Abstract
Sirtuin 1 (SIRT1), a NAD+-dependent histone deacetylase, is involved in a wide array of cellular processes including glucose homeostasis, energy metabolism, proliferation and apoptosis, and immune response. However, it is unknown whether SIRT1 plays any physiological role in the regulation of intestinal homeostasis and motility. Thus the aim was to define SIRT1 expression and function in the gastrointestinal (GI) tract under physiological conditions. Forty 12–14-wk-old SIRT1 knockout (KO) and wild-type (WT) mice were fasted 21 h and/or refed 3 h. Fasted mice were injected intraperitoneally with bromodeoxyuridine (120 mg/kg body wt) 2 h before euthanasia. SIRT1 protein was localized to gastric and intestinal epithelial nuclei and was responsive to the nutritional status. SIRT1 was required for intestinal epithelial homeostasis. The SIRT1 KO mice showed enhanced crypt proliferation and suppressed villous apoptosis, resulting in increased intestinal villous height. In the SIRT1 KO intestine, the abundance of Forkhead box protein O1 and p53 protein decreased, whereas the subcellular localization of β-catenin protein accumulated mainly in the crypts. The SIRT1 KO mice showed accelerated gastric emptying rate with increased abundance of ghrelin mRNA and protein in the stomach. Moreover, the SIRT1 KO mouse intestine showed enhanced ex vivo spontaneous contraction. We concluded that, SIRT1 plays a critical role in the control of intestinal homeostasis (by promoting apoptosis and inhibiting proliferation) and gastrointestinal motility (by reducing gastric emptying and intestinal contractile activity), implicating a novel role for SIRT1.
Keywords: sirtuin 1, epithelial homeostasis, intestinal contraction, gastric emptying, ghrelin
the nad+-dependent deacetylase sirtuin 1 (SIRT1) is involved in the regulation of diverse biological processes including cell proliferation, differentiation, and apoptosis, as well as glucose homeostasis and energy metabolism, insulin secretion and sensitivity, and immune response. SIRT1 silences gene transcription by deacetylating histone and nonhistone proteins (2). Although SIRT1 function has been intensively investigated in the brain and peripheral tissues, its physiological relevance remains largely unknown in the gastrointestinal (GI) tract, which plays a key role in the control of energy homeostasis.
As the major contributor of energy, glucose uptake by the gut is influenced by gastric emptying (21). Gastric emptying is fine-tuned by metabolic, neuronal, and hormonal signals. Gastric emptying may serve as a key factor in the control of energy homeostasis (8). Circadian rhythms govern a variety of metabolic and physiological functions (27, 33), which is controlled by the circadian clock. SIRT1 is a component of CLOCK-BMAL1 transcription complex and acts as a negative regulator of the clock gene expression (e.g., Per2, a key repressor of the circadian clock) (26). In addition, SIRT1 can regulate clock gene expression through deacetylation and degradation of Per2 (1) and is required for high-magnitude circadian rhythms underlying fine-tuning gastric emptying and intestinal contraction (11, 29). Thus we wanted to test whether SIRT1 deficiency in mice impairs the GI motility.
It is controversial whether SIRT1 inhibits apoptosis and promotes survival in epithelial cells. In fact, SIRT1 has shown both oncogenic and tumor-suppressive effects (19). SIRT1-mediated suppression of apoptosis depends on the status of tumor suppressor p53 acetylation in a tissue-specific manner (2, 4, 25). SIRT1 directly deacetylates and inactivates p53, downregulating p53-mediated growth arrest and apoptosis (7, 20, 35). On the other hand, however, depletion of SIRT1 in mice increases genomic instability, suggesting that SIRT1 acts as a tumor suppressor (7). Importantly, ectopic induction of SIRT1 suppresses intestinal tumorigenesis in vivo (9). SIRT1 deacetylates β-catenin, promotes cytoplasmic localization, and suppresses its ability to activate transcription and to drive cell proliferation (9). Moreover, inhibition of SIRT1 stimulates proliferation (of human colon cancer HCT116 cells), indicating that SIRT1 is an inhibitor of proliferation and tumor formation in colon cancer (14). However, the physiological role of SIRT1 in intestinal homeostasis has not been defined. Intestinal homeostasis is maintained through a rapid turnover of cell proliferation and apoptosis. Cell proliferation in the crypt is enhanced upon feeding but decreased during fasting. In contrast, cell apoptosis along the villous is increased during fast but decreased upon feeding. Thus the intestinal epithelial crypt-villous unit might be an excellent model for defining the physiological role of SIRT1 in the regulation of proliferation and apoptosis simultaneously in vivo. In this report, we showed that the SIRT1 protein was exclusively expressed in the GI epithelial nuclei. As a key cellular energy sensor, the protein abundance of SIRT1 in the stomach and gut is increased during fast. We hypothesized that SIRT1 regulates proliferation and apoptosis of the gut epithelium in response to energy availability, which might underlie nutrition-mediated epithelial homeostasis.
MATERIALS AND METHODS
Experimental procedures.
All experiments were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. The SIRT1 knockout (KO) mice were provided by Dr. Wenyong Chen (City of Hope's Beckman Research Institute, Duarte, CA) upon an agreement of Dr. Frederick Alt (Children's Hospital, Harvard Medical School, Boston, MA). The mice were weaned at ∼6 wk after birth, provided free access to water and a standard rodent diet (no. 2920; Harlan Laboratories, Indianapolis, IN), and maintained under the standard 12-h:12-h light/dark cycle. The mice were genotyped using primers (forward: 5′-CTT GCA CTT CAA GGG ACC AA-3′; reverse 1: 5′-GTA TAC CCA CCA CAT CTG AG-3′; and reverse 2: 5′-CTA CCA CTC CTG GCT ACC AA-3′). Homozygous male mice (SIRT1−/− = KO; SIRT1+/+ = wild-type, WT) were used in the study. For the fast-refeeding experiment, mice at the age of ∼12–14 wk were fasted for 21 h and/or then refed for 3 h (n = 10 each group). Mice were injected intraperitoneally with bromodeoxyuridine (BrdU) (Sigma-Aldrich, St. Louis, MO) at a dosage of 120 mg/kg body wt 2 h before euthanasia under isoflurane anesthesia. The mouse intestine and stomach were removed and flushed with ice-cold saline. The intestine (excluding the duodenum) was divided into two equal portions (proximal = jejunum, and distal = ileum). The tissue samples were snap-frozen immediately in liquid nitrogen and stored at −80°C for molecular analysis, or fixed in 10% neutral buffered formalin and embedded in paraffin for morphometry and immunohistochemistry.
