Insulin signaling is reduced in the diabetic retina, and the reduction occurs concurrently with decreased β-adrenergic receptor signaling. This study demonstrated that stimulation of β-adrenergic receptors on retinal Müller cells can restore insulin signaling through reduced TNF-α levels
Abstract
Purpose.
The goal of this study was to determine the relationship of TNF-α and the downregulation of insulin receptor signaling in retinal Müller cells cultured under hyperglycemic conditions and the role of β-adrenergic receptors in regulating these responses.
Methods.
Retinal Müller cells were cultured in normal (5 mM) or high (25 mM) glucose until 80% confluent and then were reduced to 2% serum for 18 to 24 hours. The cells were then treated with 10 μM salmeterol followed by Western blot analysis or ELISA. For TNF-α inhibitory studies, the cells were treated with 5 ng/mL of TNF-α for 30 minutes or by a 30-minute pretreatment with TNF-α followed by salmeterol for 6 hours. In the TNF-α short hairpin (sh)RNA experiments, the cells were cultured until 90% confluent, followed by transfection with TNF-α shRNA for 18 hours.
Results.
TNF-α-only treatments of Müller cells resulted in significant decreases of tyrosine phosphorylation of the insulin receptor and Akt in high-glucose conditions. Salmeterol (10 μM), a β-2-adrenergic receptor agonist, significantly increased phosphorylation of both insulin receptor and Akt. TNF-α shRNA significantly decreased phosphorylation of IRS-1Ser307, which was further decreased after salmeterol+TNF-α shRNA. Both TNF-α shRNA and salmeterol significantly reduced death of the retinal Müller cells.
Conclusions.
These studies demonstrate that β-adrenergic receptor agonists in vitro can restore the loss of insulin receptor activity noted in diabetes. By decreasing the levels of TNF-α and decreasing the phosphorylation of IRS-1Ser307 while increasing tyrosine phosphorylation of insulin receptor, these results suggest a possible mechanism by which restoration of β-adrenergic receptor signaling may protect the retina against diabetes-induced damage.
Diabetes is a growing epidemic and is caused by excess glucose levels and the body's inability to produce or regulate insulin.1 Although there is a significant amount of ongoing research on the regulation of insulin, very little of that research has focused on insulin regulation in the eye, as it may relate to diabetic retinopathy. Diabetic retinopathy is the leading cause of vision loss in people of working age in North America.1,2 It is unknown whether altered insulin actions in the retina are involved in the pathogenesis of diabetic retinopathy.1,2 The normal mechanism of insulin action is mediated by binding the insulin receptor, which is located on the cell membrane.3 This receptor is made up of two α subunits and two β subunits, which are connected by disulfide bonds.4,5 The actions of insulin are initiated when the receptor is bound on the α subunits, causing phosphorylation of tyrosine kinases of the β subunits and inducing a conformational change.4,5
Previous work on insulin signaling in the retina has suggested that phosphorylation of the insulin receptor can activate insulin receptor substrate complexes (IRS1–4) and lead to phosphorylation of Akt.5 Further research efforts in adipose and retinal tissues have suggested that phosphorylation on specific serine sites (Ser 307) on IRS-1 reduces the tyrosine phosphorylation of the insulin receptor6 (Walker et al., manuscript submitted). The cytokine TNF-α has been suggested to regulate IRS-1 through the serine 307 site. TNF-α-induced inhibition of IRS-1 signaling would decrease Akt phosphorylation and potentially induce apoptosis.
Our laboratory has shown that Müller cells increase TNF-α levels in a hyperglycemic environment, which was reduced after β-adrenergic receptor stimulation.7 Since β-adrenergic receptors can decrease TNF-α and TNF-α negatively regulates insulin receptor signaling to induce apoptosis, we sought to determine the mechanisms by which β-adrenergic receptors modulate insulin receptor signaling and apoptosis in retinal Müller cells.
Previous investigations have concentrated on insulin signaling in the retina,4,5,8–12 with less work focusing on the role of β-adrenergic receptor regulation of insulin signaling in the retina. Our recent work with IRS-1 (Walker et al., manuscript submitted) has suggested that β-adrenergic receptors on retinal Müller cells play a role in insulin signaling, as it relates to diabetic retinopathy. Since β-adrenergic receptors have been reported to interact with insulin receptor signaling in other targets, it suggests that β-adrenergic receptor regulation of insulin signaling is involved in the antiapoptotic actions observed after β-adrenergic receptor agonist stimulation.13
In the present study, we proposed that treatment with a selective β-2-adrenergic receptor agonist, salmeterol, would decrease levels of TNF-α, leading to increased insulin receptor signaling. In additional studies we used TNF-α shRNA to further characterize the function of TNF-α in the regulation of insulin receptor signaling. The findings will allow us to further understand the regulation of insulin signaling in the retina, specifically in retinal Müller cells.
