Abstract
Purpose
Adherence to evidence-based treatment guidelines has been proposed as a measure of cancer care quality. We sought to determine rates of and factors associated with adherence to the National Comprehensive Cancer Network (NCCN) treatment guidelines for colon cancer.
Patients and Methods
Patients within the National Cancer Data Base treated for colon adenocarcinoma (2003 to 2007) were identified. Adherence to stage-specific NCCN guidelines was determined based on disease stage. Hierarchical regression analyses were performed to identify factors predictive of adherence, overtreatment, and undertreatment.
Results
A total of 173,243 patients were included in the final cohort, 123,953 (71%) of whom were treated according to NCCN guidelines. Patients with stage I disease were more likely to receive guideline-based treatment (96%) than patients with stage II (low risk, 66%; high risk, 36%), III (71%), or IV (73%) disease (P < .001). Adherence to consensus-based guidelines increased over time. Factors associated with adherence across all stages included age, Charlson-Deyo comorbidity index score, later year of diagnosis, and insurance status. Among patients with high-risk stage II or stage III disease, older patients with pre-existing comorbidities and patients with lower socioeconomic status were less likely to be offered adjuvant chemotherapy. Among patients with stage I and II disease, young, healthy patients were more likely to be recommended chemotherapy, in discordance with NCCN guidelines.
Conclusion
Significant variation exists in the treatment of colon cancer, particularly in treatment of high-risk stage II and stage III disease. The impact of nonadherence to guidelines on patient outcomes needs to be further elucidated.
INTRODUCTION
In 1999, a seminal report from the Institute of Medicine revealed the existence of significant variation between ideal and observed cancer care across the United States.1 This variation, defined in part by the overuse, underuse, or misuse of diagnostic and treatment modalities, has generated increasing demand for up-to-date evidence-based clinical practice guidelines and appropriate performance measures.2–12 These benchmarks are currently assuming even greater importance in this era of value-based purchasing programs because US hospitals will increasingly be required to publicly disclose and in turn receive financial remuneration based on adherence to quality indices.13–17
Variation in the management of colonic adenocarcinoma has been reported,18,19 although there is a paucity of data on the sources and distribution of this variation at the national level. Despite this lack of data, there is a high degree of consensus among several guideline-generating agencies, including the National Comprehensive Cancer Network (NCCN), the American Society of Clinical Oncology, and the National Quality Forum, on specific standards of care for colon cancer treatment.3,4,20,21 Such information could provide an opportunity to standardize care for the approximately 100,000 patients in the United States who are diagnosed annually with this malignancy.22
In this study, we sought to determine hospital-based, stage-specific rates of adherence to NCCN treatment guidelines for colon cancer using the National Cancer Data Base (NCDB). In addition, we attempted to elucidate potential factors associated with adherence as well as nonadherence with these guidelines, in terms of both undertreatment and overtreatment. Our central hypothesis is that the stage-specific adherence to NCCN treatment guidelines varies and is most strongly associated with comorbidity and insurance status.
PATIENTS AND METHODS
Data Sources
The NCDB is a hospital-based cancer registry that is a joint program of the American College of Surgeons Commission on Cancer and the American Cancer Society. The NCDB is the largest cancer registry of its kind; it captures approximately 70% of all cancer diagnoses annually within the United States from more than 1,400 hospitals accredited by the Commission on Cancer. Data are collected by trained abstracters, and data quality assessments are carried out using a combination of electronic and site-specific methods.23,24
Definition of Patient Cohort
