Abstract
Epidermal growth factor (EGF) and tumor necrosis factor-α (TNF-α) signaling are critical for effective proliferative and apoptotic actions; however, little is known about the codependency of these signaling pathways in the intestinal epithelium. Because total parenteral nutrition (TPN) is associated with loss of intestinal epithelial cell (IEC) proliferation and increased apoptosis, we utilized a mouse model to explore these transactivation pathways in small bowel epithelium. Mice underwent intravenous cannulation and were given enteral nutrition or TPN for 7 days. Outcomes included IEC proliferation, apoptosis, and survival. To address transactivation or dependence of EGF and TNF on IEC physiology, TNF-α receptor knockout (KO) mice, TNFR1-KO, R2-KO, or R1R2-double KO, were used. Exogenous EGF and pharmacological blockade of ErbB1 were performed in other groups to examine the relevance of the ErB1 pathway. TPN increased IEC TNFR1 and decreased EGF and ErbB1 abundance. Loss of IEC proliferation was prevented by exogenous EGF or blockade of TNFR1. However, EGF action was prevented without effective TNFR2 signaling. Also, blockade of TNFR1 could not prevent loss of IEC proliferation without effective ErbB1 signaling. TPN increased IEC apoptosis and was due to increased TNFR1 signaling. Exogenous EGF or blockade of TNFR1 could prevent increased apoptosis, and both pathways were dependent on effective ErbB1 signaling. Exogenous EGF prevented increased apoptosis in mice lacking TNFR2 signaling. TPN mice had significantly decreased survival vs. controls, and this was associated with the TNFR1 signaling pathway. We concluded that these findings identify critical mechanisms that contribute to TPN-associated mucosal atrophy via altered TNF-α/EGF signaling. It emphasizes the importance of both TNFR1 and TNFR2 pathways, as well as the strong interdependence on an intact EGF/ErbB1 pathway.
Keywords: tumor necrosis factor
total parenteral nutrition (TPN) is vital for the support of patients who cannot tolerate enteral nutrition. However, TPN has been associated with a number of clinical problems including a decline in intestinal epithelial cell (IEC) proliferation and increased apoptosis, and together these processes can lead to intestinal mucosal atrophy (19, 23). These changes may have clinical implications, as TPN in humans is associated with increased septic and infectious complications (19). The mechanisms contributing to atrophy are not completely clear. Using a mouse model, our laboratory previously showed that TPN led to decreased p-Akt expression (8, 9). Epidermal growth factor (EGF) is a major upstream factor that can drive phosphatidylinositol 3-kinase (PI3K)/P-Akt signaling (26). EGF signaling occurs via one of four tyrosine kinase receptors, namely ErbB1–4, and these receptors are essential for modulating cell survival, proliferation, and differentiation in various tissues (4). ErbB1 has been shown to be critical for mediating both IEC proliferation and antiapoptotic actions (10). Recent evidence demonstrates that EGF signaling is dependent on intact tumor necrosis factor (TNF)-α signaling (29).
The cytokine TNF-α is produced as a 26-kDa homotrimeric, transmembrane precursor that is cleaved by metalloproteinases into a soluble 17-kDa polypeptide (2, 3, 24). TNF-α binds to two distinct transmembrane receptors: the 55-kDa low-affinity TNF receptor (TNFR1), and the 75-kDa high-affinity TNFR2 (13, 15, 20). Normally the proinflammatory action via TNFR1 signaling is balanced by a protective action via TNFR2 (6).
Recently, it has been appreciated that TNF-α and ErbB family can transactivate a number of similar intracellular pathways including Akt and MAP/ERK (10, 21, 32). TNF-α has typically been viewed as mediating inflammation and apoptosis. However, many of these actions are balanced or modulated via EGF signaling. Loss of EGFR expression or inhibition of its kinase activity lead to increased TNF-induced apoptosis (32). Additionally, IEC apoptosis predominates in the absence of effective EGFR signaling, presumably via unopposed TNF signaling. Conversely, TNF-α can also sustain IEC proliferation (15), upregulate ErbB2, as well as promote EGFR phosphorylation (21, 32). What is not known within the gastrointestinal epithelium is the relative importance of the TNFR1 vs. TNFR2 signaling pathways and transactivation of TNF signaling via EGF.
How these changes in IEC proliferation/apoptosis occur vary greatly depending on tissue type and duration of stimuli. Interestingly, our mouse TPN model represents a state of increased TNF-α expression, as well as increased IEC apoptosis and reduced IEC proliferation (30, 33, 34). This model, therefore, offers a unique opportunity to study mechanisms contributing to villus atrophy in the absence of overt inflammatory changes or epithelial destruction in inflammatory bowel disease models. These observations led us to hypothesize that derangement in the TNF-α/EGF transactivation signaling machinery mechanistically contributes to TPN-associated mucosal atrophy.
The present study demonstrates for the first time key mechanisms responsible for TPN-associated atrophy, including increased TNF-α signaling via TNFR1 and a TNF-α-independent mechanism via loss of EGF signaling. Conversely, exogenous EGF can prevent the marked increase in TNFR1 occurring with TPN. We further demonstrate the novel finding that EGF signaling was partly dependent on an intact TNFR2 pathway to maintain IEC proliferation. Additionally, prevention of TPN-associated atrophy via blockade of TNFR1 is dependent on an intact EGF signaling. Finally, the marked loss of survival in TPN mice is predominately due to increased signaling via the TNFR1 pathway.
MATERIALS AND METHODS
Animals.
C57BL/6 male, specific pathogen-free 10-wk-old mice (Jackson Laboratory, Bar Harbor, ME) were maintained under controlled temperature, humidity, and light conditions. Body weight-matched and age-matched adult male TNFR1R2 double knockout (DKO; Tnfrsf1atm1Imx Tnfrsf1btm1Imx/J ), TNFR1-KO (TNFR1KO Tnfrsf1atm1Imx/J ), and TNFR2-KO mice (R2KO Tnfrsf1btm1Mwm/J), with C57BL6J background (Jackson) were also studied. Mice were housed in metabolic cages to prevent coprophagia. Studies conformed to approval by our University Committee on Use and Care of Animals (Approval: 7703). Catheterized mice initially received 5% dextrose in 0.45 normal saline with 20 meq KCl/l at 4.8 ml/day. After 24 h, mice were randomized to enterally fed control or TPN groups.
TPN model.
Protocols are as described previously (35, 37). Enteral controls received intravenous crystalloid, standard laboratory chow, and water ad libitum. TPN mice received intravenous TPN at 4.8 ml/day. The TPN + EGF group received EGF at 25 μg/kg per 12 h (in 200 μl saline; Sigma-Aldrich, St. Louis, MO) via oral gavage, starting 1 day postcannulation (18). ErbB1 inhibitor, gefitinib (2.5 mg/ml in 1% aqueous Tween-80, 200 μl; LC Laboratories, Woburn, MA) was administered via oral gavage twice daily, starting 3 days before intravenous cannulation and continuing until mice were killed (12, 17, 25). Nitrogen and energy delivery was matched between groups (isonitrogenous/isocaloric) (38); mice were killed 7 days postcannulation using CO2. Mice would be euthanized before death if their health deteriorated to a level felt not acceptable for the welfare and wellbeing of the animal; all the procedures were in accordance with University Laboratory Animal Committee.