Methods
Rat Retinal Müller Cell Culture
Rat retinal Müller cells (rMC-1, courtesy of Vijay Sarthy, Northwestern University) were grown in 5 or 25 mM glucose DMEM (HyClone Laboratories, Logan, UT). The medium was supplemented with 10% FBS and antibiotics. The cells were cultured to 80% confluence (2–4 days) and then were transferred to medium with 2% FBS for 18 to 24 hours, to eliminate any residual growth factors in the serum. After serum reduction, the cells were treated with either 10 μM isoproterenol (nonselective β-adrenergic receptor agonist) or 10 μM salmeterol (β-2-adrenergic receptor agonist) for 6 hours. For all studies, some cells received no treatments (NT) in 5 or 25 mM glucose to serve as controls. In TNF-α inhibitory studies, cells were treated with 5 ng/mL of TNF-α alone for 30 minutes or a 30-minute pretreatment with TNF-α followed by salmeterol for 6 hours.
Immediately after treatments, the cells were lysed with lysis buffer, containing (1.58 g Tris base, 150 mL sterile water, 1.80 g NaCl, 20 mL 10% Igepal-40, 5 mL 10% Na-deoxycholate, 2 mL 100 mM EDTA, and 1 μg protease inhibitors, (Sigma-Aldrich, St. Louis, MO) and harvested at each of the treatment time points.
shRNA Studies
Synthesis of shRNA was performed according to a previous protocol (Walker et al., manuscript submitted). For knockdown studies of TNF-α, cells were cultured until 90% estimated confluence, followed by transfection of cells with shRNA against TNF-α for 18 hours. For TNF-α shRNA+salmeterol samples, the cells were first treated with TNF-α shRNA for 18 hours, followed by 6 hours of exposure to 10 μM salmeterol.
β-Adrenergic Receptor Antagonist Treatment
In inhibitory studies, cells were treated with 300 nM of CGP 20712A (β-1-adrenergic receptor antagonist) or 50 nM ICI 118551 (β-2-adrenergic receptor antagonist)14 for 30 minutes. After 30 minutes of antagonist treatment, five dishes of cells were designated as controls and harvested with no further treatment, and the remaining cells were treated with 10 μM isoproterenol for 1, 6, 12, and 24 hours, with five dishes collected at each time point.
Western Blot Analysis
Protein concentrations in cell lysates were determined by Bradford assay (Thermo Fisher Scientific, Rockford, IL). Once protein concentrations were determined, 100 μg of protein was combined with denaturing sample buffer (1 mL 2× GDW, 640 μL 1 M Tris-HCl [pH 6.8], 420 μL 30% glycerol, 250 μL β-mercaptoethanol, 200 μL 0.05% bromophenol blue, and 0.125 g recrystallized SDS), separated on 4% to 20% precast tris-glycine gels (Lonza, Rockland, MD), and transferred to nitrocellulose membranes after separation. In a 5% BSA solution, the membranes were allowed to block with the antibody of interest: total and phosphorylated insulin receptor-β Tyr 1150/1151 (diluted 1:250; Cell Signaling, Danvers, MA). All blots were washed and incubated at room temperature with anti-rabbit secondary antibodies combined with horseradish peroxidase at 1:5000 dilution. After the secondary antibodies were applied, the blots were washed and placed into enhanced chemiluminescence reagent (Thermo Fisher Scientific). The chemiluminescence of the immunoreactive bands was viewed (ImageStation 4000MM; Kodak, Rochester, NY), and the mean densitometry was calculated the for individual bands. Results were expressed as a ratio of phosphorylated to total protein levels in densitometric units with comparisons between untreated versus drug-treated samples.
For detection of Akt, analyses were performed with an infrared imaging system (Odyssey; LiCor, Lincoln, Nebraska). Protein analyses were the same as above with the exception that, membranes were placed in a blocking solution (Odyssey; LiCor) with total or phosphorylated AktSer473 (diluted 1:500; Cell Signaling, Danvers, MA). All blots were washed and then incubated at room temperature with goat anti-rabbit secondary antibodies (IRDye 800CW; LiCor, Lincoln, NE), at 1:5000 dilution in blocking solution (Odyssey; LiCor) with 1% Tween. After treatment with secondary antibodies, the blots were washed and viewed using the infrared imaging system (LiCor, Lincoln, NE) with interval intensities being calculated for individual bands. Results are expressed as the ratio of phosphorylated to total protein levels in densitometric units with comparisons between 5 and 25 mM glucose, 25 mM glucose and TNF-α shRNA treatments, and salmeterol and TNF-α shRNA treatments.