Morphometry, immunohistochemistry, and BrdU labeling.
One set of paraffin sections was cut at 7.5 μM, deparaffinized, rehydrated, and stained with hematoxylin and eosin for morphometry. Jejunum villous height and crypt depth were quantified under an Axiophot microscope (Zeiss, Jena, Germany) by a blinded examiner using the NIH image software (v1.60) in at least 20 vertically well-oriented villous-crypt units each sample.
Another set of paraffin sections were then microwaved for 20 min in 10 mM citrate buffer (pH 6.0) for antigen retrieval and permeabilized in 0.1% Triton X-100 in PBS (PBS-T) for 3 × 15 min. After being blocked for 1 h (in 10% normal donkey serum in PBS-T), sections were incubated at 4°C overnight with primary antibodies (1:100; antibodies against β-catenin and cleaved caspase 3 from Cell Signaling Technology, Danvers, MA; antibody against ghrelin from Santa Cruz Biotechnology, Santa Cruz, CA; antibody against SIRT1 from Millipore, Billerica, MA; or nonspecific isotype IgG as negative control). Note that the ghrelin antibody recognizes both acyl ghrelin and des-acyl ghrelin. After washing, sections were incubated for 2 h with FITC-conjugated donkey anti-mouse or anti-rabbit IgG (1:1,000; Jackson ImmunoResearch Laboratories, Bar Harbor, ME). Meanwhile, BrdU antibody conjugated with FITC (1:100; BD Bioscience, San Jose, CA) was used to directly stain BrdU-positive labeled cells. The nuclei were counterstained with TOPRO-3 (Invitrogen, Carlsbad, CA). The sections were mounted with 30% glycerol in PBS and visualized via a laser confocal microscopy. Finally, the proportion of proliferating crypt cells was quantified by counting the number of BrdU-labeled nuclei in at least 20 vertically well-oriented crypts in each sample and expressing this as a percentage of total nuclei per crypt. In addition, fractional distribution of ghrelin-positive cells was estimated by immunohistochemistry and counted per image.
Western blotting.
Proteins were extracted from the mouse gut. Tissue samples were powdered in liquid nitrogen and homogenized and sonicated on ice in RIPA buffer (50 mM Tris·HCl at pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mM sodium orthovanadate, 1 mM sodium fluoride) and centrifuged at 10,000 g for 15 min at 4°C. Protein concentration was determined using BCA protein assay (Pierce, Rockford, IL). Samples were boiled at 100°C for 10 min in 2× sample buffer. Total protein (100 μg) of the tissue protein extracts was loaded and electrophoresed in running buffer on an ∼7.5–12% Tris-glycine SDS-polyacrylamide gel. Following the SDS PAGE, proteins were transferred to methanol presoaked PVDF membrane at 100 V, 4°C for 60 min. After being blocked in PBS-T containing 5% milk, the membrane was incubated at 4°C overnight with primary antibodies [1:500; against cleaved caspase 3 and p53 from Cell Signaling Technology; against SIRT1 from Millipore; and against Forkhead box protein O1 (FoxO1) and β-actin from Santa Cruz Biotechnology]. After being washed, the membrane was incubated with anti-rabbit (or mouse) IgG-conjugated horseradish-peroxidase (1:3,000; Bio-Rad Laboratories, Hercules, CA) and reacted with ECL-Plus chemiluminescent reagent (Amersham Biosciences, Piscataway, NJ). Image on the membrane was captured by ChemiDoc XRS and analyzed with Quantity One 4.6.6 (Bio-Rad Laboratories). Finally, the densitometry of protein abundance was normalized by the loading control (namely β-actin).
qRT-PCR.
Total RNA was extracted from the frozen tissue samples using RNeasy Mini kit (Qiagen, Valencia, CA). The RNA concentration was determined by NanoDrop 1000 (NanoDrop, Wilmington, DE). Reverse transcription was performed with 1 μg RNA, random hexamers, and oligo (dT)12–18 using SuperScript-III-First-Strand-Synthesis-SuperMix kit (Invitrogen). mRNA abundance was quantified by real-time qRT-PCR using SYBR Green I dye. Forward and reverse primers were 5′-TCCAAGAAGCCACCAGCTAA and 5′- AACATCGAAGGGAGCATTGA for ghrelin (NM_ 021488); 5′-ATGCTCGCCATCCACAAGA and 5′-GCGGAATCGAATGGGAGAAT for Per2 (NM_011066.3); and 5′-GCACAGTCAAGGCCGAGAAT and 5′-GCCTTCTCCATGGTGGTGAA for Gapdh (NM_008084.2). Assays were performed in triplicate with an ABI 7900HT Fast real-time PCR system (Applied Biosystems, Foster City, CA). qPCR conditions were as follows: 10 min at 95°C, then 40 cycles of 30 s at 95°C and 60 s at 60°C. Data were normalized to Gapdh or 18S ribosomal RNA (ΔΔCT analysis) as indicated.
13C-octanoic acid breath test.
