The transition zone between healthy and severely affected regions of the retina differ in structural abnormalities in patients with choroideremia, Stargardt disease, or retinitis pigmentosa.
Abstract
Purpose.
To describe the structural changes across the transition zone (TZ) in choroideremia (CHM) and Stargardt disease (STGD) and to compare these to the TZ in retinitis pigmentosa (RP).
Methods.
Frequency-domain (Fd)OCT line scans were obtained from seven patients with CHM, 20 with STGD, and 12 with RP and compared with those of 30 previously studied controls. A computer-aided manual segmentation procedure was used to determine the thicknesses of the outer segment (OS) layer, the outer nuclear layer plus outer plexiform layer (ONL+), the retinal pigment epithelium plus Bruch's membrane (RPE+BM), and the outer retina (OR).
Results.
The TZ, while consistent within patient groups, showed differences across disease groups. In particular, (1) OS loss occurred before ONL+ loss in CHM and RP, whereas ONL+ loss occurred before OS loss in STGD; (2) ONL+ was preserved over a wider region of the retina in CHM than in RP; (3) RPE+BM remained normal across the RP TZ, but was typically thinned in CHM. In some CHM patients, it was abnormally thin in regions with normal OS and ONL+ thickness. In STGD, RPE+BM was thinned by the end of the TZ; and (4) the disappearances of the IS/OS and OLM were more abrupt in CHM and STGD than in RP.
Conclusions.
On fdOCT scans, patients with RP, CHM, and STGD all have a TZ between relatively healthy and severely affected retina. The patterns of changes in the receptor layers are similar within a disease category, but different across categories. The findings suggest that the pattern of progression of each disease is distinct and may offer clues for strategies in the development of future therapies.
In retinal degenerative diseases, there is a transition zone (TZ)1–3 between relatively normal retina containing healthy photoreceptors and severely affected retina with extreme photoreceptor atrophy. Through the use of frequency-domain optical coherence tomography (fdOCT), the TZ can now be visualized and quantitatively assessed.1,2 A better understanding of the TZ is of importance for two reasons. First, knowledge of which retinal layers are affected across the TZ will aid in determining the potential efficacy and optimal injection locations for gene therapy.1,4 Second, the TZ offers a potential model of disease progression. That is, it is plausible to assume that the sequence and nature of structural changes observed spatially across the TZ at one specific time (that of fdOCT acquisition) also occur at each location in the retina as the disease progresses over time.
Thus far, fdOCT studies of the TZ have been limited mainly to retinitis pigmentosa (RP) and Usher syndrome.1,2 A consistent pattern of changes in the outer retina has emerged from these studies. Specifically, Hood et al.2 found that structural damage (thinning) in RP occurs first in the outer segment (OS) layer and then in the outer nuclear layer (ONL), but that retinal pigment epithelium (RPE) thickness remains relatively normal across the TZ. This pattern is consistent with the disease process associated with many genetic forms of RP, where the defect often originates with an abnormal OS protein.
Our main purpose in the present study was to determine whether the pattern of changes in the photoreceptor/RPE layers within the TZ differs in the various diseases that affect the receptors. That is, to what extent is the pattern of loss specific to the disease entity? To answer this question, we chose two disease entities, one, like RP, which typically starts outside the fovea, and one that starts within the fovea. Choroideremia (CHM), an X-linked retinal disorder, causes degeneration of the choroid and outer retina. Because of the nature of the progression of this disease, beginning in the midperiphery and moving centrally, patients with CHM typically have a central retina that is both structurally and functionally near normal, but the peripheral retina is severely affected. This pattern is similar to the pattern often seen in patients with RP. Stargardt disease (STGD), an autosomal recessive disorder, is a form of juvenile macular degeneration characterized by atrophy of the retinal pigment epithelium (RPE) and photoreceptor layer. In contrast to CHM and RP, STGD usually first affects the central retina and then progresses toward the periphery. Thus, patients with STGD typically have a diseased central retina with loss of central vision, but a near-normal peripheral retina. TZs are therefore present between the healthier and affected regions in both CHM and STGD.
