 8-week washout period.
Results
Of the 503 enrolled women, 32.4% discontinued initial AI therapy within 2 years because of adverse effects; 24.3% discontinued specifically because of musculoskeletal symptoms. Median time to treatment discontinuation as a result of any symptom was 6.1 months (range, 0.1 to 21.2 months) and was significantly shorter in patients randomly assigned to exemestane (hazard ratio [HR], 1.5; 95% CI, 1.1 to 2.1; P = .02). Younger age and taxane-based chemotherapy were associated with higher likelihood of treatment discontinuation (HR, 1.4; 95% CI, 1.02 to 1.9; P = .04; and HR, 1.9; 95% CI, 1.00 to 3.6; P = .048, respectively). Of the 83 patients who chose to switch to the second AI, 38.6% continued the alternate AI for a median of 13.7 months.
Conclusion
Premature discontinuation of initial AI therapy as a result of symptoms is common, although more than one third of patients may be able to tolerate a different AI medication. Additional research is needed to identify predictive tools and interventions for AI-associated treatment-emergent symptoms.
INTRODUCTION
Treatment with an aromatase inhibitor (AI) improves disease-free survival compared with tamoxifen1 and is recommended for inclusion in the treatment regimen for postmenopausal women with early-stage, hormone receptor (HR) –positive breast cancer.2 Cross-trial, indirect comparisons suggest that the three commercially available AIs, the azoles (letrozole and anastrozole) and the steroidal compound exemestane, have similar benefits and toxicities when compared with tamoxifen,3–7 and recently reported results demonstrate that the safety and efficacy of anastrozole are nearly identical to exemestane.4
Although aromatase inhibition was initially thought to be well tolerated, subsequent research and clinical experience have demonstrated that AIs are associated with frequently occurring toxicities that negatively impact persistence with therapy.8–10 Of these, musculoskeletal toxicities are the most common, occurring in up to 50% of patients.9 The etiology of AI-associated musculoskeletal symptoms remains unclear but may be a result, in part, of estrogen deprivation.9 Although AI-associated musculoskeletal symptoms seem to be a class effect, in one study, women who developed intolerable musculoskeletal symptoms while taking anastrozole were enrolled onto a clinical trial of letrozole therapy. Surprisingly, 71.5% of patients were able to tolerate the second AI for at least 6 months.11 These data suggest that individual patient differences may dictate intolerance to one but not another AI. Some studies have suggested that development of adverse effects may be associated with obesity, prior chemotherapy, and no prior tamoxifen therapy.10,12 However, none of these has been confirmed, and tools to predict which patients will develop AI-associated musculoskeletal symptoms are not currently available.
We prospectively enrolled patients with HR-positive breast cancer onto the Exemestane and Letrozole Pharmacogenetics (ELPh) clinical trial, in which several clinical phenotypes were carefully annotated after random assignment to either exemestane or letrozole.8 The overall primary objective of the ELPh trial was to correlate change in breast density with 2 years of AI therapy and inherited variants in the aromatase gene, CYP19. In the exploratory analysis reported in this article, we hypothesized that a proportion of women who could not tolerate exemestane could tolerate letrozole, and vice versa. The primary goal was to evaluate persistence with the second agent and to investigate clinical indicators of which patients might tolerate which of the two drugs.
PATIENTS AND METHODS
Patients
Eligible patients were recruited from August 2005 through July 2009 to the prospective ELPh trial (ClinicalTrials.gov identifier: NCT00228956). This trial was conducted by the Consortium on Breast Cancer Pharmacogenomics, which includes the Indiana University Bren and Melvin Simon Cancer Center, the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, and the University of Michigan Comprehensive Cancer Center. Detailed inclusion and exclusion criteria have previously been described.8 In brief, postmenopausal women with stage 0 to III HR-positive breast cancer were eligible. Prior tamoxifen was permitted, but women could not have previously received an AI. All indicated surgery, chemotherapy, and radiation therapy for breast cancer were completed before enrollment. The protocol was approved by the institutional review boards of all participating study sites. All enrolled patients provided written informed consent. The clinical trial was reviewed by an independent Data and Safety Monitoring Committee on a biannual basis.
Study Design
After enrollment, women were randomly assigned to treatment with exemestane (25 mg) or letrozole (2.5 mg) daily for 2 years. Random assignment was stratified based on prior tamoxifen, prior chemotherapy, and bisphosphonate therapy. Patients underwent clinical evaluation before starting treatment and after 1, 3, 6, 12, and 24 months and completed the modified Health Assessment Questionnaire (HAQ) and pain visual analog scale (VAS)8 at each time point. Patients at risk of ovarian function recovery underwent serum estradiol assessment at all study visits through month 12. Reasons for study discontinuation were recorded prospectively on a case report form by the study coordinators at each site. Patients who discontinued study participation because of adverse events were queried about the adverse effects that led to discontinuation of therapy.