Mice (∼12 wk old) were fasted overnight for assessing gastric emptying rate (GER) using 13C-octanoic acid breath test (30), which was modified and coupled with real-time measurement of CO2 production using the Comprehensive Laboratory Animal monitoring system. In brief, mice were transferred and adapted into the calorimetric chamber for 1 h before gastric administration of 13C-octanoic acid-containing meal. To minimize effects of circadian rhythm and gastric volume on the GER, mice were tested between 9:00 and 11:00 AM. A baseline breath sample was taken before the gastric tracer administration. The test meal was composed of 5% (vol/vol) 1-13C-octanoic acid (99% enrichment; Cambridge Isotope Laboratories, Andover, MA) in milk, which produced a reasonable abundance of 13C isotopic enrichment in breath CO2. This test meal was administered at 8 μl/g body wt into the stomach via orogastric gavage. After the gastric administration, breath CO2 was sampled every 3.5 min from the chamber and injected into evacuated 10-ml Exetainer tubes for 15 s. The 13C enrichment of breath samples was measured by an isotope ratio mass spectrometry (Thermo Finnigan Deltaplus XL, San Jose, CA) and expressed as atom percentage. The measured 13CO2 recovery in the breath was expressed as percentage excretion per hour of the given 1-13C-octanoic acid dose (% dose/h). Data for the 13CO2 excretion rate were fitted by a nonlinear regression model (10) using the NLIN Procedure (SAS Version 9.1; SAS Institute, Cary, NC): y = atbe−ct, where y is the percentage of 13CO2 excretion in breath per hour, t is time in hours, and a, b, and c are regression-estimated constants. The following parameters were computed (15): half-excretion time (T1/2, min) = gamma inv (0.5, b+1, 1/c); lag phase (Tlag, min) = b/c; and gastric emptying coefficient = In (a).
Intestinal contractile activity.
To measure intestinal contractile activity (34), strips (∼10 mm in length from the distal ileum) were mounted in 15-ml organ baths filled with Krebs-Ringer solution (103 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 25 mM NaHCO3, 1.1 mM NaH2PO4, and 15 mM glucose) that was buffered with BSA and gassed with 5% CO2-95% O2. Isometric force was monitored by an external force displacement transducer (Grass FT.03; Grass Instrument, Quincy, MA) connected to a PowerLab (AD Instruments, Colorado Springs, CO). A 0.4-g load was applied to the 1-cm ileal strip when it was first mounted. After a 30-min equilibration, spontaneous contractile activity was recorded under this resting load of 0.4 g, without further imposed stretch. Contractile activity was calculated as the area under the curve integrated for 10 min and/or normalized by the tissue weight.
Statistical analysis.
Data were analyzed by the MIXED Procedure (SAS Version 9.1), in which fixed effects (including genotype, treatment, and time), random effects (including individual mouse), and repeated measures (at different time points in the same mouse) were considered. A full model for ANOVA was employed including genotype, treatment, time, and their interactions. If applicable, repeated measures (such as data for gastric emptying) were included in a repeated statement. Data were expressed as means ± SE. P < 0.05 or 0.01 was considered as statistical significance.
RESULTS
SIRT1 deficiency accelerated gastric emptying and increased intestinal contraction.
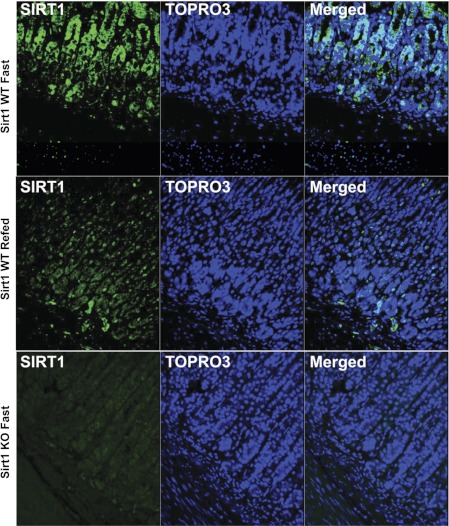
Although SIRT1 is highly expressed in the brain and peripheral tissues (including liver and pancreas), it is unknown whether SIRT1 is expressed in the stomach or gut. To explore the physiological role of SIRT1, we determined its cellular localization in the GI tract. SIRT1 protein (in green) was mainly localized to the gastric mucosal epithelium (Fig. 1). Notably, SIRT1 in the WT stomach was induced during fasting and distributed in the cytoplasm and nucleus. However, SIRT1 was less abundant and mainly localized in the nuclei during refeeding. Of note, we observed that the stomach was empty at 1 h after refeeding in the KO mice, whereas it was full in the WT mice.
Fig. 1.
Sirtuin 1 (SIRT1) protein expressed in the gastric epithelium. ∼13–14-wk-old male mice were fasted for 21 h and/or then refed for 3 h. The SIRT1 protein (in green) was mainly localized to the epithelial nuclei, where nuclei were counterstained with TOPRO-3 (in blue). KO, knockout. WT, wild-type.
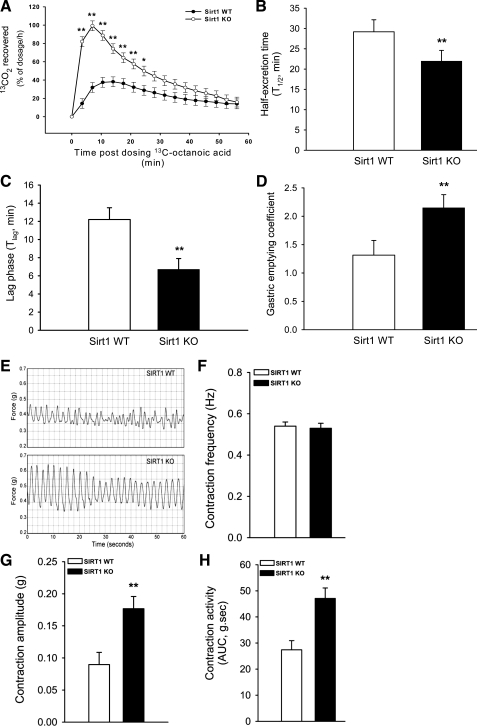
Whole-body energy balance can be determined more precisely by net intake of food energy and expenditure of body energy. Apparently, digestion and absorption of food affect net intake of food energy, which is mainly regulated by gastric emptying and intestinal motility. We hypothesized that SIRT1 might function as an energy sensor in the GI tract and modulate its motility to control net intake (input) of food energy. Thus we wanted to quantify the rate of gastric emptying in the KO mice using 13C-octanoic acid breath test. It is well established that gastric emptying is a limiting step for and positively correlated with the rate of 13CO2 release into breath (from oxidation of 13C-octanoic acid in the liver). Gastric emptying (indicated by the rate of 13CO2 recovered in breath) increased during the first 30 min in the KO mice (Fig. 2A). To derive key parameters for gastric emptying, the data were further fitted by a nonlinear regression model. Both half-excretion time and lag phase for liquid meal were shorter, whereas gastric emptying coefficient was higher in the KO mice than those in the WT mice (Fig. 2, B–D). Importantly, moreover, the ex vivo spontaneous contractile activity of the KO mouse gut increased as measured by isometric force (Fig. 2, E–H). Figure 2E shows representative traces of the spontaneous contractions from the mouse ileum strips, and Fig. 2, F and G, show statistical results of the frequency duration and amplitude of spontaneous contraction. Note that frequencies of spontaneous contractions were approximately at 0.5 Hz (= 30 times/min) and not altered between the WT and KO mice. This is consistent with a notion that the mouse ileum contracts at a very regular frequency of ∼30 times per minute. However, the amplitude of spontaneous contraction was enhanced in the KO mice compared with that in the WT. Therefore, the KO mice show higher spontaneous contractile activity (i.e., integral area under the curve in Fig. 2H) attributed to enhanced amplitude. These data indicated that gastric emptying and gut contraction were accelerated in the SIRT1 KO mice.
