Abstract
We reported previously that mechanical stretch in rat colonic obstruction induces cyclooxygenase (COX)-2 expression in smooth muscle cells. The aims of the present study were to investigate whether in vivo treatment with COX-2 inhibitor has prophylactic and therapeutic effects on motility dysfunction in colon obstruction, and if so what are the underlying mechanisms. Partial colon obstruction was induced with a silicon band in the distal colon of 6–8-wk-old Sprague-Dawley rats; obstruction was maintained for 3 days or 7 days. Daily administration of COX-2 inhibitor NS-398 (5 mg/kg) or vehicle was started before or after the induction of obstruction to study its prophylactic and therapeutic effects, respectively. The smooth muscle contractility was significantly suppressed, and colonic transit rate was slower in colonic obstruction. Prophylactic treatment with NS-398 significantly prevented the impairments of colonic transit and smooth muscle contractility and attenuated fecal collection in the occluded colons. When NS-398 was administered therapeutically 3 days after the initiation of obstruction, the muscle contractility and colonic transit still improved on day 7. Obstruction led to marked increase of COX-2 expression and prostaglandin E2 (PGE2) synthesis. Exogenous PGE2 decreased colonic smooth muscle contractility. All four PGE2 E-prostanoid receptor types (EP1 to EP4) were detected in rat colonic muscularis externa. Treatments with EP1 and EP3 antagonists suppressed muscle contractility in control tissue but did not improve contractility in obstruction tissue. On the contrary, the EP2 and EP4 antagonists did not affect control tissue but significantly restored muscle contractility in obstruction. We concluded that our study shows that COX-2 inhibitor has prophylactic and therapeutic benefits for motility dysfunction in bowel obstruction. PGE2 and its receptors EP2 and EP4 are involved in the motility dysfunction in obstruction, whereas EP1 and EP3 mediate PGE2 regulation of colonic smooth muscle contractile function in normal state.
Keywords: mechanical stretch, mechanotranscription, NS-398, colon, smooth muscle, E-prostanoid, prostaglandin E2
bowel obstruction is a significant health challenge in adults and children, occurring in both the small and large intestines. Obstruction may be acute or chronic, partial or complete, and it can be classified in general as mechanical and nonmechanical obstructions (35, 44). Mechanical obstruction may originate extrinsic to the intestine, e.g., adhesions and hernias, or intrinsic to the intestine, e.g., carcinoma and diverticulitis. The nonmechanical obstruction may be caused by neuromuscular dysfunctions, such as those in ileus and Hirschsprung's disease, or be idiopathic such as in pseudoobstruction. Bowel obstruction leads to altered motility function with suppression in smooth muscle contractility (5, 43, 53). Motility dysfunction is responsible for symptoms such as vomiting, abdominal distension, pain, and constipation (34, 46), and may lead to intestinal failure (13, 47). The molecular mechanisms underlying these changes are not known. As a result, there is no effective medical treatment for obstruction other than surgical resection or decompression (44, 46). Nevertheless, even if obstruction is surgically removed or corrected, many patients experience a prolonged disturbance of motility function in the bowel proximal to resection (10, 14, 16). Therefore, there is a great need of effective medical treatments to relieve symptoms related to motility dysfunction in obstruction.
Cyclooxygenase (COX) is an enzyme that catalyzes the major rate-limiting step of the synthesis of prostaglandins (PGs), which play important roles in mediating smooth muscle contractility in the gastrointestinal (GI) tract (10, 19, 23, 27, 33, 38, 49). Whereas COX-1 is the constitutive isoform of COX in most cell types, COX-2 is the inducible form within certain cells such as smooth muscle cells (SMC). COX-2 has long been considered an inflammatory mediator involved in motility dysfunction in various inflammatory conditions such as postoperative ileus (38), gut inflammation (3, 23), and necrotic enteritis (24). Among many COX-2-derived PGs, PGE2 is the most prominent prostaglandin in the GI tract. PGE2 is well known to affect smooth muscle contractile function (6, 7, 36, 45). Four distinct E-prostanoid (EP) receptor subtypes have been characterized and cloned, and they are classified as EP1, EP2, EP3, and EP4 (28). Each of the receptor subtypes has a unique signaling mechanism and initiates distinct cellular responses (28).
Our recent studies demonstrate that mechanical stretch in the distended segment oral to obstruction markedly induces gene expression of COX-2 in the rat colon (43). Direct stretch of colonic muscle strips or cultured SMCs in vitro also leads to marked induction of COX-2 expression (21, 43). Furthermore, the stretch-induced COX-2 plays a critical role in the impairment of colonic SMC contractility in bowel obstruction (43), as suppression of SMC contractility in obstruction was significantly attenuated in COX-2 gene-deficient mice.
In the present study, we found that prophylactic in vivo treatment with COX-2 inhibitor NS-398 prevented motility dysfunction in obstruction. Even if NS-398 was started therapeutically after the initiation of obstruction, the colonic motility function still significantly improved. We also determined whether COX-2-derived PGE2 is involved in obstruction-associated motility dysfunction and, if so, which EP receptor subtype(s) is involved. In view of adverse effects of COX-2 inhibitors in human, identification of specific EP receptor subtypes involved in motility dysfunction helps to design safe and effective treatments in obstruction.
MATERIALS AND METHODS
Rat model of partial colon obstruction and experimental design.
Male Sprague-Dawley rats weighing 200–275 g and aged between 6 and 8 wk (Harlan Sprague Dawley, Indianapolis, IN) were used for the study. The rats were housed in a controlled environment (22°C, 12-h:12-h light/dark cycle) and allowed food and water ad libitum. The Institutional Animal Care and Use Committee at University of Texas Medical Branch approved all procedures performed on the animals.
The rat model of partial colon obstruction was prepared by following procedures as previously described (5, 12, 43, 53). Rats were anesthetized with 2% isoflurane inhalation by an E-Z Anesthesia vaporizer (Palmer, PA). After midline laparotomy, a distal colon segment 3 cm proximal to the end of colon was carefully exposed. A small mesenteric window (5 × 5 mm2) was made next to the exposed colon segment. Partial colon obstruction was induced by placing a 3-mm-wide medical grade silicon band around the colon wall through the small mesenteric window. The size of the silicon band (21 mm in length) is about the size of outer circumference of the colon when the colon segment is filled with a fecal pellet, allowing a partial but not complete obstruction. The sham control rats underwent the same surgical procedure except that the band was removed immediately after implantation. Rats were euthanized 3 or 7 days after operation.