Given that CHM is considered to be allied to RP and that the two diseases are sometimes confused because of their common symptoms of night blindness and progressively decreasing peripheral vision,5,6 there are reasons to believe that their TZs are similar. However, some studies have shown that photoreceptor degeneration in CHM occurs secondary to, or at least concurrently with, atrophy of the RPE and choriocapillaris.7–10 RPE atrophy, induced by lipofuscin accumulation, is a main feature of STGD, although there is disagreement as to whether photoreceptor loss occurs before or after the RPE is affected.3,11–14 Therefore, there are also reasons to expect some differences in the sequence and occurrence of structural changes of the RPE and photoreceptor layer across the three diseases. In the present study, the thicknesses of fdOCT layers associated with the OS, ONL, and RPE were quantified in 7 patients with CHM and 20 with STGD, and the results were compared to each other and to our previously reported results for patients with RP.2
Methods
Subjects
This study included 7 patients with CHM (mean age, 38.9 ± 16.7 years; range, 18–65); 20 patients with STGD (mean age, 26.3 ± 12.5 years; range, 11–59); 12 patients with RP (mean age, 25.2 ± 14.8 years; range, 11–54); and 30 healthy controls (mean age, 35.7 ± 14.0 years; range, 11–65 years). The diagnoses of CHM, STGD, or RP were made by ophthalmologists specializing in retinal disease based on fundus appearance, clinical and family histories, visual fields, and/or full-field electroretinograms. Tables 1 and 2 contain some of the key information about the patients. In most cases, the diagnosis was confirmed by genotyping. The patients with CHM had best corrected visual acuities (BCVAs) ranging from 20/16 to 20/320, with five patients having visual acuities equal to or better than 20/32. Nineteen of the 20 patients with STGD had BCVAs equal to or better than 20/200, with a median acuity of 20/125. Three patients with STGD had an optically empty space (optical gap)15,16 in the outer retina that lacked OS and inner segment (IS) layers. These optical gaps extended ≤0.6 mm nasally and temporally from the foveal center, as observed on fdOCT. The patients were excluded if they had a history of other ocular diseases or OCT scans of poor quality.
Table 1.
Characteristics of Patients with Choroideremia
| Patient | ID | Eye | Age (y) | Sex | BCVA | Mutation (CHM) |
|---|---|---|---|---|---|---|
| P1 | 7658 | OD | 18 | M | 20/16 | EX1_8del |
| P2 | 193 | OD | 37 | M | 20/250 | R253X |
| P3* | 1770 | OD | 52 | M | 20/320 | † |
| P4 | 6697 | OS | 29 | M | 20/25 | Intron 2 146+1G>A |
| P5 | 5244 | OD | 24 | M | 20/20 | R253X |
| P6 | 7828 | OD | 47 | M | 20/25 | Y531X |
| P7 | 8472 | OD | 65 | M | 20/32 | † |
Patient had a central scotoma.
Patient did not undergo genetic testing.
Table 2.
Characteristics of Patients with STGD
| Patient | ID | Eye | Age | Sex | BCVA | Mutation(s) (ABCA4) |
|---|---|---|---|---|---|---|
| P8 | 12 | OS | 33 | F | 20/150 | G1961E |
| P9 | 2 | OS | 30 | M | 20/150 | T1253M, G1961E |
| P10 | 9817 | OS | 21 | F | 20/63 | * |
| P11 | 9 | OS | 19 | M | 20/150 | IVS20+5 G>A, G1961E |
| P12 | 6953 | OD | 49 | F | 20/50 | * |
| P13 | 11 | OS | 59 | M | 20/100 | P1380L, S1696N |
| P14 | 9831 | OD | 28 | M | 20/500 | * |
| P15 | 8813 | OD | 13 | M | 20/50 | * |
| P16 | 8 | OS | 34 | M | 20/100 | G1961E, G1961E |
| P17 | 6.1 | OD | 24 | F | 20/200 | L541P/A1038V, G1961E |
| P18 | 8833 | OS | 13 | F | 20/160 | N965S, L2229P |
| P19 | 8938 | OD | 13 | M | 20/200 | A192T, R1300Q |
| P20 | 5470 | OD | 28 | F | 20/100 | * |
| P21 | 9901 | OS | 41 | M | 20/160 | I32V |
| P22 | 9327 | OS | 11 | F | 20/63 | G863A, A1695D |
| P23 | 9386 | OS | 18 | M | 20/40 | * |
| P24 | 8862 | OD | 30 | F | 20/63 | * |
| P25 | 6.1 | OD | 21 | F | 20/150 | L541P/A1038V, G1961E |
| P26 | 6.2 | OS | 18 | F | 20/70 | L541P/A1038V, G1961E |
| P27 | 10 | OS | 23 | F | 20/150 | L541P/A1038V, I1846T |
Patient did not undergo genetic testing.
All the patients with CHM, 11 patients with STGD, and all the controls were tested at the Retina Foundation of the Southwest. The results of the 30 healthy controls have been published.2 Nine of the patients with STGD were tested at Columbia University's Department of Ophthalmology and were part of studies with different purposes.14,17 Our study adhered to the tenets of the Declaration of Helsinki, and all subjects provided written informed consent. Consent procedures were approved by the Institutional Review Boards of both UT Southwestern Medical Center and Columbia University.
Frequency-Domain OCT
All subjects were scanned with fdOCT (Spectralis HRA+OCT; Heidelberg Engineering, Vista, CA), using the eye-tracking feature (ART). A 9-mm line scan along the horizontal meridian and centered on the fovea from one eye of each individual was analyzed. If scans were available for both eyes of a subject and/or more than one scan was available for each eye, the eye and scan with the highest image quality were chosen.