Cell Death Detection ELISA
Müller cells were cultured under the conditions previously stated and were treated with TNF-α alone, salmeterol alone, TNF-α+salmeterol, or not treated (control). For TNF-α shRNA conditions, the cells were treated with TNF-α shRNA, TNF-α shRNA +salmeterol, scrambled shRNA (control), normal glucose, or high glucose. Cell lysates from these treatment groups were transferred to a streptavidin-coated microplate. The amount of DNA fragmentation was analyzed by use of a cell viability assay (Cell Death Detection ELISA; Roche, Mannheim, Germany), according to the manufacturer's instructions.
ELISA Assays
ELISA assays were performed for cleaved caspase-3 (Cell Signaling, Danvers, MA) according to the manufacturer's instructions, except that equal protein concentrations were loaded into all wells to allow for analyses based on absorbance values.
Statistical Analysis
Nonparametric tests were used in the analyses in all cell culture experiments because of the small sample size for each experiment. For these experiments, the untreated (control) group was compared to all timed drug treatment groups using the Mann-Whitney U test, with P < 0.05 considered as significantly different (all analyses: Prism 4.0; GraphPad, San Diego, CA).
Results
Stimulation of β-Adrenergic Receptors Increases Insulin Receptor Signaling
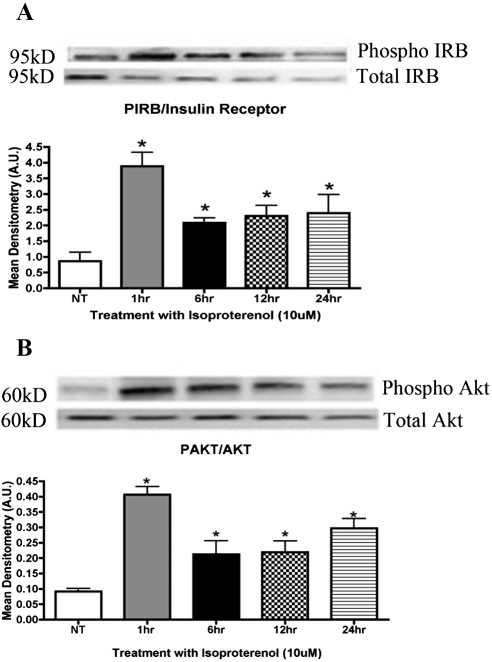
We examined the phosphorylation activity of the insulin receptor using rMC-1 cells in normoglycemic and hyperglycemic environments. Previous work has suggested a loss of retinal insulin receptor activity in diabetes.5 Our results indicate that isoproterenol treatment, a nonselective β-adrenergic receptor agonist, significantly increased phosphorylation of the insulin receptor (*P < 0.01 vs. untreated) after 1 hour of treatment (Fig. 1A). Typically, phosphorylation of the insulin receptor should increase phosphorylation of Akt. After treatment of rMC-1 cells with 10 μM isoproterenol, Akt phosphorylation levels increased significantly (*P < 0.01 vs. untreated) after 1 hour of treatment (Fig. 1B). Based on these results, insulin receptor signaling is regulated by β-adrenergic receptors.
Figure 1.
(A) Mean densitometry of protein levels of phosphorylated insulin receptor were significantly increased in Müller cells after treatment with the β-adrenergic receptor agonist isoproterenol. (B) Mean densitometry showing the ratio of Akt to phosphorylated Akt. Protein levels of Akt were significantly increased in Müller cells cultured in high-glucose (25 mM glucose) medium after treatment with 10 μM isoproterenol. Significance was determined by one-tailed, nonparametric t-tests on Western blot data. (A) *P < 0.05 vs. untreated, n = 4; (B) *P < 0.05 vs. untreated, n = 4.
Stimulation of β-Adrenergic Receptors Decreases Cleaved Caspase-3
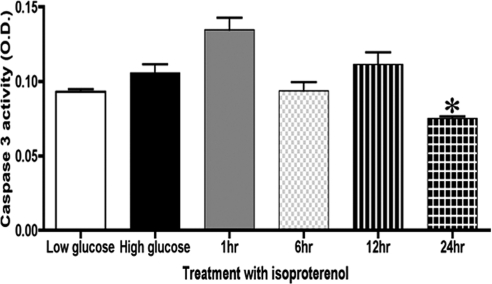
Increased phosphorylation activity of Akt contributes to significant decreases observed in apoptotic factors, such as cytochrome c, caspase-9, and caspase-3. In Figure 2, we show that treatment with 10 μM isoproterenol for 24 hours significantly decreased cleaved caspase-3 levels (*P < 0.05 vs. untreated).
Figure 2.
