Abstract
Total parenteral nutrition (TPN) is essential for patients with impaired gut function but leads to parenteral nutrition-associated liver disease (PNALD). TPN disrupts the normal enterohepatic circulation of bile acids, and we hypothesized that it would decrease intestinal expression of the newly described metabolic hormone fibroblast growth factor-19 (FGF19) and also glucagon-like peptides-1 and -2 (GLP-1 and GLP-2). We tested the effects of restoring bile acids by treating a neonatal piglet PNALD model with chenodeoxycholic acid (CDCA). Neonatal pigs received enteral feeding (EN), TPN, or TPN + CDCA for 14 days, and responses were assessed by serum markers, histology, and levels of key regulatory peptides. Cholestasis and steatosis were demonstrated in the TPN group relative to EN controls by elevated levels of serum total and direct bilirubin and also bile acids and liver triglyceride (TG) content. CDCA treatment improved direct bilirubin levels by almost fourfold compared with the TPN group and also normalized serum bile acids and liver TG. FGF19, GLP-1, and GLP-2 were decreased in plasma of the TPN group compared with the EN group but were all induced by CDCA treatment. Intestinal mucosal growth marked by weight and villus/crypt ratio was significantly reduced in the TPN group compared with the EN group, and CDCA treatment increased both parameters. These results suggest that decreased circulating FGF19 during TPN may contribute to PNALD. Moreover, we show that enteral CDCA not only resolves PNALD but acts as a potent intestinal trophic agent and secretagogue for GLP-2.
Keywords: chenodeoxycholic acid, fibroblast growth factor-15/19, glucagon-like peptide-1, glucagon-like peptide-2, total parenteral nutrition
over 30,000 patients in the United States are permanently dependent on parenteral nutrition (PN) for survival (9), and many more require PN for varying duration during hospital stay or home care. In pediatric patients, PN-associated liver disease (PNALD) is a major but still poorly understood complication of PN (19, 24). In infants that receive PN for at least 2 mo, the incidence of PNALD may be as high as 50% and can eventually lead to end-stage liver disease and need for liver transplant in those who receive total PN (TPN) for more than 3 mo (5). The clinical spectrum of PNALD includes steatosis, disruption in glucose and lipid metabolism, cholestasis (TPN-associated cholestasis), cholelithiasis, hepatic fibrosis, biliary cirrhosis, development of portal hypertension, and liver failure. The histological features of PNALD are intracellular and intracanalicular cholestasis, steatosis and periportal inflammation, and eventually fibrosis. The risk factors associated with PNALD in infants are lack of enteral feeding, developmental immaturity in the hepatic transport and metabolism of bile acids, and sepsis or infection. Despite these known risk factors, the etiology of PNALD, especially in infants and children, is not established, and presently there are no proven effective therapeutic treatments.
Among a number of proposed mechanisms to account for the pathology of PNALD is the lack of enteral feeding, leading to gut atrophy and disruption of enterohepatic circulation of bile acids. We have shown that TPN induces both intestinal mucosal atrophy and PNALD in neonatal piglets (4, 23). Intestinal mucosal atrophy induced by chronic TPN is strongly linked to the loss of enteral nutrient-mediated glucagon-like peptide-2 (GLP-2) secretion, and GLP-2 replacement markedly augments mucosal growth (3, 4). During normal enterohepatic circulation, bile acid absorption in the ileum is associated with activation of the nuclear bile acid receptor farnesoid X receptor (FXR), leading to induction of the recently described metabolic hormone fibroblast growth factor-19 (FGF19) (12). Enterohepatic secretion of FGF19 acts in the liver to repress bile acid production and improve lipid metabolism and glycemic control (8, 27). It has also recently been shown that bile acid activation of the G protein-coupled receptor TGR-5 in intestinal endocrine cells results in activation of GLP-1 secretion (25). Thus enteral bile acid activation of GLP-1 appears to contribute to an improved liver and peripheral metabolic profile but could also, via cosecreted GLP-2, result in a trophic effect in the gut. We hypothesized that disruption of the enterohepatic bile flow and gut signaling processes occur during TPN, which results in loss of FXR-mediated intestinal secretion of FGF19, GLP-1, and GLP-2 and thereby contributes to PNALD and mucosal atrophy. We therefore evaluated the role of enteral supplementation with the FXR (15) and TGR-5 (21) agonist chenodeoxycholic acid (CDCA) in a model of chronic TPN in the neonatal pig. The results show improvements in both PNALD and gut atrophy, which were associated with increases in both FGF19 and GLP-1/2 secretion in the gut.