After 132 patients had been enrolled, the protocol was amended to permit patients who reported intolerable AI-associated adverse effects to cross over to the other study medication. Patients discontinued the first AI and remained off therapy for a 2- to 8-week washout period. Patients completed the HAQ and VAS questionnaires at the time of new AI initiation and after 1 and 3 months of treatment. The protocol was subsequently amended to collect questionnaire data at the 6-month time point. Improvement or worsening of HAQ score was defined as a decrease or increase, respectively, of more than 0.22.13 Improvement or worsening of the VAS score was defined as a decrease or increase, respectively, of more than 2.0.14 Clinical data regarding treatment discontinuation after completion of cross-over participation were obtained by review of medical records, which were available for 80 of 83 patients.
Statistical Analysis
The primary end point of this descriptive study, which is an exploratory objective of the ELPh trial, is persistence with the second AI medication after discontinuation of the initial AI medication because of toxicity. Secondary analyses were performed evaluating discontinuation of initial AI therapy because of toxicity, defined as any patient-reported bothersome symptoms, as well as specifically because of musculoskeletal toxicity, defined as arthralgias, myalgias, joint pain or stiffness, tendinitis, numbness or tingling, and/or carpal tunnel syndrome.
Descriptive analyses were conducted for all continuous variables to study their underlying distribution. Comparisons of continuous variables between the two randomly assigned treatment groups (exemestane v letrozole) or other grouping variables (eg, discontinued AI for symptoms v continued AI) were made using t tests or simple logistic regression. For categorical variables, descriptors and comparisons between the groups were evaluated using contingency tables and Fisher's exact test.
The time from initiation to discontinuation of AI therapy was compared between the two treatment groups using the log-rank test, in the context of a Kaplan-Meier survival analysis. Patients who did not discontinue treatment were censored at the date of the last follow-up inquiry. Cox proportional hazards regression analysis was used to test for an independent contribution of the treatment variable, adjusting for the effects of other baseline characteristics related to time to treatment discontinuation. We report the hazard ratio (HR) and the corresponding P value for each covariate. The HR may be interpreted as a relative risk for early discontinuation of AI therapy.
RESULTS
Patient Characteristics
Baseline characteristics for all eligible patients enrolled onto this clinical trial are listed in Table 1. Three patients withdrew and were not randomly assigned. Mean follow-up was 15.5 ± 8.8 months, and all patients who remained on therapy have been observed for more than 12 months. Of the 500 eligible patients, 248 (49.6%) were randomly assigned to exemestane, and 252 (50.4%) were randomly assigned to letrozole. Almost half of randomly assigned patients had received adjuvant chemotherapy (n = 228, 45.6%), and 184 patients (36.8%) had been treated with tamoxifen for a median of 2.3 years (range, 0.2 to 12.9 years).
Table 1.
Baseline Patient Demographics or Clinical Characteristics for All Enrolled Patients, by Treatment Allocation and by Treatment Discontinuation
| Demographic or Clinical Characteristic | All Enrolled Patients (N = 500) |
Randomly Assigned to Letrozole (n = 252) |
Randomly Assigned to Exemestane (n = 248) |
Discontinued AI Because of Symptoms (n = 163) |
Continued AI (n = 294) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||||
| Median | 59 | 59 | 59 | 57 | 60 | |||||
| Range | 35-89 | 38-89 | 35-85 | 37-83 | 35-84 | |||||
| Race | ||||||||||
| White | 441 | 88.2 | 219 | 86.9 | 222 | 89.6 | 148 | 90.8 | 257 | 87.4 |
| Black | 46 | 9.2 | 27 | 10.7 | 19 | 7.7 | 12 | 7.4 | 27 | 9.2 |
| Other | 13 | 2.6 | 6 | 2.4 | 7 | 2.8 | 2 | 1.2 | 8 | 2.7 |
| Weight, kg | ||||||||||
| Mean | 79.3 | 79.2 | 79.4 | 78.7 | 80.5 | |||||
| SD | 17.4 | 17.7 | 17.2 | 16.1 | 17.9 | |||||
| BMI, kg/m2 | ||||||||||
| Mean | 29.9 | 30.0 | 29.9 | 29.7 | 30.3 | |||||
| SD | 6.4 | 6.6 | 6.2 | 6.2 | 6.5 | |||||
| Prior tamoxifen | 184 | 36.8 | 93 | 36.9 | 91 | 36.7 | 63 | 38.7 | 102 | 34.7 |
| Prior HRT | 242 | 48.1 | 114 | 45.2 | 127 | 51.2 | 79 | 48.5 | 151 | 51.4 |
| Prior chemotherapy | 228 | 45.6 | 114 | 45.2 | 114 | 46.0 | 72 | 44.2 | 129 | 43.9 |
| Time since chemotherapy, years | ||||||||||
| Median | 0.6 | 0.6 | 0.7 | 0.5 | 0.5 | |||||
| Range | 0-8.8 | 0-8.8 | 0-8.8 | 0-6.8 | 0-8.8 | |||||
| Prior taxane | 163 | 32.6 | 83 | 32.9 | 80 | 32.3 | 58 | 35.6 | 88 | 29.9 |
| Assigned AI | ||||||||||
| Letrozole | 252 | 50.4 | 252 | 100 | 0 | 0 | 72 | 44.2 | 163 | 55.4 |
| Exemestane | 248 | 49.6 | 0 | 0 | 248 | 100 | 91 | 55.8 | 131 | 44.6 |
| Baseline VAS score | ||||||||||
| Mean | 2.5 | 2.5 | 2.5 | 2.7 | 2.4 | |||||
| Range | 0-9.7 | 0-9.7 | 0-9.2 | 0-9 | 0-9.7 | |||||
| Baseline HAQ score | ||||||||||
| Mean | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | |||||
| Range | 0-2.25 | 0-2.25 | 0-1.5 | 0-1.625 | 0-2.25 | |||||
Abbreviations: AI, aromatase inhibitor; BMI, body mass index; HAQ, Health Assessment Questionnaire; HRT, hormone replacement therapy; SD, standard deviation; VAS, pain visual analog scale.