ELISA of cleaved caspase-3 in rat Müller cells showed significantly increased apoptosis in high glucose compared with low glucose. Treatment with isoproterenol significantly decreased caspase-3 activity after 24 hours of treatment of Müller cells. Significance was determined by the Mann-Whitney test (*P < 0.05 vs. untreated, n = 5).
Blocking of β-Adrenergic Receptor by Selective Inhibitors
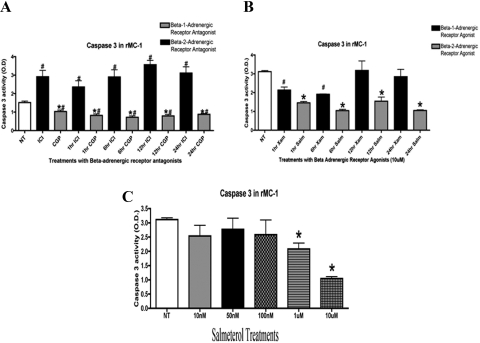
We had identified that Müller cells possess β-1- and β-2-adrenergic receptors.7 In these studies, we chose to use a pharmacologic approach to determine the dominant receptor present on Müller cells: 300 nM of CGP 20712A (a β-1-adrenergic receptor antagonist) and 50 nM of ICI 118551 (a β-2-adrenergic receptor antagonist)14 with isoproterenol as a stimulus to the receptor that was not being inhibited. Treatment with the β-1-adrenergic receptor antagonist followed by stimulation of isoproterenol for 1, 6, 12, or 24 hours showed significant decreases in cleaved caspase-3 (Fig. 3A; #P < 0.05 vs. untreated; *P < 0.01 vs. ICI samples). Inhibition of β-2-adrenergic receptors by the antagonist with stimulation of isoproterenol resulted in significant increases (#P < 0.01 vs. untreated) in cleaved caspase-3 activity (Fig. 3A). These results suggest that β-2-adrenergic receptors are responsible for regulating apoptotic signaling in retinal Müller cells.
Figure 3.
(A) ELISA showed a significant increase in caspase-3 in samples treated with ICI 118551 (a β-1-adrenergic receptor antagonist) compared with untreated samples. Caspase-3 levels were significantly decreased in samples treated with CGP 20712A (a β-2-adrenergic receptor antagonist) versus untreated (*P < 0.05 vs. NT, #ICI vs. CGP, n = 5 for ELISA assay). (B) Rat Müller cells treated with the β-1-adrenergic receptor agonist xamoterol (XAM) and the β-2-adrenergic receptor agonist, salmeterol (SAL) for 1 to 24 hours. Treatment with salmeterol significantly decreased caspase-3 activity after 1 hour of treatment, whereas treatment with xamoterol showed little effect after 6 hours. Statistical significance was determined by the Mann-Whitney test (*P < 0.05 vs. untreated; #Xam versus Sal n = 5 for ELISA assay). (C) Dose response of the selective β-2-adrenergic receptor agonist salmeterol. Treatment with 1 and 10 μM significantly decreased caspase-3 activity after 6 hours of treatment in Müller cells. Significance was determined by the Mann-Whitney test (*P < 0.05 vs. NT, n = 5).
To further confirm the findings using β-adrenergic receptor antagonists, we also treated Müller cells with β-adrenergic receptor agonists. Treatment with the selective β-1-adrenergic receptor agonist xamoterol significantly decreased caspase-3 levels after 1 and 6 hours of treatment (Fig. 3B; *P < 0.05 vs. untreated). Treatment with a β-2-adrenergic receptor agonist, salmeterol, significantly decreased cleaved caspase-3 levels at all time points (Fig. 3B; *P < 0.05 vs. untreated), which demonstrates that the β-2-adrenergic receptor is indeed a key β-adrenergic receptor subtype for apoptotic signaling in retinal Müller cells. After treatment of cells with 10-nM, 50-nM, 100-nM, 1-μM or 10-μM concentrations of salmeterol, we found that the 10-μM concentration produced the greatest decrease in cleaved caspase-3 levels compared with the other concentrations tested (Fig. 3C).
Salmeterol Significantly Reduces TNF-α While Increasing Insulin Receptor and Akt Phosphorylation
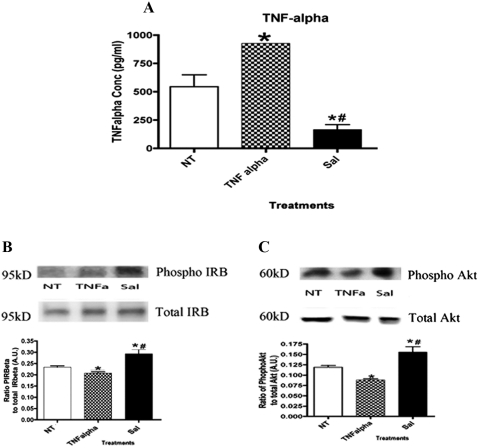
ELISA analysis revealed that Müller cells treated with TNF-α alone displayed significantly increased levels of TNF-α in a hyperglycemic environment (Fig. 4A; *P < 0.05 vs. untreated). After treatment with salmeterol, levels of TNF-α decreased significantly (Fig. 4A; *P < 0.05 vs. untreated; #P < 0.05 vs. TNF-α alone).