Fig. 2.
Gastric emptying and intestinal contraction accelerated in SIRT1 KO mice. Rates of gastric emptying (indicated by the rate of 13CO2 recovered in breath) were higher during the first 30 min in the SIRT1 KO mice than those in the WT littermates (A). The KO mice had shorter half-excretion time and lag phase but higher gastric emptying coefficient than the WT mice (B–D). Representative traces of the spontaneous contractions from ileum strips are shown in E. Due to enhanced amplitude and sustained frequency, the ex vivo spontaneous contraction activity of the ileum increased in the SIRT1 KO mice compared with that in the WT littermates (F–H). Male mice (∼12 wk-old) were fasted overnight for assessing gastric emptying rate of the test milk using 13C-octanoic acid breath test in conjunction with real-time measurement of CO2 production. Data for the 13CO2 excretion rate were fitted by a nonlinear regression model (10) (see computation in materials and methods). Spontaneous contraction of the gut was traced ex vivo by isometric force measurement (E). Data are expressed as means ± SE (n = 10 per group), *P < 0.05, **P < 0.01 denote significance (at the same time) between 2 genotypes. AUC, area under the curve.
SIRT1 deficiency increased the abundance of ghrelin mRNA but decreased that of Per2 mRNA in the stomach.
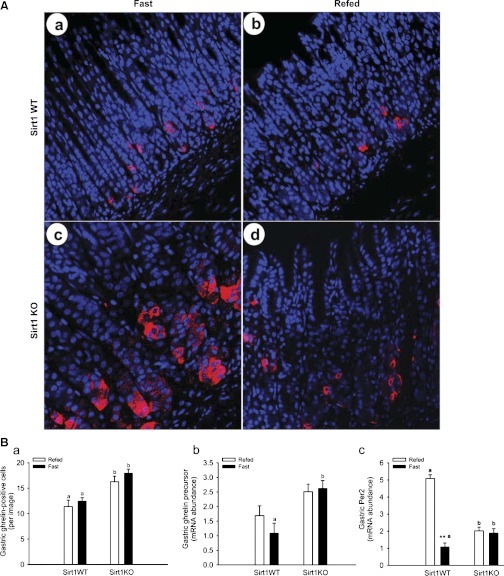
Ghrelin significantly stimulates GI motility and inversely associates with expression of circadian genes (namely Per1 and Per2). In response to energy availability, the SIRT1 represses expression of circadian genes and modulates clock outputs in the control of energy metabolism. Thus we wanted to explore whether SIRT1 deficiency affects expression of ghrelin and Per2 in the stomach (Fig. 3). Representative images for ghrelin-immunostained cells (in red) in the stomach are shown in Fig. 3A. The number of ghrelin-positive cells in the gastric epithelium was higher (P < 0.01) in the KO mice than that in the WT mice (Fig. 3B). The mRNA abundance of ghrelin precursor in the stomach was higher (P < 0.01) during fasting in the KO mice than that in the WT mice but was insignificant during the 3-h refeeding. Of note, the mRNA abundance of ghrelin precursor was not decreased by 3-h refeeding. The expression of ghrelin in the stomach is negatively correlated to that of Per2, a key circadian gene that is negatively modulated by SIRT1. The mRNA abundance of Per2 in the stomach increased (P < 0.01) under the fast status in the KO mice compared with that in the WT mice. Moreover, it was lower (P < 0.01) under the fast status than that under the refeeding status in the WT mice, but this response was abolished in the KO mice.
Fig. 3.
The abundance of ghrelin mRNA and protein increased in the SIRT1 KO mouse stomach. The ghrelin protein was expressed in the gastric oxyntic glands (A). Not only the abundance of ghrelin mRNA but also the number of ghrelin-positive cells were higher in the gastric mucosa in the SIRT1 KO mice than those in the WT littermates (B). Interestingly, the abundance of Per2 mRNA increased in the KO stomach during fasting (B). ∼13–14-wk-old male mice were fasted for 21 h and/or then refed for 3 h. The ghrelin protein (in red) was localized to endocrine cells, where nuclei were counterstained with TOPRO-3 (in blue). Gene expression was measured by real-time qRT-PCR and expressed in terms of mRNA levels relative to 18S rRNA. Data are expressed as means ± SE (n = 10 per group); **P < 0.01 denotes significance in the same genotype between fast and refeeding, whereas a,bP < 0.05 denotes significance during fast (or refeeding) between 2 genotypes.
SIRT1 deficiency stimulates cell proliferation in the gut.