Animal groups were randomly assigned to include sham surgery rats and obstruction rats each with vehicle (DMSO) or COX-2 inhibitor NS-398 (Cayman Chemical, Ann Arbor, MI) at 5 mg/kg ip daily (9, 23, 55). To determine the potential benefits of COX-2 inhibitor in the prevention and treatment of motility dysfunction in obstruction, daily treatment with NS-398 was started either prophylactically 1 h before the induction of obstruction or therapeutically after obstruction was induced. The therapeutic effect was started 24 h or 3 days after obstruction was induced for the rats being euthanized on day 3 or day 7, respectively. Once started, the inhibitor or vehicle was given daily until the time of euthanasia. The time points, days 3 and 7, were chosen to represent the early phase and late phase of obstruction, as hypertrophy and inflammatory response was not detected before day 3 but may be present by day 7 (43).
Tissue collection.
The distal colon segment (5 cm in length) oral to the site of obstruction was collected in fresh carbogenated Krebs buffer (in mmol/l: 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1 NaH2PO4, 1.2 Mgcl2, 11 d-glucose, and 25 NaHCO3). The outer circumference and the fecal content weight of the distal colons were determined. The segments were cleansed, opened along the mesenteric border, and pinned flat in a Petri dish with Sylgard base. The mucosa/submucosa and muscularis externa layers were separated by microdissection as described previously (39–41, 43). The tissue samples were processed immediately for functional study or stored at −80°C for molecular studies.
Protein extraction and Western blots.
The colonic muscularis externa tissue was homogenized on ice in lysis buffer supplemented with protease inhibitors (Sigma-Aldrich, St. Louis, MO). The compositions of lysis buffer are (in mmol/l) 20 Tris·HCl, pH 7.5, 150 NaCl, 1 EDTA, 1 ethylene glycol-bis(β-aninoethyl ether)-N,N,N′,N′-tetraacetic acid, 2.5 sodium pyrophosphate, 1 β-glycerolphosphate, 1 Na3VO4, and 1% Triton X-100, and 1 μg/ml leupeptin.
The proteins in the muscularis externa homogenates were resolved by a standard immunoblotting method as described previously (39–41, 43). Equal quantities (20 μg) of total protein were loaded and run on premade 4–12% Bis-Tris SDS-PAGE (Invitrogen, Carlsbad, CA). They were transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) for incubation with primary and secondary antibodies. The following antibodies were used in the study: primary antibodies to COX-2 (1:1,000; Cayman Chemical) and EP1, EP2, EP3, and EP4 receptors (1:200; Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies used in the study include IRDye 800-conjugated anti-mouse IgG (Rockland, Gilbertsville, PA) and Alexa Fluor 680 goat anti-rabbit IgG (Invitrogen). β-Actin (1:5,000, Sigma, St. Louis, MO) was used as loading control. The detection was done by ODYSSEY Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
RNA preparation and real-time PCR.
Total RNA was extracted from tissues using the Qiagen RNeasy kit (Qiagen, Valencia, CA). One microgram of total RNA was reverse transcribed by using the SuperScript III First-Strand Synthesis System (Invitrogen) for real-time quantitative PCR (41–43). Real-time PCR was performed by use of the Applied Biosystems 7000 real-time PCR system (Foster City, CA). The TaqMan primers and probes for detection of rat COX-2, EP1, EP2, EP3, and EP4 mRNAs were purchased from Invitrogen. For relative quantitation of gene transcription, real-time PCR was performed with 40 ng cDNA for the target genes and the endogenous control (18S rRNA). The cycling parameters for real-time PCR were as follows: uracil N-glycosylase activation at 50°C for 2 min, AmpliTaq activation at 95°C for 10 min, denaturation at 95°C for 15 s, and annealing/extension at 60°C for 1 min (repeat 40 times) on ABI7000. Duplicate cycle threshold (CT) values were analyzed in Microsoft Excel by the comparative Ct (ΔΔCT) method, as described by the manufacturer. The amount of target was obtained by normalization to endogenous reference 18S rRNA.
Enzyme immunoassay.
Rat colonic muscularis externa tissue was homogenized in cold PBS (in mmol/l 137 NaCl, 2.7 KCl, 10 Na2HPO4, KH2PO4, pH 7.4) supplemented with protease inhibitors for protein extraction. PGE2 was measured with the PGE2 enzyme immunoassay kit from Cayman Chemical by following the manufacturer's protocols (43).
Muscle bath experiments.
Freshly obtained colon segments 1∼2 cm oral to the obstruction site were obtained, opened along the mesenteric border, cleansed, and pinned flat in a Petri dish with Sylgard base in carbogenated Krebs solution. The mucosal/submucosal layers were separated and discarded by microdissection. The smooth muscle strips (4 mm × 10 mm) were mounted along the circular muscle orientation in individual muscle baths (Radnoti Glass, Monrovia, CA) filled with 10 ml of carbogenated Krebs solution at 37°C. The contractile activity was recorded as previously described (42, 43) with Grass isometric force transducers and amplifiers connected to Biopac data-acquisition system (Biopac Systems, Goleta, CA). The muscle strips were equilibrated in the muscle bath under 1 g tension for 60 min at 37°C before they were tested for contractility. The smooth muscle contractility was tested by obtaining concentration-response curves to ACh (10−6 to 10−2 M) in the muscle bath. The strips were left to equilibrate for 15 min between additions of different concentrations of ACh. The contractile response was quantified as the increase in area under contractions during 4 min after addition of ACh to the bath over the baseline area under contractions during 4 min before the addition of ACh.
Colonic transit.
The colonic transit rate was determined by the geometric center method (42) with slight modification. A Silastic catheter (1 mm inside diameter and 2.1 mm outside diameter) was implanted under general anesthesia (2% isoflurane inhalation) into the proximal colon, with its tip resting 1 cm distal to the cecum. A bolus of 1.5 ml of 1.5% methylcellulose (Fisher Scientific, Fair Lawn, NJ) containing 0.75 mg nonabsorbable phenol red was injected into the colon via the catheter, and the catheter was flushed with 0.5 ml of saline. Sixty minutes later, the rats were euthanized by CO2 inhalation. The colon segment oral to the obstruction site and distal to the tip of the catheter was removed immediately and divided into six segments of equal length. Each segment, along with its contents, was placed in 25 ml of 0.1 N NaOH and homogenized. The homogenate was kept at room temperature for 1 h. One milliliter of the supernatant was added to 0.1 ml of 20% trichloroacetic acid solution to precipitate the protein. After centrifugation at 10,000 g for 15 min, 1 ml of 0.5 N NaOH was added to the supernatant. Phenol red was determined by measuring the absorption at 550 nm by an Eppendorf Biophotometer Plus (Eppendorf North America, Westbury, NY). Colonic transit was calculated as the geometric center of distribution of phenol red described as follows: geometric center = ∑ (counts of phenol red per segment × segment number)/ ∑ (counts of phenol red per segment).