Discontinuation of AI Therapy
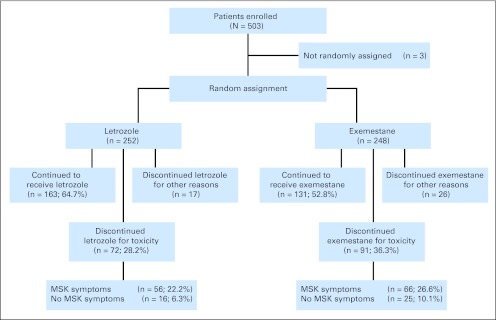
Analysis of the 500 eligible patients revealed that 163 patients (32.6%) discontinued therapy because of adverse effects (Fig 1). Ninety-one patients (36.7%) randomly assigned to exemestane, and 72 patients (28.6%) randomly assigned to letrozole discontinued therapy because of at least one treatment-emergent symptom, a difference that was statistically significant (P = .02).
Fig 1.
Patient flow and treatment discontinuation on first aromatase inhibitor. The musculoskeletal (MSK) symptoms group includes patients whose reason for treatment discontinuation included MSK symptoms.
Reasons for treatment discontinuation are listed in Appendix Table A1 (online only). Musculoskeletal symptoms were the primary patient-reported reasons for treatment discontinuation (exemestane, 66 [72.5%] of 91 patients; letrozole, 56 [77.8%] of 72 patients) and led to treatment discontinuation in 24.4% of the entire study population. Forty-three patients (8.6%) discontinued treatment for reasons other than toxicity (exemestane, 26 [10.5%] of 248 patients; letrozole, 17 [6.7%] of 252 patients), including recovery of ovarian function (n = 9). The eight exemestane-treated patients who recovered ovarian function (ages, 38 to 51 years) had recurrent menses, whereas the letrozole-treated patient (age 50 years) had an asymptomatic elevation in serum estradiol concentration.
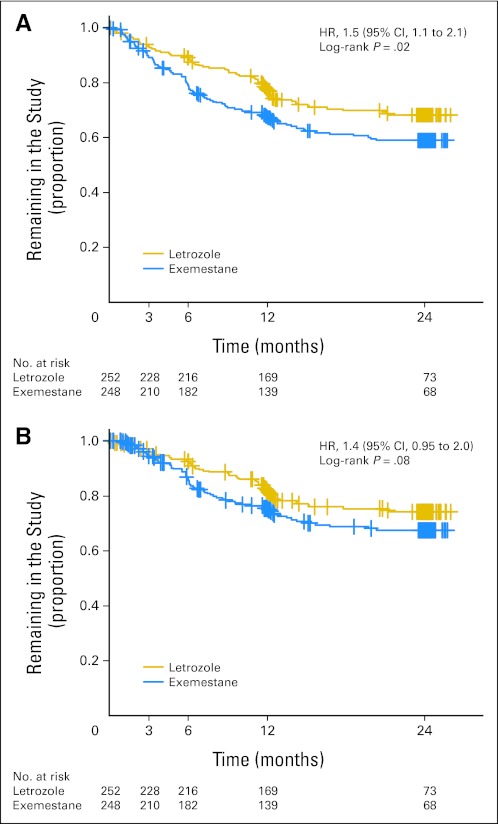
Median time to treatment discontinuation as a result of development of symptoms was 6.1 months (range, 0.1 to 21.2 months). Median time to treatment discontinuation specifically caused by musculoskeletal symptoms was 6.4 months (range, 0.1 to 21.2 months). Median time to treatment discontinuation as a result of development of symptoms was 5.8 months (range, 0.2 to 20.3 months) for patients assigned to exemestane and 8.1 months (range, 0.1 to 21.2 months) for patients assigned to letrozole. Time to treatment discontinuation as a result of any symptom was significantly shorter in patients randomly assigned to exemestane compared with letrozole (HR, 1.5; 95% CI, 1.1 to 2.1; P = .02; Fig 2A). There was a trend toward a shorter time to treatment discontinuation because of musculoskeletal symptoms for exemestane-treated patients (HR, 1.4; 95% CI, 0.95 to 2.0; P = .08; Fig 2B).
Fig 2.