The International Classification of Diseases for Oncology, third edition (ICD-03) was used to identify patients within the NCDB (2003 to 2007) who were diagnosed with adenocarcinoma of the colon (ICD-03 topography codes C180 and C182 to C199 and histology codes 8140 to 8144, 8210 to 8211, 8220 to 8221, 8260 to 8263, 8440, 8480 to 8481, and 8490) and who received their first course of treatment at the reporting facility. Patients were subsequently restaged according to the American Joint Committee on Cancer staging system (sixth edition) using pathologic data for tumor depth and number of positive nodes resected and clinical and pathologic data for the presence of distant metastases.25 Patients with stage II disease were further subcategorized into low risk and high risk based on tumor depth (T3 v T4), histologic grade (< v ≥ 3), margin status (R0 v R1), and number of nodes retrieved after lymphadenectomy (≥ v < 12 nodes). The initial cohort was further restricted to include patients who were most likely to be eligible to receive chemotherapy—those less than 80 years of age with a Charlson-Deyo comorbidity index score less than 2. In addition, in an attempt to account for potential under-reporting of chemotherapy by some hospitals, patients who were treated at institutions with a less than 20% rate of recommending adjuvant chemotherapy to patients with stage III disease were excluded.26 Adequacy of surgical resection was determined based on the most invasive treatment received, for example colectomy in a patient who underwent polypectomy followed by a colectomy. Data used in these analyses were restricted to 2003 to 2007 because of the availability of comorbidity variables.3,20
Outcomes
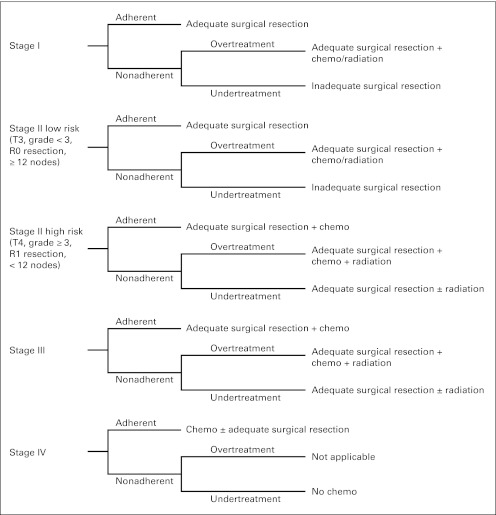
Adherence to stage-specific treatment was the primary outcome for the study (Fig 1) based on the NCCN treatment guidelines for colon cancer.20 Adherence was based on whether adjuvant chemotherapy had been recommended, independent of whether it was actually received. Furthermore, adherence for high-risk stage II colon cancer was defined as treatment with both adequate surgery and chemotherapy, which is supported by the contemporary literature27,28 and provided as an option by the NCCN.20 In addition, nonadherence to treatment guidelines was defined and examined as two separate outcomes. Specifically, the factors associated with overtreatment and undertreatment were determined.
Fig 1.
Stage-specific treatment for colon cancer based on the National Comprehensive Cancer Network guidelines.
Statistical Analyses
To determine which clinicopathologic and socioeconomic factors were associated with adherent treatment, overtreatment, or undertreatment relative to the NCCN guidelines (Fig 1), two-level, stage-specific, hierarchical regression models were created, with controlling for the heterogeneity of variance at both the patient and hospital levels.29–31 Hierarchical models predicting one of three outcomes (adherence, overtreatment, or undertreatment) as the dependent variable were assessed for adequate discrimination and fit, and forest plots were created from the resultant odds ratios (ORs) and respective 95% CIs. All available demographic and clinical data were categorized (Table 1) and examined in the models. The presence of interaction between categorical variables was tested. C statistics were calculated to assess model fit.32 Statistical analyses were performed using SAS version 9.2 for Windows (SAS Institute, Cary, NC).
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | % of Patients |
P | ||||
|---|---|---|---|---|---|---|
| Stage I(n = 36,367) | Stage II |
Stage III(n = 53,127) | Stage IV(n = 38,443) | |||
| Low Risk(n = 22,792) | High Risk(n = 22,514) | |||||
| Male | 51 | 50 | 51 | 50 | 52 | < .001 |
| Age, years | < .001 | |||||
| < 50 | 9 | 14 | 10 | 16 | 18 | |
| 50-64 | 37 | 35 | 33 | 37 | 40 | |
| 65-74 | 35 | 32 | 35 | 31 | 28 | |
| ≥ 75 | 19 | 19 | 22 | 16 | 14 | |
| Race/ethnicity | < .001 | |||||
| White | 80 | 78 | 78 | 76 | 74 | |
| African American | 11 | 12 | 12 | 14 | 16 | |
| Hispanic | 4 | 5 | 5 | 5 | 5 | |
| Asian/Pacific Islander | 3 | 3 | 3 | 3 | 3 | |
| Other | 2 | 2 | 2 | 2 | 2 | |
| Charlson-Deyo comorbidity index score | < .001 | |||||
| 0 | 77 | 77 | 77 | 79 | 81 | |
| 1 | 23 | 23 | 23 | 21 | 19 | |
| Insurance status | < .001 | |||||
| Uninsured | 2 | 4 | 4 | 4 | 6 | |
| Medicaid | 2 | 4 | 4 | 4 | 7 | |
| Medicare | 47 | 45 | 50 | 42 | 38 | |
| Government* | 1 | 1 | 1 | 1 | 1 | |
| Private | 46 | 44 | 39 | 47 | 46 | |
| Unknown | 2 | 2 | 2 | 2 | 2 | |
| Median household income | < .001 | |||||
| < $30,000 | 12 | 13 | 15 | 14 | 15 | |
| $30,000-$45,999 | 43 | 43 | 45 | 43 | 44 | |
| ≥ $46,000 | 39 | 38 | 34 | 37 | 35 | |
| Unknown | 6 | 6 | 6 | 6 | 6 | |
| Year of diagnosis | < .001 | |||||
| 2003 | 20 | 18 | 23 | 20 | 20 | |
| 2004 | 20 | 18 | 22 | 20 | 20 | |
| 2005 | 21 | 19 | 21 | 20 | 20 | |
| 2006 | 20 | 21 | 19 | 20 | 20 | |
| 2007 | 19 | 24 | 15 | 20 | 20 | |
| Treatment region | < .001 | |||||
| West | 14 | 15 | 15 | 16 | 15 | |
| Midwest | 26 | 26 | 25 | 25 | 26 | |
| Northeast | 22 | 22 | 21 | 21 | 21 | |
| South | 38 | 37 | 39 | 38 | 39 | |
| Treatment facility | < .001 | |||||
| Teaching hospital | 20 | 19 | 23 | 20 | 18 | |
| Community cancer center | 52 | 51 | 52 | 51 | 47 | |
| Community hospital | 28 | 30 | 25 | 29 | 35 | |
Includes federal insurance programs such as Veterans Affairs, TRICARE/Military, and Public Health Service.