Figure 4.
(A) ELISA of TNF-α displaying significant increases in TNF-α-alone treatment levels. Treatment with salmeterol significantly decreased levels of TNF-α. Statistical significance was determined by the Mann-Whitney test (*P < 0.05 vs. untreated; #P < 0.05 vs. TNF-α alone, n = 5). (B) Mean densitometry of protein phosphorylation ratios for insulin receptor β was significantly decreased in TNF-α-treated cells, but treatment with 10 μM salmeterol significantly increased phosphorylation of the insulin receptor in Müller cells cultured in high glucose. (C) Western blot analysis showing a significant increase in the TNF-α-alone–treated cells. Treatment with salmeterol significantly increased the phosphorylation of Akt. Statistical significance was analyzed with a nonparametric, one-tailed t-test for Western blot analysis (*P < 0.05 vs. untreated; #P < 0.05 vs. TNF-α alone, n = 5).
Further efforts to determine whether β-2-adrenergic receptor stimulation alone could increase insulin receptor signaling demonstrated a significant increase in phosphorylation after treatment with 10 μM salmeterol (Fig. 4B). TNF-α-only treatments in Müller cells resulted in a significant decrease in tyrosine phosphorylation on insulin receptor in high-glucose conditions (Fig. 4B; *P < 0.05 vs. untreated; #P < 0.05 vs. TNF-α alone). These findings suggest that β-adrenergic receptor regulation of insulin receptor phosphorylation occurs predominantly through the β-2-adrenergic receptor subtype. Similar to the results for insulin receptor regulation, selective β-2-adrenergic receptor agonist treatment increased Akt phosphorylation on serine 473 (Fig. 4C; *P < 0.05 vs. untreated; #P < 0.05 vs. TNF-α alone). A significant decrease in phosphorylation of Akt was noted with TNF-α-only treatment. Taken together, these data suggest that β-2-adrenergic receptors regulate insulin receptor and Akt actions.
Silencing of TNF-α Plays a Critical Role in Phosphorylation of Akt and IRS-1Ser 307
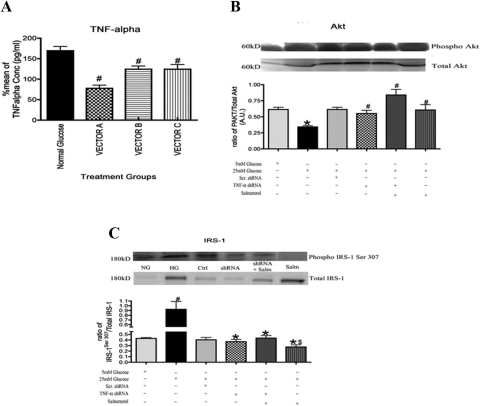
Our present data suggest that TNF-α has a modulatory role in insulin receptor signaling, specifically downstream of insulin receptor with actions on proteins such as Akt and IRS-1Ser 307. After verification of a successful knockdown by TNF-α shRNA (Fig. 5A), we found a significant decrease in Akt in rMC-1 in cells cultured in high glucose that was inhibited in cells treated with salmeterol (Fig. 5B). Treatment with TNF-α shRNA+ salmeterol also increased Akt phosphorylation, which was expected, since salmeterol alone decreases TNF-α activity (Fig. 5B).
Figure 5.
(A) ELISA of TNF-α showing significant decreases in various TNF-α shRNA vectors. Statistical significance was determined by Mann-Whitney test (*P < 0.05 vs. untreated; #P < 0.05 vs. TNF-α alone, n = 5). (B) Akt phosphorylation activity increased significantly after treatment with shRNA alone, shRNA+salmeterol, and salmeterol-alone samples. (C) Western blot analysis of phosphorylation of IRS-1Ser307 showed a significant decrease in shRNA alone and shRNA+salmeterol-treated cells compared with the high-glucose samples. Treatment with salmeterol also significantly decreased the phosphorylation of IRS-1Ser307 in Müller cells. Significance was determined by one-tailed, nonparametric Mann-Whitney tests on Western blot data (*P < 0.05 vs. 25 mM glucose, $P < 0.05 vs. salmeterol n = 4, #P < 0.05 vs. 5 mM Glucose, n = 4).