(A) Time to treatment discontinuation for any patient-reported symptom, by drug. (B) Time to treatment discontinuation for patient-reported musculoskeletal symptoms, by drug. Proportion of patients remaining on the first aromatase inhibitor medication is given on the y-axis. HR, hazard ratio.
Predictors of Discontinuation of AI Therapy
Shorter time to treatment discontinuation as a result of any symptom remained significant in multivariate analysis for patients randomly assigned to exemestane compared with letrozole (HR, 1.4; 95% CI, 1.02 to 1.9; P = .03; Table 2). Patients younger than age 55 years (HR, 1.4; 95% CI, 1.0 to 1.9; P = .04), patients with higher baseline VAS scores (HR, 1.1; 95% CI, 1.00 to 1.2, for each 1-point increase; P = .04), and patients who received taxane-containing chemotherapy (HR, 1.9; 95% CI, 0.99 to 3.6; P = .048) were more likely to discontinue AI therapy as a result of any symptom. Patients who received taxane-containing chemotherapy (HR, 2.0; 95% CI, 1.00 to 4.0; P = .045) were also more likely to discontinue AI therapy because of musculoskeletal symptoms (Table 3).
Table 2.
Univariate and Multivariate Analysis of Predictors of Time to Treatment Discontinuation as a Result of Any Patient-Reported Treatment-Emergent Symptom
| Potential Predictor | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (≤ v > 55 years) | 1.4 | 1.02 to 1.9 | .03 | 1.4 | 1.0 to 1.9 | .04 |
| Race (white v other) | 0.8 | 0.5 to 1.3 | .35 | 0.7 | 0.3 to 1.5 | .33 |
| BMI (25-30 v ≤ 25 kg/m2) | 1.0 | 0.5 to 1.9 | .93 | 1.0 | 0.4 to 2.0 | 1.00 |
| BMI (> 30 v < 25 kg/m2) | 0.9 | 0.7 to 1.2 | .41 | 0.8 | 0.5 to 1.3 | .33 |
| Drug (exemestane) | 1.5 | 1.1 to 2.1 | .03 | 1.4 | 1.02 to 1.9 | .03 |
| Prior chemotherapy (yes v no) | 1.0 | 0.5 to 1.9 | .64 | 0.8 | 0.5 to 1.3 | .32 |
| Prior chemotherapy (taxane v nontaxane) | 1.8 | 0.97 to 3.3 | .06 | 1.9 | 0.99 to 3.6 | .048 |
| Prior tamoxifen | 1.2 | 0.9 to 1.6 | .28 | 1.2 | 0.9 to 1.7 | .28 |
| Prior HRT | 0.9 | 0.7 to 1.2 | .51 | 1.0 | 0.5 to 1.9 | .99 |
| Baseline HAQ score (per 1.0 change) | 1.0 | 0.5 to 2.0 | .98 | 0.9 | 0.5 to 1.6 | .71 |
| Baseline VAS score (per 1.0 change) | 1.1 | 0.99 to 1.2 | .06 | 1.1 | 1.0 to 1.2 | .04 |
| Bisphosphonate therapy at 1 and/or 3 months | 0.9 | 0.6 to 1.4 | .74 | 0.9 | 0.6 to 1.4 | .58 |
Abbreviations: BMI, body mass index; HAQ, Health Assessment Questionnaire; HR, hazard ratio; HRT, hormone replacement therapy; VAS, pain visual analog scale.
Table 3.
Univariate and Multivariate Analysis of Predictors of Time to Treatment Discontinuation as a Result of Patient-Reported Treatment-Emergent Musculoskeletal Symptoms
| Potential Predictor | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (≤ v > 55 years) | 0.7 | 0.5 to 1.0 | .048 | 0.7 | 0.5 to 1.1 | .09 |
| Race (white v other) | 0.9 | 0.3 to 2.7 | .85 | 0.9 | 0.4 to 2.2 | .81 |
| BMI (25-30 v ≤ 25 kg/m2) | 0.9 | 0.6 to 1.4 | .76 | 0.9 | 0.6 to 1.4 | .62 |
| BMI (> 30 v < 25 kg/m2) | 0.9 | 0.6 to 1.4 | .61 | 0.9 | 0.6 to 1.2 | .58 |
| Drug (exemestane) | 1.4 | 0.95 to 2.1 | .08 | 1.4 | 0.9 to 2.1 | .09 |
| Prior chemotherapy (yes v no) | 1.2 | 0.8 to 1.8 | .36 | 1.1 | 0.6 to 2.3 | .78 |
| Prior chemotherapy (taxane v nontaxane) | 1.9 | 0.96 to 3.8 | .06 | 2.0 | 1.0 to 4.0 | .045 |
| Prior tamoxifen | 1.2 | 0.8 to 1.8 | .34 | 1.2 | 0.8 to 1.9 | .43 |
| Prior HRT | 0.9 | 0.7 to 1.2 | .43 | 1.0 | 0.5 to 1.9 | .97 |
| Baseline HAQ score (per 1.0 change) | 1.1 | 0.7 to 1.8 | .71 | 1.0 | 0.4 to 2.1 | .93 |
| Baseline VAS score (per 1.0 change) | 1.1 | 0.97 to 1.2 | .12 | 1.1 | 0.9 to 1.3 | .22 |
| Bisphosphonate therapy at 1 and/or 3 months | 0.8 | 0.5 to 1.3 | .33 | 0.8 | 0.4 to 1.3 | .30 |
Abbreviations: BMI, body mass index; HAQ, Health Assessment Questionnaire; HR, hazard ratio; HRT, hormone replacement therapy; VAS, pain visual analog scale.