RESULTS
Patient Population and Stage Distribution
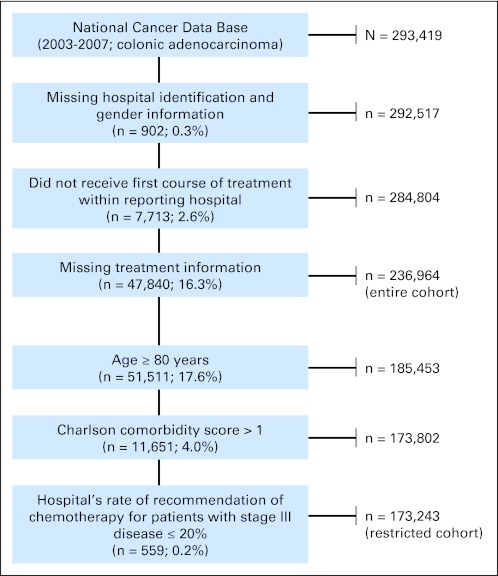
A total of 293,419 patients were identified before applying the exclusion criteria (Fig 2). The final analytic cohort included 173,243 patients with colon adenocarcinoma. The clinicopathologic characteristics of these patients are listed in Table 1. The median age was 64 years (range, 19 to 79 years), and there was a relatively even distribution for sex and year of diagnosis. There was also minimal variation (< 10%) in the distribution of patients by stage (stage I, 21%; stage II low risk, 13%; stage II high risk, 13%; stage III, 31%; and stage IV, 22%). The majority of patients were white, held private insurance, and were treated at community cancer centers. Of patients categorized as having high-risk stage II disease, 23% had T4 tumors, 30% had histologic grade ≥ 3, 8% had positive margins, and 65% had less than 12 nodes retrieved.
Fig 2.
Selection of the study cohort.
Stage-Specific Treatment and Adherence Patterns
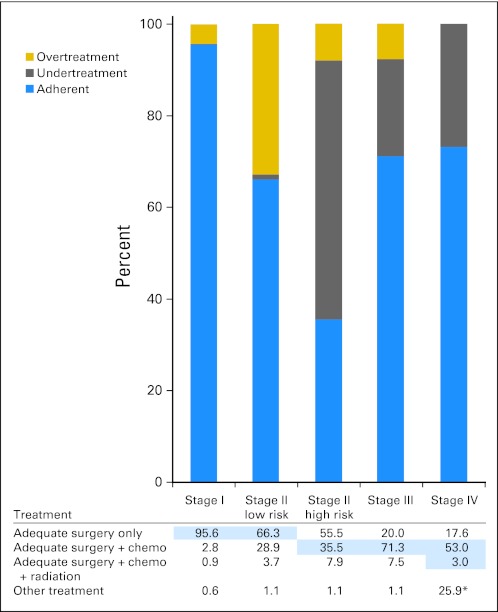
Stage-specific treatment and adherence patterns are presented in Figure 3. Of note, there was a significant variation for the treatment of stage II high-risk patients, with 56% of patients treated with adequate surgery only. This resulted in a low adherence rate for this subgroup, with only 36% of high-risk stage II patients receiving both adequate surgery and chemotherapy.
Fig 3.
Stage-specific treatment and adherence patterns. Chemo, chemotherapy. (*) Only includes undertreatment (no chemotherapy) or adherent (chemotherapy) patients; no surgery included.