Statistical analysis.
All data points are expressed as means ± SE. Statistical analysis was performed by analysis of variance with nonrepeated measures (by Student-Newman-Keuls test) for comparisons of multiple groups and Student's t-test for comparisons of two groups. A P value of ≤0.05 was considered statistically significant.
RESULTS
Prophylactic and therapeutic effects of COX-2 inhibitor NS-398 on colonic smooth muscle contractility.
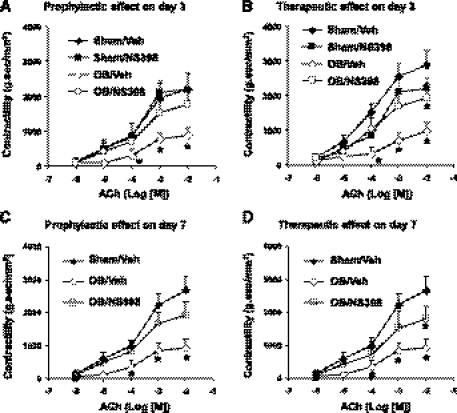
Colonic circular smooth muscle contractility in response to cholinergic muscarinic agonist ACh (10−6 to 10−2 M) was significantly suppressed in obstruction (P < 0.05 in the concentrations of 10−4, 10−3, and 10−2 M, n = 3 or 4 rats) (Fig. 1). When COX-2 inhibitor NS-398 (5 mg/kg, daily) was given to sham rats for 3 days, the smooth muscle contractility was not significantly changed (Fig. 1, A and B). When the animals were treated with NS-398 starting 1 h before induction of obstruction (prophylactic), the smooth muscle contractility was significantly restored in obstruction on both days 3 and 7 (Fig. 1, A and C). To determine whether COX-2 inhibitor is effective as a therapeutic treatment to improve smooth muscle function, NS-398 was given 1 day after the induction of obstruction. We found that NS-398 still significantly improved the smooth muscle contractility in obstruction by 3 days (Fig. 1B). Furthermore, even if NS-398 was started 3 days after the induction of obstruction, the muscle contractility still significantly improved in obstruction for 7 days (Fig. 1D).
Fig. 1.
Prophylactic and therapeutic effects of cyclooxygenase (COX)-2 inhibitor NS-398 on colonic circular muscle contractility in sham and obstruction. Rats with sham operation (sham) and obstruction (ob) were kept for 3 days (A and B) and 7 days (C and D). Daily administration of vehicle (Veh) (DMSO) or NS-398 (5 mg/kg) was given in sham or obstruction rats, starting either 1 h before operation (for prophylactic effect, A and C) or 1 day after operation (for therapeutic effect of 3-day obstruction, B) and 3 days after operation (for therapeutic effect of 7-day obstruction, D). The colonic circular smooth muscle contractile response to acetylcholine (ACh) (10−6 ∼ 10−2 M) was determined in muscle baths. (N = 3 or 4 rats for each group. *P < 0.05 vs. sham treated with vehicle).
Prophylactic and therapeutic effects of COX-2 inhibitor NS-398 on colonic transit in obstruction.
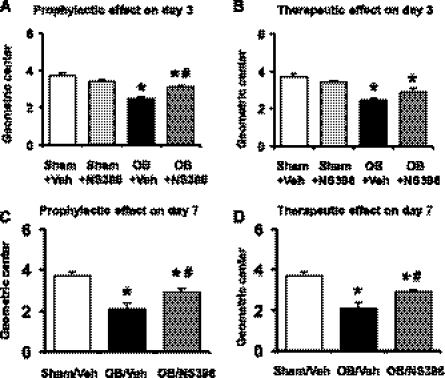
To quantitatively determine the in vivo motor function, we measured the geometric center (GC) of the colonic transit of a phenol red-contained cellulose bolus in the 10-cm-long colon segment proximal to the site of obstruction. The GC value was 3.7 ± 0.2 (n = 6) in the colons of sham control rats treated with vehicle (Fig. 2, A and B). Treatment with COX-2 inhibitor NS-398 did not significantly change colonic transit rate in sham controls. The GC value was 3.4 ± 0.2 (P > 0.05 vs. sham with vehicle) after 3 days treatment with NS-398 (Fig. 2, A and B). The colonic transit was markedly delayed in obstruction, with GC values of 2.5 ± 0.1 (n = 4, Fig. 2 A and B) and 2.1 ± 0.3 (n = 4, Fig. 2, C and D) on days 3 and 7 of obstruction, respectively (P < 0.05 vs. sham with vehicle). When NS-398 was started before the induction of obstruction (for prophylactic effect), the GC value was increased to 3.1 ± 0.1 in the 3-day obstruction group (P < 0.05 vs. obstruction control, Fig. 2A), and to 2.9 ± 0.2 in the 7-day group (P < 0.01 vs. obstruction control, Fig. 2C). Furthermore, even if NS-398 was started 3 days after the induction of obstruction (as a therapeutic trial), the colonic motor function was still significantly improved on day 7. The GC value was increased to 2.9 ± 0.1 (n = 4, P < 0.01 vs. obstruction control) in the 7-day group (Fig. 2D). When NS-398 was started 1 day after the induction of obstruction and the colonic transit was determined on day 3 of obstruction, the GC value changed to 2.9 ± 0.2 (n = 5) from 2.5 ± 0.1 (n = 4, Fig. 2B). However, the difference of the GC values between the two groups (obstruction with vehicle and obstruction with NS-398) was not statistically significant (P = 0.064).
Fig. 2.
Prophylactic and therapeutic effects of COX-2 inhibitor NS-398 on colonic transit in obstruction. Rats with sham operation and obstruction were kept for 3 days (A and B) and 7 days (C and D). Daily vehicle (DMSO) or NS-398 (5 mg/kg) was administered in sham or obstruction rats, starting either 1 h before operation (for prophylactic effect, A and C) or 1 day after operation (for therapeutic effect, B) and 3 days after operation (for therapeutic effect of 7-day obstruction, D). The colon transit rate was determined by calculating the geometric center of the phenol red meal. (N = 4 to 6. *P < 0.05 vs. sham/veh, #P < 0.05 vs. obstruction/veh).
Effect of COX-2 inhibitor NS-398 on intraluminal fecal accumulation in the obstructed colons.