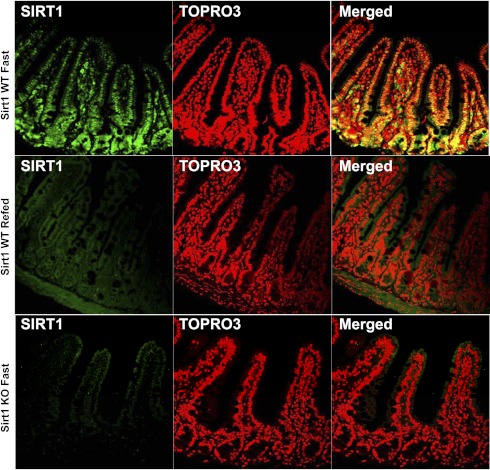
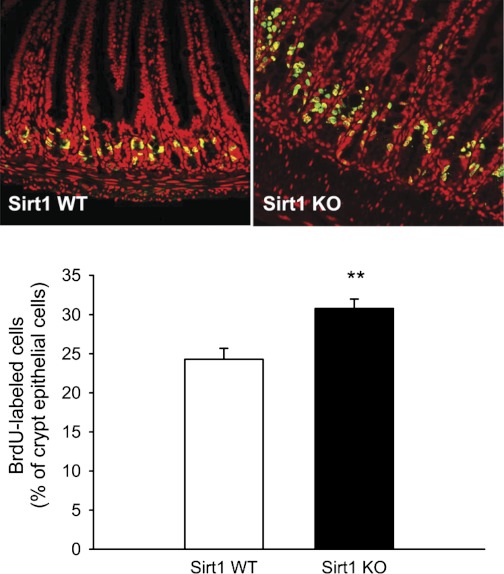
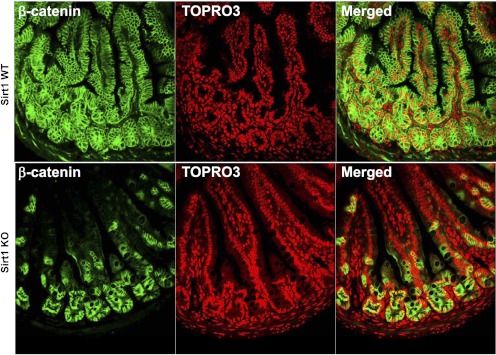
We further determined SIRT1 protein expression and localization in the gut (Fig. 4). Similarly, SIRT1 protein expression was responsive to the nutritional switching from fasting to refeeding. SIRT1 was expressed in the epithelium and exclusively localized to the nuclei. We wanted to define whether SIRT1 plays a physiological role in the maintenance of intestinal homeostasis. Using in vivo 2-h BrdU pulse-chase labeling, we found that BrdU-labeled cells (in green in Fig. 5) were mainly localized to the crypt, a highly proliferating zoon, and at a higher percentage (of crypt epithelial cells, P < 0.01) in the SIRT1 KO mouse gut, indicating that SIRT1 might suppress intestinal cell proliferation under a normal, physiological state. Because SIRT1 gain-of-function suppresses β-catenin signaling in the gut, we wanted to express whether SIRT1 physiologically inhibits β-catenin signaling underlying suppression of cell proliferation. We found that the total β-catenin was expressed along the entire mucosal crypt-villous unit and mainly localized to the cytoplasm (and plasma membrane) in the WT mice (Fig. 6). However, β-catenin was highly preserved in the crypt and mainly localized to the nucleus (and cytoplasm) in the KO mice. This cellular distribution of β-catenin might indicate that SIRT1 regulates β-catenin shuttling between the cytoplasm and nucleus or β-catenin gene expression and protein turnover per se.
Fig. 4.
SIRT1 protein expressed in the intestinal epithelial nuclei. ∼13–14-wk-old male mice were fasted for 21 h and/or then refed for 3 h. The SIRT1 protein (in green) was mainly localized to the epithelial nuclei, where nuclei were counterstained with TOPRO-3 (in red).
Fig. 5.
SIRT1 deficiency stimulates cell proliferation in the intestinal crypt. Intestinal cell proliferation was determined by bromodeoxyuridine (BrdU) labeling. BrdU labeling images and quantitative results of individual samples are shown. Mice were fasted for 21 h and then injected intraperitoneally with BrdU (120 mg/kg body wt) 2 h before euthanasia. Whereas the nuclei were counterstained with TOPRO-3 (in red), BrdU-positive labeled cells (in green) were quantified as a percentage of total nuclei per crypt. Data are expressed as means ± SE (n = 10 per group), **P < 0.01 denote significance between 2 genotypes.
Fig. 6.
SIRT1 deficiency preserves β-catenin retention in the intestinal crypt. ∼13–14-wk-old male mice were fasted for 21 h. The β-catenin protein (in green) was mainly localized to the intestinal epithelium, where nuclei were counterstained with TOPRO-3 (in red).
SIRT1 deficiency suppresses cell apoptosis in the gut.
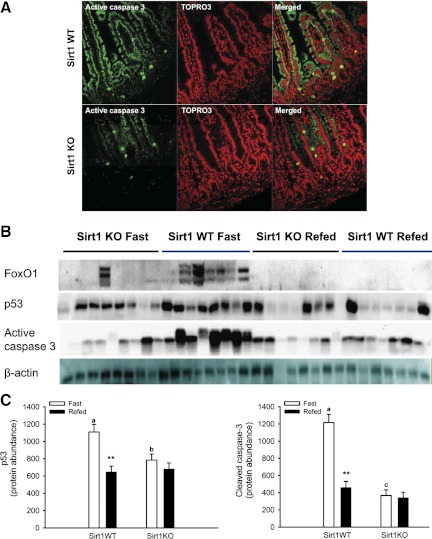
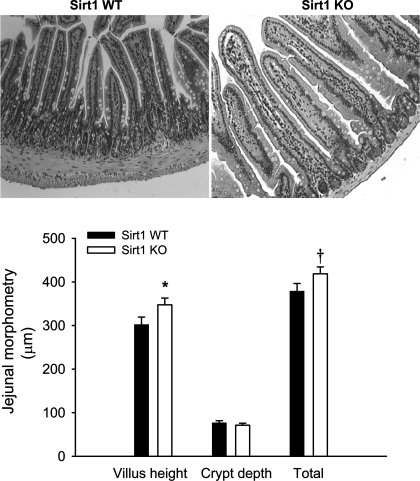
Intestinal epithelial homeostasis is determined by proliferation and cell death (mainly apoptosis under physiological conditions). We wanted to define whether SIRT1 physiologically modulates intestinal epithelial apoptosis. We found that SIRT1 deficiency suppressed proapoptotic signaling in the gut (Fig. 7). Clearly, active caspase 3 (in green, a marker for cells undergoing apoptosis) was mainly expressed in the villous epithelial cytoplasm. Notably, active caspase 3 protein was less abundant in the SIRT1 KO gut during fasting. These results suggest that SIRT1 might promote the intestinal epithelial cell apoptosis under fasting. Furthermore, we found that protein abundance of transcription factor FoxO1 and tumor suppresser p53 in the gut, which are downstream targets of SIRT1 and key positive regulators of cell apoptosis, was decreased in the KO mice compared with that in the WT mice. Clearly, protein abundance of FoxO1 and p53 in the WT gut was increased under the fast status compared with that under the refed status. But this was not the case in the KO gut. Except for posttranslational modification (i.e., deacetylation), SIRT1 might suppress its degradation by ubiquitination and/or enhance its expression per se. As a result of SIRT1-mediated suppression of proliferation and promotion of apoptosis, villous height was increased in the SIRT1 KO jejunum although crypt depth was not different (Fig. 8).