Overall, 123,845 (72%) of 173,243 patients in the cohort received adherent treatment. Patients with stage I disease were more likely to receive guideline-based treatment (96%) than patients with stage II (low risk, 66%; high risk, 36%), III (71%), or IV (73%) disease (P < .001; Fig 3).
Nonadherence within stage I was largely attributed to overtreatment (4% of patients); only 0.4% of patients with stage I disease were undertreated (with inappropriate use of polypectomy or lack of surgical resection; Fig 3). A majority of patients with stage III (71%) and stage IV (73%) disease received recommendations for chemotherapy in accordance with the NCCN guidelines.
Factors Associated With Adherence to Treatment Guidelines
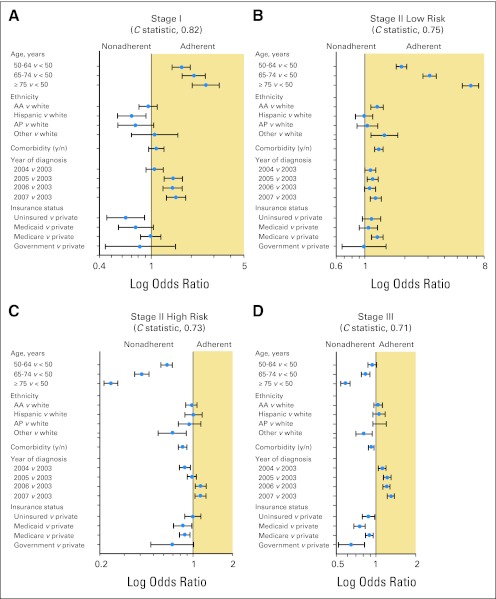
Forest plots generated from hierarchical regression models depicting the factors associated with stage-specific adherence to NCCN guidelines are presented in Figure 4. For patients with stage I and low-risk stage II disease, increasing age was independently associated with a greater likelihood of receiving adherent treatment. For age 50 to 64 years versus age less than 50 years, ORs were as follows: stage I, 1.67 (95% CI, 1.43 to 1.96); and low-risk stage II, 1.90 (95% CI, 1.73 to 2.07). Similarly, for patients with low-risk stage II disease, a Charlson-Deyo comorbidity index score of greater than 0 was associated with a greater likelihood of receiving adherent treatment (OR, 1.28; 95% CI, 1.18 to 1.38). An opposite trend was observed for high-risk stage II and stage III disease, with older patients and those with a greater number of comorbid conditions being more likely to receive nonadherent treatment (Fig 4). Stage III patients without private insurance (OR, 0.87; 95% CI, 0.78 to 0.97) were less likely than insured patients to receive guideline-based treatment. Across all stages, a later year of diagnosis was independently associated with an increased likelihood of receiving guideline-based care; for 2007 versus 2003, ORs were as follows: stage I, 1.53 (95% CI, 1.30 to 1.81); low-risk stage II, 1.21 (95% CI, 1.10 to 1.33); high-risk stage II, 1.14 (95% CI, 1.04 to 1.26); stage III, 1.29 (95% CI, 1.21 to 1.38); and stage IV, 1.26 (95% CI, 1.17 to 1.36).
Fig 4.
Forest plot of factors predicting adherent treatment for (A) stage I, (B) stage II low-risk, (C) stage II high-risk, and (D) stage III disease. Odds ratios (blue dots) are shown with associated 95% CIs (horizontal lines). Models were also adjusted for sex, education, income, geographic region, and facility type as appropriate. Government includes federal insurance programs such as Veterans Affairs, TRICARE/Military, and Public Health Service. AA, African American; AP, Asian/Pacific Islander.
Factors Associated With Undertreatment and Overtreatment
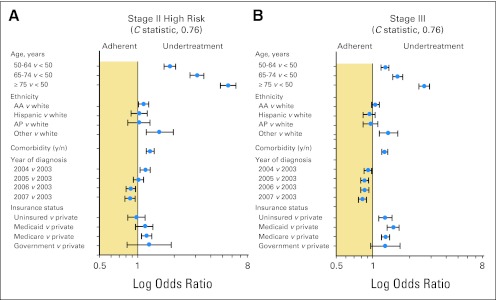
To delineate factors independently associated with undertreatment or overtreatment relative to the NCCN guidelines, separate hierarchical models were constructed (Fig 5). For patients with high-risk stage II and stage III disease, increasing age and comorbidity score were independently associated with undertreatment relative to the NCCN guidelines (Fig 5). Lack of insurance and earlier year of diagnosis were also independently associated with undertreatment (Fig 5). For patients with stage I and low-risk stage II disease, the following factors were associated with a lower likelihood of overtreatment: increasing age compared with age less than 50 years (50 to 64 years: OR, 0.54; 95% CI, 0.50 to 0.59; 65 to 74 years: OR, 0.35; 95% CI, 0.32 to 0.39; ≥ 75 years: OR, 0.18; 95% CI, 0.16 to 0.21); increasing Charlson-Deyo comorbidity index score (OR, 0.83; 95% CI, 0.78 to 0.89); nonwhite versus white race/ethnicity (OR, 0.77; 95% CI, 0.62 to 0.94); and diagnosis in 2007 versus 2003 (OR, 0.79; 95% CI, 0.73 to 0.86).