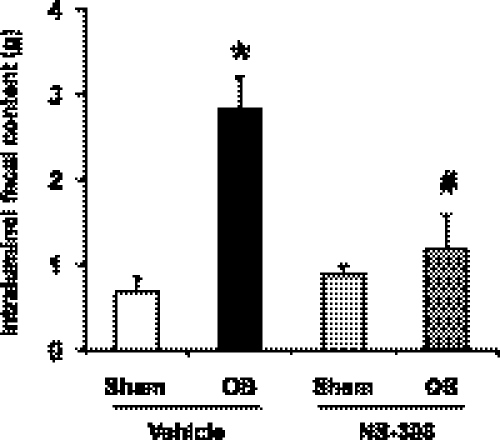
Motility dysfunction in colon obstruction is responsible for symptoms such as abdominal distension and constipation due to intraluminal content accumulation oral to the obstruction site (35, 44, 46). An objective measurement of fecal output in the model of colon obstruction was not practical. The fecal output was 2 to 3 pellets in the first 24 h of obstruction. By 2 to 3 days, there was no fecal output in pellet form. Instead the fecal discharge became liquid, and spread around the para-anal area. We measured the weight of intraluminal content of the 5-cm distal colon segment oral to the obstruction site. Colon obstruction led to a significant accumulation of intraluminal content (2.83 ± 0.38 g vs. 0.69 ± 0.17 g in sham/vehicle, P < 0.05) in obstruction on day 7 (Fig. 3). NS-398 administration did not affect the value in sham rats but markedly improved the lumen distension in obstruction with the intraluminal accumulation of only 1.19 ± 0.39 g (P < 0.05 vs. obstruction with vehicle, Fig. 3).
Fig. 3.
Effect of NS-398 on the intraluminal fecal content in the colon. Daily administration of vehicle (20% DMSO) or NS-398 (5 mg/kg in 200 μl, ip) was started 1 h before the operation. Sham operated or obstructed rats with vehicle or NS-398 treatment were euthanized 7 days later for determination of intraluminal fecal content in the 5-cm colon segment oral to the obstruction band. N = 3 or 4 for each group. *P < 0.05 vs. sham in vehicle control group. #P < 0.05 vs. OB in vehicle control group.
Effect of COX-2 inhibitor NS-398 on the increase of PGE2 level in obstruction.
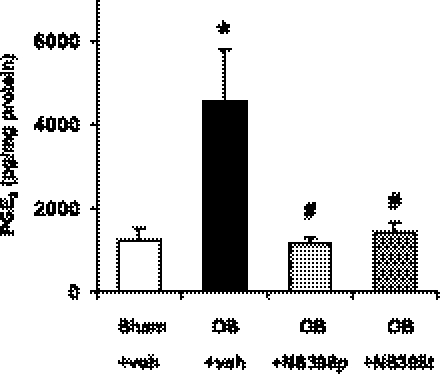
To confirm that NS-398 at 5 mg/kg is effective in blocking COX-2 activity in vivo, we measured the PGE2 levels in the colonic muscularis externae in rats with sham operation and colon obstruction for 3 days treated with vehicle or NS-398. The PGE2 level increased significantly (P < 0.05) from 1,236 ± 259 (pg/mg of protein) in sham to 4,577 ± 1,249 (pg/mg of protein) in the colon-obstruction group (Fig. 4). Prophylactic use of NS-398 almost completely blocked the increase of PGE2 in obstruction (P < 0.05 vs. obstruction with vehicle, Fig. 4). Furthermore, therapeutic use of NS-398 still significantly decreased the level of PGE2 in obstruction (Fig. 4). These data indicate that the increased PGE2 was attributable to increased COX-2 in obstruction and that in vivo administration of NS-398 at 5 mg/kg was effective in blocking COX-2 activity induced by mechanical stretch in obstruction.
Fig. 4.
COX-2 inhibitor NS-398 (5 mg/kg, daily) blocked the increase of prostaglandin E2 (PGE2) in obstruction. The PGE2 level in the colonic muscularis externae increased significantly in colon obstruction (3 days), and this increase was blocked by prophylactic or therapeutic treatments with NS-398 at 5 mg/kg. (n = 4 to 7 rats in each group, *P < 0.05 vs. sham/veh, #P < 0.05 vs. obstruction/veh.)
Inhibitory effect of PGE2 on colonic circular muscle contractility.
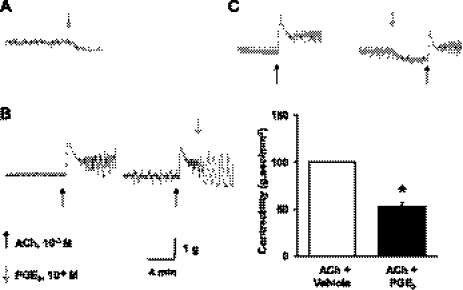
To determine whether PGE2 is inhibitory to smooth muscle contractile activity in rat colon, we tested the effects of exogenous PGE2 in naïve colonic circular muscle strips in vitro. When exogenous PGE2 at 10−6 M was added to the bath solution, the baseline smooth muscle tone was decreased (Fig. 5A). When the muscle strips were under the effect of cholinergic muscarinic agent ACh (10−3 M), addition of exogenous PGE2 (10−6) instantly decreased the smooth muscle contractile activity (Fig. 5B). On the other hand, pretreatment of muscle strips with PGE2 (10−6) significantly decreased the smooth muscle contractility evoked by ACh by 52 ± 3% (n = 8 strips/3 rats, P < 0.05 vs. ACh control) (Fig. 5C).
Fig. 5.
Inhibitory effect of exogenous PGE2 on rat colonic circular muscle contractility. Exogenous PGE2 (10−6M) inhibited spontaneous activity of smooth muscle tone (A) and ACh (10−3 M)-induced muscle contractions (B). Pretreatment of muscle strips with PGE2 also significantly inhibited smooth muscle contractile response to ACh (C). (n = 8 strips from 3 rats, *P < 0.05 vs. control).
Expression of PGE2 receptors EP1, EP2, EP3, and EP4 in the colonic muscularis externae.
