Abstract
The synthesis of a unique isoindoline- and tetrahydroisoquinoline (THIQ)-containing tricyclic sultam library, utilizing a Heck-aza-Michael (HaM) strategy is reported. Both isoindoline and THIQ rings are installed through a Heck reaction on a vinylsulfonamide, followed by one-pot deprotection and intramolecular aza-Michael reaction. Subsequent cyclization with either paraformaldehyde condensation or 1,1'-carbonyldiimidazole coupling generates a variety of tricyclic sultams. Overall, a 160-member library of these sultams, together with their isoindolines/THIQ and secondary sulfonamides precursors, were constructed using this strategy.
Introduction
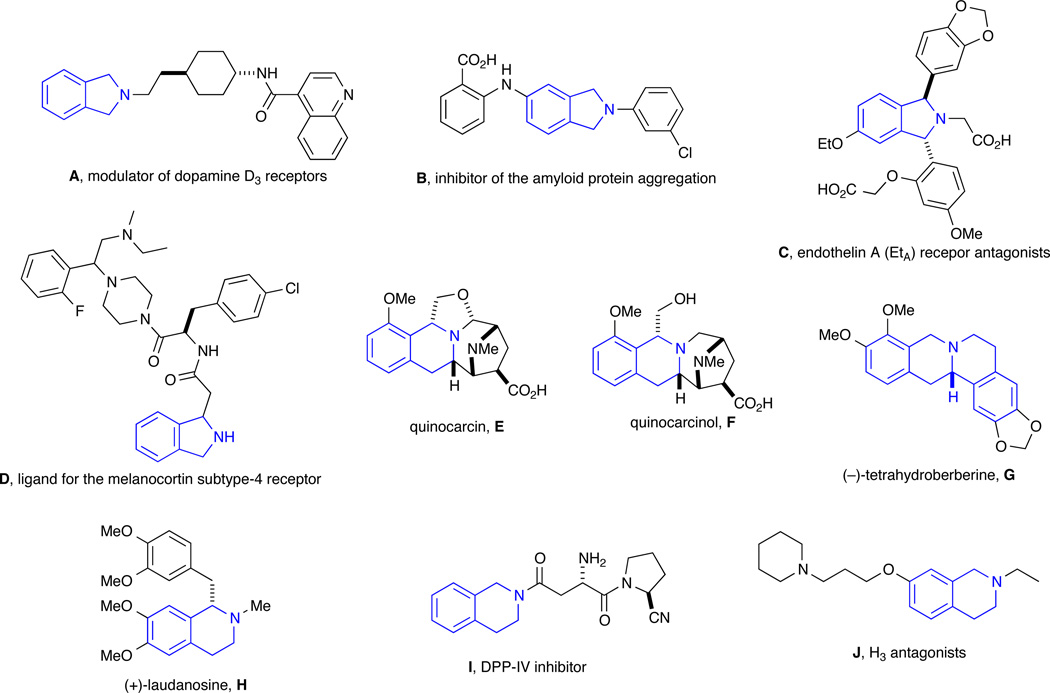
The growing demand for diverse small molecule libraries for the development of therapeutic agents requires efficient methodologies to provide access to structurally unique drug-like molecules.1,2 Substituted isoindolines and THIQ are heterocyclic motifs presented in numerous natural products and bioactive small molecules displaying a wide array of biological activity. In particular, isoindolines have been shown to exhibit a number of potent properties, including: (i) modulation of the dopamine D3 receptor and hence potential as antipsychotic agents (A, Figure 1),3 (ii) inhibition of amyloid protein aggregation indicating a potential capacity in the treatment of Alzheimer's disease (B),4 (iii) selective antagonism of the ETA receptor and thus may play a pathophysiological role in a large number of diseases related to the heart and lungs (C),5 and (iv) ligand affinity for the melanocortin subtype-4 receptor (MC4R) (D).6 Additional disparate activities have also been seen in a family of THIQ-containing anti-tumor antibiotics, including a number of naturally occurring alkaloids such as quinocarcin (E) and quinocarcinol (F).7 (–)-Tetrahydroberberine (G) also shows a variety of bioactivities such as insecticidal activity against Drosophila melanogaster,8 anti-tumor promoter (inhibitory effect on Epstein-Barr virus),9 while (+)-Laudanosine (H), which occurs naturally in opium, has been shown to interact with GABA receptors, opioid receptors, and nicotinic acetylcholine receptors.10 THIQ I was discovered as a potent and selective inhibitor of prolyl dipeptidase DPP8 and therefore has potential in the treatment of Type II diabetes.11 As a final note, compound J displays histamine H3 receptor antagonism and thus may be useful in the treatment of a variety of CNS disorder.12 Taken collectively, isoindolines and THIQ-containing compounds have emerged as attractive targets in organic and medicinal chemistry, thus spurring on methods development for their synthesis.13,14
Figure 1.