Real-time PCR.
RNA extraction of mucosal scrapings were as previously described (7, 34). Oligomers were designed using an optimization program (www.premierbiosoft.com) (Table 1). Real-time PCR was performed and normalized to β-actin (7).
Table 1.
Primers used in this experiment
| Gene | GenBank Accession No. | Forward | Reverse | Size, bp |
|---|---|---|---|---|
| Amphiregulin | NM_009704 | GGTCTTAGGCTCAGGCCATTA | CGCTTATGGTGGAAACCTCTC | 137 |
| EGF | NM_010113 | TTCTCACAAGGAAAGAGCATCTC | GTCCTGTCCCGTTAAGGAAAAC | 175 |
| TGF-α | NM_031199 | CACTCTGGGTACGTGGGTG | CACAGGTGATAATGAGGACAGC | 136 |
| HBEGF | NM_010415 | CGGGGAGTGCAGATACCTG | TTCTCCACTGGTAGAGTCAGC | 102 |
| Epiregulin | NM_007950 | CTGCCTCTTGGGTCTTGACG | GCGGTACAGTTATCCTCGGATTC | 115 |
| Betacellulin | NM_007568 | AATTCTCCACTGTGTGGTAGCA | GGTTTTCACTTTCTGTCTAGGGG | 121 |
| Nrg1 | NM_178591 | TCAGCAAGTTAGGAAACGACAG | ACATAAGGTCTTTCAGTTGAGGC | 100 |
| Nrg3 | NM_008734 | TTACGCTGTAGCGACTGCATC | GCCTACCACGATCCATTTAAGC | 123 |
| Nrg4 | NM_032002 | CACGCTGCGAAGAGGTTTTTC | CGCGATGGTAAGAGTGAGGA | 104 |
| EGFR (ErbB1) | NM_207655 | GCATCATGGGAGAGAACAACA | TCAGGAACCATTACTCCATAGGT | 100 |
| TNFR1 | NM_100609 | CCGGGAGAAGAGGGATAGCTT | TCGGACAGTCACTCACCAAGT | 113 |
| TNFR2 | NM_011610 | ACACCCTACAAACCGGAACC | AGCCTTCCTGTCATAGTATTCCT | 66 |
| Bax | NM_007527 | TGAAGACAGGGGCCTTTTTG | AATTCGCCGGAGACACTCG | 140 |
| Bcl-2 | NM_009741 | ATGCCTTTGTGGAACTATATGGC | GGTATGCACCCAGAGTGATGC | 120 |
| β-Actin | NM_007614 | ATGGAGCCGGACAGAAAAGC | CTTGCCACTCAGGGAAGGA | 108 |
EGF, epidermal growth factor; TGF, transforming growth factor; HBEGF, heparin-binding EGF-like growth factor; Nrg, neuregulin; TNFR1, tumor necrosis factor receptor superfamily, member 1a; TNFR2, tumor necrosis factor receptor superfamily, member 1b.
Western blotting.
For protein analysis, a more precise isolation of IEC was performed (11). Immunoblots were performed as described (7). Results are expressed as a ratio to β-actin. Primary antibodies were as follows: rabbit anti-ErbB1, rabbit anti-phospho-P38, rabbit anti-P38, mouse anti-TNFR1, and mouse anti-TNFR2 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-ErbB2, mouse anti-phospho-SAPK/JNK, mouse anti-SAPK/JNK, mouse anti-P-Akt, mouse anti-Akt, mouse anti-P-ERK, mouse anti-ERK (Cell Signaling Technology, Beverly, MA). Secondary antibody was either the corresponding goat anti-mouse-horseradish peroxidase or goat anti-rabbit antibody (Santa Cruz).
Immunofluorescent/immunohistochemistry microscopy.
Sections of jejunum were embedded in an optimum cutting temperature compound (Tissue-Tek; Sakura Finetechnical, Tokyo, Japan) and frozen, and immunofluorescent staining was as previously described (7). Immunohistochemistry used standard techniques and manufacturer's guidelines (Santa Cruz). Concentration of anti-TNFR1 antibody was 1:100.
Intestinal morphology assessment.
Villus height and crypt depth were measured in at least 10 well-oriented full-length crypt-villus units per specimen and averaged. Data were analyzed using commercially available digital image analysis software (NIS-Elements, AR 3.0).
Epithelial cell proliferation and apoptosis.
For IEC proliferation, immunofluorescent staining for proliferating cell nuclear antigen (PCNA) was performed as previously described (7). Results are expressed as number of PCNA-positive cells per crypt and then converted to percentage of controls (control values = 100%). PCNA was also expressed at the relative expression in immunoblots. Immunofluorescent staining for active caspase-3 was performed to assess IEC apoptosis, as previously described (8). Results are expressed as an Apoptotic Index, (% active caspase-3-positive cells/villi using a mean of 10 villi per mouse).
Data analysis.
Data are expressed as means ± SD. Statistical analysis used t-test for comparing two groups and one-way ANOVA for multiple groups (with Bonferroni post hoc analysis). Chi-square was used for categorical data. Significance was defined as P < 0.05. Kaplan-Meier curves were generated to define survival differences.
RESULTS
TPN administration increased intestinal IEC TNF-α and TNFR1 abundance.
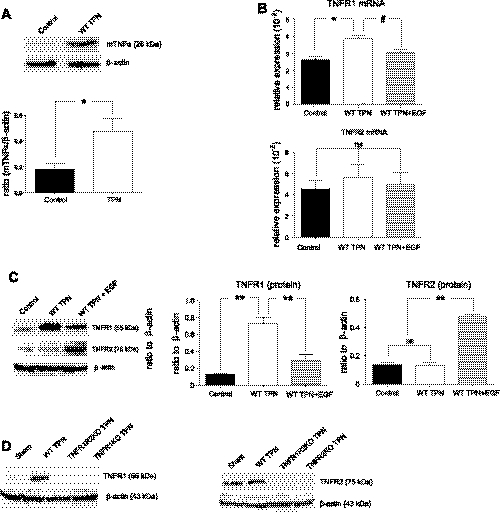
Our group previously demonstrated TPN-associated increased mucosal TNF-α mRNA expression (31). To examine this further, we showed that IEC-membrane-bound pro-TNF-α significantly increased with TPN (control: 0.18 ± 0.09 vs. TPN: 0.43 ± 0.16, P < 0.05) (Fig. 1A). Additionally, expression of both TNFR1 mRNA and protein increased 1.6-fold and sevenfold, respectively, in wild-type TPN mice vs. enteral controls (Fig. 1, B and C). In contrast, TNFR2 expression did not significantly change. Confirmation of the specificity of the antibodies to TNFR1 and TNFR2 is shown in Fig. 1D.
Fig. 1.
