The so-called IS/OS border visible on OCT scans is now thought to be the ellipsoids of the inner segment. This band of increased reflectivity is visible, but less intense, in patients with markedly reduced cone function secondary to achromatopsia and cone dystrophy.
Abstract
Purpose.
The integrity of the inner segment ellipsoid (ISe) band, previously called the inner segment/outer segment (IS/OS) border, seen on optical coherence tomography (OCT) scans is of clinical significance. To better understand the influence of cones on the appearance of this band, the intensity of its signal in patients with diminished cone function was examined.
Methods.
Horizontal line scans through the fovea of 30 healthy controls, 10 patients with achromatopsia (A), and six with cone dystrophy (CD) were obtained with frequency domain (fd) OCT. The fdOCT borders were segmented with a computer-aided manual technique. The ISe was divided into regions 60.1 μm wide and 19.5 μm deep. The relative ISe intensity of each region was defined as its intensity divided by the intensity of a local region, which extended in depth from the choroid to the retinal ganglion cell/retinal nerve fiber layer.
Results.
Except for the central fovea, all patients had a clear ISe band across the region studied, ± 3 mm from the foveal center. However, the relative ISe intensity was significantly lower (P < 0.0001) in patients (A: 1.14 ± 0.14; CD: 1.27 ± 0.14), than in controls (1.61 ± 0.16). There were no differences in the relative intensity of the other retinal layers.
Conclusions.
Although present, the intensity of this ISe band is lower in patients with diminished cone function than it is in healthy controls. This is consistent with the hypothesis that both rod and cone receptors must be absent or damaged for the ISe band to be missing.
With frequency domain optical coherence tomography (fdOCT), it is now possible to visualize the outer retinal layers of the human retina in vivo. One structure of particular clinical importance is the so-called inner segment (IS)/outer segment (OS) border. The IS/OS border is a highly reflective band clearly visible on fdOCT scans (see Fig. 1A). Because the IS/OS border is missing and/or disrupted in diseases of the outer retina,1–7 the integrity of this border is being used clinically as a sign of disease of the receptors and/or choroid. Further, because the edge of the IS/OS border appears to coincide with the edge of the visual field in patients with retinitis pigmentosa (RP),8 it has been suggested as an endpoint in clinical trials.
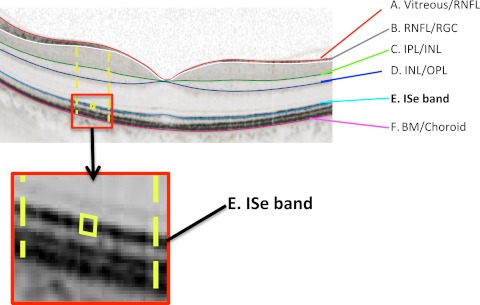
Figure 1.
An fdOCT horizontal scan through the fovea of a healthy control subject. The layers and bands manually segmented are indicated. Inset, the ISe band is shown without the segmentation line. BM, Bruch's membrane; INL, inner nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; RGC, retinal ganglion cell; RNFL, retinal nerve fiber layer.
Given the clinical relevance of the IS/OS border, it is important to understand the origin of this fdOCT signal. A recent report by Spaide and Curcio9 provides a summary of the evidence against the IS/OS border being the source of this fdOCT signal. In addition, they designed an anatomic model of the outer retina based on published histology. By quantitatively comparing the bands seen on fdOCT to this model, they concluded that the band typically called the IS/OS border aligns with the ellipsoids of the IS. An earlier study by Fernández et al.10 came to a similar conclusion based on a qualitative comparison of retinal anatomy to fdOCT images obtained with adaptive optics and high resolution fdOCT. Thus, we will refer to the heretofore IS/OS border as the IS ellipsoid (ISe) band.