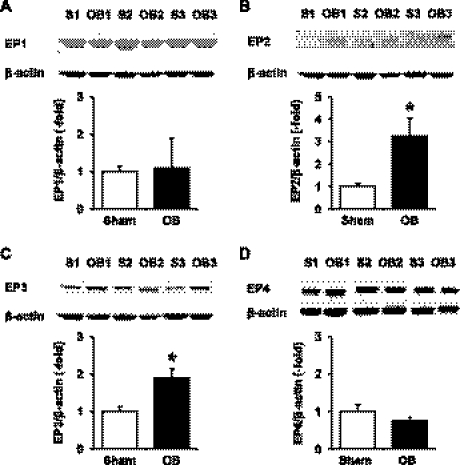
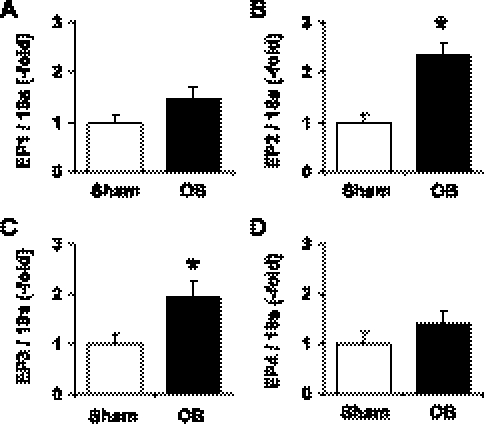
Western blot showed that all the PGE2 receptor subtypes EP1, EP2, EP3, and EP4 were present in the rat colonic muscularis externae (Fig. 6). The mRNAs encoding each of the receptor subtypes were also readily detected in the rat colonic muscularis externa by real-time PCR (Fig. 7). On the basis of the Ct values of the real-time PCR results, EP3 mRNA is the most abundant in the sham rats (Ct: 27.68 ± 0.17, n = 6), followed by EP4 (Ct: 28.05 ± 0.18, n = 6), and EP1 mRNAs (Ct: 30.06 ± 0.21, n = 6). The EP2 mRNA is the least abundant among the four types of EP receptors (Ct: 32.08 ± 0.32, n = 6). We determined whether the expression of these receptor subtypes was altered in obstruction. The expression of EP1 and EP4 receptor mRNA and protein was not changed in the obstruction group (day 3) compared with sham controls (Figs. 6 and 7). On the contrary, the expression of EP2 and EP3 proteins and mRNAs was significantly upregulated in obstruction (Figs. 6 and 7). The EP2 and EP3 receptor mRNA levels increased 2.4- and 1.9-fold in obstruction on day 3 compared with the sham control (n = 4 or 5, both P < 0.05 vs. sham).
Fig. 6.
Expression of PGE2 receptor subtypes (E-prostanoid, EP1, EP2, EP3, and EP4) in rat colon muscularis externae in sham and obstruction. Colonic muscularis externae was isolated from the midcolons of rats with sham operation and obstruction for 3 days. Western blot was applied to detect the receptor subtypes with antibodies specific to EP1 (A), EP2 (B), EP3 (C), and EP4 (D), respectively. 3 out of 4 or 5 sham and obstruction samples were shown in the upper panel of each graph. (*P < 0.05 vs. sham).
Fig. 7.
Expression of mRNAs for PGE2 receptor subtypes (EP1, EP2, EP3, and EP4) in rat colon muscularis externae in sham and obstruction. Colonic muscularis externae was isolated from the midcolons of rats with sham operation and obstruction for 3 days. Total RNA was isolated for real-time PCR quantitation of mRNA for EP1 (A), EP2 (B), EP3 (C), and EP4 (D), respectively. (n = 4 in each group, *P < 0.05 vs. sham).
Involvement of EP receptor subtypes in the regulation of colonic circular muscle contractility in normal and obstruction.
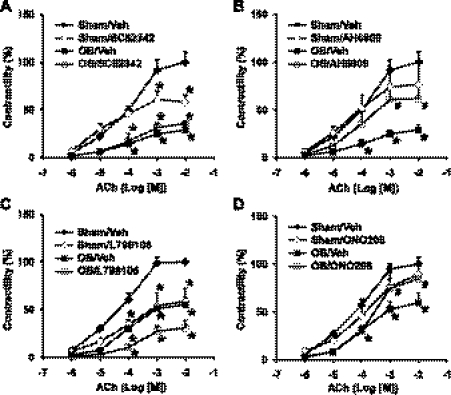
There is no universal EP receptor blocker. However, selective antagonists to the receptor subtypes are available. To determine whether PGE2 and specific EP receptor(s) are involved in the impairments of muscle contractility encountered in obstruction, circular muscle strips were isolated from sham and obstructed colons (3 day). The muscle strips were incubated for 24 h with vehicle control (DMSO), and the specific antagonists to EP1 (SC-52342, Enzo, Plymouth Meeting, PA), EP1/EP2 (AH6809, Enzo), EP3 (l-798106, Tocris Bioscience, Ellisville, MO) and EP4 (ONO-AE 3-208, Haoyuan Chemexpress, Shanghai, China), separately. The concentration of 10−5 M was chosen for all the antagonists because this was found effective in blocking respective EP receptor subtypes (4, 18, 32, 48). Incubation with EP2 or EP4 antagonists did not significantly change colonic circular muscle contractility in sham control tissue (Fig. 8). However, treatment with EP1 or EP3 antagonists suppressed the contractile response in control tissue, indicating an endogenous PGE2 influence via EP1 and EP3 in normal state (Fig. 8). The circular muscle contractility was significantly decreased in the obstruction tissue, and treatment with EP1 antagonist SC-52342 and EP3 antagonist l-798106 did not improve the smooth muscle contractility. However, treatment with EP1/EP2 antagonist AH6809 and EP4 antagonist ONO-AE 3-208 significantly restored the smooth muscle contractile response to ACh in the tissue taken from obstruction group (Fig. 8). These data indicate that EP2 and EP4 are involved in the COX-2- and PGE2-mediated impairment of smooth muscle contractility in obstruction.
Fig. 8.
Effects of EP1, EP2, EP3, and EP4 receptor antagonists on colonic circular muscle contractility in obstruction. The colonic circular muscle strips from the sham and obstruction rats (3 days) were incubated with DMSO or individual antagonists SC-52342 (A), AH-6809 (B), L798106 (C), and ONO-AE3-208 (D). The contractility of the muscle strips was determined 24 h later and was expressed as percent change of the contractility relative to the maximal response in sham with vehicle treatment. (n = 4, *P < 0.05 vs. sham/veh, #P < 0.05 vs. ob/veh).