Segmentation Procedure
A previously described2,18,19 manual segmentation procedure, aided by a computer program (MatLab, ver. 7.4; The MathWorks, Natick, MA) was used to segment six borders. These borders, labeled 1 through 6 in Figure 1B, were (1) the border between the inner plexiform layer (IPL) and inner nuclear layer (INL); (2) the border between the INL and outer plexiform layer (OPL); (3) the outer limiting membrane (OLM); (4) the border between the IS and OS layers of the receptors; (5) the border between the OS layer and RPE; and (6) the border between the Bruch's membrane (BM) and the choroid. The line scans were flattened after segmentation and before thickness analysis to eliminate the effect of scan curvature on thickness results. As shown in Figure 1B, we defined four retinal layers by using the six boundaries:
ONL+: distance between 2 (INL/OPL) and 3 (OLM). This layer includes the nuclei of the receptors, the fibers of Henle, and the OPL.2
OS: distance between 4 (IS/OS) and 5 (OS/RPE).
RPE+BM: distance between 5 (OS/RPE) and 6 (BM/choroid).
Outer retina (OR): distance between 1 (IPL/INL) and 6 (BM/choroid).
Figure 1.
(A) FdOCT scan of the horizontal meridian of a normal retina. (B) Same scan as in (A), but showing the segmented borders and thicknesses derived from them.
Retinal flecks20 were found in four of the patients with STGD (P13, P19, P21, and P24). Segmenting around the flecks was difficult, and so the flecks were ignored during segmentation, and the thicknesses of the outer retinal layers in the regions containing flecks were excluded. One entire hemifield of P13 with STGD was excluded because of significant flecks. In addition, border 2 was difficult to discern in the central region of three of the patients with STGD (P15, P16, and P18) because of pigment migration (see the Results section). Thicknesses obtained using this boundary were excluded in the small region where the boundary was unclear. Last, P8 with STGD and P6 with CHM each had some localized macular pathology, and so these regions were excluded from the analysis.
Results
Choroideremia
The fdOCT scan of P1 with CHM is shown in Figure 2. This patient's OS, ONL+, RPE+BM, and OR thicknesses (colored curves) are plotted as functions of distance from the foveal center in Figures 2C–F, with the mean (black bold trace) and 95% confidence intervals (CI) (black thin traces) of the controls. The OS, ONL+ and RPE+BM z-scores (the number of standard deviations above or below the mean of the controls) in the patients with CHM are shown in Figure 3. (The z-scores were obtained by relating the thickness of each region of each hemifield of each patient to the distribution of the 30 control thicknesses for the same regional width.) Each patient is represented by a different color (P1 is red). Table 3 summarizes our framework for categorizing the structural changes occurring in the transition from healthy to affected retina in CHM, as described below.
Figure 2.
(A) FdOCT scan of the horizontal meridian of P1 with CHM. Red arrows: the locations at which the OPL formed an interlaminar bridge4; yellow arrow: the location of a tubular structure.21,22 (B) Same scan as in (A), but showing the segmented borders. Blue circles: the points at which the OLM disappeared. (C) OS thickness profiles across the retina in the patient in (A) (dark blue trace) and the mean (bold black trace) and ±2 SD (thin black traces) of the 30 controls. Purple circles: the locations at which the OS thickness fell below the 95% CI; red circles: points at which the OS thickness approached 0. (D) ONL+ thickness profiles across the retina in the patient in (A) (light blue trace) and for the mean and ±2 SD of the 30 controls. (E) RPE+BM thickness profiles across the retina in the patient in (A) (pink trace) and for the mean and ±2 SD of the 30 controls. (F) OR thickness profiles across the retina in the patient in (A) (green trace) and for the mean and ±2 SD of the 30 controls. Orange circles: the locations at which the OR thickness approached an asymptotic level. Colored bars, bottom: the extent of each region; black arrows: the direction of disease progression.
Figure 3.
(A) Colored symbols: the OS layer thicknesses in the patients with CHM, shown as z-scores (the number of standard deviations above or below the mean of the controls), which were obtained by relating the thickness of each region of each hemifield of each patient to the distribution of the 30 control thicknesses for the same region width. (◆) The mean ±1 SE of the patients. Dashed horizontal lines: controls' mean and ±2 SD (CI boundaries); (*) regions in which the OS layer was not present. (B) Same as in (A), but for ONL+ thickness. (C) Same as in (A), but for RPE+BM thickness. Solid lines: the z-score equivalent of the minRPE+BM thickness found in the patients with severe RP.
Table 3.