For all representative images in the figures, a minimum of 3 experiments and images were used for each selection where not indicated, and results are the means ± SD and N = 6–7 per group. Compared with wild-type (WT) TPN: #P < 0.05; *P < 0.01; **P < 0.001. Compared with control mice: ^P < 0.001, &P < 0.01. ns, not significantly different. Small intestine, mucosal membrane-bound pro-tumor necrosis factor (TNF)-α and TNF receptor-1 (TNFR1) increased with total parenteral nutrition (TPN), whereas, TNFR2 expression did not change. C57BL6/J mice received 7 days of TPN vs. enteral chow. Exogenous epidermal growth factor (EGF) (25 μg/kg per 12 h; via oral gavage, starting 1 day postcannulation; Ref. 18) was given to an additional group of TPN mice. mRNA was isolated from mucosal extracts, and protein analysis was performed from isolated intestinal epithelial cells (IECs); both real-time PCR and immunoblots were expressed as a ratio to β-actin. A: pro-TNF-α isolated from small bowel IEC. B: mRNA abundance of TNFR1 and TNFR2. C: protein abundance of TNFR1 and TNFR2. D: confirmatory immunoblots in TNFR1 and TNFR2 knockouts (KO). Results are the means ± SD and N = 6–7 per group. For representative immunoblots, a minimum of 6 experiments and images were used for each selection. Compared with WT TPN: #P < 0.05; *P < 0.01; **P < 0.001. ns, not significant.
We next assessed the effects of exogenous EGF on TNF-α and its receptors. EGF given to TPN mice almost completely reversed the increase in TNFR1, whereas, at the protein level, EGF led to markedly increased TNFR2 (Fig. 1C). This marked difference in response to exogenous EGF on TNFR1 and TNFR2 suggested distinct roles for TNF-α/EGF transactivation between these two receptors.
Abundance of ErbB ligands decreased in wild-type TPN mice.
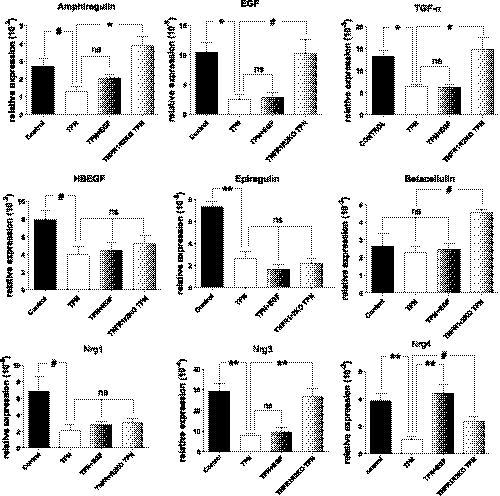
mRNA expression of jejunal mucosal ErbB ligands EGF, transforming growth factor (TGF)-α, amphiregulin, heparin-binding EGF-like growth factor (HBEGF), epiregulin, and neuregulins (Nrg1, 3, 4) were all significantly decreased with TPN (Fig. 2). We next examined whether ligand loss could be prevented with exogenous EGF but found that only the TPN-associated decline in Nrg4 was significantly reversed (Fig. 2). Interestingly, abrogation of TNF signaling during TPN using TNFR1R2-DKO mice prevented the decline in EGF, TGF-α, amphiregulin, betacellulin, and Nrg3 expression compared with wild-type TPN mice, and values in DKO mice were not significantly different than enteral controls. This suggests that the increase in TNF-α signaling with TPN accounts for much of the decline in EGF ligand expression.
Fig. 2.
Most ErbB ligands were significantly decreased with TPN. Differential mRNA expression of ligands were determined with real-time PCR of mucosal scrapings in control, TPN, TPN in TNFR1R2DKO mice, and TPN + EGF groups. Results are the means ± SD and N = 6–7 per group. Compared with WT TPN: #P < 0.05; *P < 0.01; **P < 0.001. ns, not significant. HBEGF, heparin-binding EGF; Nrg, neuregulin.
TPN led to a decline in ErbB1 expression.
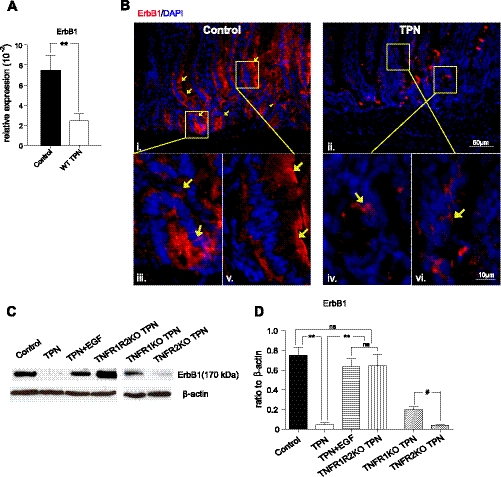
TPN led to a significant decline in expression of ErbB1 (3-fold mRNA; Fig. 3A). ErbB1 immunofluorescent staining was predominately located in crypts and the lower one-third of villi (Fig. 3B). A marked decrease in fluorescence intensity was noted in wild-type, TPN mice. Additionally, in controls, ErbB1 was located on the cell membrane; however, with TPN administration both ErbB1 density declined and an internalization of ErbB1 were observed.
Fig. 3.
ErbB1 abundance was markedly altered with TPN administration. A: mRNA abundance from isolated mucosal scrapings. B: immunofluorescent staining was performed on IEC from WT control (i, iii, and v) and TPN (ii, iv, and vi) mice with anti-ErbB1 and counterstained with 4,6-diamidino-2-phenylindole (DAPI) nuclear stain. Note the strong expression along the cellular membrane of crypts and in the lower one-third of villi in controls and very weak expression, and predominately cytoplasmic presence in IECs from TPN mice (yellow arrows). C and D: mRNA abundance of ErbB1 in enterally fed mice (control) compared with TPN, TPN + EGF and TPN in TNFRKO mice. C: representative immunoblots of isolated small bowel IECs are given from each indicated group. D: mean mRNA abundances for each study group. For representative images, a minimum of 3 experiments and images were used for each selection, and for immunoblots a minimum of 6 experiments and images were used. Results are the means ± SD and N = 6–7 per group. Compared with WT TPN: #P < 0.05; **P < 0.001.
Immunoblot analysis of IEC isolates confirmed that ErbB1 protein expression significantly decreased (>10-fold) in wild-type TPN mice vs. controls (Fig. 3, C and D). Exogenous EGF in TPN mice prevented the loss of abundance of ErbB1. Interestingly, in TNF-α receptor-KO mice, a distinct difference in ErbB family expression was observed. TNFR1R2-DKO TPN mice maintained control levels of ErbB1, whereas TNFR1-KO TPN mice only partially prevented ErbB1 loss, and TNFR2-KO TPN mice had levels similar to wild-type TPN mice (Fig. 3D). Quite interestingly, these results show that aberrations in TNF signaling with TPN administration contributed to the TPN-associated loss of ErbB1. These data suggest that TNFR1 signaling directly leads to a loss of ErbB1 abundance in this TPN model.
TPN downregulated phospho-Akt abundance and altered other downstream intracellular signals.