DISCUSSION
Bowel obstruction alone accounts for 1.7–4.0% of medical emergency visits with abdominal pain (35, 44, 51). In addition, many GI disorders present as functional obstructions with a distended lumen, such as achalasia, pyloric stenosis, pseudoobstruction, and idiopathic megacolon. Motility dysfunction is a common pathological phenomenon in these obstructive bowel disorders (OBDs) and accounts for symptoms such as vomiting, abdominal distension, pain, and constipation (35, 44, 46). Because the pathophysiological mechanisms of these disorders are not clear, there has been no effective medical treatment (35, 44, 46). In rat models of obstruction in the colon as well as esophagus, stomach, and small intestine, we found that stretch-induced gene expression (mechanotranscription) of COX-2 is a common pathophysiological mechanism in lumen distension throughout the gut (22, 43). The stretch-induced COX-2 plays a critical role in the impairments of smooth muscle contractile function in these conditions (22, 43). The present study investigated whether in vivo use of COX-2 inhibitor has prophylactic and therapeutic benefits for motility dysfunction in obstruction. We found that NS-398 at 5 mg/kg daily effectively inhibits COX-2 activity in obstruction, as COX-2-derived PGE2 is almost completely blocked. Prophylactic treatment with NS-398 prevented impairments of smooth muscle contractility and improved colonic motor function and lumen distension in obstruction. Furthermore, even if NS-398 was started 3 days after colon obstruction had been established, the COX-2 inhibitor still significantly improved smooth muscle contractility and colon transit in obstruction on day 7. These data therefore demonstrate that COX-2 inhibitor has prophylactic and therapeutic benefits in the treatment of OBDs.
Among all the obstructive conditions, chronic partial obstruction (such as in Hirschsprung's disease and colon cancer-related lumen narrowing) and functional obstruction (such as in pseudoobstruction and achalasia) may serve as ideal pathological conditions for clinical trials of COX-2 inhibitors. In these conditions, the proximal bowel segment is distended, therefore triggering mechanotranscription of COX-2, and the distal lumen is not completely occluded, therefore allowing for passage of intraluminal contents. In the case of pseudoobstruction, there is no physical lumen narrowing at all. Therefore, an improvement of smooth muscle contractility by COX-2 inhibitor eliminating COX-2 activity is expected to restore motor function and to normalize intestinal transit. Cancer is the most common cause of colon obstruction in the United States (11, 44, 46, 51); the 5-year survival rate of patients with obstructed colon cancer following surgery is significantly worse than that of their counterparts without obstruction (31, 52). Given that COX-2 is involved in the development of colon cancer (20, 50), our finding that the mechanically sensitive COX-2 gene is robustly induced in obstruction may explain why obstructed colon cancer has a worse prognosis. Therefore, COX-2 inhibitor could be particularly beneficial to patients with obstructed colon cancer, as it acts on two distinct mechanisms, i.e., preventing the cancer-promoting effect of COX-2 and restoring motility function impaired by obstruction-induced COX-2.
In view of adverse effects of current COX-2 inhibitors in humans, safer and more specific agents for motility dysfunction in obstruction are sought to block affects of stretch-induced COX-2 on gut smooth muscle contractility. Furthermore, although COX-2 plays a critical role in motility dysfunction in inflammation (3, 23, 38, 49) and obstruction (43), the mechanism whereby COX-2 impairs motility function is not well understood. We found that COX-2-derived PGE2 plays an important role in the impairment of smooth muscle function in obstruction. Exogenous or pathologically increased PGE2 is clearly inhibitory to colonic circular muscle contractility in rats. In the muscle bath, exogenous PGE2 suppressed both the spontaneous tonic contractions and the active smooth muscle contraction evoked by ACh. PGE2 pretreatment also significantly inhibited smooth muscle contraction in response to ACh. Four distinct receptor subtypes for PGE2 have been cloned and pharmacologically characterized (17, 28). We therefore determined the EP receptor subtypes involved in COX-2-mediated smooth muscle dysfunction. Each of the four receptor subtypes has unique signal transduction mechanisms. Activation of the EP1 receptor leads to an increase in intracellular calcium, possibly via G-protein Gq11 (17). Various EP3 isoforms, arising from differential splicing of the EP3 gene, have been identified (1, 30). These EP3 isoforms may couple to Gi, Gq, and Gs proteins (30). On the other hand, both EP2 and EP4 receptors couple to Gs, and activation of these receptor subtypes results in stimulation of adenylyl cyclase and increases in intracellular cAMP (28). Increases in intracellular cAMP will lead to smooth muscle relaxation in the gut (37). We found that all four EP receptor subtypes are expressed in rat colonic muscularis externae. Our data suggest that PGE2 at physiological levels or pathological levels acts very differently on smooth muscle contractile function through distinct EP receptors. Endogenous PGE2 helps to maintain smooth muscle contractile function in the normal state. EP1 and EP3 are the dominant EP subtypes involved in the PGE2 regulation of smooth muscle homeostasis in the normal state, as treatments with EP1 and EP3 antagonists, but not EP2 and EP4 antagonists, suppressed smooth muscle contractility. However, in bowel obstruction, stretch-induced COX-2 leads to marked increase of PGE2. This pathologically increased release of PGE2 suppresses colonic smooth muscle contractility in obstruction. This inhibitory effect is mainly through receptors EP2 and EP4. Incubation with EP4 antagonist ONO-AE3-208, but not EP1 and EP3 antagonists, significantly improved smooth muscle contractility in obstruction. At the present time, a strictly selective EP2 antagonist is not available. However, AH6809 as an antagonist has the highest affinity for EP2 receptor in the rodents although it also acts as a weak ligand at the EP1 receptor (17). To differentiate the effects of EP2 and EP1, many studies compared the effects of AH6809 with selective antagonists to EP1 (37, 49, 50). In the present study, we found that EP1 antagonist SC-19220 had no effect in restoring smooth muscle contractility in the muscle strips of the obstructed colon. However, AH6809 clearly improved muscle contractility in these tissues, indicating that EP2, along with EP4, is involved in the suppression of smooth muscle contractile function in obstruction.
Our studies demonstrate that stretch-induced COX-2 plays a critical role in OBDs, and blockade of COX-2-derived PGE has therapeutic benefits in the treatment of obstruction-related motility dysfunction. Identification of other mechanically responsive genes may further our understanding of pathophysiological mechanisms in OBD. In the present study, we found that mechanical stretch in obstruction markedly induced expression of EP2. However, the expression of EP1 and EP4 is not altered in obstruction. It appears that mechanical stretch is a highly selective regulator of gene expression in gut SMCs as in other types of cells (2, 15). It is worth noting that the level of EP2 receptor in the colonic smooth muscle was low in the normal state. The low level of EP2 receptor may explain why EP2 is not involved in the PGE2 mediation of smooth muscle contractile function in the control tissue. However, the expression of EP2 receptor is markedly upregulated in obstruction, and the stretch-induced EP2 may be accounted for by motility impairment in obstruction, as AH6809 significantly improved smooth muscle function in the obstruction tissue.