CHM Framework
| Region | Characteristics |
|---|---|
| IC | OS and ONL+ within CI; RPE+BM may be below CI |
| IIC | OS below CI; ONL+ within (or above) CI; RPE+BM may be below CI |
| IIIC | No OS layer (or IS/OS line); ONL+ within CI nasally and near normal temporally; RPE+BM may be below CI |
| IVC | No IS layer (or OLM); OPL has separated (forming an “interlaminar bridge”4); RPE+BM below CI; ONL+ is abnormal or not detectable; no OS layer |
| VC | Asymptotic OR thickness; no OS or IS layers; RPE+BM below CI; ONL+ is abnormal or not detectable |
In four of the seven patients with CHM, there was a central region, denoted region IC, in which OS thickness was within the normal CI, as seen by points falling within the dashed black lines in IC of Figure 3A. This and other regions of the TZ were determined from plots like Figure 2C, where the purple circles indicate the locations on the nasal and temporal sides of fixation where P1's OS thickness fell below the CI. Region IC in P1 is denoted by the purple bar at the bottom of Figure 2F. Three CHM patients (P2, P3, and P4) did not have a central region with a normal OS thickness. In all four of the CHM patients containing region IC, ONL+ thickness was within or above normal limits in this region (Fig. 3B). In addition, in two of the four patients containing a region IC, the RPE+BM was abnormally thin (thickness less than −2 SD from the control mean) in at least one hemifield (Fig. 3C). In the four patients with a region IC, the median extent of this region was 115 μm (nasal) and 255 μm (temporal), with ranges of 10 to 470 μm (nasal) and 70 to 2060 μm (temporal). (In Fig. 2, distances and thicknesses are shown in millimeters.)
Region IIC, found in all seven patients, was defined as having a clearly present but abnormally thin OS layer (Fig. 3A) and an ONL+ that was normal or near normal in thickness. In particular, five patients had normal or above normal ONL+ thickness in region IIC, with the other two patients having slightly below normal ONL+ thickness (Fig. 3B). Interestingly, five patients had an abnormally thin RPE+BM in region IIC in at least one hemifield (Fig. 3C). The red bar at the bottom of Figure 2F marks region IIC in P1. The locations of region IIC's borders on the nasal and temporal sides of fixation, indicated by the red circles in Figure 2C, are defined as the points at which the OS thickness decreased to 0 (i.e., where the IS/OS junction disappeared; dark blue border, Fig. 2B). Note that in P1, the ONL+ was within normal limits and the RPE+BM thickness was borderline abnormal within region IIC. In all seven patients, the median extent of region IIC was 485 μm (nasal) and 1460 μm (temporal), with ranges of 210 to 1930 μm (nasal) and 220 to 2260 μm (temporal).
The nasal and temporal locations of the disappearance of the OLM (light blue segmented border in Fig. 2B) are shown for P1 by the blue circles in Figure 2B. Region IIIC was defined as the centralmost region without a measurable OS layer, but with a visible OLM. The light blue bar at the bottom of Figure 2F marks this region in P1. Region IIIC was found in all seven patients. In that region, the ONL+ was typically of near-normal thickness. In particular, the ONL+ was within normal limits in three of the four nasal hemifields with a measurable ONL+ and in three of the six temporal hemifields with a measurable ONL+ (Fig. 3B). (We did not measure ONL+ thickness in the very narrow part of the scan in which the OLM abruptly disappeared, to prevent an inaccurate spike in ONL+ thickness. Because of the small width of region IIIC in some patients, this area of excluded ONL+ coincided with the entire region IIIC in three nasal hemifields and one temporal hemifield, making it impossible to measure ONL+ in these hemifields.) In addition, RPE+BM thickness was below the CI in five patients in region IIIC in at least one hemifield (Fig. 3C). The median extent of region IIIC was 90 μm (nasal) and 420 μm (temporal), with ranges of 40 to 500 μm (nasal) and 50 to 1230 μm (temporal).
Interestingly, in at least one hemiretina of all seven patients, the OPL appeared to separate at the same location where the OLM disappeared, and part of the OPL approached the RPE, forming what Jacobson et al.4 called an “interlaminar bridge” (Fig. 2A, red arrow). Beyond region IIIC, the ONL was either unrecognizable and too difficult to measure (i.e., the distinctions between the ONL+ and other retinal layers became indiscernible) or simply no longer present.
Beyond region IIIC, OR thickness continued to decrease after the OLM disappeared in all seven patients. However, it reached an asymptotic minimum in each hemifield, as illustrated by the orange circles in Figure 2F for P1. The point at which the OR reached an asymptotic thickness marked the outer limit of region IVC. The orange bar at the bottom of Figure 2F denotes region IVC for P1. All seven patients had an abnormally thin RPE+BM in region IVC (Fig. 3C). The median extent of region IVC was 1060 μm (nasal) and 640 μm (temporal), with ranges of 200 to 2960 μm (nasal) and 350 to 3120 μm (temporal).
Last, region VC, the region beyond region IVC, which continued to the end of the scan (Fig. 2F, black bar), represents the most affected retina. All patients had an approximately constant minimum OR thickness and thinned RPE+BM thickness (Fig. 3C) in region VC. In addition, “outer retinal tubulation”21,22 (Fig. 2A, yellow arrow) was found in region IVC and/or VC in at least one hemifield of five patients with CHM.