While the general location of the ISe band now appears to be established, the role played by the rod and cone receptors in generating this fdOCT signal is less clear. Fernández et al.10 suggested that they were visualizing individual cone ISe regions, which formed the band visualized on the fdOCT image. In particular, they proposed that the ISe band was due to light scattered by the mitochondria in the ellipsoids situated in the distal portion of the IS. As the ellipsoids are considerably larger in cones than rods, they suggested that the signal is largely due to the cone ellipsoids. Spaide and Curcio9 do not discuss the relative contributions of the rods and cones, although figure 7 in their report implies the participation of both.
A recent study by Birch et al.11 suggests that both rods and cones contribute to the ISe band. For example, a prominent ISe band was seen in patients with cone dystrophy even in regions with little or no cone activity, as long as rod sensitivity was within −10 dB or so of normal. However, if the ISe band depends on the cones, even in part, it should not be normal in patients with little or no cone function. To better understand the influence of the cones on the appearance of the ISe band, we examined the intensity of the signal from the ISe band in patients with diminished cone function.
Methods
Subjects
We used OCT scans from one eye of 30 control subjects (35.7 ± 14.0 years) with normal healthy vision, 10 patients (33.8 ± 15.3 years) with achromatopsia, and six with cone dystrophy (44.3 ± 20.3 years). All control subjects and patients with cone dystrophy were tested at the Retina Foundation of the Southwest; the 10 achromats were tested at the Department of Ophthalmology, Columbia University. All were part of previous studies (Chen RWS, et al. IOVS 2011;52:ARVO E-Abstract 5008).11 The patients with cone dystrophy had clear evidence of progressive cone loss, severely depressed or nondetectable cone ERGs, and normal or near-normal rod ERG amplitudes.11 All but one of the 10 achromats had full-field ERG tests; all nine showed a normal rod response, while the cone response was nondetectable (Chen RWS, et al. IOVS 2011;52:ARVO E-Abstract 5008).
The tenets of the Declaration of Helsinki were followed and written informed consent was obtained from all participants. Consent procedures were approved by the Institutional Review Board of UT Southwestern Medical Center and the Committee of the Institutional Board of Research Associates of Columbia University.
OCT Testing
All individuals were scanned with a spectral-domain OCT confocal scanning laser ophthalmoscope (Spectralis HRA+OCT; Heidelberg Engineering, Vista, CA) using the eye-tracking feature (ART). A 9-mm line scan along the horizontal meridian, and centered on the fovea, was obtained as previously described (see Fig. 1A).12 When scans of both eyes were available, the better OCT image was selected.
Analysis of the ISe Band (Formerly IS/OS Border)
A manual segmentation procedure, aided by a computer program, (MATLAB, ver. 7.4; Mathworks, Natick, MA), was used to segment six borders, as previously described.12,13 These borders are labeled A through F in Figure 1. The ISe band (Fig. 1E) is the focus of this study. The other borders used to define regions serving as controls in the analysis are: the vitreous/RNFL, RNFL/RGC, IPL/INL, INL/OPL, and BM/choroid borders.
To obtain a measure of the intensity of the ISe band:
All scans were resized to have a lateral spatial resolution of 6.1 μm per pixel, and the vertical resolution was 3.87 μm per pixel;
The center of the fovea was marked by hand and all images aligned at their centers;
The ISe band was divided into segments 5 pixels (19.5 μm) in depth and 10 pixels (60.1 μm) in width (yellow box in Fig. 1 inset); and
A “local region” of an ISe segment (between yellow dashed lines in Fig. 1) was defined as the area between the BM/choroid and the RNFL/RGC border (between B and F in Fig. 1) and extending ± 275 μm in width.
Based on these definitions, the “relative intensity of an ISe segment” was taken as the average intensity of the ISe segment divided by the average intensity of the local region.
Note that the depth (5 pixels) of the ISe segment was chosen to encompass the thickest ISe band seen. Therefore, technically this analysis cannot distinguish between a less intense versus thinner ISe band. In any case, making the ISe segment smaller (3 pixels in depth) actually slightly increased the differences between groups.