Incidence of Cross Over From One AI to the Other
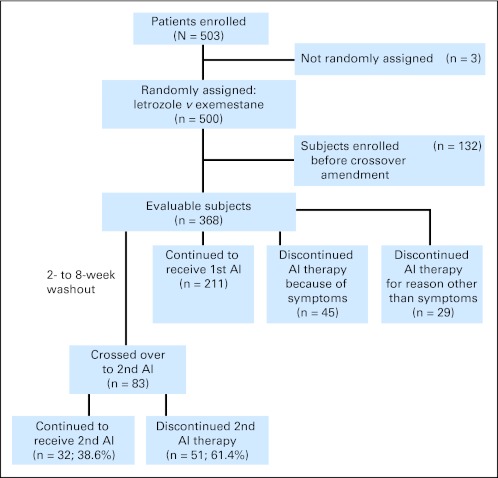
Because the option to cross over from the first to the second AI as a result of toxicity was added in an amendment, only the 368 patients who enrolled after the amendment was approved at the individual sites were eligible for the cross-over analysis (Fig 3). Of the evaluable cross-over cohort, 128 patients (34.8%) discontinued initial AI therapy because of intolerable symptoms after a median of 6.1 months (range, 0.2 to 21.2 months). Of those, 83 patients (63.8%) chose to cross over to the other drug, whereas the remainder chose to discontinue AI therapy and study participation. Characteristics of the cohort of patients who crossed over to the other AI are listed in Appendix Table A2 (online only). Forty-nine patients (26.8%) initially treated with exemestane switched to letrozole, whereas 34 patients (18.4%) initially treated with letrozole switched to exemestane.
Fig 3.
Patient flow diagram for aromatase inhibitor (AI) crossover in the Exemestane and Letrozole Pharmacogenetics trial.
Patients were analyzed for change in symptoms between discontinuation of the first AI and initiation of the second drug. Mean decreases in VAS and HAQ scores during the washout period were 1.23 and 0.07, respectively. On the basis of change in VAS score, 36.7% of patients experienced improvement in pain, 51.9% were unchanged, and 11.4% had worsening. Change in HAQ score revealed that 23.8% of patients experienced improvement in functional status, 55% were unchanged, and 21.2% had worsening.
Persistence With Second AI Treatment
Of the 83 patients who crossed over to the second AI, 51 (61.4%) discontinued the second AI medication after a median of 3.5 months (range, 0.2 to 27.8 months), 34 (66.7%) of whom discontinued therapy because of musculoskeletal symptoms. Seventy-eight percent of patients reported that the symptoms that led to discontinuation of the first AI also led to discontinuation of the second AI. Of the 32 patients (38.6%) who remained on AI therapy at their last clinic follow-up, the median duration of treatment with the second AI was 13.7 months (range, 2.8 to 38.8 months).
More of the patients originally treated with letrozole (23 of 34 patients, 67.6%) discontinued exemestane after cross over, compared with the reverse (28 of 49 patients, 57.1%), although this difference was not statistically significant. In univariate and multivariate Cox regression analysis, no potential predictors of discontinuation of the second AI medication were statistically significant (Appendix Table A3, online only).
DISCUSSION
In this prospective analysis of AI treatment discontinuation, we demonstrated that approximately one third of patients developed intolerable adverse effects, primarily musculoskeletal in nature, during AI therapy. In addition, we prospectively observed that more than one third of patients who developed severe symptoms while taking the first AI were able to tolerate a different AI. We further determined that young age, taxane chemotherapy, and pre-existing pain are predictors of discontinuation of adjuvant AI therapy.
Our data are consistent with previously published studies that have demonstrated poor adherence and persistence with adjuvant endocrine therapy. Significant strengths of our study include prospective data collection from patients enrolled at the initiation of AI therapy, comparison of patient experiences on two different AIs, and preplanned switching to the other AI if patients developed intolerance to the first AI. Our study design permits analysis of each patient's entire experience with AI therapy and includes a switch between nonsteroidal and steroidal AI therapy, whereas the other reported trial of switching from one AI to another only enrolled patients at the time of initiation of the second AI medication and only evaluated nonsteroidal AI medications.11
Unique to this study, we demonstrated that exemestane was associated with a shorter time to discontinuation of initial AI therapy compared with letrozole. We observed the same trend in higher early treatment discontinuation rates with exemestane in the cross-over population. These observations are in contrast to reports from the MA.27 randomized clinical trial of anastrozole versus exemestane for adjuvant breast cancer therapy, in which similar treatment discontinuation rates were reported for the two AI-treated cohorts.4 In addition, our observations are in contrast to findings in the MAP.3 randomized clinical trial of exemestane versus placebo for breast cancer prevention.15 In MAP.3, the treatment discontinuation rate because of drug-related toxicity was 15.6%, which was 4.6% higher than what was reported in the MAP.3 placebo arm but less than half of the discontinuation rate that we are reporting in our ELPh trial. There are differences in study design and conduct between our study and the MA.27 and MAP.3 studies that could account for the different findings. In MAP.3 in particular, the patient population was substantially different from the ELPh trial. The women enrolled onto the prevention trial were older and had not previously been diagnosed with or treated for cancer, which are all factors that could potentially influence tolerability. In addition, in the ELPh trial, we focused on time to treatment discontinuation, as opposed to simple discontinuation rates. Finally, although both ELPh and MA.27 evaluated a nonsteroidal versus a steroidal AI, the nonsteroidal AIs in the two studies were different. Although the difference in tolerability of the two medications demonstrated in our study has potentially significant implications for clinical practice, our finding requires replication to determine its true clinical importance.