Fig. 7.
SIRT1 deficiency suppresses proapoptotic signaling in the intestine. In fasted mice, active caspase 3 protein (in green in A) was mainly localized to the intestinal epithelium, where nuclei were counterstained with TOPRO-3 (in red). Blotting images and quantitative results of individual samples are shown in B and C, respectively. ∼13–14-wk-old male mice were fasted for 21 h and/or then refed for 3 h. Protein abundance in the gut was quantified by Western blotting and normalized by β-actin protein. Data are expressed as means ± SE (n = 10 per group), **P < 0.01 denotes significance in the same genotype between fast and refeeding, whereas a,bP < 0.05 and a,cP < 0.01 denote significance during fast between 2 genotypes.
Fig. 8.
SIRT1 deficiency stimulates intestinal mucosal growth. Jejunum villous height and crypt depth were quantified using the NIH image software. Morphological images and quantitative results of individual samples are shown. Mice were fasted for 21 h. Data are expressed as means ± SE (n = 10 per group), †P < 0.10, *P < 0.05 denote significance between 2 genotypes.
DISCUSSION
SIRT1 is induced to adjust energy metabolism in response to caloric restriction and probably mediates mammalian longevity. The NAD+-dependent SIRT1 plays a pivotal role in metabolic control in response to energy availability (28). The GI integrity and function depend on homeostatic control of cell proliferation and apoptosis in response to luminal nutrition, microbiome, injury, or inflammation. SIRT1, a NAD+-dependent class III histone deacetylase, is involved in a wide array of cellular processes including energy metabolism, proliferation and apoptosis, and immune response. Although energy homeostasis has been focused on energy expenditure and food intake, dietary net energy varies with wide-ranging efficiency rates of digestion and absorption, which is determined by intestinal motility and absorptive surface. However, it is unknown whether SIRT1 plays a physiological role in the regulation of intestinal homeostasis. Thus we wanted to test whether energy-sensitive SIRT1 promotes the whole-body energy homeostasis at the first pass by fine-tuning the intestinal motility and homeostasis. In the present study, we showed that SIRT1-null mice had accelerated gastric emptying and gut contraction in conjunction with increased abundance of ghrelin mRNA and protein in the stomach. Moreover, the intestinal homeostasis was disrupted in the SIRT1-null mice, resulting in increased cell proliferation and decreased apoptosis. Furthermore, fast-induced proapoptotic signaling in the gut was abolished in the SIRT1-null mice. Therefore, the energy-sensitive SIRT1 deacetylase finely tunes gastric emptying and intestinal mucosal homeostasis in response to the nutritional status.
Gastric emptying is fine-tuned by metabolic, neuronal, and hormonal signals. In fact, gastric emptying may serve as a key factor in the control of energy homeostasis. In the present study, 13C-octanoic acid was used in liquid milk for breath test. Of note, 13CO2 excretion in the mouse breath test is insignificant after 60 min for liquid meal, but it is measurable until 180 min for solid meal (32). Half-excretion time (T1/2 = 29 min) in the WT mice was similar as reported T1/2 (= 28 min) for the mouse gastric emptying of liquid meal (32). The T1/2 in the KO mice was shorter than that in the WT, suggesting that SIRT1 deficiency accelerates GER of liquid meal. However, it would be interesting to determine whether there is any difference in gastric emptying of solid meal. Interestingly, we noticed that the SIRT1 KO mice had higher food intake per unit of body weight in the present study, which might result from accelerated gastric emptying. In the present study, SIRT1 deficiency accelerated GER and rate of intestinal contraction and increased the abundance of ghrelin mRNA and protein in the stomach. Ghrelin is produced predominantly by the X/A cells within the gastric mucosa (6). Ghrelin mediates gastric phase III-like contractions, enhances gastric emptying, and stimulates small intestinal transit (3). Importantly, the gastric abundance of ghrelin mRNA and protein was higher in the SIRT1 KO mice, and this is associated with accelerated gastric emptying and intestinal motor activity. It is demonstrated that there are two subpopulations of ghrelin cells in the stomach. Whereas acyl ghrelin- and des-acyl ghrelin-immunopositive reactions overlap in closed-type round cells, des-acyl ghrelin-immunopositive reaction occurs in open-type cells where acyl ghrelin is immunonegative (22). The ghrelin antibody (used in the present study) recognizes both acyl ghrelin and des-acyl ghrelin. Thus further studies are warranted to define which form (acyl vs. des-acyl) ghrelin is regulated by SIRT1. Ghrelin accelerates gastric emptying mainly via activation of the vagus and to some extent by activation of peripheral receptors on enteric neurons but not on smooth muscle. It is unlikely that the SIRT1 deficiency-induced enhancement in the ex vivo spontaneous contractility of the ileal strip is mediated via a direct action of ghrelin. However, further studies are warranted to determine whether ghrelin is the key mediator for SIRT1 action on gastric emptying and intestinal contraction. For example, it would be interesting to determine whether specific antagonists of ghrelin receptor can block SIRT1 deficiency-enhanced gastric emptying and gastric contractility.