Biologically active compounds containing isoindolines and THIQs.
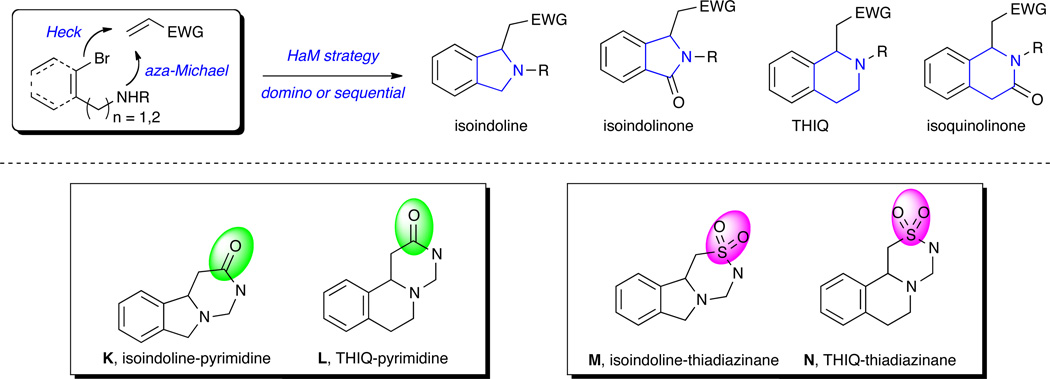
The generation of a variety of substituted isoindolines and THIQs,6,15 isoindolinones and isoquinolinones16 utilizing both transition metal-catalyzed coupling and aza-Michael cyclization steps in domino or sequential Heck-aza-Michael (HaM) cascades has emerged in a number of highly efficient pathways (Figure 2). In spite of the prevalent usage of acrylate, the utilization of vinylsulfonamide derivatives in HaM strategies leading to isoindolines/THIQ-based tricyclic sultam products is void in the literature. More interestingly, a brief literature survey shows 4 compounds containing isoindolinepyrimidine substructure K and roughly 250 compounds with THIQ-pyrimidine substructure L, while isoindoline/THIQ-thiadiazinane substructures M and N represent completely new molecular chemotypes (Figure 2). As part of a larger program aimed at DOS strategies toward sultam libraries and their comparison with amides, we herein report the facile synthesis of a library of tricyclic sultam heterocycles containing isoindolines/THIQ motifs utilizing a HaM reaction protocol.
Figure 2.
HaM strategy in the synthesis of isoindolines and THIQ, isoindolinones and isoquinolinones.
Results and Discussion
We recently reported the synthesis of sultams using vinylsulfonamides as reliable and efficient precursors, which undergo facile hetero-Michael reactions and IMDA.17 In addition, domino HaM protocols have been developed in our laboratory for the synthesis of a library of functionalized 1,1-dioxido-1,2-benzisothiazolines.18 Along these lines, we envisioned the construction of a tricyclic sultam library via integration of a HaM protocol with vinylsulfonamides, as outlined in Scheme 1. In this approach, the secondary sulfonamide linchpin 3 can easily be synthesized via a Heck reaction of substituted bromobenzene 1 and vinylsulfonamide 2. Subsequent one-pot, sequential deprotection and intramolecular aza-Michael reaction on 3 results in generation of the central alkaloid ring bearing a secondary amine and a proximal secondary sulfonamide, which can be utilized for further transformation into tricyclic ring systems 5 and 6.
Scheme 1.
Library outline.