MATERIALS AND METHODS
Animals.
The study protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals [DHHS publication no. (NIH) 85–23, revised 1985, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205]. Newborn (2-day-old), crossbred pigs were obtained from the Texas Department of Criminal Justice (Huntsville, TX), transported to the animal facility at the Children's Nutrition Research Center (Houston, TX) and immediately placed in cages in a heated room (∼30°C) until surgery the following day.
Surgery.
The piglets were anesthetized using isoflurane general anesthesia, and silastic catheters were inserted into the jugular vein and carotid artery as previously described (4, 23). Intragastric and intraduodenal catheters were also implanted. Catheters were secured in pockets of a jacket that was attached to a tether, which allowed free movement and secure administration of TPN to the piglets in their cages. During the initial 24 h postoperatively, all pigs received TPN at 50% of full intake providing (in g·kg−1·day−1) 12.5 glucose, 6.5 L-amino acids, 2.5 lipid, and 412 kJ·kg−1·day−1 at a volume of 120 ml·kg−1·day−1. Thereafter, intakes were increased to 100% within 48 h according to their respective treatment. Pre- and postoperatively on each day, piglets received enrofloxacin (2.5 mg/kg; Bayer, Shawnee Mission, KS). Postoperatively, each piglet received one dose of analgesic (0.1 mg/kg butorphenol tartrate; Fort Dodge Laboratories, Fort Dodge, IA).
Study design.
Piglets (N = 15) were randomly allocated to three groups, enteral nutrition (EN), TPN, and TPN + CDCA group. Each group received nutrition for 14 days as follows. EN pigs were fed a liquid cow's milk-based formula (Litter Life; Merrick, Middleton, WI) at a rate of 50 g·kg body wt−1·day−1, suspended in 240 ml water providing in g·kg body wt−1·day−1, 25 lactose, 12.5 protein, 5 fat, and electrolytes, (trace) minerals, and vitamins (815 kJ·kg body wt−1·day−1). TPN piglets were administered TPN providing per 240 ml fluid in g·kg body wt−1·day−1, 25 glucose, 13 amino acids, 5 lipid (Intralipid 20%; Fresenius Kabi, Bad Homburg, Germany), and electrolytes, (trace) minerals, and vitamins (823 kJ·kg body wt−1·day−1). TPN + CDCA piglets were administered TPN as described above and also received CDCA via the duodenal catheter at 30 mg·kg body wt−1·day−1, in doses of 10 mg/kg body wt dissolved in ethanol at 0.15 ml/kg body wt every 8 h. TPN pigs above were administered ethanol alone via duodenal catheter. EN was administered every 4 h intragastrically, and TPN was administered continuously. Piglets were weighed daily, and their intake was adjusted accordingly.
Intravenous glucose tolerance test.
On days 7 and 14 after an 8-h fast, pigs received an intravenous glucose tolerance test (IVGTT, 1.0 g glucose/kg body wt) with arterial blood sampled over 60 min for assay of plasma glucose and insulin as described previously (23).
Plasma analysis.