Results
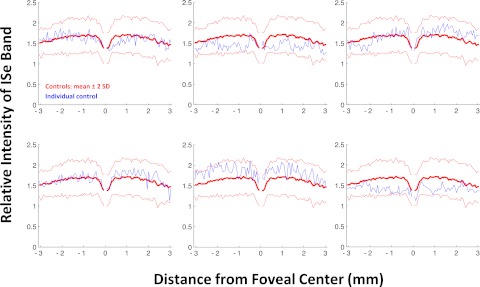
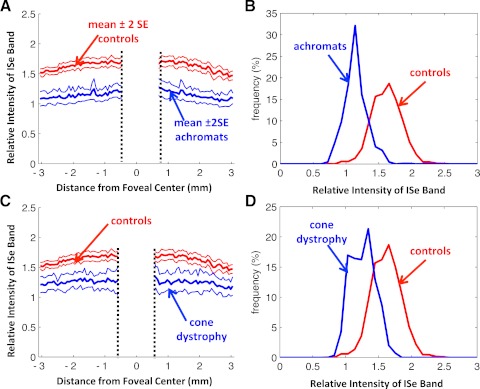
The average relative intensity of the ISe band for the 30 healthy control subjects is shown as the bold red curve in Figure 2. On average, the relative intensity in the center of the fovea, ± 375 μm (approximately ± 1.3°), is lower than outside the central fovea, where the ISe intensity is relatively constant across the ± 3 mm, about ± 10.4°, shown. However, the relative ISe intensity exhibits considerable variability among controls as can be seen in Figure 2 where the thin red lines are the ± 2 SD limits and the blue curves show the results for six representative controls.
Figure 2.
The relative intensity of the ISe band is shown in blue for 12 typical controls. The bold red curve is the mean of the controls shown with ± 2 SD limits (thin red lines).
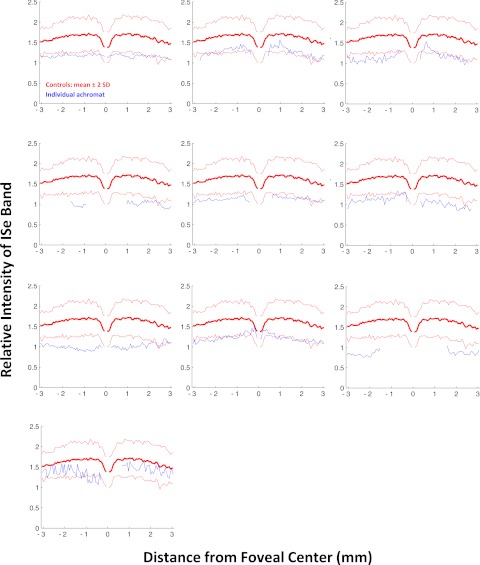
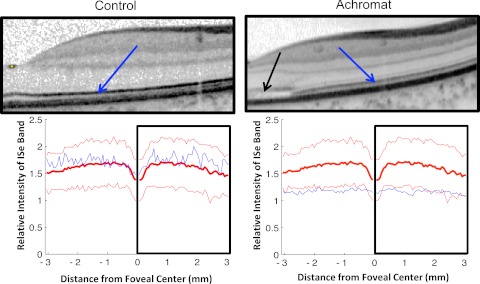
Figure 3 shows the results for the 10 achromats. As in Figure 2, the blue curves show the individual results and the red curves are the control mean ± 2 SD. The relative ISe intensity falls below the mean of the control subjects for all 10 patients with achromatopsia and often falls below the −2 SD limit as well. To provide a qualitative sense of this effect, Figure 4 shows the results for one of the control subjects and one of the achromats, each fairly typical of their group. The difference in relative intensity of the ISe band (blue arrows) can be seen in the scans, which are associated with the portion of the lower panel shown within the black rectangles.
Figure 3.
The relative intensity of the ISe band is shown in blue for 10 patients with achromatopsia. The bold red curve is the mean of the control subjects shown with ± 2 SD limits (thin red lines).
Figure 4.