The etiology of the musculoskeletal symptoms is unknown.16,17 Therefore, we were unable to assess mechanism-specific predictors of development of toxicity. Rather, we focused on nonmechanism-based, clinical predictive factors of toxicity, which could be used to guide individualized treatment decision making at the time of endocrine therapy initiation or to direct specific modifying interventions as they become available.18 Our observation that younger age and prior taxane-based chemotherapy conferred a greater likelihood of treatment discontinuation is consistent with prior reports of the same factors potentially being predictive of development of AI-associated toxicity.10,19 However, in contrast to published data of predictors of toxicity, prior tamoxifen therapy, prior hormone replacement therapy, and body mass index did not predict for persistence with therapy in our trial.10,12 One possible explanation for the discrepancies between the previously published studies and this report is the difference in clinical end point.
One of the most important observations from this study is the ability of more than one third of women to tolerate a second AI when they could not tolerate the first. Although cross over from exemestane to letrozole seemed slightly more successful than the converse, this difference was not statistically significant. A substantial limitation of our trial design is that participation in the cross-over portion of the study was not required. Therefore, patients who opted to switch to the other AI may have been more motivated, resulting in a higher percentage of patients able to tolerate the second AI. Regardless, this is still a reasonable strategy to use in the clinic, because our results demonstrate that a subset of patients will be able to continue AI therapy despite intolerance of the initial AI.
The reason for tolerance of a second AI when a patient is unable to tolerate the first AI is unclear. The majority of patients in our trial had no change in musculoskeletal symptoms during the washout period, so improvement off therapy was not predictive of persistence with the second AI. We were unable to identify any predictors of persistence with the second AI, although our ability to detect differences was limited by the small sample size and relatively brief follow-up. More than 95% of patients had detectable plasma drug concentrations during the cross-over study period (data not shown), suggesting that patients were actually taking the second AI. One possible explanation of the ability to tolerate one AI but not the other includes differences in activity or toxicity of different AI medications or different classes of AI therapy. Differences in degree of inhibition of aromatase activity between drugs are unlikely, however, because patients originally taking letrozole could subsequently tolerate exemestane, and vice versa.
Approximately half of the women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial who developed joint symptoms on anastrozole experienced improvement in symptoms during 6 months of continued therapy.20 The authors hypothesized that tissues may adjust to the low estrogen concentrations over time, resulting in improvement in symptoms. This same phenomenon may permit patients intolerant of the first AI to tolerate the second AI, even though estrogen deprivation was interrupted during the brief washout period. Prospective clinical evaluation of this hypothesis would require a randomized clinical trial of continuing the initial AI versus switching to a different AI, which would be challenging to conduct in a highly symptomatic population.
In summary, at least one third of patients prematurely discontinue adjuvant AI therapy, and although age, prior treatments, and pre-existing pain may impact toxicity, none is an absolute predictor. More importantly, more than one third of patients who switch drugs may tolerate the second AI. Additional biochemical and genetic studies designed to refine existing predictors of these adverse effects for individual patients are warranted. A better understanding of the mechanisms underlying development of AI-associated toxicities is important, because this could yield clues to more accurate predictors of development of toxicity and guide future interventional symptom prevention or management strategies. Overall, this additional information may help improve tolerance of the medications, thereby improving quality of life and persistence with therapy and, by extension, breast cancer outcomes.
Support
Supported in part by Pharmacogenetics Research Network Grant No. U-01 GM61373 (D.A.F.) and Clinical Pharmacology Training Grant No. 5T32-GM08425 (D.A.F.) from the National Institute of General Medical Sciences, National Institutes of Health (NIH), Bethesda, MD, and by Grants No. M01-RR000042 (University of Michigan), M01-RR00750 (Indiana University), and M01-RR00052 (Johns Hopkins University) from the National Center for Research Resources (NCRR), a component of the NIH. N.L.H. is a Damon Runyon-Lilly Clinical Investigator supported (in part) by the Damon Runyon Cancer Research Foundation (CI#53-10). In addition, these studies were supported by grants from Pfizer (D.F.H.), Novartis Pharma AG (D.F.H.), and the Fashion Footwear Association of New York/QVC Presents Shoes on Sale (D.F.H.).