Library Design
A full matrix library was designed using in silico analysis, literature precedence, and observed synthetic results for compounds 5 and 6, together with their precursors 3 and 4,.19 A virtual library incorporating all possible building block combinations of bromobenzene 1 and vinylsulfonamide 2 was constructed for each scaffold (3, 4, 5, and 6) (Scheme 2). Physico-chemical property filters were applied, guiding the elimination of undesirable building blocks that led to products with undesirable in silico properties.20 These metric filters included standard Lipinski Rule of 5 parameters (molecular weight <500, ClogP <5.0, number of H-acceptors <10, and number of H-donors <5), in addition to consideration of the number of rotatable bonds (<5) and polar surface area. Absorption, distribution, metabolism, and excretion (ADME) properties were calculated along with a diversity analysis using standard H-aware 3D BCUT descriptors comparing against the MLSMR screening set (ca. 7/2010; ~330,000 unique chemical structures). Guided by this library design analysis, the corresponding bromobenzene 1 and vinylsulfonamide 2 were chosen to generate the proposed sultam libraries.
Scheme 2.
Preparation of Heck reaction precursors 1 and 2.
Library construction
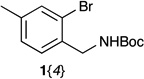
We started from the preparation of substituted (2-bromophenyl)methanamine and 2-(2-bromophenyl)ethanamine, which are either commercially available, obtained through NH3 substitution of substituted benzyl bromide,21 or through BH3 reduction of the corresponding cyanides22 (Scheme 2). Without further purification, the resulting primary amines were Boc-protected thus generating the desired substituted t-butyl 2-bromobenzylcarbamates 1{1–4} and t-butyl 2-bromophenethylcarbamate 1{5}. With these substituted bromobenzenes in hand, we were able to form unsubstituted isoindolines (from 1{1}), isoindolines with electron donating (from 1{2}, 1{4}) and electron withdrawing groups (from 1{3}), as well as THIQ derivatives (from 1{5}).
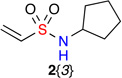
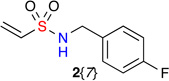
Vinylsulfonamides 2{1–8} were synthesized from 2-chloroethanesulfonyl chloride and the corresponding primary amines through sulfonylation and in situ β-elimination of HCl (Scheme 2). The scope of substituents on 2{1–8} includes linear, branched and cyclic alkyl groups 2{1–4}, as well as unsaturated groups 2{5–8}, all of which were prepared in one-step on multi-gram scale with good yield. The validation of the Heck reaction was performed using tert-butyl 2-bromobenzylcarbamate 1{1} and N-benzylethenesulfonamide 2{5} (Table 1). The initial screening of Pd-catalysts found Pd(PPh3)2Cl2 to give optimal yield (entries 1–5), while a quick survey of different solvent and catalyst loading parameters failed to improve the yield (entries 6 and 7). Microwave conditions were also attempted, and although they reduced the reaction time the yield was comparable to conventional oil bath conditions. With these parameters in hand, the library was synthesized employing optimized oil bath conditions as outlined in entry 5, using 10 mol% of Pd(PPh3)2Cl2 in toluene. Using these conditions, no regioisomer was observed and the products were of exclusively E-configuration as evident by a 1H-NMR coupling constant (15.3 Hz, which was in agreement with literature reports).23
Table 1.
Optimization of the Heck Reaction.a
 | ||||||
|---|---|---|---|---|---|---|
| entry | catalyst | loading (mol%) | additive | equiv | solvent | yield (%)b |
| 1 | Pd(OAc)2 | 10 | PPh3 | 0.2 | toluene | 30 |
| 2 | PdCl2 | 10 | PPh3 | 0.2 | toluene | 42 |
| 3 | Pd(PPh3)4 | 10 | - | - | toluene | 40 |
| 4 | Pd(dppf)Cl2 | 10 | - | - | toluene | 47 |
| 5 | Pd(PPh3)2Cl2 | 10 | - | - | toluene | 80 |
| 6 | Pd(PPh3)2Cl2 | 10 | - | - | DMF | 40 |
| 7 | Pd(PPh3)2Cl2 | 5 | - | - | toluene | 57 |
General procedure: A mixture of 1.1 equiv of 1{1}, 1.0 equiv of 2{5}, Pd catalyst, additive, 3 equiv of Et3N in toluene (0.1 M) was heated in a sealed tube at 120 °C for 14 hrs.