Fig 5.
Forest plot of factors predicting undertreatment for (A) high-risk stage II and (B) stage III disease. Odds ratios (blue dots) are shown with associated 95% CIs (horizontal lines). Models were also adjusted for sex, education, income, geographic region, and facility type as appropriate. Government includes federal insurance programs such as Veterans Affairs, TRICARE/Military, and Public Health Service. AA, African American; AP, Asian/Pacific Islander.
DISCUSSION
Using a large, nationally representative hospital-based cancer registry, we have found significant variation in the stage-specific management of colon cancer with adherence to NCCN guidelines estimated for stage I (96%), stage II low-risk (66%), stage II high-risk (36%), stage III (71%), and stage IV (73%) disease. Factors associated with adherence across all stages included age, comorbidity, later year of diagnosis, and insurance status.
In this analysis, we defined adherence for high-risk stage II colon cancer as treatment with both adequate surgery and chemotherapy, which resulted in a low adherence rate of 36% for this subgroup. The nonadherence was predominantly attributable to undertreatment in the form of lack of recommended adjuvant chemotherapy, especially for older patients with pre-existing comorbidities. However, given the mixed results from contemporary reports as to the benefit of adjuvant therapy in nonstratified patients with stage II disease, this variation in practice may reflect deliberate, carefully considered deviation from the guidelines.33–39 Indeed, although numerous retrospective and population-based reports suggest that there may be a survival benefit from systemic therapy in high-risk cohorts, most randomized trials to date have not been designed to address or been adequately powered to detect a survival difference in patients with node-negative disease.33,37,40,41 Both the NCCN and American Society of Clinical Oncology suggest that adjuvant chemotherapy be considered in patients with stage II disease who have pathologically defined high-risk features.4,20,21 Although we were able to stratify patients according to these criteria, other proposed high-risk features, such as lymphovascular invasion or perforation, are not currently collected within the NCDB, and the lack of information about these features may have resulted in the misclassification of some patients as having low-risk stage II disease. Despite this limitation, our observation that a significantly greater proportion of patients with high-risk stage II disease than of patients with low-risk stage II disease received recommendations for adjuvant chemotherapy implies that some clinicians believe that there is a clinical benefit from adjuvant chemotherapy for this high-risk cohort. Moreover, two recent pooled analyses of at least seven randomized trials have indicated that with the exception of inadequate lymphadenectomy, the pathologic factors that are used to define high-risk stage II disease have potential value in predicting response to chemotherapy, suggesting that guideline adherence is consistent with current best evidence.34,37 Controversy still exists, however, given that data from recent randomized controlled trials, such as Eastern Cooperative Oncology Group trial 5202, which specifically aimed to identify subsets of patients with stage II disease who are most likely to respond to adjuvant therapy, will require several years to mature.42 Although practice guidelines should be based on evidence, they are not necessarily always derived from high-quality, level I data, and therefore, caution should be applied when attempting to equate guideline adherence with quality of care.43 A recent report by Poonacha and Go44 noted that many of the NCCN guidelines were based on lower levels of scientific evidence.
However, there is strong evidence supporting the use of adjuvant chemotherapy in patients with stage III disease.33–38,45 In this analysis, we observed that only 71.3% of patients with stage III disease were recommended adjuvant chemotherapy. It is important to emphasize that adherence was based on whether adjuvant chemotherapy was recommended by the clinician, independent of whether it was actually received. As may be encountered in any large cancer registry, however, there may have been under-reporting of recommendations for adjuvant chemotherapy within the NCDB, resulting in an underestimation of true adherence rates.26,46 Our results are consistent with two observational studies based on Surveillance, Epidemiology, and End Results–Medicare data and Cancer Care Outcomes Research and Surveillance Consortium registry data, which reported similar rates for the receipt of adjuvant chemotherapy among elderly patients with stage III colon cancer.18,46 In contrast to our results, a more recent study that examined chemotherapy or medical oncology evaluation for stage III colon cancer using Surveillance, Epidemiology, and End Results–Medicare data reported a 96.8% concordance for the aggregate patient-level analyses and 71.5% concordance for the hospital-level analysis.19 This discrepancy in patient-level concordance could be related to a number of factors including comparison of cohorts, which vary by demographic or socioeconomic factors, or study exclusion criteria, which limited the final analytic cohort from 10,712 to 6,112 patients with stage III colon cancer.