Blood samples were obtained on day 14 for plasma analysis for liver parameters, which was done by automated analyzer at Baylor College of Medicine core facility. Plasma FGF19 levels were assayed using ELISA (Quantitative ELISA Kit by R&D Systems, Minneapolis, MN). For GLP-1 and GLP-2 assays, blood samples were collected into chilled tubes containing EDTA, kept on ice, and centrifuged within 30 min at 3,000 revolution/min for 10 min. Plasma was collected and analyzed for GLP-1 by radioimmunoassay using antiserum 89 390 reacting with the amidated COOH terminus of GLP-1, and therefore mainly reacting with fully processed GLP-1. In the circulation, GLP-1 is metabolized by the enzyme dipeptidyl peptidase IV to the NH2-terminally truncated metabolite GLP-1 9–36 amide; this metabolite crossreacts 100% in the GLP-1 assay. The assay measures the sum of degraded and undegraded GLP-1 as described previously (17). Plasma GLP-2 concentrations were quantified as described previously using an antibody raised against human GLP-2 that recognizes both the human and porcine full length GLP-2 (1–33) peptide (4).
Tissue collection.
At the end of the study, all pigs were euthanized with an intravenous injection of pentobarbital sodium (50 mg/kg) and phenytoin sodium (5 mg/kg; Beutanasia-D; Schering-Plough Animal Health, Kenilworth, NJ). The abdomen was opened, and the entire small intestine distal to the ligament of Treitz to the ileocecal junction was immediately flushed with ice-cold saline. After being flushed with ice-cold saline, the small intestine was divided into two equal portions; the proximal half was designated the jejunum, and the distal half, the ileum. Liver and small intestine were weighed, and tissue was snap-frozen in liquid nitrogen and stored at −80°C until further analysis.
Histological analysis and morphometry.
A 2–3-cm segment of fresh tissue from the proximal and distal small intestine was fixed in 4% buffered formalin for 24 h and then stored in 70% ethanol at room temperature for 24 h. Samples were embedded in paraffin, sectioned (5 mm), and stained with eosin and hematoxylin. The mean villus height and crypt depth were quantified by an Axiophot microscope (Zeiss, Jena, Germany) and NIH Image 1.60 (National Institutes of Health, Bethesda, MD) in at least 15 vertically well-oriented villus-crypt columns as described previously (4).
RESULTS
We have previously described the development of hepatic steatosis and inflammation in neonatal piglets maintained on TPN (23, 28). To test the potential effects of bile acid supplementation in this model, we compared groups of piglets (n = 5 per group) that were fed enterally or parenterally with or without CDCA (EN, TPN, TPN + CDCA). CDCA was administered via a duodenal catheter at 30 mg·kg body wt−1·day−1, in doses of 10 mg/kg body weight every 8 h. EN was administered every 4 h intragastrically, and TPN was administered continuously. Piglets were weighed daily, and their intake was adjusted accordingly.
At baseline, all three groups EN, TPN, TPN + CDCA were comparable for age and weight with mean weight in grams and standard deviation (± SD) of 2,063 ± 302, 2,092 ± 460, and 2,015 ± 501, respectively, for EN, TPN, TPN + CDCA. At the end of 14 days, mean weight gain (g/day) was 51.1 ± 3.7, 45.3 ± 5.7, and 44.7 ± 5.2, respectively, for the three groups. The liver weights (g/kg body wt) were lower (P < 0.05) in EN vs. TPN and TPN + CDCA (27.8 ± 3.8 vs. 38.0 ± 5.3 and 40.0 ± 6.7, respectively). Although isocaloric nutrition was provided to each group, the EN group had the highest weight gain. Diarrhea was observed in two of the five piglets in the CDCA treatment group. No other adverse effects were documented.
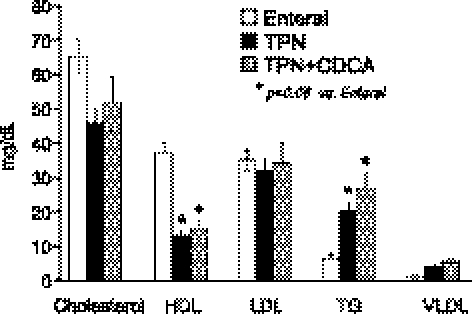
Plasma lipid profiles were determined for each of the groups. The EN group had higher (P < 0.05) total cholesterol and HDL levels compared with the TPN group or the TPN + CDCA group (Fig. 1). No differences in LDL levels were evident in either group. Plasma triglycerides were higher (P < 0.05) in the TPN and TPN + CDCA compared with the EN group (Fig. 1). No significant differences were documented in alanine aminotransferase or albumin values in any group.