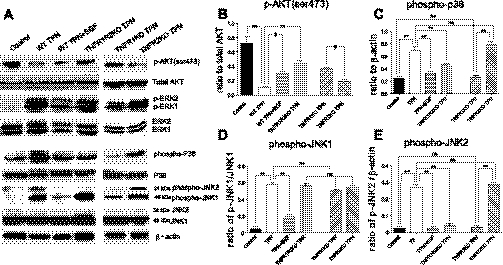
We have previously identified a loss of p-Akt expression with TPN administration (8) and confirmed this in the present study (Fig. 4B). Interestingly, in the present study, loss of p-Akt was partially prevented in TPN + EGF mice. More strikingly, TNFR1R2-DKO or TNFR1-KO TPN-mice also prevented p-Akt loss to an even greater degree (Fig. 4B). No change in p-Akt expression was noted in TNFR2-KO TPN mice (Fig. 4B). These results suggest that TNF-α signaling via TNFR1 is responsible for loss of PI3K/p-Akt signaling with TPN administration.
Fig. 4.
Several downstream signaling pathways were markedly altered with TPN. Summary of Erk is not shown, as no differences between TPN groups were detected. Protein derived from IEC isolates of small bowel from indicated study groups. A: representative immunoblots for Akt, Erk, p38, and JNK for active forms and total expression of each protein. B: p-Akt abundance expressed as a ratio to total Akt expression. C: phosphorylated p38 (pp38) abundance expressed as a ratio to β-actin. D and E: p-JNK1 and p-JNK2 abundance expressed as a ratio to total JNK1 and JNK2, respectively. For representative immunoblots, a minimum of 6 experiments and images were used for each selection. Results are the means ± SD and N = 6–7 per group. Compared with WT TPN: #P < 0.05; **P < 0.001.
In contrast to the results with p-Akt, phospho-ERK (MAPK/ERK) expression was upregulated with TPN (Fig. 4A). Neither exogenous EGF nor use of TNFR-KO mice prevented this increased expression. To further investigate the downstream signaling involved with TPN, we measured phospho-p38 (pp38), phospho-SAPK/JNK (P-SAPK/JNK), and IKK-βα expression in IECs (Fig. 4, C–E). TPN resulted in increased pp38 and p-JNK1/JNK2 expression in wild-type mice, whereas TPN + EGF prevented these increases. Upregulation of pp38 was also prevented in TNFR1R2-DKO and TNFR1-KO mice receiving TPN. Interestingly, only the upregulation of phospho-JNK2 expression was prevented in TNFR1R2D-KO and TNFR1-KO TPN mice, whereas ERK and phospho-JNK1 remained unchanged (Fig. 4, D and E).
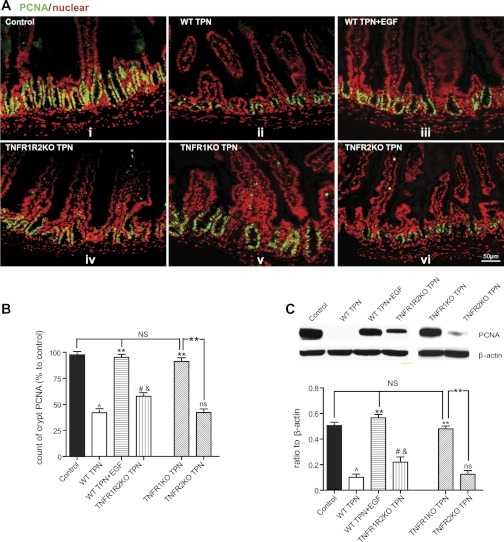
TPN-associated decline in IEC proliferation is due to loss of EGF and increased TNF-α signaling through TNFR1.
Baseline IEC proliferation was similar in all chow-fed transgenic groups compared with wild-type mice (data not shown). TPN in wild-type mice resulted in a significant decline in numbers of PCNA-stained IECs and PCNA by Western immunoblot (5-fold; Fig. 5, A–C), and this was completely prevented with exogenous EGF or in TNFR1-KO TPN mice. A partial prevention in IEC proliferation loss was seen in TNFR1R2-DKO TPN mice (2-fold higher than wild-type TPN mice); however, no change was noted in the TNFR2-KO TPN mice vs. wild-type-TPN mice (Fig. 5, B and C). Taken together with the findings of exogenous EGF leading to a loss of TNFR1 expression, increase in TNFR2 and TNFR1 mice having increased EGF abundance demonstrate the tight interrelation of TNF and EGF signaling on the mechanism, leading to loss of IEC proliferation during TPN. Additionally, the finding that IEC proliferation was only partly recovered in DKO mice but totally recovered in TNFR1-KO TPN mice also supports the critical role TNFR2 has toward supporting IEC proliferation.
Fig. 5.
Epithelial cell proliferation. A: epithelial cell proliferation was measured by immunofluorescent proliferating cell nuclear antigen (PCNA) staining (green) with nuclear counterstain DAPI (red). B: immunofluorescent staining is summarized by the means ± SD percent of PCNA-positive crypt cells in relation to the number identified in enteral controls. C: PCNA is also measured by Western immunoblotting and normalized to β-actin abundance in IEC small bowel isolates samples. For representative images, a minimum of 3 experiments and images were used for each selection, and for immunoblots a minimum of 6 experiments and images were used. Results are the means ± SD and N = 6–7 per group. Compared with WT TPN: #P < 0.05; **P < 0.001. Compared with control mice: ^P < 0.001, P < 0.01. ns, not significantly different.
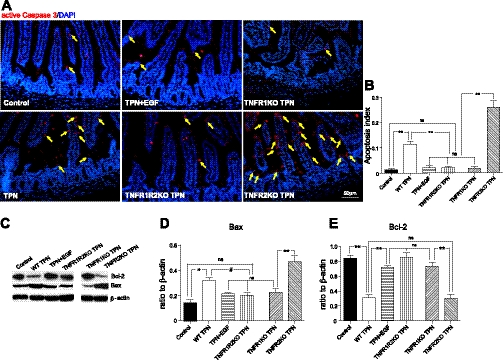
TPN-associated IEC apoptosis is related to loss of EGF and increased TNF-α signaling through TNFR1.
Baseline apoptosis rates were similar in all chow-fed transgenic groups compared with wild-type mice (data not shown). TPN administration led to significantly increased small bowel IEC apoptosis (Fig. 6, A–B). Active caspase-3 staining was predominately located in the villi and to a lesser extent in crypts. The number of active caspase-3 enterocytes significantly decreased in TPN + EGF mice (to control levels). Even with low EGF levels in TPN mice, reduction in IEC apoptosis was also found in TNFR1R2-DKO and TNFR1-KO TPN groups. Very interestingly, loss of TNFR2 in TPN mice led to significantly higher rates of IEC apoptosis than in wild-type-TPN mice, almost all of which occurred in the villi. Removal of TNFR1 led to an increase in TNFR2 (Fig. 7A, iii), whereas use of TNFR2-KO TPN mice led to an increase in TNFR1 (Fig. 7A, ii). Together this strongly suggests that the balance of TNFR1 and TNFR2 signaling is critically important for preventing apoptosis.
Fig. 6.
Epithelial cell apoptosis. A: IEC apoptosis measured by immunofluorescent active caspase-3 staining (red, and highlighted with yellow arrows) with DAPI (blue) counterstain. B: summary of apoptosis given as apoptosis index, expressed as means ± SD of active caspase-3-positive IEC per all counted IECs. C–E: immunoblot summary of Bax and Bcl-2, expressed as a ratio to β-actin in IEC small bowel isolates. For representative images, a minimum of 3 experiments and images were used for each selection, and for immunoblots a minimum of 6 experiments and images were used. Results are the means ± SD and N = 6–7 per group. Compared with WT TPN: #P < 0.05; **P < 0.001. ns, not significantly different.