Isolated yield.
With optimized Heck reaction conditions being developed, the one-pot, deprotection and intramolecular aza-Michael reaction was next examined by treating 3{1,5} with 5N HCl in EtOAc, followed by neutralizing to pH 8 with addition of NaHCO3 (saturated aqueous, Scheme 3). Isoindoline product 4{1,5} was obtained in 91% yield, and was clean enough to go through next step without further purification. Cyclization was accomplished through paraformaldehyde condensation and 1,1'-carbonyldiimidazole (CDI) coupling between the secondary amine and sulfonamide presented in 4{1,5}, and provided the desired products 5{1,5} and 6{1,5} with satisfactory yield.
Scheme 3.
One-pot Deprotection, Intramolecular aza-Michael Reaction and Cyclization.
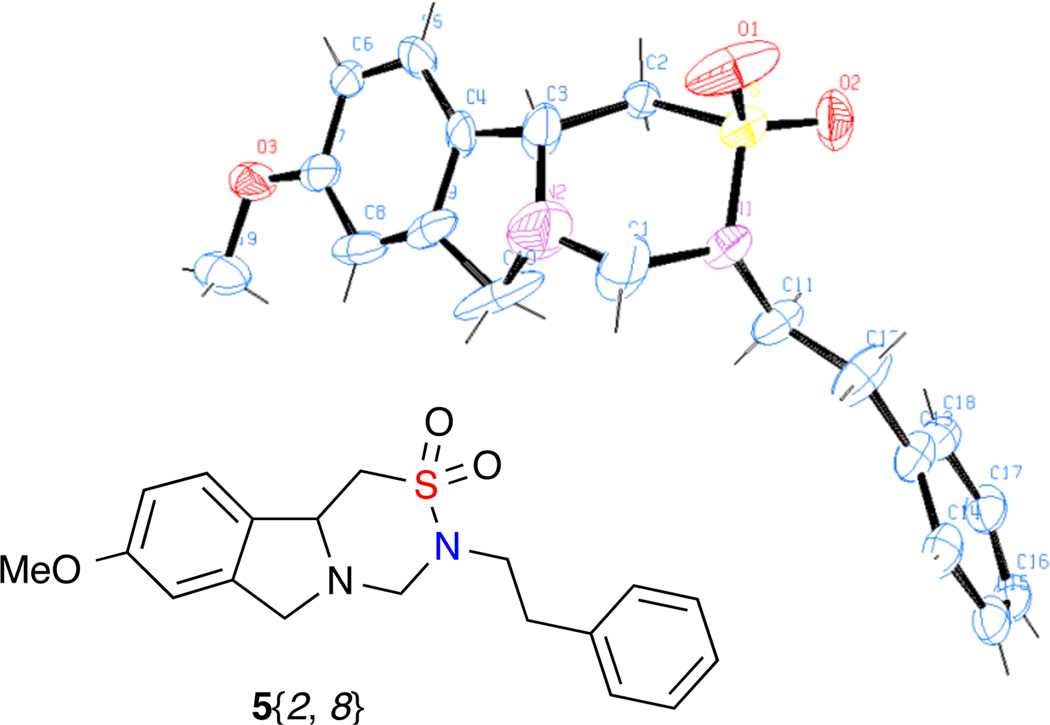
After the preparation of starting materials 1{1–5} and 2{1–8} and optimized route toward tricyclic sultams 5 and 6 were developed, the proposed library was generated according to plan. The results of the synthesis of 3{1–5, 1–8}, 4{1–5, 1–8}, 5{1–5, 1–8}, 6{1–5, 1–8} are summarized in Table 2. The Heck reaction was carried out in a Radley Carousel parallel synthesizer where the yield of products 3{1–5, 1–8} ranged from 51% to 99%, with an average yield of 71%. The aza-Michael reactions were run in 1-dram vials on an aluminum reaction block. Yields were generally good, as the average yield for compounds 4{1–5, 1–8} as high as 90%. Although the intermediate products were not purified by flash chromatography, they were pure enough as shown in NMR and used in the next step without purification. The formaldehyde condensation and CDI couplings were also run in 1-dram vials on an aluminum reaction block and provided moderate to good yield, with average yields of 57% and 75%, respectively. The structure of 5{2, 8} was confirmed via X-ray crystallography (Figure 4). Overall, a total of 80 tricyclic sultams 5{1–5, 1–8} and 6{1–5, 1–8}, 40 isoindolines and THIQs 4{1–5, 1–8}, as well as 40 secondary sulfonamides 3{1–5, 1–8}, were successfully synthesized and samples of each compound with quantities ≥60 mg and with purity >90% (determination by UV area percent from HPLC analysis) were submitted to the KU-CMLD.