Fig. 1.
Plasma lipid profile in neonatal pigs fed enteral nutrition (EN), total parenteral nutrition (TPN) or TPN + chenodeoxycholic acid (CDCA). Treatments were for 14 days with N = 5 pigs/group; results show plasma cholesterol and lipoprotein fraction after 14 days of treatment (mg/dl). HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; VLDL, very-low-density lipoprotein.
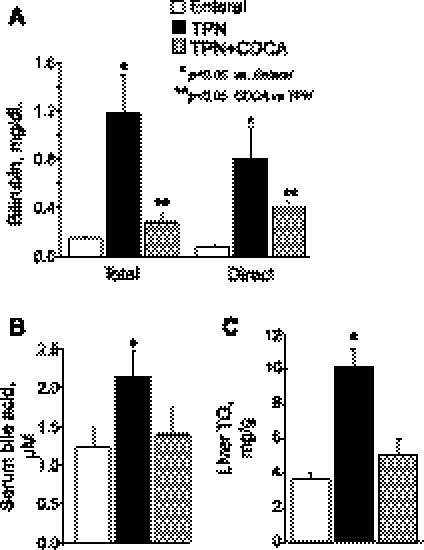
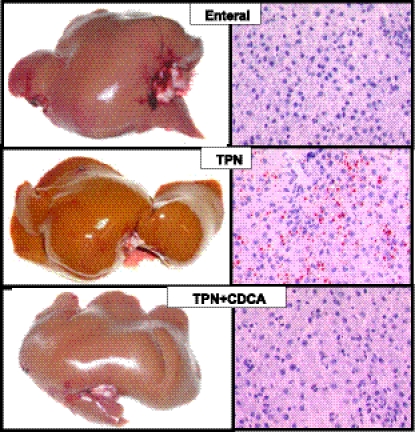
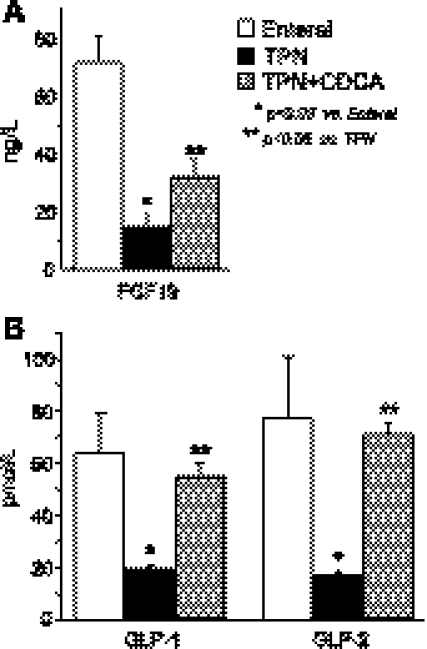
As expected, the TPN group showed steatosis and liver dysfunction after 2 wk, as indicated by elevated liver triglycerides and elevated plasma levels of direct and total bilirubin, as well as bile acids (Fig. 2). Cholestasis and steatosis in the TPN group were also evident macroscopically in yellow liver appearance and microscopically in microvesicular lipid droplets by oil-red O staining, whereas EN and TPN + CDCA groups appeared normal (Fig. 3). In accord with our initial hypothesis that TPN would decrease, and bile acid signaling would increase expression of FGF19 and GLP-1 and GLP-2, levels of all three peptides were significantly decreased in plasma from the TPN group and increased in the TPN + CDCA group (Fig. 4).
Fig. 2.
Plasma markers of liver cholestasis and liver triglyceride content in neonatal pigs fed EN, TPN, or TPN + CDCA. Treatments as in Fig. 1. Serum levels of bilirubin (A) and bile acids (B) and liver levels of triglycerides (C) are shown. Differences between groups are based on one-way ANOVA and Tukey's test; *P < 0.05 vs. enteral, **P < 0.05 CDCA vs. TPN.