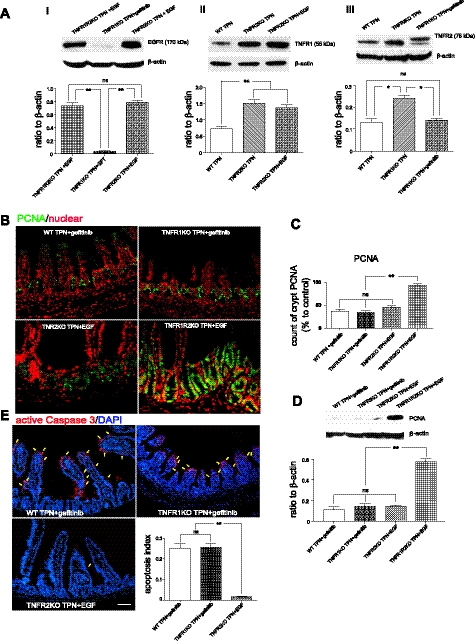
Fig. 7.
A: EGFR (i), TNFR1 (ii) and TNFR2 (iii) protein expression. B: PCNA (green) immunofluorescent staining, with DAPI counterstain (red). C: summary of PCNA-positive cells (as % of all crypt IECs). D: PCNA immunoblot results expressed as a ratio to β-actin. E: representative immunofluorescent staining of active caspase-3 staining (red, with highlighted cells with yellow arrows) with DAPI (blue) counterstain from the indicated groups. Inset: apoptosis index of the groups shown. Note, gefitinib was dosed at 2.5 mg/ml in 1% aqueous Tween-80, 200 μl, and given via oral gavage twice daily, starting 3 days before intravenous cannulation and continuing until mice were killed. For representative images, a minimum of 3 experiments and images were used for each selection, and for immunoblots a minimum of 6 experiments and images were used. Results are the means ± SD and N = 6–7 per group. Compared with WT TPN: *P < 0.001; **P < 0.001. ns, not significantly different.
Bax mRNA expression was upregulated, and Bcl-2 mRNA expression was downregulated in wild-type TPN mice (data not show). Bax protein abundance increased twofold in wild-type TPN mice, and this was completely prevented by administration of EGF (Fig. 6D). TPN in TNFR1R2-DKO and TNFR1-KO mice, but not TNFR2-KO mice, also prevented this increase in Bax. Bcl-2 protein expression declined threefold in wild-type TPN mice vs. controls (Fig. 6E). Markedly increased levels of Bax and decreased levels of Bcl-2 were observed in TNFR2-KO TPN mice, suggesting that Bax and Bcl-2 levels may contribute to the much higher apoptosis rates in TNFR2-KO mice. Although variations in differences in expression were seen between mRNA and protein expression, Bax and Bcl-2 protein values returned to control values in TNFR1R2-DKO and TNFR1-KO TPN groups. Furthermore, EGF partially prevented Bax increases and completely returned Bcl-2 levels to control values (Fig. 6C). This suggests one potential mechanism for how EGF and blockade of TNFR1 mediate their prevention of IEC apoptosis potentially through the Bcl-2 family.
ErbB1 signaling must occur to allow normal epithelial cell proliferation, and blockade of ErbB1 signaling markedly increased apoptosis with TPN.
To further investigate the interdependence of these signaling pathways, EGFR was blocked with the EGFR-specific kinase inhibitor gefitinib (Fig. 7). Gefitinib given to TPN mice resulted in a marked decline in IEC proliferation compared with control groups (Fig. 7, B–D), similar to wild-type TPN group. Although blockade of TNFR1 prevented loss of proliferation and increase in IEC apoptosis with TPN, this was prevented with concomitant gefitinib treatment and was associated with reduced ErbB1 expression (Fig. 7A, i) and increased TNFR1 expression (Fig. 7A, ii). This suggested that the protection of IEC proliferation and apoptosis from blockade of TNFR1 was highly dependent on EGF signaling. Interestingly, although EGF treatment could also prevent loss of proliferation in wild-type TPN mice, this prevention was observed in the TNFR1R2 DKO TPN group, but not in TNFR2-KO mice (Fig. 7, B–D), where there was increased TNFR1 expression in TNFR2KO TPN group (Fig. 7A, ii) and increased TNFR2 expression in TNFR1KO TPN group (Fig. 7A, iii). This demonstrated the critical role of the balance of TNFR1 and TNFR2 pathway for maintaining IEC proliferation. As shown in Fig. 6, A and B, TNFR2-KO TPN mice had profound rates of IEC apoptosis. Interestingly, the importance of EGF-mediated prevention of apoptosis is further demonstrated by the ability of exogenous EGF to counter this marked increase in IEC apoptosis seen in TNFR2-KO TPN mice (Fig. 7E). This may be via prevention in the associated loss of EGFR with TPN (Fig. 7 A, i).
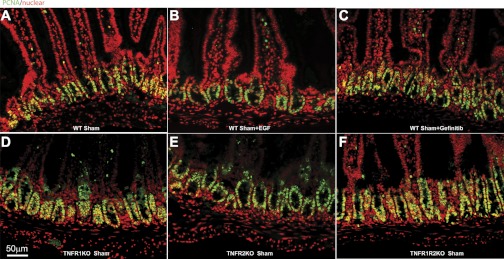
As proliferation rates could markedly vary with these transgenic mice, as well as with exogenous EGF and gefitinib treatment, Fig. 8 shows the changes in PCNA staining in Sham (enterally fed) control mice. The results show very little change in number of PCNA cells in any of these groups of control mice. To further insure that baseline abundance of TNF receptors was not influenced in the experiments, as well as EGF ligands, Table 2 shows alterations in these respective abundances with sham mice given either gefitinib or exogenous EGF. Note that abundances of EGF and Nrg3 were increased with exogenous EGF, a finding previously described (36); however, the majority of abundances remain quite stable.
Fig. 8.
Epithelial cell proliferation was measured by immunofluorescent PCNA staining (green) with nuclear counterstain DAPI (red). A: WT Sham. B: WT Sham + EGF. C: WT Sham + Gefitinib. D: TNFR1KO Sham. E: TNFR2 Sham. F: TNFR1R2KO Sham. For representative images, a minimum of 3 experiments and images were used for each selection.
Table 2.