For studies on IRS-1Ser307, we found that high-glucose culturing conditions significantly increased IRS-1Ser307 phosphorylation in rMC-1 cells (Fig. 5C), similar to what is noted in adipose tissues.15 This response was inhibited when cells were treated with salmeterol. Treatment of cells with TNF-α shRNA+salmeterol also reduced IRS-1Ser307, although not as much as that caused salmeterol alone, suggesting that salmeterol's effect on IRS-1 involves more than regulation of TNF-α (Fig. 5C; *P < 0.05 vs. 5 mM glucose; #P < 0.05 vs. 25 mM glucose samples).
TNF-α Is a Mediator of Apoptosis in rMC-1 Cells
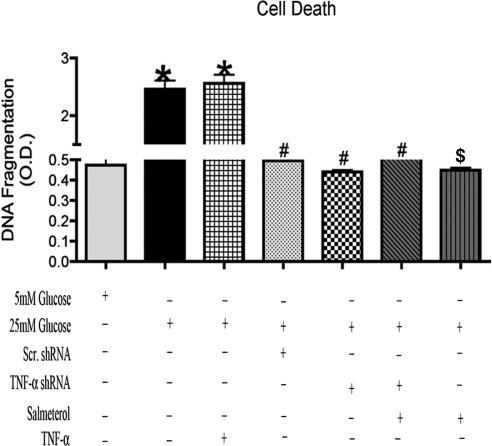
In Müller cells, apoptosis was characterized with a cell viability ELISA, in which the addition of TNF-α in a hyperglycemic environment resulted in an increase in cell death. Cellular transfections with TNF-α shRNA and TNF-α shRNA+salmeterol treatments showed significant decreases in apoptosis compared with TNF-α-only–treated samples (Fig. 6). These results suggest that TNF-α plays a major role in the induction of apoptosis in Müller cells, since the inhibition of TNF-α significantly reduced apoptosis (Fig. 6; *P < 0.05 vs. 5 mM glucose; #P < 0.05 vs. 25 mM glucose; and $P < 0.05 vs. TNF-α alone).
Figure 6.
Analysis of apoptosis showed an increase with treatment of TNF-α, but after transfection with TNF-α shRNA, levels of cell death decreased significantly and further decreased in TNF-α shRNA+salmeterol and salmeterol-alone treatment. (*P < 0.05 vs. 5 mM glucose, #P < 0.05 vs. 25 mM glucose, n = 5; $P < 0.05 vs. TNF-α alone).
Discussion
The insulin receptor–insulin receptor substrate (IRS)–Akt pathway is known to mediate numerous effects of insulin at the cellular level, such as inhibition of apoptosis, maintenance of glucose levels, and synthesis of proteins.4,5 Signaling through the first steps of this pathway is highly complex and can produce >1000 possible signal pathway variations.16 Insulin receptor signaling is initiated by the phosphorylation of β subunits on specific tyrosine sites that signal farther downstream to a complex of insulin receptor substrates (IRS 1–4) that also become phosphorylated.4–6,17 Insulin receptor substrates are docking proteins that are responsible for eliciting insulin actions throughout the body.6 One protein that can lie downstream of the IRS complex is the antiapoptotic factor, Akt. Phosphorylation of Akt after insulin stimulation has been suggested to inhibit the caspases, specifically caspase-3 and -9, which results in reduced apoptosis.
Our results demonstrate that insulin receptor phosphorylation is reduced in rat Müller cells cultured in a hyperglycemic environment compared with untreated samples, which agrees with previous findings.4,5,9 Furthermore, our results show that stimulation of β-adrenergic receptors with isoproterenol significantly increases phosphorylation of Tyr1150/1151 on insulin receptor.4 In addition to increased phosphorylation of the insulin receptor, treatment with isoproterenol significantly increased Akt activity, leading to decreased cleavage of caspase-3.
Other studies have identified that β-2-adrenergic receptors are the dominant receptors present on Müller cells for the regulation of insulin receptor signaling. Stimulation of β-2-adrenergic receptors with salmeterol, a specific β-2-adrenergic receptor agonist, showed significant increases in tyrosine phosphorylation of the insulin receptor, as well as increased phosphorylation of the antiapoptotic factor, Akt. The reduction of cell death is likely through an Akt-mediated pathway, since inhibitors to phosphatidylinositol-3-kinase also inhibited the actions of insulin.4 The results from the retinal explants agree well with our findings in retinal Müller cells.