Fig. 3.
Macroscopic and microscopic images of liver and steatosis in pigs fed EN, TPN, or TPN + CDCA. Treatments as in Fig. 1. Steatosis is evident from microvesicular lipid droplets stained by oil-red-O (right panels).
Fig. 4.
Plasma concentrations of fibroblast growth factor-19 (FGF19) and glucagon-like peptide (GLP)-1 and GLP-2 in neonatal pigs fed EN, TPN, or TPN + CDCA. Treatments as in Fig. 1. Levels of FGF19 (A) and GLP-1 and -2 (B) are shown. Differences between groups are based on one-way ANOVA and Tukey's test; *P < 0.05 vs. enteral, **P < 0.05 CDCA vs. TPN.
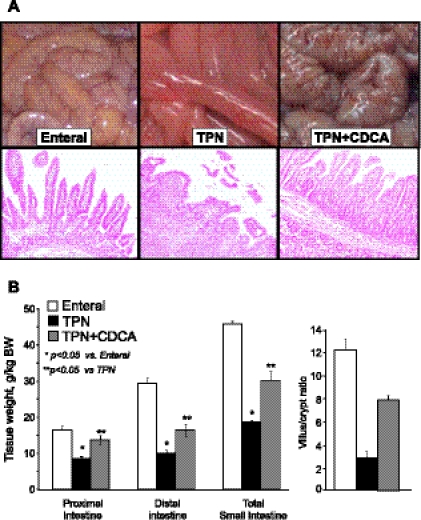
The restoration of gut peptide secretion was associated with marked differences in small intestinal weight and morphology. Small bowel from piglets in the TPN + CDCA group was remarkably preserved compared with their thin and friable counterparts from the TPN group (Fig. 5). Total small bowel weight in the TPN + CDCA group was almost double that of the TPN group (P < 0.05). These differences were more prominent in the proximal small bowel. Histological assessment of distal small intestine showed evidence of severe villus blunting in the TPN group. Morphometric analysis of the distal small bowel revealed a villus/crypt ratio (V/C) that was significantly reduced in the TPN group compared with EN group. However, the CDCA treatment markedly (P < 0.05) improved this effect (Fig. 5).
Fig. 5.
Small intestinal weight and morphology in neonatal pigs fed EN, TPN, or TPN + CDCA. Treatments as in Fig. 1. A: macroscopic appearance and representative eosin-stained sections from distal intestine of each group. B: tissue weight and villus/crypt ratio measurements. Villus/crypt results are from the ileum. Differences between groups are based on one-way ANOVA and Tukey's test; *P < 0.05 vs. enteral, **P < 0.05 CDCA vs. TPN.
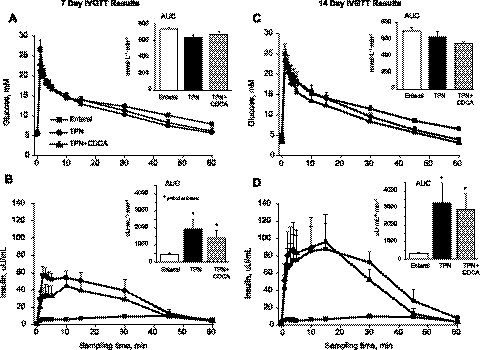
Given our previous evidence that TPN induces insulin resistance and that CDCA-augmented FGF19 and GLP-1 secretion might improve this condition, we performed IVGTT in all piglets at 7 and 14 days (Fig. 6). As expected, the results show that TPN resulted in insulin resistance compared with EN pigs at both time points marked by increased mean insulin AUC values. However, CDCA treatment did not improve this outcome at either time point.
Fig. 6.