Stargardt Disease
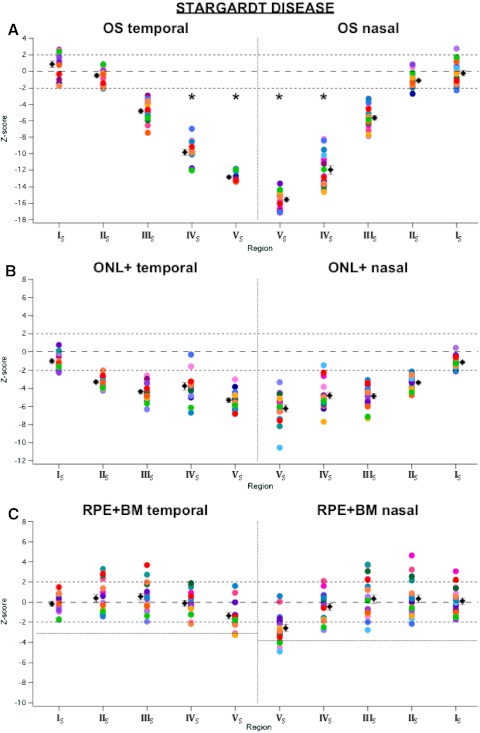
Figure 4 shows the fdOCT scan and corresponding OS, ONL+, and RPE+BM thickness profiles of one of the patients with STGD (P11), in a format analogous to that in Figure 2. The z-scores for patients with STGD are shown in Figure 5, where P11 is bright green. Our working framework for the STGD TZ, described below, is summarized in Table 4.
Figure 4.
(A) FdOCT scan of the horizontal meridian of a patient with STGD. Red arrows: pigment migration. (B) Same scan as in (A), but showing the segmented borders. Blue circles: the locations at which the OLM disappeared. (C) OS thickness profiles across the retina in the patient in (A) (dark blue trace) and for the mean (bold black trace) and ±2 SD (thin black trace) of the 30 controls. Purple circles: the locations at which the OS thickness fell below the 95% CI; red circles: the locations at which OS thickness approached 0. (D) ONL+ thickness profiles across the retina in the patient in (A) (light blue trace) and for the mean and ±2 SD of the 30 controls. Green circles: the locations at which ONL+ thickness fell below the 95% CI. (E) RPE+BM thickness profiles the across retina in the patient in (A) (pink trace) and for the mean and ±2 SD of the 30 controls. Colored bars, bottom: the extent of each region; black arrows: the direction of disease progression. (All data in this figure are presented as if from a right eye.)
Figure 5.
Same as in Figure 3, but for patients with STGD.
Table 4.
STGD Framework
| Region | Characteristics |
|---|---|
| IS | OS, ONL+, and RPE+BM within CI |
| IIS | ONL+ below CI; OS and RPE+BM within CI |
| IIIS | OS and ONL+ below CI, but present; RPE+BM within CI |
| IVS | No OS layer (or IS/OS line); ONL+ below CI; RPE+BM within CI |
| VS | No IS layer (or OLM); RPE+BM below control mean; pigment migration; no OS layer; ONL+ below CI |
In the patients with STGD, region IS was the peripheral region, within which the outer retinal layers had approximately normal thickness. In at least one hemifield of all 20 patients with STGD (specifically, in 33 of the 39 analyzed hemifields), there was a peripheralmost region (of the part of the retina visualized in the scan), denoted region IS, in which the ONL+, OS, and RPE+BM thicknesses were within normal limits (Figs. 5A–C). This region is shown as the green bar at the bottom of Figure 4E for P11.
Region IIS was defined as the region with an abnormally thinned ONL+, but an OS thickness within normal limits. This region is denoted by the purple bar at the bottom of Figure 4E for P11. Region IIS began in the periphery where the ONL+ thickness fell below the normal CI (Fig. 4D, green circles) and ended at the more central point where the OS thickness fell below the normal CI (Fig. 4C, purple circles). In 36 of the 39 analyzed hemifields, the OS thickness fell below normal limits at a more central location than did the ONL+ thickness (Figs. 5A, 5B). Therefore, 92% of the hemifields had a region IIS. RPE+BM thickness was within normal limits in 27 (75%) of the 36 hemifields containing a region IIS (Fig. 5C). For 30 of the hemifields with a region IIS (excluding the six hemifields in which the peripheral boundary of region IIS occurred outside of the scan region), the median extent of this region was 660 μm (nasal) and 1020 μm (temporal), with ranges of 40 to 1820 μm (nasal) and 30 to 4890 μm (temporal).
Region IIIS was defined as the region with clearly present, yet abnormally thin OS layer and ONL+. In P11, this region is denoted by the red bar at the bottom of Figure 4E. The region's more peripheral boundary was the point at which the OS thickness fell below the normal CI (Fig. 4C, purple circles) and its more central border was where the OS thickness decreased to 0 (Fig. 4C, red circles). A region IIIS was found in all 39 hemifields (Figs. 5A, 5B). Again, the RPE+BM thickness was within the CI in the majority (31/39) of these hemifields (Fig. 5C). The median extent of region IIIS was 165 μm (nasal) and 220 μm (temporal), with ranges of 10 to 1060 μm (nasal) and 10 to 1210 μm (temporal).