Portions of the horizontal scans (upper panels) from a control subject (left panels) and a patient with achromatopsia (right panels) are shown along with the relative intensity profiles (bottom panels).
The mean relative ISe intensity for all 10 achromats is clearly lower than that of the control subjects as seen in Figure 5A. The central portion of Figure 5A is partially occluded to alert the reader that some of the achromats were missing receptor outer segments and ISe bands in the center of the fovea as shown by the black arrow in Figure 4 and previously reported by others.14,15 Figure 5B displays the group data as histograms, where the frequency as a percentage for all the regions of all individuals is plotted as a function of the relative ISe intensity. Note that the distribution for the control subjects peaks at approximately 1.6 (mean 1.61 ± 0.16), indicating that the relative intensity of the ISe band is 60% greater than the average of the local region of the retina. On the other hand, the mean ± SD for the achromats is 1.14 ± 0.14, significantly lower than that of the control subjects (two-sample t-test; P < 0.0001).
Figure 5.
(A) The mean ± 2 SE of the relative intensity of the ISe band for the 30 control subjects (red) and 10 achromats (blue). (B) Frequency distribution of relative intensity of the ISe band for the 30 control subjects (red) and 10 achromats (blue). (C) The mean ± 2 SE of the relative intensity of the ISe band for the 30 control subjects (red) and six patients with cone dystrophy (blue). (D) Frequency distribution of ISe band intensity for the 30 control subjects (red) and six patients with cone dystrophy (blue).
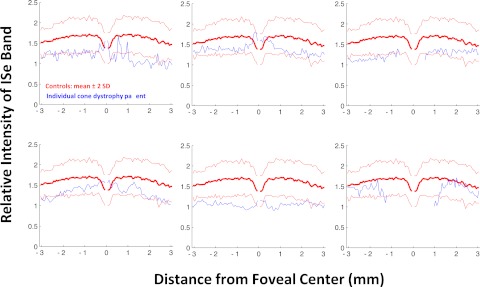
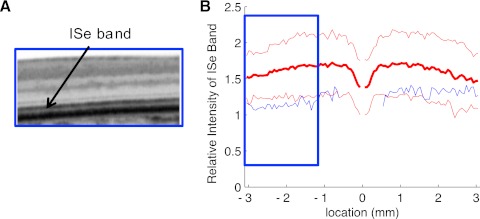
The results for the six patients with cone dystrophy were similar to those for the achromats. The relative intensity of the ISe band for the individual patients all fell below the mean as seen in Figure 6. Figure 7A, from Birch et al.,11 shows the fdOCT scan from a portion of the retina of a patient with cone dystrophy. In this portion of the retina, the rod threshold was normal, but the cone threshold was −16 to −19 dB below normal. Notice in Figure 7B that the relative ISe intensity falls below the −2 SD boundary in this region (blue rectangle).
Figure 6.
The relative intensity of the ISe band is shown in blue for six patients with cone dystrophy. The bold red curve is the mean of the control subjects shown with ± 2 SD limits (thin red lines).
Figure 7.
(A) Portion of an fdOCT horizontal scan of a cone dystrophy patient (from Ref. 11). (B) The relative intensity of the ISe band in (A) is shown as the blue curve within the blue rectangle.
The group data in Figures 5C and 5D show clear differences between the controls and the patients with cone dystrophy. The overall mean ± SD for this group, 1.27 ± 0.14, was slightly higher than that of the group with achromatopsia, but still clearly smaller than controls (two-sample t-test; P < 0.0001).
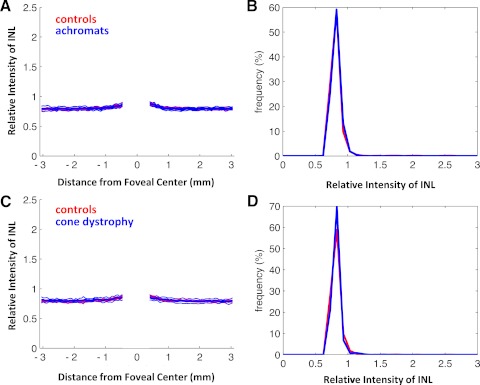
To assure that the change in relative intensity was unique to the ISe band, a similar analysis was performed on the INL, RGC+IPL, and RNFL. For all three regions, and both patient populations, the patient group was nearly identical with the control group. An example of this can be seen in Figure 8 for the INL. (The central region is blanked out in Figs. 8A and 8C because there is little or no INL in the very center of the fovea in control subjects or patients.)