Intravenous glucose tolerance (IVGTT) test performed after 7 and 14 days of treatment in neonatal pigs fed EN, TPN, or TPN + CDCA. Treatments as in Fig. 1. Arterial plasma glucose (A and C) and insulin (B and D) concentrations measured during 60 min after intravenous glucose bolus (1.0 g/kg) performed at day 7 (right) and day 14 (left). Insets: respective estimates of area-under-the-curve (AUC). Differences between groups are based on one-way ANOVA and Tukey's test; *P < 0.05 vs. enteral.
DISCUSSION
Enteral nutrition protects against the development of PNALD (14, 22, 30). This study was based on the hypothesis that TPN associated pathologies, particularly PNALD and gut atrophy, are at least in part a consequence of the loss of the normal enterohepatic circulation of bile acids that occurs with enteral nutrition. This hypothesis is based on the ability of bile acids to induce gut expression and secretion of key signaling peptides, including FGF19 and GLP-1 and GLP-2. The FGF19 pathway is based on activation of the bile acid nuclear receptor FXR, which directly induces FGF19 gene expression and secretion in enterocytes (12, 13). The GLP-1 and GLP-2 pathway is based on activation of the bile acid membrane, G protein-coupled receptor TGR-5, which induces GLP-1/2 secretion by enteroendocrine cells (25).
FGF19 has recently emerged as a key regulator of hepatic bile acid production (6). Moreover, FGF19 treatment of obese mice prevented or reversed diabetes, improved glycemic control, and reduced triglyceride levels (8, 10, 18, 29). Thus we predicted that restoration of FGF19 expression in response to bile acid treatment would improve pathologies associated with PNALD. In addition, evidence of gut mucosal atrophy was observed upon initiation of TPN, with effects evident as early as 24 h in neonatal pigs (16). Because GLP-2 is a well established, gut-trophic factor, we predicted that the reported induction of GLP-1 and -2 in response to bile acid treatment would prevent such atrophy and perhaps the insulin resistance we previously showed in this TPN piglet model (4, 23).
To test these hypotheses, we treated neonatal pigs in a well-established model of TPN administration (16) with or without enteral infusion of the bile acid CDCA. Unlike ursodeoxycholic acid, CDCA activates both FXR and TGR-5. Our results confirmed that TPN markedly decreased levels of FGF19, GLP-1, and GLP-2 in the circulation, whereas CDCA treatment significantly induced all three peptides (Fig. 4). In accord with our predictions, liver pathologies were markedly improved, as indicated by decreased steatosis and decreased plasma levels of bile acids and bilirubin. The finding that TPN results in reduced FGF19 secretion is novel and may provide a mechanism to explain the cholestasis and steatosis observed with PNALD. Because FGF19 production in the small intestine causes suppression of CYP7A1 in hepatocytes (12), diminished FGF19 levels in TPN could increase CYP7A1 expression in the liver, resulting in persistent activation of bile acid synthesis and accumulation in hepatocytes.
A clear prediction of our results is that systemic treatment with FGF19 should result in similar beneficial effects, particularly with respect to the liver. Because we have found that the injected protein is quite unstable, we have used increasing doses but so far have not observed such responses to our treatments with recombinant FGF19. Although this negative result may be due to technical limitations, it raises the possibility that additional factors are required to account for the beneficial effects of CDCA.
Despite this negative result, results from mice suggest that bile acid induction of FGF19 could also contribute to the prevention of steatosis. Overexpression or infusion of FGF19 in mice reduced adiposity, serum triglycerides, and hepatic acetyl coenzyme A carboxylase and increased metabolic rate and glycemic control (8, 27). Conversely, FGF receptor 4-deficient mice exhibit increased white adipose tissue mass, glucose intolerance, insulin resistance, and hyperlipidemia, further confirming an important role of FGF19 in glucose and lipid metabolic regulation (10). The effect of FGF19 on hepatic lipid metabolism is poorly understood but likely increases fatty acid oxidation and suppresses triglyceride synthesis, both outcomes that could reduce hepatic steatosis.