Another important finding of our study was that among patients with stage III disease, older age was associated with undertreatment, independent of pre-existing comorbidities as well as other clinicopathologic and socioeconomic factors. Our analysis excluded patients older than 80 years, which likely resulted in an overestimation of adherence for the older age group. A recent prospective survey of American oncologists revealed that in the absence of secondary comorbid conditions, more than 90% of respondents would recommend adjuvant therapy to an 80-year-old patient with stage III colon cancer.47 Despite the reported survey results, there is a large body of literature demonstrating that older persons are less likely to receive or complete chemotherapy and are more likely to experience higher adverse events.
The consensus among clinicians is consistent with results of a pooled analysis of seven randomized controlled trials, which demonstrated a similar survival benefit from chemotherapy in patients older than 70 years and their younger counterparts.40 However, the majority of individual studies are known to have either underenrolled or excluded patients older than 75 years. A recent observational study reported results consistent with our findings: Elderly patients with stage III disease were less likely to receive adjuvant therapy despite having fewer long-term adverse events relative to their younger counterparts.18 It is clear that assessment of treatment adherence in older patients is complex given the inter-related nature of comorbidities, performance status, and clinician bias.
Although undertreatment of patients with pathologically confirmed or potential node-positive disease has become a focus of the National Quality Forum and some value-based purchasing programs, overtreatment of patients with early-stage disease, especially those with stage I disease, has not received similar consideration as a contributor to lower quality of care.4,48 We found that nonadherence in patients with stage I and low-risk stage II disease was primarily attributable to overtreatment in the form of recommendations for adjuvant chemotherapy, especially for young, healthy, insured patients. To our knowledge, there is no high- level evidence suggesting improved survival outcomes after receipt of systemic therapy within this cohort. Moreover, given the adverse effect profile of current chemotherapy regimens, the potentially deleterious effects of overtreatment, both to the patient and to an overburdened health care system seeking high-quality, low-cost interventions, suggest that overtreatment of patients with early-stage adenocarcinoma of the colon may be a valuable, albeit relatively understudied, opportunity for quality improvement. In stage III patients, overtreatment was noted for patients receiving radiation therapy in addition to surgical resection and chemotherapy. It is possible that this finding is related to misclassification of a high rectal or rectosigmoid cancer as a sigmoid cancer or appropriate treatment of a perforated tumor, which cannot be defined within these data.
Limitations of the NCDB data are that treatment data were missing for 16% of patients and coding errors may exist, which are likely not random and may have led to overestimation or underestimation of the exact proportion of patients receiving adherent or nonadherent treatment. Despite these limitations, a significant deviation in adherence to NCCN clinical practice guidelines for colon cancer was still detected. Given the variation in the level of evidence used to construct these guidelines, whether adherence to the guidelines correlates with improved survival or patient-reported outcomes or is an appropriate quality measure is somewhat contentious.5,10–17 It is essential that guidelines be updated frequently to integrate current best evidence and that guidelines indicate the strength of the recommendations based on the quality of this evidence.43,49 In addition, the generalizability of these findings is uncertain with respect to hospitals outside of the Commission on Cancer, particularly given that 90% of the NCDB patients were noted to have health insurance.
Although the rate of adherence to guidelines increased steadily over the 5-year period examined, there was still marked variation in treatment patterns among hospitals accredited by the American College of Surgeons Commission on Cancer, particularly for patients with high-risk stage II and stage III colon cancer. To determine the true impact of nonadherence to guidelines, treatment-associated disease-specific survival outcomes must be examined. Furthermore, value-based purchasing programs that provide financial incentives in an attempt to promote adherence to clinical practice guidelines or other surrogate quality measures will undoubtedly have to account for the subjectivity inherent in the generation of these benchmarks, as well as for other sources of variation, including patient preference.
Acknowledgment
We acknowledge Dr Thomas Feeley for his financial support of the research project.
Footnotes
Supported by the Institute for Cancer Care Excellence at The University of Texas MD Anderson Cancer Center and by Grant No. CA016672 from the National Institutes of Health.