Although insulin signaling has been well studied throughout various tissues in the body, less work has focused on the regulation of insulin signaling in the retina, specifically the relationship of inflammation and insulin receptor signaling. One cell type in the retina that may be susceptible to changes in this proposed relationship is the Müller cell. Müller cells serve as structural support cells for the retina and span the entire thickness of the retina.18 Müller cells become activated and express increased glial fibrillary acidic protein levels in diabetes,18 which suggests that the cells are nonhomeostatic and will alter their regulation of inflammatory markers, glucose transport, oxidative stress, growth factors, and finally, cell survival.19,20
Previous studies from our laboratory have shown that Müller cells display increased inflammatory markers (specifically TNF-α) in a hyperglycemic environment, which was reduced after β-adrenergic receptor stimulation.7 TNF-α has been shown to play a major role in the induction of apoptosis in retinal cells, specifically during the onset of diabetic retinopathy.21 Use of the TNF-α inhibitor (etanercept) produced significant reductions in DNA fragmentation and activation of caspases, further demonstrating that TNF-α is important in early diabetic abnormalities in the retina.21
In insulin receptor signaling, TNF-α can play an inhibitory role by directly phosphorylating IRS-1 at Ser 307, which produces a loss of insulin receptor signal transduction, leading to the increased apoptosis noted in the diabetic retina.3,22,23 Our findings in Müller cells confirm previous work in our laboratory in β1-adrenergic receptor KO mice in which we found that phosphorylation of IRS-1 at Ser 307 is significantly increased in comparison to their wild-type littermates.23 These studies also found a significant decrease in Akt and significant increases in cleaved caspase-3 levels, as well as TNF-α levels in the β1-adrenergic receptor knockout mice.23 Our present findings with salmeterol suggest that β-2-adrenergic receptor stimulation may inhibit cytokine release in retinal Müller cells cultured in a hyperglycemic environment, resulting in elimination of IRS-1Ser307 phosphorylation. This would allow the antiapoptotic actions of insulin to be properly transduced to Akt, producing a reduction in apoptosis. To further confirm these findings regarding the role of TNF-α in retinal Müller cells, we demonstrated that reduction of TNF-α levels with shRNA significantly reduced the phosphorylation of serine 307 on IRS-1, leading to increased Akt activity.
These studies demonstrate that treatment with a β-adrenergic receptor agonist in vitro can restore the loss of insulin receptor activity noted in diabetic retinal Müller cells. These results match well in vivo studies with topical isoproterenol, in which isoproterenol negated deleterious changes in phosphorylation of the insulin signaling pathway during diabetes in rats.24
Although our findings confirm our hypothesis of TNF-α modulation in insulin receptor signaling, we also recognize that our findings could be the result of phosphatases such as PTP1B that were previously suggested by Rajala et al.,25,26 which would decrease insulin receptor activity leading to a decrease in Akt activity.25,26 It is well known that the insulin receptor mediates the activation of caspase-3 by signaling through the PI3K/Akt pathway as well as the MAPK pathway.25,26 Our future studies will focus more in detail on the relationship of the PI3K and MAPK pathways in retinal Müller cells.
Further explanation of Akt modulation of cell death presented in the present study can be explained by previous findings by Gottlob et al.,27 who suggested that Akt mediates hexokinase activity in the mitochondria of cells. An upregulation of hexokinase in the mitochondria was found to inhibit apoptosis. Further findings by Majewski et al.28 suggested that this antiapoptotic action of hexokinase was the result of its ability to inhibit cytochrome c release once placed in the mitochondria. These previous findings are in line with our laboratory's findings in retinal Müller cells that showed an increase in the release of cytochrome c after silencing of IRS-1, this loss of IRS-1 led to a decrease in Akt (Walker et al. manuscript submitted).
In conclusion, the β-2-adrenergic receptor was the dominant receptor subtype for mediating the effects of insulin in retinal Müller cells. Furthermore, a selective β-2-adrenergic receptor agonist, salmeterol, increased insulin receptor signal transduction, leading to decreased apoptosis. Salmeterol increased the actions of insulin through inhibition of TNF-α in retinal Müller cells cultured in high glucose. In this manner, salmeterol inhibited the TNF-α-mediated increased phosphorylation of IRS-1Ser307, allowing the effects of insulin to be transduced to Akt, thus preventing apoptosis. These results provide a potential mechanism of action for β-adrenergic receptor therapies for diabetic retinopathy.
Footnotes
Supported by Career Development Awards 2-2008-1044 and 1-2011-597 from the Juvenile Diabetes Research Foundation (JJS), the Oxnard Foundation (JJS), the International Retinal Research Foundation (JJS), National Institutes of Health NRSA Minority Grant F31EY019240-02 (RJW), and HEI Research to Prevent Blindness Award, and National Eye Institute Vision Core Grant PHS 3P30 EY013080.