In our rat model of colon obstruction, the colonic smooth muscle contractility was markedly restored in both 3-day and 7-day groups with prophylactic and therapeutic treatments with NS-398. However, the colonic transit rate, although significantly improved, never returned to the sham control level. In the case of therapeutic trial of NS-398 for the 3-day obstruction group, where NS-398 was started 1 day after the induction of obstruction and the colonic transit was determined on day 3 of obstruction, the GC value did not show statistically significant improvement with NS-398 treatment. We know that NS-398 is effective in blocking COX-2 activity and that blocking of COX-2 and COX-2-derived PGs is effective in restoring smooth muscle contractile function. But why could COX-2 inhibitor NS-398 not completely restore motor function in obstruction? First of all, mechanical stretch in obstruction may alter expression of other genes in addition to that encoding COX-2, and these mechanically sensitive genes may also be involved in the impairment of motility function in obstruction. For instance, we found that PGE2 receptors EP2 and EP3 are upregulated in obstruction and that EP2 is utilized in the PGE2-mediated suppression of gut smooth muscle contractility. Therefore, a full inhibition of COX-2 activity may not warrant a complete restoration of motor function in obstruction. Another reason is that prostaglandins and other lipid metabolites are well known to possess genomic effects as well as nongenomic effects (8, 19, 27, 29, 55). PGE2 through its four distinct receptors may cause long-term cellular effects. This may explain why therapeutic treatment with NS-398 significantly improved colon transit in the 7-day obstruction model, but not in the 3-day model.
In conclusion, COX-2 inhibitor has prophylactic and therapeutic benefits for motility dysfunction in bowel obstruction in rats. In addition, we found that all four PGE2 receptors are present in rat colonic smooth muscle. EP2 and EP4 receptors are involved in COX-2- and PGE2-mediated suppression of smooth muscle function in bowel obstruction. However, in the normal state, EP1 and EP3 receptors help to maintain smooth muscle contractile function. These findings may help to design safe and selective therapeutic agents for motility dysfunction in OBDs.
GRANTS
This work was supported in part by the National Institute of Health (R01DK082563 to X.-Z. Shi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Adam M, Boie Y, Rushmore TH, Muller G, Bastien L, McKee KT, Metters KM, Abramovitz M. Cloning and expression of three isoforms of the human EP3 prostanoid receptor. FEBS Lett 338: 170–174, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Adam RM, Eaton SH, Estrada C, Nimgaonkar A, Shih SC, Smith LE, Kohane IS, Bägli D, Freeman MR. Mechanical stretch is a highly selective regulator of gene expression in human bladder smooth muscle cells. Physiol Genomics 20: 36–44, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Barbara G, DeGiorgio R, Deng Y, Vallance B, Blennerhassett P, Collins SM. Role of immunologic factors and cyclooxygenase 2 in persistent postinfective enteric muscle dysfunction in mice. Gastroenterology 120: 1729–1736, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Bassil AK, Borman RA, Jarvie EM, McArthur-Wilson RJ, Thangiah R, Sung EZH, Lee K, Sange GJ. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol 154: 126–135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoni S, Gabella G, Ghizzardi P, Ballabeni V, Impicciatore M, Lagrasta C, Arcari ML, Barocelli E. Motor response of rat hypertrophic intestine following chronic obstruction. Neurogastroenterol Motil 16: 365–374, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Botella A, Delvaux M, Fioramonti J, Frexinos J, Bueno L. Stimulatory (EP1 and EP3) and inhibitory (EP2) prostaglandin E2 receptors in isolated ileal smooth muscle cells. Eur J Pharmacol 237: 131–137, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Botella A, Delvaux M, Fioramonti J, Frexinos J, Bueno L. Receptor subtypes involved in dual effects induced by prostaglandin E2 in circular smooth muscle from dog colon. J Pharmacol Exp Ther 273: 1008–1014, 1995 [PubMed] [Google Scholar]
- 8.Cong P, Pricolo V, Biancani P, Behar J. Abnormalities of prostaglandins and cyclooxygenase enzymes in female patients with slow transit constipation. Gastroenterology 133: 445–453, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Ferrario A, Fisher AM, Rucher N, Gomer CJ. Celecoxib and NS-398 enhance photodynamic therapy by increasing in vitro apoptosis and decreasing in vivo inflammatory and angiogenic factors. Cancer Res 65: 2551–2556, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Fornai M, Blandizzi C, Colucci R, Antonioli L, Bernardini N, Seqnani C, Baraqatti B, Baroqi S, Berti P, Spisni R, Del Tacca M. Role of cyclooxygenases 1 and 2 in the modulation of neuromuscular functions in the distal colon of humans and mice. Gut 54: 608–616, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser ID, Condon RE, Schulte WJ, DeCrosse JJ, Cowles VE. Intestinal motility changes in experimental large bowel obstruction. Surgery 87: 677–682, 1980 [PubMed] [Google Scholar]
- 12.Gabella G. Hypertrophy of visceral smooth muscle. Anat Embryol 182: 409–424, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology 130, Suppl 1: S16–S28, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Grosfeld JL, Rescorla FJ. Duodenal atresia and stenosis: reassessment of treatment and outcome based on antenatal diagnosis, pathologic variance, and long-term follow-up. World J Surg 17: 301–309, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Kanefsky J, Lenburg M, Hai CM. Cholinergic receptor and cyclic stretch-mediated inflammatory gene expression in intact ASM. Am J Respir Cell Mol Biol 34: 417–425, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HY, Kim JH, Jung SE, Lee SC, Park KW, Kim WK. Surgical treatment and prognosis of chronic intestinal pseudo-obstruction in children. J Pediatr Surg 40: 1753–1759, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol 122: 217–224, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kita K, Takahashi K, Ohashi Takasuka H Y, Aihara E, Takeuchi K. Phosphodiesterase isozymes involved in regulation of HCO3- secretion in isolated mouse stomach in vitro. J Pharmacol Exp Ther 326: 889–896, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Krause W, DuBois RN. Eicosanoids and the large intestine. Prostaglandins Other Lipid Mediat 61: 145–61, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Krause W, Dubois RN. The Molecular basis for prevention of colorectal cancer. Clin Colorectal Cancer 1: 47–54, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Li F, Sarna SK, Shi XZ. Intracellular signaling mechanism of stretch-induced expression of COX-2 in colonic smooth muscle cells (Abstract). Gastroenterology 140: S120, 2011 [Google Scholar]