Table 2.
Yield of 3{1–5, 1–8}, 4{1–5, 1–8}, 5{1–5, 1–8}, 6{1–5, 1–8}.
 | |||||
| 1{1–5} |  |
 |
 |
 |
 |
| 2{1–8} | |||||
 |
92, 73, 98, 58a,b |
51, 98, 80, 56 |
80, 96, 84, 56 |
66, 99, 70, 55 |
67, 95, 56, 60 |
 |
83, 98, 99, 60 |
74, 93, 77, 67 |
51, 98, 64, 40 |
81, 94, 72, 43 |
66, 82, 88, 60 |
 |
90, 74, 76, 46 |
67, 94, 35, 70 |
63, 97, 86, 70 |
75, 97, 93, 40 |
58, 92, 93, 57 |
 |
86, 74, 82, 63 |
58, 94, 71, 62 |
62, 99, 73, 58 |
82, 53, 45, 35 |
71, 98, 58, 52 |
 |
80, 99, 63, 48 |
47, 92, 58, 70 |
50, 97, 64, 57 |
71, 99, 59, 57 |
66, 92, 94, 61 |
 |
65, 98, 84, 65 |
55, 98, 67, 62 |
75, 64, 46, 81 |
75, 81, 76, 39 |
69, 84, 93, 22 |
 |
70, 94, 68, 58 |
88, 79, 69, 50 |
75, 60, 78, 71 |
99, 99, 92, 32 |
75, 86, 76, 74 |
 |
58, 99, 72, 88 |
59, 95, 88, 30 |
76, 94, 86, 65 |
92, 92, 74, 66 |
72, 92, 98, 60 |
Numbers refer to the respective stepwise yield of 3{X, Y}, 4{X, Y}, 5{X, Y}, 6{X, Y} from corresponding starting materials.
Isolated yield for 3, 5 and 6; crude yield for 4.
Figure 4.
ORTEP diagram of 5{2, 8}.
In conclusion, we successfully completed the production of an 80-member library of unique tricyclic sultams through a HaM pathway with variable substituents. These compounds, together with their isoindolines/THIQ and secondary sulfonamides precursors, are in the process of being distributed to a number of biological collaborators within the NIH Molecular Libraries Probe Center Network (MLPCN).
Experimental Procedures
General procedure for the Heck reaction to synthesize sulfonamide 3
To pressure tube containing substituted bromobenzene 1 (2.2 mmol, 1.1 equiv) was added sulfonamide linchpin 2 (2.0 mmol, 1.0 equiv) and toluene (20 mL, 0.1 M). To this solution was added Et3N (6.0 mmol, 3.0 equiv) and Pd(PPh3)2Cl2 (0.2 mmol, 0.1 equiv) and the mixture was stirred at 120°C for 6–12 hours. The reaction mixture was concentrated and the residue was purified using flash chromatography.
General procedure for one-pot deprotection, intramolecular aza-Michael reaction to synthesize sulfonamide 4
To a solution of sulfonamide 3 (1.4 mmol, 1.0 equiv) in EtOAc (14 mL, 0.1 M) was added 5N HCl (1.4 mL, 5.0 equiv) and the reaction mixture was stirred at room temperature for 48 hours. Aqueous NaHCO3 was carefully added to the reaction mixture until pH 8. The mixture was stirred for another 24 hours, and then extracted with EtOAc. The combined organic layers were washed with brine, dried over Na2SO4 to afford the crude product, which is used in next step without further purification.