Figure 8.
Same as Figure 5 for an analysis of the relative intensity of the INL.
Discussion
Based on recent work, what has been called the “IS/OS border” appears to be located in the ellipsoid region of the IS.9,10 To better understand the influence of the cones on the appearance of the ISe band, we examined the intensity of the signal from the ISe band in patients with diminished cone function. The relative intensity of the ISe band was significantly lower in patients with achromatopsia or cone dystrophy, conditions known to decrease the number of functioning cones. Thus, cone photoreceptors are necessary for an ISe border of normal intensity.
Clinical Implication
There are two clinical implications. First, while an abnormal appearing ISe band undoubtedly indicates outer retinal problems, patients with a relatively normal appearing band on visual inspection can have abnormal cone function. Only with a quantification of the relative intensity of this band was an abnormality detected. For example, the patient in Figure 7 showed a decrease in cone sensitivity of between 16 and 19 dB (1.6 to 1.9 log units) in the region shown in the blue rectangle. The ISe band appeared normal in this region, but the relative intensity clearly was below normal. The second implication concerns the agreement recently reported between the edge of the ISe band and the edge of the visual field in patients with RP.8 The visual fields are largely dependent on the cones, but we have shown that the ISe band does not disappear unless both rods and cones are affected. However, this does not diminish the usefulness of the ISe contour for clinical trials with RP patients, as the effects on the rods in RP are typically greater than, or equal to, the effects on the cones. Because the loss of the ISe band implies a loss of both rod and cone function, there should be, as there is,8 a good correspondence between the edge of the cone driven visual field sensitivity and the ISe contour.
Caveat and Unanswered Questions
First, this study does not speak to the proximal source of the ISe signal. The role mitochondria, as well as other structures in this region,16 play in producing this signal is still to be determined. Second, as mentioned in Methods, our measurement does not distinguish between bands of lower reflectance versus thinner bands. However, our analyses with thinner ISe segments and visual inspection clearly indicate that while the patients' ISe bands may be thinner, they are also less intense. Third, we cannot be sure that residual cones are not contributing to the ISe band seen in the achromats and cone dystrophy patients. Many achromats have some functioning cones, while patients with cone dystrophy can maintain some cone function as well, although we have shown that the ISe band is present even when cone thresholds are markedly elevated.11 Fourth, and related to this point, we do not know if the ISe band would have a normal relative intensity if a patient lost all rod receptors, but maintained normal cone function. We have seen a lower relative ISe intensity in regions of the retina in RP. However, we cannot be sure the cones are functioning normally in these regions without further work with two-color threshold measurements.11 Finally, it remains to be seen if the intensity of the ISe band is affected under other conditions. For example, we are particularly interested in patients with clear functional loss as documented on visual fields and multifocal ERGs, but who have reasonably normal appearing fdOCT scans.17,18
Summary
The ISe band, previously called the IS/OS border, appears intact in patients with achromatopsia and cone dystrophy. However, the intensity of this ISe band is lower in these patients with diminished cone function than it is in healthy control subjects.
Footnotes
Supported by National Eye Institute Grants R01-EY-09076 (DCH), EY-018213 (SHT), and P30-EY-019007; Foundation Fighting Blindness; and an unrestricted grant to the Department of Ophthalmology, Columbia University, from Research to Prevent Blindness.