- 22.Lin YM, Li F, Sarna SK, Shi XZ. Mechanotranscription is a common pathophysiological mechanism throughout the gastrointestinal tract (Abstract). Gastroenterology 140: S706, 2011 [Google Scholar]
- 23.Linden DR, Sharkey KA, Ho W, Mawe GM. Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J Physiol 557: 191–205, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lugo B, Ford HR, Grishin A. Molecular signaling in necrotizing enterocolitis: regulation of intestinal COX-2 expression. J Pediatr Surg 42: 1165–1171, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Matsuo M, Yoshida N, Zaitsu M, Ishii K, Hamasaki Y. Inhibition of human glioma cell growth by a PHS-2 inhibitor, NS398, and a prostaglandin E receptor subtype EP1-selective antagonist, SC51089. J Neurooncol 66: 285–92, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Menezes M, Corbally M, Puri P. Long-term results of bowel function after treatment for Hirschsprung's disease: a 29-year review. Pediatr Surg Int 22: 987–990, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Mohajer B, Ma TY. Eicosanoids and the small intestine. Prostaglandins Other Lipid Mediat 61: 125–143, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Narumiya S, Suqimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, functions. Physiol Rev 79: 1193–1226, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol 24: 1542–1548, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Negishi M, Sugimoto Y, Namba T, Irie A, Narumiya S, Ichikawa A. Signal transductions of three isoforms of mouse prostaglandin E receptor EP3 subtype. Adv Prostaglandin Thromboxane Leukot Res 23: 255–257, 1995 [PubMed] [Google Scholar]
- 31.Ohman U. Prognosis in patients with obstructing colorectal carcinoma. Am J Surg 143: 742–747, 1982 [DOI] [PubMed] [Google Scholar]
- 32.Piazuelo E, Jimenez P, Strunk M, Santander S, Garcia A, Esteva F, Lanas A. Effects of selective PGE2 receptor antagnosis in esophageal adenocarcinoma cells derived from Barrett's esophagus. Prostaglandins Other Lipid Mediat 81: 150–161, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Porcher C, Horowitz B, Ward SM, Sanders KM. Constitutive and functional expression of cyclooxygenase 2 in the murine proximal colon. Neurogastroenterol Motil 16: 785–799, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Prihoda M, Flatt A, Summers RW. Mechanisms of motility changes during acute intestinal obstruction in the dog. Am J Physiol Gastrointest Liver Physiol 247: G37–G42, 1984 [DOI] [PubMed] [Google Scholar]
- 35.Russell JC, Welch JP. Pathophysiology of bowel obstruction. In: Bowel Obstruction, ed. Welch JP. Philadelphia, PA: W. B. Saunders, 1990, pp. 28–58 [Google Scholar]
- 36.Sanders KM. Evidence that prostaglandins are local regulatory agents in canine ileal circular muscle. Am J Physiol Gastrointest Liver Physiol 246: G361–G371, 1984 [DOI] [PubMed] [Google Scholar]
- 37.Sarna SK, Shi XZ. Function and regulation of colonic contractions in health and disease. In: Physiology of the Gastrointestinal Tract, ed. Johnson LR. Amsterdam: Elsevier Academic, 2006, 965–993 [Google Scholar]
- 38.Schwarz NT, Kalff JC, Turler A, Engel BM, Watkins SC, Billiar TR, Bauer AJ. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology 121: 1354–1371, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Shi XZ, Lindholm PF, Sarna SK. NF-kappa B activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterology 124: 1369–1380, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterology 129: 1518–1532, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Shi XZ, Sarna SK. Transcriptional regulation of inflammatory mediators secreted by human colonic circular smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 289: G274–G284, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Shi XZ, Sarna SK. Gene therapy with VIP, VIP receptor agonists and VIP receptor antagonists: a novel approach to designing pro-motility and anti-motility agents. Am J Physiol Gastrointest Liver Physiol 295: G187–G196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi XZ, Lin YM, Powell DR, Sarna SK. Pathophysiology of motility dysfunction in bowel obstruction: Role of stretch-induced COX-2. Am J Physiol Gastrointest Liver Physiol 300: G99–G108, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silen W. Acute intestinal obstruction. In: Harrison's Principles of Internal Medicine (16th Edition), ed. Braunwald E, Isselbacher KJ, Petersdorf RG, Wilson JD, Martin JB, Fauci AS. New York, NY: McGraw-Hill, 2005, pp. 1803–1805 [Google Scholar]
- 45.Smid SD, Svensson KM. Inhibition of COX-2 and EP1 receptor antagonism reduces human colonic longitudinal muscle contractility in vitro. Prostaglandins Other Lipid Mediat 88: 117–121, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Summers RW. Approach to the patient with ileus and obstruction. In: Textbook of Gastroenterology, ed. Yamada, Alpers, Laine, Owyang, and Powell. Philadelphia, PA: Lippincott Williams & Wilkins, 1999, pp. 842–858 [Google Scholar]
- 47.Thompson JS. Overview of etiology and management of intestinal failure. Gastroenterology 130, Suppl 1: S3–S4, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Tokanovic S, White CW, Malone DT, Exintaris B, Ventura S. Characterization of the prostanoid receptor mediating inhibition of smooth muscle contractility in the rat prostate gland. Naunyn Schmiedebergs Arch Pharmacol 381: 321–328, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Wallace JL. COX-2: a pivotal enzyme in mucosal protection and resolution of inflammation. Scientific World Journal 6: 577–588, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 29: 781–788, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welch JP. General considerations and mortality. In: Bowel Obstruction, ed. Welch JP. Philadelphia, PA: Saunders, 1990, pp. 59–95 [Google Scholar]
- 52.Wolmark N, Wieand HS, Rockette HE, Fisher B, Glass A, Lawrence W, Lerner H, Cruz AB, Volk H, Shibata H, Evans J, Prager D, other NSABP Investigators. The prognostic significance of tumor location and bowel obstruction in dukes B and C colorectal cancer. Findings from the NSABP clinical trials. Ann Surg 198: 743–752, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Won KJ, Suzuki T, Hori M, Ozaki H. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol Motil 18: 53–61, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Yang C, Kwan YW, Au AL, Poon CC, Zhang Q, Chan SW, Lee SM, Leung GP. 14,15-Epoxyeicosatrienoic acid induces vasorelaxation through the prostaglandin EP(2) receptors in rat mesenteric artery. Prostaglandins Other Lipid Mediat 93: 44–51, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Xiao ZL, Biancani P, Behar J. Effects of progesterone on motility and prostaglandin levels in the distal guinea pig colon. Am J Physiol Gastrointest Liver Physiol 297: G886–G893, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]