Appendix
Table A1.
Reasons for Treatment Discontinuation, by Drug
| Reason for Treatment Discontinuation | No. of Patients |
|
|---|---|---|
| Letrozole | Exemestane | |
| Patient-reported symptoms | ||
| Musculoskeletal symptoms | 55 | 65 |
| Neuropathy/CTS | 7 | 10 |
| Depression, anxiety, cognitive dysfunction | 8 | 9 |
| Fatigue, insomnia | 9 | 9 |
| GI (eg, nausea, diarrhea, constipation, bloating) | 3 | 9 |
| Headache | 4 | 4 |
| Gynecologic (decreased libido, vaginal dryness) | 2 | 3 |
| Weight gain | 2 | 2 |
| Rash | 2 | 2 |
| Hot flashes | 1 | 3 |
| Dizziness | 2 | 1 |
| Total patient-reported symptoms | 72 | 91 |
| Other reasons for study discontinuation | ||
| Ovarian function recurrence | 1 | 8 |
| Noncompliance | 3 | 7 |
| Patient/physician preference | 9 | 7 |
| Breast cancer recurrence | 4 | 4 |
| Total other reasons | 17 | 26 |
NOTE. Patients could report more than one symptom leading to treatment discontinuation.
Abbreviation: CTS, carpal tunnel syndrome.
Table A2.
Demographics and Clinical Characteristics of Patients Eligible for Cross Over
| Demographic or Clinical Characteristic | Crossed Over to Second AI (n = 83) |
Declined Cross Over (n = 45) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 60 | 55 | ||
| Range | 37-80 | 41-83 | ||
| Race | ||||
| White | 76 | 91.6 | 39 | 86.7 |
| Black | 6 | 7.2 | 5 | 11.1 |
| Other | 1 | 1.2 | 1 | 2.2 |
| Weight, kg | ||||
| Mean | 80.6 | 74 | ||
| SD | 16.5 | 12.3 | ||
| BMI, kg/m2 | ||||
| Mean | 30.5 | 27.9 | ||
| SD | 6.2 | 4.3 | ||
| Prior tamoxifen | 25 | 30.1 | 19 | 42.2 |
| Prior HRT | 46 | 55.4 | 16 | 35.6 |
| Prior chemotherapy | 37 | 44.6 | 19 | 42.2 |
| Prior taxane | 32 | 38.6 | 16 | 35.6 |
| Assigned AI | ||||
| Letrozole | 34 | 41.0 | 22 | 48.9 |
| Exemestane | 49 | 59.0 | 23 | 51.1 |
| Baseline VAS score | ||||
| Mean | 3.0 | 2.0 | ||
| Range | 0-9.0 | 0-7.8 | ||
| Baseline HAQ score | ||||
| Mean | 0.2 | 0.2 | ||
| Range | 0-1.50 | 0-1.375 | ||
| VAS score at discontinuation of initial AI | ||||
| Mean | 5.7 | 4.8 | ||
| Range | 0-10 | 0-8.5 | ||
| HAQ score at discontinuation of initial AI | ||||
| Mean | 0.250 | 0.313 | ||
| Range | 0-2.125 | 0-1.375 | ||
Abbreviations: AI, aromatase inhibitor; BMI, body mass index; HAQ, Health Assessment Questionnaire; HRT, hormone replacement therapy; SD, standard deviation; VAS, pain visual analog scale.
Table A3.
Univariate and Multivariate Analysis of Predictors of Time to Discontinuation of the Second AI as a Result of Any Patient-Reported Treatment-Emergent Symptom for the Cross-Over Cohort
| Potential Predictor | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (≤ v > 55 years) | 0.8 | 0.2 to 4.3 | .54 | 0.5 | 0.2 to 1.3 | .13 |
| Race (white v other) | 0.9 | 0.7 to 1.1 | .79 | 2.0 | 0.5 to 7.8 | .31 |
| BMI (25-30 v ≤ 25 kg/m2) | 1.5 | 0.8 to 2.9 | .36 | 2.2 | 0.9 to 5.4 | .08 |
| BMI (> 30 v < 25 kg/m2) | 0.6 | 0.2 to 1.9 | .22 | 0.8 | 0.4 to 1.7 | .54 |
| Drug (exemestane) | 0.8 | 0.02 to 41.3 | .38 | 0.9 | 0.5 to 1.5 | .69 |
| Prior chemotherapy (v no chemotherapy) | 1.0 | 0.4 to 2.1 | .91 | 0.6 | 0.3 to 1.3 | .17 |
| Prior tamoxifen | 1.3 | 0.6 to 2.6 | .38 | 1.7 | 0.8 to 3.6 | .16 |
| Prior HRT | 1.3 | 0.6 to 2.9 | .46 | 1.9 | 0.7 to 4.8 | .17 |
| Baseline HAQ score (per 1.0 change) | 0.7 | 0.3 to 2.0 | .52 | 1.4 | 0.2 to 8.6 | .71 |
| Baseline VAS score (per 1.0 change) | 1.0 | 0.5 to 2.1 | .49 | 0.9 | 0.7 to 1.1 | .36 |
| Time on first AI | 1.0 | 0.5 to 2.0 | .72 | 1.0 | 0.4 to 2.2 | .18 |
| Duration of washout between first and second AI | 1.0 | 0.4 to 2.4 | .50 | 1.0 | 0.5 to 2.1 | .10 |
| Change in HAQ score between first and second AI | 0.7 | 0.2 to 3.2 | .38 | 0.6 | 0.2 to 1.8 | .34 |
| Change in VAS score between first and second AI | 1.0 | 0.5 to 2.1 | .64 | 1.1 | 0.8 to 1.5 | .50 |
Abbreviations: AI, aromatase inhibitor; BMI, body mass index; HAQ, Health Assessment Questionnaire; HR, hazard ratio; HRT, hormone replacement therapy; VAS, pain visual analog scale.