Alternations in abundance of TNF receptors
| Sham + Gefitinib | Sham + EGF | P value | |
|---|---|---|---|
| Amphiregulin(10-2) | 3.25 ± 1.64 | 3.67 ± 0.69 | ns |
| EGF(10-3) | 3.99 ± 2.04* | 9.76 ± 4.52 | P = 0.014 |
| TGFα(10-3) | 12.96 ± 2.51 | 12.66 ± 2.95 | ns |
| HBEGF(10-2) | 7.52 ± 2.71 | 9.21 ± 1.85 | ns |
| Epiregulin(10-3) | 7.72 ± 2.14 | 8.40 ± 1.83 | ns |
| Betacellulin(10-3) | 4.47 ± 1.06 | 4.53 ± 2.24 | ns |
| Nrg1(10-3) | 4.24 ± 0.72 | 4.88 ± 1.42 | ns |
| Nrg3(10-4) | 19.3 ± 8.19 | 28.8 ± 6.25 | P = 0.07 |
| Nrg4(10-4) | 4.38 ± 1.96 | 4.58 ± 1.40 | ns |
| ErbB1(10-3) | 5.43 ± 1.21 | 7.64 ± 3.10 | ns |
| TNFR1(10-2) | 2.59 ± 0.43 | 2.17 ± 0.33 | ns |
| TNFR2(10-2) | 4.20 ± 1.42 | 4.44 ± 1.92 | ns |
Values are means ± SE.
P < 0.01; ns, no significant difference.
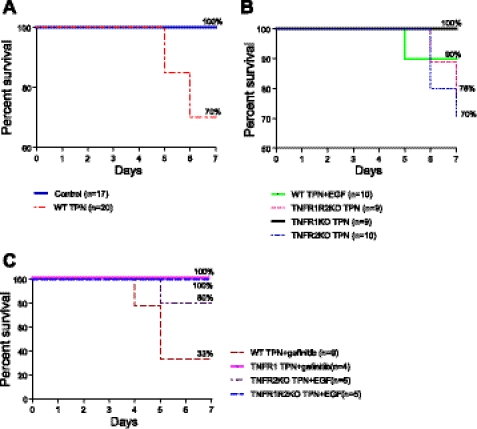
Mouse survival with TPN improves with EGF or blockade of TNFR1 and adversely affected with loss of TNFR2.
TPN in a clinical setting is associated with increased morbidity and mortality (19, 22), and this correlates in our mouse model with a high rate of septicemia and mortality (16, 29). Survival curves were generated to address whether modulation of EGF/TNF signaling impacted mortality (Fig. 9). Survival was 100% at 7 days in control mice, whereas 30% of mice died before 7 days in wild-type TPN mice (Fig. 9A, P = 0.046). In the TNFR1- KO TPN group, a 100% survival was observed (P = 0.058 vs. wild-type TPN). The TPN + EGF group showed a moderate improvement in outcome compared with wild-type TPN mice. However, the survival of the TNFR2-KO TPN group approached that of wild-type TPN mice, whereas the combined TNFR1R2-DKO TPN group had a much worse outcome compared with TNFR1KO TPN mice (Fig. 9B), suggesting that blockade of the TNFR2 signaling pathway adversely affected survival. These data suggest that an imbalance of TNFR1 vs. R2 signaling affects survival and may relate to the marked increase in apoptosis that occurred in this TPN group. The survival rate in wild-type TPN mice was 70%; however, this decreased to 33% for wild-type TPN mice receiving gefitinib (Fig. 9C). Interestingly, although administration of gefitinib eliminated the benefits to IEC proliferation and apoptosis in TNFR1-KO mice, it did not adversely affect survival in this strain (Fig. 9C). The mechanisms accounting for this preserved survival may relate to other factors, potentially preserved epithelial barrier function, and will need to be examined in future studies.
Fig. 9.
Kaplan-Meier survival curves for each study group; study N per group and survival percentages for each group are shown. TPN administration reduced survival percentages, and this was improved with either EGF supplementation, or with elimination of TNFRs, with 100% survival in the TNFR1-KO TPN group. A: comparison between control and TPN groups. B: comparison between TPN + EGF and TPNR-KO groups. C: comparison of survival curves among other study groups from Fig. 7. Survival was significantly lower in TPN vs. control group (P = 0.046). Survival approached significant difference for TNFR1-KO TPN vs. WT TPN groups (P = 0.058).
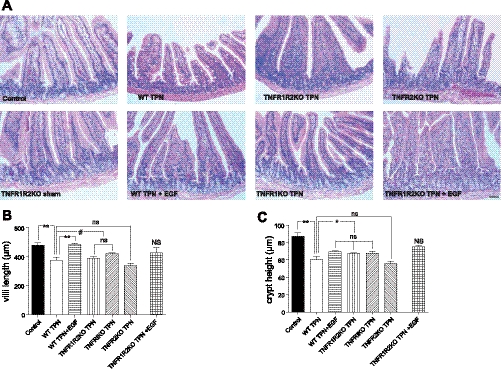
TPN-associated mucosal atrophy was prevented by exogenous EGF, as well as by blocking the TNFR1 signaling pathway.
Histological appearance of jejunal specimens are shown in Fig. 10. TPN resulted in a loss of villus length and crypt depth compared with enteral controls. Administration of EGF to TPN mice prevented loss of villus height but did not significantly impact loss of crypt depth. Use of TNFR-KO mice showed that blockade of the TNFR2 pathway failed to impact morphology, whereas blockade of the TNFR1 pathway significantly prevented much of the loss of villus height. Combining EGF administration and TNFR1R2-DKO mice resulted in virtually normal (control) histological appearance (crypt depth and villus height).
Fig. 10.
Microscopic appearance of villi and crypts (A) from each study group. Mean villus height (B) and crypt depth (C) are shown. Results show that both parameters are decreased for WT TPN mice compared with controls. Loss of villus height is prevented with EGF administration or use of TNFR1R2 TPN or TNFR1 TPN mice. No improvement in these measures were noted in the TNFR2 TPN group. For representative images, a minimum of 3 experiments and images were used for each selection. Results are the means ± SD and N = 6–7 per group. **P < 0.001; *P < 0.01; #P < 0.05.
DISCUSSION
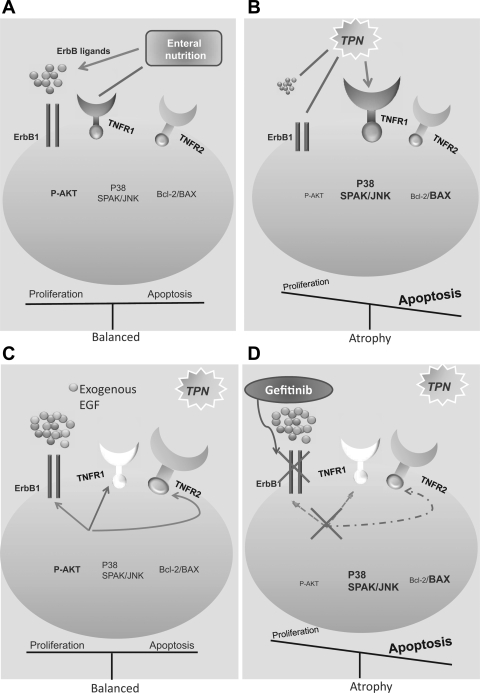
Most of the interactions between TNF-α and EGF have been studied in vitro or in models of inflammatory bowel disease (IBD). Although these are quite informative, the use of this TPN model allows for an examination of these factors in intact intestine and during a process of crypt-villus architecture remodeling. In the present study, we showed that TNFR1 and TNF-α were significantly upregulated on small IEC during TPN, whereas ErbB1 was downregulated. This suggested that removal of enteral nutrition results in a proinflammatory state. By using TNFR1/R2-KO mice and blocking ErbB1, we showed that TNFR1 signaling was proapoptotic, whereas TNFR2 had distinct proproliferation effects, demonstrating that a critical balance of the TNFR1 and TNFR2 pathways is needed for IEC homeostasis. Furthermore, elimination of TNFR1 prevented the TPN-associated loss of IEC proliferation; however, this was strongly codependent on effective EGF signaling. Although administration of EGF could not prevent the loss of IEC proliferation in TNFR2-KO TPN mice, this may have been due to a dominant expression of TNFR1. With removal of both TNFR1 and TNFR2, exogenous EGF completely prevented the loss of proliferation in TPN mice. Interestingly, despite loss of TNFR2 signaling leading to a marked increase in apoptosis, the antiapoptosis effect of EGF was not dependent on TNFR2 signaling. A summation of the complex interactions of these two signaling pathways is shown in Fig. 11.