Region IVS was defined as the one without a measureable OS layer, but with a visible OLM. For P11, this region is denoted by the blue bar at the bottom of Figure 4E. The nasal and temporal locations at which the OLM disappeared are indicated by the blue circles in Figure 4B. The region between the red and blue circles is IVS. A region IVS was found in 33 hemifields. In the three patients with an optically empty space in the fovea, the OLM did not disappear, and so their TZs ended with region IIIS. RPE+BM thickness was again within the CI for most hemifields containing region IVS (27/32; flecks prevented RPE+BM measurement in one hemifield of one patient; Fig. 5C). For the 33 hemifields with a region IVS, the median extent of this region was 160 μm (nasal) and 135 μm (temporal), with ranges of 60 to 860 μm (nasal) and 80 to 470 μm (temporal).
Finally, the most central, most affected region was denoted region VS (Fig. 4E, black bar). Region VS, found in all 33 hemifields containing region IVS, extends to the foveal center. The RPE seemed to have finally decreased by region VS, as the RPE+BM thickness was below −2 SD of the controls in 16 of 33 hemifields, and below −1 SD of the controls in 11 of the remaining 17 hemifields (Fig. 5C). In addition, pigment migration (Fig. 4A, red arrow) was found in this most-affected area in 15 of the 17 eyes containing region VS.
Discussion
As in the case of the patients with RP (Table 5),2 progressive degeneration of the photoreceptors occurred across the TZs of patients with CHM and STGD, as shown by the decreasing OS thickness (z-scores) from the healthier to the more affected regions (Figs. 3A, 5A, 6A). However, there are interesting differences among the three diseases; these are highlighted in Table 6.
Table 5.
RP Framework2
| Region | Characteristics |
|---|---|
| IR | OS, ONL+, and RPE+BM within CI |
| IIR | OS below CI; ONL+ and RPE+BM within CI |
| IIIR | OS and ONL+ below CI, but present; RPE+BM within CI |
| IVR | No OS layer (or IS/OS line); ONL+ abnormal and decreasing; RPE+BM within CI |
| VR | Asymptotic ONL+, no OS layer (or IS/OS line), RPE+BM within CI |
Figure 6.
Same as in Figure 3, but for 12 patients with RP (who had Humphrey visual field diameters ≥10°, foveal sensitivities ≥32 dB, and BCVAs between 20/15 and 20/50).
Table 6.
Summary of Changes across the TZ
↑, Above normal limits; ⊙, within normal limits; ↓, below normal limits; x, not present; ■, cannot be measured (too abnormal or not present). 1. The Roman numerals I–V are equivalent to the letters A–E used in prior work for the RP TZ.2
First, in both CHM and RP, there was a region (regions IIC and IIR, respectively) with near-normal ONL+ thickness, but an abnormally thin OS layer (Figs. 3A, 3B, 6A, 6B). Conversely, in STGD, there was a region (region IIS) with a normal OS thickness, but an abnormally thin ONL+ (Figs. 5A, 5B; Table 6, red rectangles), which suggests that loss of photoreceptor OSs occurs before the loss of cell bodies in both CHM and RP, but that the cell bodies are affected before the OS layer in STGD. Of course, these structural differences may not predict the actual pathophysiological changes. However, if they do, then, although the recovery of the OS layer is the appropriate focus of therapies for CHM and RP, treatments specifically aimed at rescuing photoreceptor cell bodies may also be successful in STGD.
Second, while the thinning of the ONL+ layer occurred just beyond the location at which the OS layer became thinner in RP, the ONL+ thickness was preserved over a larger retinal region in the patients with CHM (especially nasally). The asterisks in the OS plots in Figures 3A, 5A, and 6A signify that the OS layer was not present in the given region (the IS/OS junction was not present). In the healthiest region lacking OSs in RP (region IVR), ONL+ thickness was significantly below normal limits, whereas in the healthiest region lacking OSs in CHM (region IIIC), the ONL+ thickness was usually within the CI nasally (and more preserved temporally than in RP; Figs. 3A, 3B, 6A, and 6B; Table 6, green rectangles). Perhaps in CHM, unlike RP, cells bodies are able to survive for some time without a detectable OS layer.