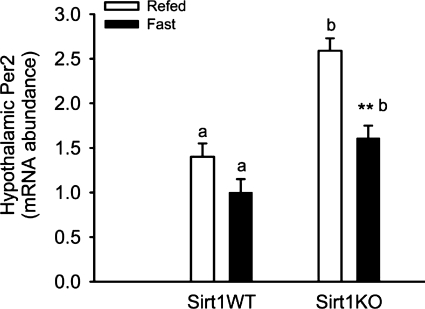
The transcriptional activator clock is a histone acetyl transferase, whereas the NAD+-dependent SIRT1 functions as a histone deacetylase, which links the circadian clock with energy metabolism (23, 27). SIRT1 enhances the deacetylation and degradation of Per2 (1). Both SIRT1 gain-of-function and SIRT1 activators repress the Per2 promoter activity, whereas SIRT1 inhibitors have the opposite effect. Ghrelin and Per2 are rhythmically but inversely expressed in stomach X/A cells and synchronized by food-entrainable circadian clock (16). Without little effect of SIRT1-mediated repression, abundance of ghrelin mRNA and protein increased in the KO mouse stomach. Under the fast status, SIRT1-mediated repression of Per2 expression was alleviated in the SIRT1 KO mouse stomach and hypothalamus (Fig. 9). Interestingly, the abundance of Per2 mRNA in the hypothalamus was increased under the SIRT1 deficiency. Thus SIRT1 deficiency might enhance the circadian oscillation in the stomach and the circadian clock in the hypothalamus, probably amplifying circadian rhythm of gastric empting and gut contraction. In the present study, however, we cannot conclude whether Per2 directly regulates ghrelin expression and signaling and whether ghrelin is the key mediator for SIRT1-induced action on gastric emptying and intestinal contraction. Further studies are warranted to elucidate how SIRT1 mediates gastric emptying and intestinal homeostasis. For example, it would be important 1) to define whether Per2 directly regulates ghrelin expression using ghrelin promoter-driven luciferase assay, 2) to determine whether ghrelin is the key mediator for SIRT1-induced action on gastric emptying using ghrelin receptor antagonists, and 3) to dissect molecular mechanisms underlying SIRT1-induced modulation of gastric emptying using tissue-specific deletion or overexpression of SIRT1 mouse models.
Fig. 9.
The abundance of Per2 mRNA increased in the SIRT1 KO mouse hypothalamus. The abundance of Per2 mRNA in the hypothalamus increased in the SIRT1 KO mice than that in the WT littermates. ∼13–14-wk-old male mice were fasted for 21 h and/or then refed for 3 h. Gene expression was measured by real-time qRT-PCR and expressed in terms of mRNA levels relative to 18S rRNA. Data are expressed as means ± SE (n = 10 per group), ** or a,bP < 0.01 denote significance in the same genotype between fast and refeeding, or significance during fast (or refeeding) between 2 genotypes.
SIRT1 acts as an energy-sensitive growth suppressor in certain cell types (24). SIRT1-mediated deacetylation of substrates (namely p53 and β-catenin) is involved in the regulation of cell proliferation and apoptosis. However, it is unknown whether SIRT1 plays a physiological role in the control of intestinal epithelial homeostasis. In this study, we found that SIRT1 was expressed in the GI epithelial nuclei was and responsive to the nutritional status. The abundance of SIRT1 protein in the stomach and gut was increased during fast but undetectable during refeeding. Moreover, SIRT1 deficiency in mice showed increased villous height (with stimulated proliferation and suppressed apoptosis), suggesting that SIRT1 plays a physiological role in the control of intestinal epithelial homeostasis.
Tumor suppressor p53 transactivates proapoptotic genes and induces apoptosis through direct action on mitochondria (5, 17). SIRT1 directly deacetylates p53 and negatively regulates p53-mediated transcriptional activation, attenuating p53-mediated functions (such as apoptosis) (9, 14, 20). In addition, SIRT1 might enhance p53 deacetylation-mediated apoptosis through its mitochondrial translocation (12). In the present study, the protein abundance of p53 decreased under the fasting status in the SIRT1 KO gut, which was associated with suppressed apoptosis. Moreover, the SIRT1 KO mice showed enhanced cell proliferation and accumulated nucleus β-catenin in the crypts, suggesting that SIRT1 suppresses cell proliferation of intestinal epithelium through altering β-catenin subcellular localization and/or protein turnover. In the intestine, the Wnt/ β-catenin signaling plays a crucial role in driving epithelial cell proliferation. As a key signal transducer, β-catenin can mediate transcription activation of Wnt target genes, thus promoting cell proliferation of the crypt epithelium (14, 18). Inhibition of SIRT1 inactivates β-catenin in colorectal carcinoma cells (13). However, overexpression of SIRT1 in the intestinal epithelium suppresses the ability of β-catenin to drive transcription and proliferation (9). In the present study, β-catenin was accumulated in the KO intestinal crypts, which might account for higher proliferation, supporting the notion that SIRT1 deacetylates β-catenin and reduces transcriptional activity, thus suppressing proliferation in vivo. In this present study, we show that SIRT1 signaling was responsive to the nutritional status and essential for the intestinal epithelial homeostasis. However, further studies are warranted to dissect molecular mechanisms underlying SIRT1-mediated modulation of intestinal homeostasis in response to nutrition.
In summary, we demonstrate that SIRT1 inhibits gastric emptying and intestinal contraction, which is associated with dysregulated expression of Per2 and ghrelin in the stomach; SIRT1 suppresses cell proliferation with unstabilizing cytosolic β-catenin in the intestinal crypts; and SIRT1 promotes cell apoptosis with enhancing p53 function in the intestinal villi. Our study suggests that SIRT1 activation is essential for the circadian control of gastric emptying and intestinal contraction, and for the nutritional modulation of intestinal mucosal homeostasis.