Footnotes
Written on behalf of the Consortium on Breast Cancer Pharmacogenomics.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00228956.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: Daniel F. Hayes, Oncimmune Honoraria: Vered Stearns, AstraZeneca; Anna Maria Storniolo, Pfizer Research Funding: N. Lynn Henry, AstraZeneca, Lilly; David A. Flockhart, Pfizer, Novartis; Vered Stearns, Pfizer, Novartis; Daniel F. Hayes, AstraZeneca, Pfizer, Novartis, Veridex; Anna Maria Storniolo, Pfizer, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: N. Lynn Henry, David A. Flockhart, Vered Stearns, Daniel F. Hayes, Anna Maria Storniolo
Financial support: David A. Flockhart, Daniel F. Hayes
Administrative support: Anne T. Nguyen, Karineh Tarpinian
Provision of study materials or patients: N. Lynn Henry, Vered Stearns, Daniel F. Hayes, Anna Maria Storniolo
Collection and assembly of data: N. Lynn Henry, Faouzi Azzouz, Anne T. Nguyen, Suzanne Lemler, Jill Hayden, Karineh Tarpinian, Elizabeth Yakim
Data analysis and interpretation: N. Lynn Henry, Faouzi Azzouz, Zereunesay Desta, Lang Li, David A. Flockhart, Vered Stearns, Daniel F. Hayes, Anna Maria Storniolo
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 2.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goss PE, Ingle JN, Pater JL, et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol. 2008;26:1948–1955. doi: 10.1200/JCO.2007.11.6798. [DOI] [PubMed] [Google Scholar]
- 4.Goss PE, Ingle JN, Chapman J-AW, et al. Final analysis of NCIC CTG MA.27: A randomized phase III trial of exemestane versus anastrozole in postmenopausal women with hormone receptor positive primary breast cancer. 33rd Annual San Antonio Breast Cancer Symposium; December 8-12, 2010; San Antonio, TX. abstr S1-1. [Google Scholar]
- 5.Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 6.Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): A randomised controlled trial. Lancet. 2007;369:559–570. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 7.Coates AS, Keshaviah A, Thürlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1-98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 8.Henry NL, Giles JT, Ang D, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111:365–372. doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry NL, Giles JT, Stearns V. Aromatase inhibitor-associated musculoskeletal symptoms: Etiology and strategies for management. Oncology (Williston Park) 2008;22:1401–1408. [PubMed] [Google Scholar]
- 10.Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 11.Briot K, Tubiana-Hulin M, Bastit L, et al. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: The ATOLL (articular tolerance of letrozole) study. Breast Cancer Res Treat. 2010;120:127–134. doi: 10.1007/s10549-009-0692-7. [DOI] [PubMed] [Google Scholar]
- 12.Sestak I, Cuzick J, Sapunar F, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: A retrospective, exploratory analysis. Lancet Oncol. 2008;9:866–872. doi: 10.1016/S1470-2045(08)70182-7. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe F, Michaud K, Strand V. Expanding the definition of clinical differences: From minimally clinically important differences to really important differences—Analyses in 8931 patients with rheumatoid arthritis. J Rheumatol. 2005;32:583–589. [PubMed] [Google Scholar]
- 14.Farrar JT, Pritchett YL, Robinson M, et al. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: Analyses of data from clinical trials of duloxetine in pain disorders. J Pain. 2009;11:109–118. doi: 10.1016/j.jpain.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 16.Henry NL, Pchejetski D, A'Hern R, et al. Inflammatory cytokines and aromatase inhibitor-associated musculoskeletal syndrome: A case-control study. Br J Cancer. 2010;103:291–296. doi: 10.1038/sj.bjc.6605768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingle JN, Schaid DJ, Goss PE, et al. Genome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J Clin Oncol. 2010;28:4674–4682. doi: 10.1200/JCO.2010.28.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry NL, Banerjee M, Wicha M, et al. Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer. 2011;117:5469–5475. doi: 10.1002/cncr.26230. [DOI] [PubMed] [Google Scholar]
- 19.Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115:3631–3639. doi: 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buzdar AU. Clinical features of joint symptoms observed in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial. J Clin Oncol. 2006;24(suppl 1):15s. abstr 551. [Google Scholar]