Fig. 11.
Summary of epithelial cell EGF/TNF signaling interactions in the TPN model. A: enteral nutrition is associated with a sustained EGF signaling and low levels of TNFR1 abundance/signaling, resulting in preserved p-Akt abundance and balanced IEC proliferation and apoptosis. B: TPN administration leads to a loss of EGF signaling and upregulated TNFR1 signaling, resulting in an imbalance between TNFR1/TNFR2, and subsequently decreased p-Akt activity and altered Bcl-2/BAX ratio, increased SAPK/JNK, and p38 signaling. All of this contributes to reduced IEC proliferation and elevated IEC apoptosis. C: TPN mice supplemented with exogenous EGF resulted in increased EGF signaling, decreased TNFR1 expression, and increased TNFR2 abundance. Each of these results in a rebalancing of IEC proliferation and apoptosis. D: TPN in mice given gefitinib have markedly reduced IEC proliferation and increased apoptosis. Note that removal of TNFR1 can prevent loss of IEC proliferation but not with blockade of ErbB1 signaling; demonstrating a strong transactivation of these 2 signaling pathways. Note also that administration of gefitinib to TNFR1-KO mice led to diminished TNFR2 abundance (inhibitory signal to this receptor).
Intestinal IEC growth is critical for adaptive proliferation after intestinal resection (18, 28) and response to damage (5), and EGFR (ErbB-1) is a major mediator of these responses (27). In our study, TPN resulted in a marked loss of ErbB1 and several ligands. Exogenous EGF partially reversed TPN-associated TNFR1 upregulation and partially prevented ErbB1 downregulation. This resulted in a prevention of increased pp38 and p-SAPK/JNK expression, preserved IEC proliferation, and returned apoptosis to control rates. Furthermore, the marked shift in Bcl-2 and Bax expression may be the common downstream signals to contributing mechanistically to these observed changes, as has been previously suggested (14). This common response suggests that the mechanistic cause of mucosal atrophy during TPN is due to downregulation of ErbB1 and/or upregulation of TNFR1 signaling; both of which may converge via phosphorylation of p38 and SAPK/JNK signaling. This is further supported by the findings wherein TNFR1-KO TPN mice did not regain normal IEC proliferative levels when EGF signaling was blocked. EGF administration protected the TPN-associated loss of p-Akt abundance; similar results were observed in mice without TNFR1, but not in TNFR2-KO TPN mice. This suggested that EGF and TNFR1 may interact through a common intracellular path via p-Akt to affect IEC proliferation and apoptosis. Conversely, TNFR2 has been reported to mediate IEC proliferation (1); however, in our study, we did not detect any change in the expression of TNFR2 between control and TPN mice; however, in TPN + EGF mice there was an increase in TNFR2 protein expression and a complete recovery of IEC proliferation. The fact that blocking TNFR1 (TNFR1-KO) signaling while preserving TNFR2 similarly prevented loss of IEC proliferation suggested that preserved TNFR2 signaling has a key role in driving enterocytic proliferation. Further support for the role of TNFR2 signaling in IEC proliferation was seen by the failure to alter IEC proliferation in TNFR2-KO TPN mice compared with wild-type TPN mice. Such results are consistent with previous in vivo studies of septic shock. (6)
IEC apoptosis was also significantly increased with TPN. It was interesting that exogenous EGF or removal of TNFR1 signaling were very effective at preventing this rise in apoptosis. Furthermore, these results correspond to other proapoptotic signals in small bowel IECs, such as resection-induced apoptosis (18). Conversely and even more dramatic was the observation of a marked increase in IEC apoptosis in TNFR2-KO TPN mice vs. wild-type TPN mice. This finding suggests that TNFR2 signaling in IECs has a particularly strong antiapoptotic function and that a balance between TNFR1, TNFR2, and EGF signaling is critical in modulating IEC apoptotic rates. The fact that Bax was increased and Bcl-2 was decreased in the TNFR2-KO group suggests a possible mechanism for this antiapoptotic action.
In our present study, blockade of ErbB1 signaling significantly decreased survival in TPN mice and was associated with a significant increase in IEC apoptosis and decreased proliferation. This suggests that loss of ErbB1 signaling is a key factor in the development of TPN-associated atrophy. Additionally, removing the TNFR1 signaling pathway could not prevent this observed loss of proliferation without an intact ErbB1 path. The decreased abundance of ErbB1 in wild-type TPN mice increased with EGF supplementation, and this may be one way in which EGF prevents TPN-associated atrophy, potentially via ErbB1-ErbB2 heterodimerization. Frey et al. (10) reported elevated ErbB4 levels in inflammatory bowel disease; as this disease process is characterized by an increase in TNF-α, future work will be needed to examine the interaction of ErbB1 and the other ErbB family members, ErbB2, 3 and 4, in this TPN model. Additionally, EGF/TNF signaling may affect the immunocyte infiltration into the small bowel of TPN mice and will also need to be examined in future studies.
In conclusion, TPN led to an increase in IEC apoptosis and a decline in proliferation. This study demonstrates that this occurs through increased TNF-α signaling and loss of ErbB signaling. EGF and TNF-α signaling were tightly interdependent, and TPN-associated atrophy may occur via both an imbalance of TNF-α receptors and decline in EGF. The TPN model is novel in that previous attempts to understand this complex TNF/EGF interrelation have been confined to either in vitro settings or use of IBD models, which may have confounding processes of severe epithelial destruction, a finding not observed in the TPN model. A greater understanding of this process may have tremendous benefit to the safety of clinical delivery of TPN.