Third, whereas the RPE+BM thickness in the RP patients was approximately within normal limits across the TZ (Fig. 6C), the RPE+BM thickness in patients with CHM was abnormally thin by region IVC and was sometimes thin even in the more central regions IC to IIIC (Fig. 3C). Patients with STGD usually have a thin RPE+BM in their most affected region (region VS; Fig. 5C; blue rectangles in Table 6). It is important to emphasize that we think that we were measuring the RPE+BM thickness, not the RPE thickness by itself. The RPE+BM thickness that we measured in the control eyes (average 19 μm) was similar to that measured in a recent human histologic study.23 Further, in regions of the retina of patients with severe RP and a missing RPE on fundus examination, a thin RPE+BM band remained. The thinning of the RPE+BM by the end of the TZs in CHM and STGD was close to that in patients with severe RP. In particular, the RPE+BM thickness of the CHM patients in regions IVC and VC (≈10 μm) was close to the residual RPE+BM thickness of 9 μm (minRPE+BM) that we found in a group of patients with severe RP with measurable damage to the RPE. Our observation of a region IC with normal OS and ONL+ thickness, but thin RPE+BM thickness in some CHM patients lends support to the possibility proposed in recent papers7–10 that the RPE is the primary site of disease in CHM, followed by the photoreceptors. Nonetheless, thinning of the RPE+BM is seen at the end of the TZ in all patients with CHM, including those whose OS layer appears to be affected before the RPE. This suggests that therapies targeting the RPE may also be of use in CHM. Although a thin RPE+BM was found in most patients with STGD by region VS, RPE+BM thickness was found to be relatively normal in STGD patients at less severe locations in the TZ with already thinned ONL+ and OS layer. It therefore appears that photoreceptor loss can occur before RPE loss in STGD, consistent with some findings in the literature,3,13,14 but in contrast to others.11,12
Fourth, there were qualitative differences in the appearance of the TZs. In particular, the disappearances of the IS/OS junction and OLM were much more abrupt in CHM and STGD than in RP. As illustrated in Figure 7, the disappearances of the IS/OS line (red arrows) and OLM (blue arrows) were gradual (shallower slope) in RP, yet were nearly vertical (steeper slope) in CHM and STGD. These abrupt changes agree with previous findings of abrupt structural and functional losses in CHM,10,24–26 and with the distinct borders between areas of hypo- and hyperfluorescence on fundus autofluorescence in STGD.27 Also, as mentioned above, the OLM disappeared in a more affected location than did the IS/OS junction in almost all CHM and STGD patients, similar to RP.2 However, the distance between the disappearance of the IS/OS line and the OLM was significantly shorter in CHM (mean, 345 ± 388 μm) and STGD (mean, 221 ± 170 μm), compared to that in RP (mean, 676 ± 471 μm) (two-tailed P < 0.05 for each, compared with RP).
Figure 7.
(A) Temporal hemifield of the CHM patient's scan in Figure 2A. (B) Temporal hemifield of the STGD patient's scan in Figure 4A. (C) Temporal hemifield of an RP patient's scan. Red arrows: the location where the IS/OS line disappears; blue arrows: the location where the OLM disappears.
In addition, our findings largely support the model of CHM progression proposed by Jacobson et al.4 In particular, we both found that loss of OSs, sometimes concurrent with RPE thinning, occurred early in the disease, although we believe that the ONL is preserved to a greater extent than their model suggests. Jacobson et al. also found thickening of the total retina to be a key early event in CHM, and we believe this to be possible, due to the increased thickness of the inner retinal layers (retinal nerve fiber layer, retinal ganglion cell layer plus IPL, INL; data not shown) and the ONL seen in some of our patients.
In conclusion, on fdOCT scans, patients with RP, CHM, and STGD all have a TZ between the relatively healthy and the severely affected retina. The patterns of changes in the receptor layers (e.g., OS, ONL, and RPE) are similar within a disease category, but clearly different across categories. The observations suggest that the pattern of progression of each disease is distinct and may offer clues for strategies in the development of future therapies.
Acknowledgments
The authors thank Ali Raza and Xian Zhang for helpful comments and Rando Allikmets, Gaetano Barile, Stanley Chang, Gary Fish, Theodore Smith, Rand Spencer, and Stephen Tsang for diagnosing and referring the patients.
Footnotes
Supported National Eye Institute Grants R01-EY-09076, EY-018213, and P30-EY-019007; the Foundation Fighting Blindness; and an unrestricted grant to the Department of Ophthalmology, Columbia University, from Research to Prevent Blindness.