GRANTS
This work is supported by the USDA/ARS under Cooperative Agreement No. 6250-51000-043, NIH Grants DK075489 and DK084125, and the National Natural Science Foundation of China Grant 30728016 (X. Guan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y. Wang, X. Shi, J. Qi, X. Li, and X. Guan contributed to the study concept and design; acquisition of data; statistical analysis; interpretation of data; and drafting the manuscript. In addition, X. Guan obtained funding, supervised the study, and finalized the manuscript. K. Uray contributed to acquisition, analysis, and interpretation of ex vivo contraction data; and critical revision of the manuscript.
ACKNOWLEDGMENTS
The authors thank Qiang Tong, Zhuo Yang, Maria Truong, Fuguo Zhou, Aijun Zhang, Firoz Vohra, and Shaji Chacko at Baylor College of Medicine for scientific and technical support. We also thank Shinil Shah at the University of Texas Health Science Center at Houston for technical support. This work is a publication of the United States Department of Agriculture, Agricultural Research Service (USDA/ARS) Children's Nutrition Research Center, Departments of Pediatrics and Medicine, Baylor College of Medicine, and Texas Children's Hospital, Houston, TX. The contents of this publication do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES
- 1. Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134: 317–328, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer 9: 123–128, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen CY, Asakawa A, Fujimiya M, Lee SD, Inui A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol Rev 61: 430–481, 2009. [DOI] [PubMed] [Google Scholar]
- 4. Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA 100: 10794–10799, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303: 1010–1014, 2004. [DOI] [PubMed] [Google Scholar]
- 6. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141: 4255–4261, 2000. [DOI] [PubMed] [Google Scholar]
- 7. Deng CX. SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci 5: 147–152, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duggan JP, Booth DA. Obesity, overeating, and rapid gastric emptying in rats with ventromedial hypothalamic lesions. Science 231: 609–611, 1986. [DOI] [PubMed] [Google Scholar]
- 9. Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de CR, Fuchs C, Hahn WC, Guarente LP, Sinclair DA. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLos One 3: e2020, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghoos YF, Maes BD, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, Vantrappen G. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 104: 1640–1647, 1993. [DOI] [PubMed] [Google Scholar]
- 11. Goo RH, Moore JG, Greenberg E, Alazraki NP. Circadian variation in gastric emptying of meals in humans. Gastroenterology 93: 515–518, 1987. [DOI] [PubMed] [Google Scholar]
- 12. Han MK, Song EK, Guo Y, Ou X, Mantel C, Broxmeyer HE. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell 2: 241–251, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holloway KR, Calhoun TN, Saxena M, Metoyer CF, Kandler EF, Rivera CA, Pruitt K. SIRT1 regulates Dishevelled proteins and promotes transient and constitutive Wnt signaling. Proc Natl Acad Sci USA 107: 9216–9221, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kabra N, Li Z, Chen L, Li B, Zhang X, Wang C, Yeatman T, Coppola D, Chen J. SirT1 is an inhibitor of proliferation and tumor formation in colon cancer. J Biol Chem 284: 18210–18217, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kocelak P, Zahorska-Markiewicz B, Jonderko K, Olszanecka-Glinianowicz M, Zak-Golab A, Holecki M, Kaminska M, Szymszal M. Long-term effects of lipase inhibition by orlistat on gastric emptying and orocecal transit time of a solid meal. J Gastroenterol 43: 609–617, 2008. [DOI] [PubMed] [Google Scholar]
- 16. LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci USA 106: 13582–13587, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol 6: 443–450, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Li Z, Nie F, Wang S, Li L. Histone H4 Lys 20 monomethylation by histone methylase SET8 mediates Wnt target gene activation. Proc Natl Acad Sci USA 108: 3116–3123, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu T, Liu PY, Marshall GM. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res 69: 1702–1705, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107: 137–148, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Macdonald IA. Physiological regulation of gastric emptying and glucose absorption. Diabet Med 13: S11–S15, 1996. [PubMed] [Google Scholar]
- 22. Mizutani M, Atsuchi K, Asakawa A, Matsuda N, Fujimura M, Inui A, Kato I, Fujimiya M. Localization of acyl ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am J Physiol Gastrointest Liver Physiol 297: G974–G980, 2009. [DOI] [PubMed] [Google Scholar]
- 23. Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134: 329–340, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Narala SR, Allsopp RC, Wells TB, Zhang G, Prasad P, Coussens MJ, Rossi DJ, Weissman IL, Vaziri H. SIRT1 acts as a nutrient-sensitive growth suppressor and its loss is associated with increased AMPK and telomerase activity. Mol Biol Cell 19: 1210–1219, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peck B, Chen CY, Ho KK, Di FP, Myatt SS, Coombes RC, Fuchter MJ, Hsiao CD, Lam EW. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther 9: 844–855, 2010. [DOI] [PubMed] [Google Scholar]
- 26. Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324: 651–654, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer 9: 886–896, 2009. [DOI] [PubMed] [Google Scholar]
- 28. Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab 7: 104–112, 2008. [DOI] [PubMed] [Google Scholar]
- 29. Suzuki A, Asahina M, Ishikawa C, Asahina KM, Honma K, Fukutake T, Hattori T. Impaired circadian rhythm of gastric myoelectrical activity in patients with multiple system atrophy. Clin Auton Res 15: 368–372, 2005. [DOI] [PubMed] [Google Scholar]
- 30. Symonds E, Butler R, Omari T. Noninvasive breath tests can detect alterations in gastric emptying in the mouse. Eur J Clin Invest 32: 341–344, 2002. [DOI] [PubMed] [Google Scholar]
- 32. Symonds EL, Butler RN, Omari TI. Assessment of gastric emptying in the mouse using the [13C]-octanoic acid breath test. Clin Exp Pharmacol Physiol 27: 671–675, 2000. [DOI] [PubMed] [Google Scholar]
- 33. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uray KS, Laine GA, Xue H, Allen SJ, Cox CS., Jr Intestinal edema decreases intestinal contractile activity via decreased myosin light chain phosphorylation. Crit Care Med 34: 2630–2637, 2006. [DOI] [PubMed] [Google Scholar]
- 35. Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature 451: 587–590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]