We also expected that enteral bile acid induction of GLP-1 secretion might improve the dysregulation of glucose homeostasis and insulin resistance that we recently reported in piglets given chronic TPN. GLP-1 is a potent incretin hormone that is known to augment pancreatic insulin secretion and insulin sensitivity, and expression of its precursor glucagon is well known to be stimulated by nutrients (2). In addition, it was possible that increased circulating FGF19 could improve glucose homeostasis by suppression of hepatic gluconeogenesis via a recently described mechanism that functions in parallel with insulin (18). Surprisingly, despite the fact that both GLP-1 and FGF19 secretion were substantially restored by CDCA treatment, this did not translate into improved insulin sensitivity based on IVGTT results.
The other striking observation in this study was the dramatic stimulation of intestinal growth and reversal of mucosal atrophy in piglets treated with CDCA. This was evident by macroscopic appearance, as well as a significant improvement in histology documented by the strong increase in V/C ratio. The idea that bile and bile acids in particular can stimulate intestinal mucosal growth has been reported previously. Studies in rodents show that feeding bile salts induced mucosal proliferation, and biliary diversion after small bowel resection leads to impaired intestinal adaptation and growth (1, 20, 26); we have also shown that bile acid administration induces precocious gut maturation (11). Interestingly, differences in intestinal adaptation associated with pancreaticobiliary diversion were ascribed to changes in circulating levels of enteroglucagon, the previously described hormone whose activity is now known to be due to GLP-2 (1). Thus our results suggest a novel mechanism for bile acid-induced mucosal growth that we suggest involves activation of enteroendocrine L cell secretion of the intestinal trophic peptide GLP-2. In addition, however, we have not reproduced the trophic effect with GLP-2 treatment alone, and bile acids have been shown to induce proliferation of cultured intestinal epithelial cells (7, 26). Thus we cannot rule out more direct effects or additional factors in the bile acid induced responses.
We conclude that the FXR-FGF19 axis is disrupted by TPN in a neonatal piglet model, as is the TGR-5-GLP axis. We predict that FXR activation by exogenous CDCA induces FGF19, which exerts beneficial metabolic effects. Similarly, we predict that TGR-5 activation may mimic nutrient responses to induce expression of both GLP-1 and -2, with the former complementing the metabolic effects of FGF19 and the latter exerting well-known local intestinal trophic effects. The net result of these responses is a significant improvement in liver function, as revealed by decreased serum bilirubin and bile acids as well as liver triglycerides, and also markedly stimulated intestinal mucosal growth in a piglet TPN model. These initial studies provide a firm basis for further studies to explore the molecular mechanisms for the beneficial responses observed. They also raise the provocative prospect that CDCA or other bile acids, which are generally thought of as toxic agents but have recently emerged as key signaling molecules, may have therapeutic utility in the clinical context of various diseases such as PNALD and short-bowel syndrome in patients receiving PN.
GRANTS
This work was supported by a grant NIH R01 DK068804 and R.P. Doherty, Jr. Welch Chair, (Q-0022) to D. D. Moore. A. Jain was supported in part by a grant from the American Liver Foundation. This work is a publication of the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children's Hospital, Houston, TX. This work was supported by federal funds from the U.S. Department of Agriculture, Agricultural Research Service under Cooperative Agreement Number 58-6250-6-001, and the Texas Medical Center Digestive Diseases Center (NIH Grant P30 DK-56338). The contents of this publication do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors. AKJ, BS, DGB, and DDM were involved in study concept and design; AKJ, BS, DGB, and JJH in acquisition of data; all authors were involved in analysis and interpretation of data; AKJ, BS, DGB, and DDM were involved in drafting of the manuscript; AKJ, DGB, and DDM obtained funding.
ACKNOWLEDGMENTS
The authors acknowledge Liwei Cui, Xiaoyan Chang, Nancy Benight, and the staff of the Texas Medical Center Digestive Disease Center Cellular for technical assistance.