Disclosure: M.A. Lazow, None; D.C. Hood, Topcon, Inc. (C); R. Ramachandran, None; T.R. Burke, None; Y.-Z. Wang, None; V.C. Greenstein, None; D.G. Birch, None
References
- 1. Jacobson SG, Aleman TS, Sumaroka A, et al. Disease boundaries in the retina of patients with Usher syndrome caused by MYO7A gene mutations. Invest Ophthalmol Vis Sci. 2009;50:1886–1894 [DOI] [PubMed] [Google Scholar]
- 2. Hood DC, Lazow MA, Locke KG, Greenstein VC, Birch DG. The transition zone between healthy and diseased retina in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen Y, Ratnam K, Sundquist SM, et al. Cone photoreceptor abnormalities correlate with vision loss in patients with Stargardt disease. Invest Ophthalmol Vis Sci. 2011;52:3281–3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jacobson SG, Cideciyan AV, Sumaroka A, et al. Remodeling of the human retina in choroideremia: Rab escort protein 1 (REP-1) mutations. Invest Ophthalmol Vis Sci. 2006;47:4113–4120 [DOI] [PubMed] [Google Scholar]
- 5. Lee TK, McTaggart KE, Sleving PA, et al. Clinical diagnoses that overlap with choroideremia. Can J Ophthalmol. 2003;38:364–372 [DOI] [PubMed] [Google Scholar]
- 6. Davis WT, Sheppard E. Gyrate atrophy of the retina and choroid following retinitis pigmentosa. Arch Ophthalmol. 1940;23:1252–1256 [Google Scholar]
- 7. Krock BL, Bilotta K, Perkins DB. Noncell-autonomous photoreceptor degeneration in a zebrafish model of choroideremia. Proc Natl Acad Sci U S A. 2007;104:4600–4605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tolmachova T, Wavre-Shapton ST, Barnard AR, MacLaren RE, Futter CE, Seabra MC. Retinal pigment epithelium defects accelerate photoreceptor degeneration in cell type-specific knockout mouse models of choroideremia. Invest Ophthalmol Vis Sci. 2010;51:4913–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mura M, Sereda C, Jablonski MM, MacDonald IM, Iannaccone A. Clinical and functional findings in choroideremia due to complete deletion of the CHM gene. Arch Ophthalmol. 2007;125:1107–1113 [DOI] [PubMed] [Google Scholar]
- 10. Flannery JG, Bird AC, Farber DB, Weleber RG, Bok D. A histopathologic study of a choroideremia carrier. Invest Ophthalmol Vis Sci. 1990;31:229–236 [PubMed] [Google Scholar]
- 11. Glazer LC, Dryja TP. Understanding the etiology of Stargardt's disease. Ophthalmol Clin North Am. 2002;15:93–100 [DOI] [PubMed] [Google Scholar]
- 12. Cideciyan AV, Aleman TS, Swider M, et al. Mutations in ABCA4 result in accumulation of lipofuscin before slowing of the retinoid cycle: a reappraisal of the human disease sequence. Hum Mol Genet. 2004;13:525–534 [DOI] [PubMed] [Google Scholar]
- 13. Gomes NL, Greenstein VC, Carlson JN, et al. A comparison of fundus autofluorescence and retinal structure in patients with Stargardt disease. Invest Ophthalmol Vis Sci. 2009;50:3953–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burke TR, Rhee DW, Smith T, et al. Quantification of peripapillary sparing and macular involvement in Stargardt Disease (STGD1). Invest Ophthalmol Vis Sci. 2011;52:8006–8015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cella W, Greenstein VC, Zernant-Rajang J, et al. G1961E mutant allele in the Stargardt disease gene ABCA4 causes bull's eye maculopathy. Exp Eye Res. 2009;89:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thiadens AA, Somervuo V, van den Born LI, et al. Progressive loss of cones in achromatopsia: an imaging study using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:5952–5957 [DOI] [PubMed] [Google Scholar]
- 17. Burke TR, Allikmets R, Smith RT, Gouras P, Tsang SH. Loss of peripapillary sparing in non-group I Stargardt disease. Exp Eye Res. 2010;91:592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:2328–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hood DC, Cho J, Raza AS, Dale EA, Wang M. Reliability of a computer-aided manual procedure for segmenting optical coherence tomography scans. Optom Vis Sci. 2011;88:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Voigt M, Querques G, Karim A, et al. Analysis of retinal flecks in fundus flavimaculatus using high-definition spectral-domain optical coherence tomography. Am J Ophthalmol. 2010;150:330–337 [DOI] [PubMed] [Google Scholar]
- 21. Zweifel SA, Engelbert M, Laud K, Margolis R, Spalde RF, Freund KB. Outer retinal tubulation: a novel optical coherence tomography finding. Arch Ophthalmol. 2009;127:1596–1602 [DOI] [PubMed] [Google Scholar]
- 22. Pennesi ME, Weleber RG. High-resolution optical coherence tomography shows new aspects of Bietti crystalline retinopathy. Retina. 2010;30:531–532 [DOI] [PubMed] [Google Scholar]
- 23. Curcio CA, Messinger JD, Sloan KR, et al. Human chorioretinal layer thicknesses measured in macula-wide, high-resolution histological sections. Invest Ophthalmol Vis Sci. 2011;52:3943–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacDonald IM, Russell L, Chan CC. Choroideremia: new findings from ocular pathology and review of recent literature. Surv Ophthalmol. 2009;54:401–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katz BJ, Yang Z, Payne M, et al. Fundus appearance of choroideremia using optical coherence tomography. Adv Exp Med Biol. 2006;572:5761. [DOI] [PubMed] [Google Scholar]
- 26. Potter MJ, Wong E, Szabo SM, McTaggart KE. Clinical findings in a carrier of a new mutation in the choroideremia gene. Ophthalmology. 2004;111:1905–1909 [DOI] [PubMed] [Google Scholar]
- 27. Anastasakis A, Fishman GA, Lindeman M, Genead MA, Zhou W. Infrared scanning laser ophthalmoscope imaging of the macula and its correlation with functional loss and structural changes in patients with Stargardt disease. Retina. 2011;31:949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]