Presented at the Plenary Session of the 64th Annual Cancer Symposium of the Society of Surgical Oncology, March 2-5, 2011, San Antonio, TX.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Ryaz Chagpar, Yan Xing, Barry W. Feig, George J. Chang, Y. Nancy You, Janice N. Cormier
Collection and assembly of data: Ryaz Chagpar, Yan Xing, Barry W. Feig, George J. Chang, Y. Nancy You, Janice N. Cormier
Data analysis and interpretation: Ryaz Chagpar, Yan Xing, Yi-Ju Chiang, George J. Chang, Y. Nancy You, Janice N. Cormier
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Hewitt ME, Simone JV. National Cancer Policy Board: Ensuring Quality Cancer Care. Washington, DC: National Academy Press; 1999. [Google Scholar]
- 2.Somerfield MR, Einhaus K, Hagerty KL, et al. American Society of Clinical Oncology clinical practice guidelines: Opportunities and challenges. J Clin Oncol . 2008;26:4022–4026. doi: 10.1200/JCO.2008.17.7139. [DOI] [PubMed] [Google Scholar]
- 3.Desch CE, McNiff KK, Schneider EC, et al. American Society of Clinical Oncology/National Comprehensive Cancer Network Quality Measures. J Clin Oncol . 2008;26:3631–3637. doi: 10.1200/JCO.2008.16.5068. [DOI] [PubMed] [Google Scholar]
- 4.National Quality Forum Measure Details: National Voluntary Consensus Standards for Quality of Cancer Care. http://www.qualityforum.org/Publications/2009/05/National_Voluntary_Consensus_Standards_for_Quality_of_Cancer_Care.aspx.
- 5.Blayney DW, McNiff K, Hanauer D, et al. Implementation of the Quality Oncology Practice Initiative at a university comprehensive cancer center. J Clin Oncol . 2009;27:3802–3807. doi: 10.1200/JCO.2008.21.6770. [DOI] [PubMed] [Google Scholar]
- 6.Agency for Healthcare Research and Quality: Homepage. http://www.qualityindicators.ahrq.gov/ [DOI] [PubMed]
- 7.The Joint Commission's Annual Report on Quality and Safety: Improving America's hospitals. http://www.jointcommission.org/assets/1/18/2010_Annual_Report.pdf.
- 8.Stulberg JJ, Delaney CP, Neuhauser DV, et al. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA . 2010;303:2479–2485. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson JO, Neuss MN, McNiff KK, et al. Improvement in oncology practice performance through voluntary participation in the Quality Oncology Practice Initiative. J Clin Oncol. 2008;26:1893–1898. doi: 10.1200/JCO.2007.14.2992. [DOI] [PubMed] [Google Scholar]
- 10.Bach PB. Using practice guidelines to assess cancer care quality. J Clin Oncol . 2005;23:9041–9043. doi: 10.1200/JCO.2005.03.6111. [DOI] [PubMed] [Google Scholar]
- 11.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med . 2003;348:2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 12.Smith TJ, Hillner BE. Ensuring quality cancer care by the use of clinical practice guidelines and critical pathways. J Clin Oncol . 2001;19:2886–2897. doi: 10.1200/JCO.2001.19.11.2886. [DOI] [PubMed] [Google Scholar]
- 13.Kahn JM, Scales DC, Au DH, et al. An official American Thoracic Society policy statement: Pay-for-performance in pulmonary, critical care, and sleep medicine. Am J Respir Crit Care Med . 2010;181:752–761. doi: 10.1164/rccm.200903-0450ST. [DOI] [PubMed] [Google Scholar]
- 14.Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med . 2009;360:2390–2393. doi: 10.1056/NEJMp0900963. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services. http://www.cms.gov/home/rsds.asp. [DOI] [PubMed]
- 16.Fung CH, Lim YW, Mattke S, et al. Systematic review: The evidence that publishing patient care performance data improves quality of care. Ann Intern Med . 2008;148:111–123. doi: 10.7326/0003-4819-148-2-200801150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Lindenauer PK, Remus D, Roman S, et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med . 2007;356:486–496. doi: 10.1056/NEJMsa064964. [DOI] [PubMed] [Google Scholar]
- 18.Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA . 2010;303:1037–1045. doi: 10.1001/jama.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg CC, Lipsitz SR, Neville B, et al. Receipt of appropriate surgical care for Medicare beneficiaries with cancer. Arch Surg . 2011;146:1128–1134. doi: 10.1001/archsurg.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 21.Benson AB, 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol . 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute: SEER Cancer Statistics Review 1975-2008. http://seer.cancer.gov/csr/1975_2008/results_merged/sect_06_colon_rectum.pdf.