REFERENCES
- 1. Al-Mukhtar MY, Sagor GR, Ghatei MA, Bloom SR, Wright NA. The role of pancreatico-biliary secretions in intestinal adaptation after resection, and its relationship to plasma enteroglucagon. Br J Surg 70: 398–400, 1983 [DOI] [PubMed] [Google Scholar]
- 2. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Burrin DG, Stoll B, Guan X, Cui L, Chang X, Holst JJ. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology 146: 22–32, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Burrin DG, Stoll B, Jiang R, Chang X, Hartmann B, Holst JJ, Greeley GH, Jr, Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr 71: 1603–1610, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Carter BA, Shulman RJ. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat Clin Pract Gastroenterol Hepatol 4: 277–287, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 50: 1955–1966, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeRubertis FR, Craven PA, Saito R. Bile salt stimulation of colonic epithelial proliferation. Evidence for involvement of lipoxygenase products. J Clin Invest 74: 1614–1624, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145: 2594–2603, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Howard L, Ament M, Fleming CR, Shike M, Steiger E. Current use and clinical outcome of home parenteral and enteral nutrition therapies in the United States. Gastroenterology 109: 355–365, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Huang X, Yang C, Luo Y, Jin C, Wang F, McKeehan WL. FGFR4 prevents hyperlipidemia and insulin resistance but underlies high-fat diet induced fatty liver. Diabetes 56: 2501–2510, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Hwang ST, Henning SJ. Ontogenic regulation of components of ileal bile acid absorption. Exp Biol Med (Maywood) 226: 674–680, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217–225, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Jones S. Mini-review: endocrine actions of fibroblast growth factor 19. Mol Pharm 5: 42–48, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology 130: S70–77, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 284: 1362–1365, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Niinikoski H, Stoll B, Guan X, Kansagra K, Lambert BD, Stephens J, Hartmann B, Holst JJ, Burrin DG. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets. J Nutr 134: 1467–1474, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 43: 535–539, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, Kliewer SA. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab 13: 729–738, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rager R, Finegold MJ. Cholestasis in immature newborn infants: is parenteral alimentation responsible? J Pediatr 86: 264–269, 1975 [DOI] [PubMed] [Google Scholar]
- 20. Roy CC, Laurendeau G, Doyon G, Chartrand L, Rivest MR. The effect of bile and of sodium taurocholate on the epithelial cell dynamics of the rat small intestine. Proc Soc Exp Biol Med 149: 1000–1004, 1975 [DOI] [PubMed] [Google Scholar]
- 21. Sato H, Macchiarulo A, Thomas C, Gioiello A, Une M, Hofmann AF, Saladin R, Schoonjans K, Pellicciari R, Auwerx J. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem 51: 1831–1841, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Sigalet DL. Short bowel syndrome in infants and children: an overview. Semin Pediatr Surg 10: 49–55, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Stoll B, Horst DA, Cui L, Chang X, Ellis KJ, Hadsell DL, Suryawan A, Kurundkar A, Maheshwari A, Davis TA, Burrin DG. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J Nutr 140: 2193–2200, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teitelbaum DH. Parenteral nutrition-associated cholestasis. Curr Opin Pediatr 9: 270–275, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toledo A, Yamaguchi J, Wang JY, Bass BL, Turner DJ, Strauch ED. Taurodeoxycholate stimulates intestinal cell proliferation and protects against apoptotic cell death through activation of NF-kappaB. Dig Dis Sci 49: 1664–1671, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, Stewart TA. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 143: 1741–1747, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Wang H, Khaoustov VI, Krishnan B, Cai W, Stoll B, Burrin DG, Yoffe B. Total parenteral nutrition induces liver steatosis and apoptosis in neonatal piglets. J Nutr 136: 2547–2552, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Yu C, Wang F, Kan M, Jin C, Jones RB, Weinstein M, Deng CX, McKeehan WL. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J Biol Chem 275: 15482–15489, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Zamir O, Nussbaum MS, Bhadra S, Subbiah MT, Rafferty JF, Fischer JE. Effect of enteral feeding on hepatic steatosis induced by total parenteral nutrition. JPEN J Parenter Enteral Nutr 18: 20–25, 1994 [DOI] [PubMed] [Google Scholar]