Disclosure: R.J. Walker, None; N.M. Anderson, None; Y. Jiang, None; S. Bahouth, None; J.J. Steinle, None
References
- 1. Flynn H. Diabetes and Ocular Disease. San Francisco: Foundation of the American Academy of Ophthalmology; 2000 [Google Scholar]
- 2. Klein R. Diabetic retinopathy. Annu Rev Public Health. 1996;17:137–158 [DOI] [PubMed] [Google Scholar]
- 3. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96 [DOI] [PubMed] [Google Scholar]
- 4. Reiter CEN, Sandirasegarane L, Wolpert EB, et al. Characterization of insulin signaling in rat retina in vivo and ex vivo. Am J Physiol Endocrinol Metab. 2003;285:E763–E774 [DOI] [PubMed] [Google Scholar]
- 5. Reiter CEN, Wu X, Sandirasegarane L, et al. Diabetes reduces basal retinal insulin receptor signaling. Diabetes. 2006;55:1148–1156 [DOI] [PubMed] [Google Scholar]
- 6. Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes: central role of tumor necrosis factor-α. J Clin Invest. 1994;94:1543–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker R, Steinle J. Role of beta-adrenergic receptors in inflammatory marker expression in Müller cells. Invest Ophthalmol Vis Sci. 2007;48:5276–5281 [DOI] [PubMed] [Google Scholar]
- 8. Barber AJ, Nakamura M, Wolpert EB, et al. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem. 2001;276:32814–32821 [DOI] [PubMed] [Google Scholar]
- 9. Bronson SK, Reiter CEN, Gardner TW. An eye on insulin. J Clin Investig. 2003;111:1817–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das A, Pansky B, Budd G. Demonstration of insulin-specific mRNA in cultured rat retinal glial cells. Invest Ophthalmol Vis Sci. 1987;28:1800–1810 [PubMed] [Google Scholar]
- 11. Das APB, Budd GC, Kollarits CR. Immunocytochemistry of mouse and human retina with antisera to insulin and S-100 protein. Curr Eye Res. 1984;3:1397–1403 [DOI] [PubMed] [Google Scholar]
- 12. Kondo T, Kahn CR. Altered insulin signaling in retinal tissue in diabetic states. J Biol Chem. 2004;279:37997–38006 [DOI] [PubMed] [Google Scholar]
- 13. Williams KP, Steinle JJ. Maintenance of beta-adrenergic receptor signaling can reduce fas signaling in human retinal endothelial cells. Exp Eye Res. 2009;89:448–455 [DOI] [PubMed] [Google Scholar]
- 14. Kitagawa Y, Adachi-Akahane S, Nagao T. Determination of beta-adrenoceptor subtype on rat isolated ventricular myocytes by use of highly selective beta-antagonists. Br J Pharmacol. 1995;1:1635–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sykiotis GP, Papavassiliou AG. Serine phosphorylation of insulin receptor substrate-1: a novel target for the reversal of insulin resistance. Mol Endocrinol. 2001;15:1864–1869 [DOI] [PubMed] [Google Scholar]
- 16. White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–E422 [DOI] [PubMed] [Google Scholar]
- 17. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science. 1996;271:665–670 [DOI] [PubMed] [Google Scholar]
- 18. Mizutani M, Gerhardinger C, Lorenzi M. Müller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449 [DOI] [PubMed] [Google Scholar]
- 19. Fletcher EL, Downie LE, Ly A, et al. A review of the role of glial cells in understanding retinal disease. Clin Exp Optom. 2008;91:67–77 [DOI] [PubMed] [Google Scholar]
- 20. Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Müller cells. Invest Ophthalmol Vis Sci. 2002;43:3109–3116 [PubMed] [Google Scholar]
- 21. Joussen AM, Doehmen S, Le ML, et al. TNF-α mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. 2009;15:1418–1428 [PMC free article] [PubMed] [Google Scholar]
- 22. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 23. Panjala SR, Jiang Y, Kern TS, Thomas SA, Steinle JJ. Increased tumor necrosis factor-alpha, cleaved caspase-3 levels and insulin receptor substrate-1 phosphorylation in the beta-adrenergic receptor knockout mouse. Mol Vis. 2011;17:1822–1828 [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang Y, Walker RJ, Kern TS, Steinle JJ. Application of isoproterenol inhibits diabetic-like changes in the rat retina. Exp Eye Res. 2010;91:171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rajala RV, Tanito M, Neel BG, Rajala A. Enhanced retinal insulin receptor-activated neuroprotective survival signal in mice lacking the protein-tyrosine phosphatase-1B gene. J Biol Chem. 2010;285:8894–8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajala RV. Phosphoinositide 3-kinase signaling in the vertebrate retina. J Lipid Res. 2010;51:4–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Majewski N, Nogueira V, Bhaskar P, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830 [DOI] [PubMed] [Google Scholar]