- 23.Winchester DP, Stewart AK, Phillips JL, et al. The national cancer data base: Past, present, and future. Ann Surg Oncol . 2010;17:4–7. doi: 10.1245/s10434-009-0771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: A powerful initiative to improve cancer care in the United States. Ann Surg Oncol . 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Joint Committee on Cancer: AJCC Cancer Staging Manual (ed 6) Chicago, IL: Springer; 2002. [Google Scholar]
- 26.Cress RD, Zaslavsky AM, West DW, et al. Completeness of information on adjuvant therapies for colorectal cancer in population-based cancer registries. Med Care . 2003;41:1006–1012. doi: 10.1097/01.MLR.0000083740.12949.88. [DOI] [PubMed] [Google Scholar]
- 27.Bastos DA, Ribeiro SC, de Freitas D, et al. Combination therapy in high risk stage II or stage III colon cancer: Current practice and future prospects. Ther Adv Med Oncol . 2010;2:261–272. doi: 10.1177/1758834010367905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dotan E, Cohen SJ. Challenges in the management of stage II colon cancer. Semin Oncol . 2011;38:511–520. doi: 10.1053/j.seminoncol.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai J, Li Z, Rocke D. Hierarchical logistic regression modeling with SAS GLIMMIX. http://www.lexjansen.com/wuss/2006/Analytics/ANL-Dai.pdf.
- 30.O'Connell AA, McCoach DB. Applications of hierarchical linear models for evaluations of health interventions: Demystifying the methods and interpretations of multilevel models. Eval Health Prof . 2004;27:119–151. doi: 10.1177/0163278704264049. [DOI] [PubMed] [Google Scholar]
- 31.Cohen ME, Dimick JB, Bilimoria KY, et al. Risk adjustment in the American College of Surgeons National Surgical Quality Improvement Program: A comparison of logistic versus hierarchical modeling. J Am Coll Surg . 2009;209:687–693. doi: 10.1016/j.jamcollsurg.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Peng C, Lee K, Ingersoll G. An introduction to logistic regression analysis and reporting. J Educ Res . 2002;96:3–14. [Google Scholar]
- 33.Quasar Collaborative Group. Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 34.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol . 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 35.International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. J Clin Oncol . 1999;17:1356–1363. [PubMed] [Google Scholar]
- 36.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol . 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 37.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J Clin Oncol. 2004;22:1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson NW, Yothers G, Lopa S, et al. Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: Pooled analysis of NSABP C-01 through C-05—A baseline from which to compare modern adjuvant trials. Ann Surg Oncol . 2010;17:959–966. doi: 10.1245/s10434-009-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figueredo A, Charette ML, Maroun J, et al. Adjuvant therapy for stage II colon cancer: A systematic review from the Cancer Care Ontario Program in evidence-based care's gastrointestinal cancer disease site group. J Clin Oncol . 2004;22:3395–3407. doi: 10.1200/JCO.2004.03.087. [DOI] [PubMed] [Google Scholar]
- 40.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med . 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 41.Compton CC. Optimal pathologic staging: Defining stage II disease. Clin Cancer Res . 2007;13:6862s–6870s. doi: 10.1158/1078-0432.CCR-07-1398. [DOI] [PubMed] [Google Scholar]
- 42.National Cancer Institute: Phase III randomized study of oxaliplatin, leucovorin calcium, and fluorouracil with versus without bevacizumab in patients with resected stage II colon cancer and at high risk for recurrence based on molecular markers. http://www.cancer.gov/clinicaltrials/ECOG-E5202.
- 43.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol . 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 44.Poonacha TK, Go RS. Level of scientific evidence underlying recommendations arising from the National Comprehensive Cancer Network clinical practice guidelines. J Clin Oncol . 2011;29:186–191. doi: 10.1200/JCO.2010.31.6414. [DOI] [PubMed] [Google Scholar]
- 45.Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol . 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu CY, Delclos GL, Chan W, et al. Assessing the initiation and completion of adjuvant chemotherapy in a large nationwide and population-based cohort of elderly patients with stage-III colon cancer. Med Oncol . 2011;28:1062–1074. doi: 10.1007/s12032-010-9644-7. [DOI] [PubMed] [Google Scholar]
- 47.Keating NL, Landrum MB, Klabunde CN, et al. Adjuvant chemotherapy for stage III colon cancer: Do physicians agree about the importance of patient age and comorbidity? J Clin Oncol . 2008;26:2532–2537. doi: 10.1200/JCO.2007.15.9434. [DOI] [PubMed] [Google Scholar]
- 48.Centers for Medicare and Medicaid Services: Medicare program; hospital inpatient value-based purchasing program. Final rule. Fed Regist . 2011;76:26490–26547. [PubMed] [Google Scholar]
- 49.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]