GRANTS
This work was supported by National Institutes of Health 2R01AI-44076-12. The work was also supported with the help of the National Cancer Institute through the University of Michigan's Cancer Center Support Grant (5 P03 CA46592).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Al-Lamki RS, Sadler TJ, Wang J, Reid MJ, Warren AY, Movassagh M, Lu W, Mills IG, Neal DE, Burge J, Vandenebeele P, Pober JS, Bradley JR. Tumor necrosis factor receptor expression and signaling in renal cell carcinoma. Am J Pathol 177: 943–954, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beutler B, Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature 320: 584–588, 1986 [DOI] [PubMed] [Google Scholar]
- 3. Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385: 729–733, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Citri A, Yarden Y. EGF-ErbB signaling: Towards the systems level. Nat Rev Mol Cell Biol 7: 505–516, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Dekaney CM, Gulati AS, Garrison AP, Helmrath MA, Henning SJ. Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. Am J Physiol Gastrointest Liver Physiol 297: G461–G470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ebach DR, Riehl TE, Stenson WF. Opposing effects of tumor necrosis factor receptor 1 and 2 in sepsis due to cecal ligation and puncture. Shock 23: 311–318, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Feng Y, McDunn J, Teitelbaum D. Decreased phospho-Akt signaling in a mouse model of total parenteral nutrition (TPN): A potential mechanism for the development of intestinal mucosal atrophy. Gastroenterology 136: A-96, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng Y, McDunn JE, Teitelbaum DH. Decreased phospho-Akt signaling in a mouse model of total parenteral nutrition: a potential mechanism for the development of intestinal mucosal atrophy. Am J Physiol Gastrointest Liver Physiol 298: G833–G841, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feng Y, Sun X, Yang H, Teitelbaum DH. Dissociation of E-cadherin and beta-catenin in a mouse model of total parenteral nutrition: a mechanism for the loss of epithelial cell proliferation and villus atrophy. J Physiol 587: 641–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frey MR, Edelblum KL, Mullane MT, Liang D, Polk DB. The ErbB4 growth factor receptor is required for colon epithelial cell survival in the presence of TNF. Gastroenterology 136: 217–226, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grossmann J, Maxson JM, Whitacre CM, Orosz DE, Berger NA, Fiocchi C, Levine AD. New isolation technique to study apoptosis in human intestinal epithelial cells. Am J Pathol 153: 53–62, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hare KJ, Hartmann B, Kissow H, Holst JJ, Poulsen SS. The intestinotrophic peptide, glp-2, counteracts intestinal atrophy in mice induced by the epidermal growth factor receptor inhibitor, gefitinib. Clin Cancer Res 13: 5170–5175, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Heller RA, Kronke M. Tumor necrosis factor receptor-mediated signaling pathways. J Cell Biol 126: 5–9, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jarboe MD, Juno RJ, Bernal NP, Knott AW, Zhang Y, Erwin CR, Warner BW. Bax deficiency rescues resection-induced enterocyte apoptosis in mice with perturbed EGF receptor function. Surgery 136: 121–126, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Kaiser GC, Polk DB. Tumor necrosis factor alpha regulates proliferation in a mouse intestinal cell line. Gastroenterology 112: 1231–1240, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Kang W, Gomez FE, Lan J, Sano Y, Ueno C, Kudsk KA. Parenteral nutrition impairs gut-associated lymphoid tissue and mucosal immunity by reducing lymphotoxin Beta receptor expression. Ann Surg 244: 392–399, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kiristioglu I, Teitelbaum DH. Alteration of the intestinal intraepithelial lymphocytes during total parenteral nutrition. J Surg Res 79: 91–96, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Knott AW, O'Brien DP, Juno RJ, Zhang Y, Williams JL, Erwin CR, Warner BW. Enterocyte apoptosis after enterectomy in mice is activated independent of the extrinsic death receptor pathway. Am J Physiol Gastrointest Liver Physiol 285: G404–G413, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Kudsk K, Croce M, Fabian T, Minard G, Tolley E, Poret H, Kuhl M, Brown R. Enteral vs. parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg 215: 503–511, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loetscher H, Schlaeger EJ, Lahm HW, Pan YC, Lesslauer W, Brockhaus M. Purification and partial amino acid sequence analysis of two distinct tumor necrosis factor receptors from HL60 cells. J Biol Chem 265: 20131–20138, 1990 [PubMed] [Google Scholar]
- 21. McElroy SJ, Frey MR, Yan F, Edelblum KL, Goettel JA, John S, Polk DB. Tumor necrosis factor inhibits ligand-stimulated EGF receptor activation through a TNF receptor 1-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 295: G285–G293, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moore F, Feliciano D, Andrassy R, McArdle A, Booth F, Morgenstein-Wagner T, Kellum J, Welling R, Moore E. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg 216: 172–183, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moore F, Moore E, Jones TN, McCroskey BL, Peterson VM. TPN versus TPEN following major abdominal trauma: reduced septic morbidity. J Trauma 29: 916–923, 1989 [DOI] [PubMed] [Google Scholar]
- 24. Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Warner J, Willard D, Becherer JD. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature 385: 733–736, 1997 [DOI] [PubMed] [Google Scholar]
- 25. O'Brien DP, Nelson LA, Williams JL, Kemp CJ, Erwin CR, Warner BW. Selective inhibition of the epidermal growth factor receptor impairs intestinal adaptation after small bowel resection. J Surg Res 105: 25–30, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Rodrigues G, Falasca M, Zhang Z. A novel positive feedback loop mediated by the docking protein Gab 1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol 20: 1448–1459, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sheng G, B KQ, Guo J, Warner B. Epidermal growth factor receptor-mediated proliferation of enterocytes requires p21waf1/cip1 expression. Gastroenterology 131: 153–156, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Shin CE, Falcone RA, Jr, Kemp CJ, Erwin CR, Litvak DA, Evers BM, Warner BW. Intestinal adaptation and enterocyte apoptosis following small bowel resection is p53 independent. Am J Physiol Gastrointest Liver Physiol 277: G717–G724, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Sun X, Yang H, Nose K, Nose S, Haxhija EQ, Koga H, Feng Y, Teitelbaum DH. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol 294: G139–G147, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Wildhaber B, Yang H, Spencer A, Drongowski R, Teitelbaum D. Lack of enteral nutrition-effects on the intestinal immune system. J Surg Res 123: 8–16, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Wildhaber BE, Yang H, Teitelbaum DH. Total parenteral nutrition-induced apoptosis in mouse intestinal epithelium: modulation by keratinocyte growth factor. J Surg Res 112: 144–151, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Yamaoka T, Yan F, Cao H, Hobbs SS, Dise RS, Tong W, Polk DB. Transactivation of EGF receptor and ErbB2 protects intestinal epithelial cells from TNF-induced apoptosis. Proc Natl Acad Sci USA 105: 11772–11777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gammadelta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol 172: 4151–4158, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Yang H, Fan Y, Teitelbaum DH. Intraepithelial lymphocyte-derived interferon-γ evokes enterocyte apoptosis with parenteral nutrition in mice. Am J Physiol Gastrointest Liver Physiol 284: G629–G637, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Yang H, Gumucio DL, Teitelbaum DH. Intestinal specific overexpression of interleukin-7 attenuates the alternation of intestinal intraepithelial lymphocytes after total parenteral nutrition administration. Ann Surg 248: 849–856, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yusta B, Holland D, Koehler JA, Maziarz M, Estall JL, Higgins R, Drucker DJ. ErbB signaling is required for the proliferative actions of GLP-2 in the murine gut. Gastroenterology 137: 986–996, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Zhang C, Feng Y, Yang H, Koga H, Teitelbaum D. Bone morphogenetic protein signaling pathway is up-regulated in a mouse model of total parenteral nutrition. J Nutr 137: 1315–1321, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang C, Feng Y, Yang H, Koga H, Teitelbaum DH. The bone morphogenetic protein signaling pathway is upregulated in a mouse model of total parenteral nutrition. J Nutr 139: 1315–1321, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]