Disclosure: D.C. Hood, Topcon, Inc. (F); X. Zhang, Topcon, Inc. (C); R. Ramachandran, None; C.L. Talamini, None; A. Raza, None; J.P. Greenberg, None; J. Sherman, Optovue (F), Zeiss (F), Topcon, Inc. (F); S.H. Tsang, None; D.G. Birch, None
References
- 1. Chang LK, Koizumi H, Spaide RF. Disruption of the photoreceptor inner segment-outer segment junction in eyes with macular holes. Retina. 2008;28:969–975 [DOI] [PubMed] [Google Scholar]
- 2. Spaide RF, Koizumi H, Freund KB. Photoreceptor outer segment abnormalities as a cause of blind spot enlargement in acute zonal occult outer retinopathy-complex diseases. Am J Ophthalmol. 2008;146:111–120 [DOI] [PubMed] [Google Scholar]
- 3. Wang NK, Chou CL, Lima LH, et al. Fundus autofluorescence in cone dystrophy. Doc Opthalmol. 2009;119:141–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oh J, Smiddy WE, Flynn HW, Jr, Gregori G, Lujan B. Photoreceptor inner/outer segment defect imaging by spectral domain OCT and visual prognosis after macular hole surgery. Invest Ophth Vis Sci. 2010;51:1651–1658 [DOI] [PubMed] [Google Scholar]
- 5. Inoue M, Morita S, Watanabe Y, et al. Inner segment/outer segment junction assessed by spectral-domain optical coherence tomography in patients with idiopathic epiretinal membrane. Am J Ophthalmol. 2010;150:834–839 [DOI] [PubMed] [Google Scholar]
- 6. Hood DC, Lazow MA, Locke KG, Greenstein VC, Birch DG. The transition zone between healthy and diseased retina in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kitaguchi Y, Kusaka S, Yamaguchi T, Mihashi T, Fujikado T. Detection of photoreceptor disruption by adaptive optics fundus imaging and fourier-domain optical coherence tomography in eyes with occult macular dystrophy. Clin Ophthalmol. 2011;5:345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hood DC, Ramachandran R, Holopigian K, Lazow MA, Birch DG, Greenstein VS. Method for deriving visual field boundaries from OCT scans of patients with retinitis pigmentosa. Biomed Opt Exp. 2011;2:1106–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography. Retina. 2011;31:1609–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernández EJ, Hermann B, Povazay B, et al. Ultrahigh resolution optical coherence tomography and pancorrection for cellular imaging of the living human retina. Opt Exp. 2008;16:11083–11094 [DOI] [PubMed] [Google Scholar]
- 11. Birch DG, Wen Y, Locke K, Hood DC. Rod sensitivity, cone sensitivity and photoreceptor layer thickness in retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2011;52:7141–7147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:2328–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hood DC, Cho J, Raza AS, Dale EA, Wang M. Reliability of a computer-aided manual procedure for segmenting optical coherence tomography scans. Optom Vis Sci. 2011;88:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varsanyi B, Somfai GM, Lesch B, Vamos R, Farkas A. Optical coherence tomography of the macula in congenital achromatopsia. Invest Ophthalmol Vis Sci. 2007;48:2249–2253 [DOI] [PubMed] [Google Scholar]
- 15. Barthelmes D, Sutter FK, Kurz-Levin MM, et al. Quantitative analysis of OCT characteristics in patients with achromatopsia and blue-cone monochromatism. Invest Ophthalmol Vis Sci. 2006;47:1161–1166 [DOI] [PubMed] [Google Scholar]
- 16. Jonnal RS, Besecker JR, Derby JC, et al. Imaging outer segment renewal in living human cone photoreceptors. Opt Exp. 2010;18:5257–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dale EA, Hood DC, Greenstein VC, Odel JG. A comparison of multifocal ERG and frequency domain OCT changes in patients with abnormalities of the retina. Doc Ophthalmol. 2009;120:175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Talamini CL, Raza AS, Dale EA, Greenstein VC, Odel JG, Hood DC. Abnormal multifocal ERG findings in patients with normal-appearing retinal anatomy. Doc Ophthalmol. 2011;123:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]