General procedure for paraformaldehyde cyclization to synthesize sultam 5
To a solution of sulfonamide 4 (0.3 mmol, 1.0 equiv) in CH2Cl2 (1.5 mL, 0.2 M) was added paraformaldehyde (0.9 mmol, 3.0 equiv) and Na2SO4 (0.9 mmol, 3.0 equiv) and the reaction mixture was stirred at 40 °C for overnight. The solid was removed by filtration and filtrate was concentrated under reduced pressure to afford the crude product, which was purified using flash chromatography.
General procedure for CDI cyclization to synthesize sultam 6
To a solution of sulfonamide 4 (0.3 mmol, 1.0 equiv) in THF (3 mL, 0.1 M) was added Cs2CO3 (0.6 mmol, 2.0 equiv) followed by CDI (0.36 mmol, 1.2 equiv) and the reaction mixture was stirred at 40 °C for overnight. The solid was removed through filtration and filtrate was concentrated under reduced pressure to afford the crude product, which was purified using flash chromatography.
Supplementary Material
Acknowledgment
Financial support of this work was provided by the National Institute of General Medical Sciences and is gratefully acknowledged (P50-GM069663 and P41-GM076302). The authors also thank Dr. Victor Day for crystal structure data.
Footnotes
Supporting Information Available. Experimental procedures and full characterization for representative compounds is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Austin CP. The Completed Human Genome: Implications for Chemical Biology. Curr. Opin. Chem. Biol. 2003;7:511–515. doi: 10.1016/s1367-5931(03)00083-8. [DOI] [PubMed] [Google Scholar]; (b) Hopkins AL, Groom CR. The Druggable Genome. Nat. Rev. Drug Discovery. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]; (c) Drews J, Ryser S. Genomic Sciences and the Medicine of Tomorrow. Nat. Biotechnology. 1996;14:1516–1518. doi: 10.1038/nbt1196-1516. [DOI] [PubMed] [Google Scholar]
- 2.(a) Dolle RE, Bourdonnec BL, Worm K, Morales GA, Thomas CJ, Zhang W. Comprehensive Survey of Chemical Libraries for Drug Discovery and Chemical Biology: 2009. J. Comb. Chem. 2010;12:765–806. doi: 10.1021/cc100128w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dolle RE, Bourdonnec BL, Goodman AJ, Morales GA, Thomas CJ, Zhang W. Comprehensive Survey of Chemical Libraries for Drug Discovery and Chemical Biology: 2007. J. Comb. Chem. 2008;10:753–802. doi: 10.1021/cc800119z. [DOI] [PubMed] [Google Scholar]; (c) Dolle RE, Bourdonnec BL, Goodman AJ, Morales GA, Salvino JM, Zhang W. Comprehensive Survey of Chemical Libraries for Drug Discovery and Chemical Biology: 2006. J. Comb. Chem. 2007;9:855–902. doi: 10.1021/cc700111e. [DOI] [PubMed] [Google Scholar]
- 3.Johnson CN, Stemp G. 2,3-Dihydro-1H-isoindole Derivatives Useful As Modulators of Dopamine D3 Receptors (Antipsychotic Agents) PCT Int. Appl. WO 0021950 A1. 2000 [Google Scholar]
- 4.Augelli-Szafran CE, Lai Y, Sakkab AT, Walker LC. Method of Inhibiting Amyloid Protein Aggregation and Imaging Amyloid Deposits Using Isoindoline Derivatives. PCT Int. Appl. WO 0076969 A1. 2000 [Google Scholar]
- 5.(a) Kukkola PJ, Bilci NA, Ikeler TJ. A Novel Regio- and Stereoselective Synthesis of Isoindolines. Tetrahedron Lett. 1996;37:5065–5068. [Google Scholar]; (b) Kukkola PJ, Bilci NA, Ikler T, Savage P, Shetty SS, DelGrande D, Jeng AY. Isoindolines: A New Series of Potent and Selective Endothelin-A Receptor Antagonists. Bioorg. Med. Chem. Lett. 2001;11:1737–1740. doi: 10.1016/s0960-894x(01)00273-6. [DOI] [PubMed] [Google Scholar]
- 6.Shi Q, Ornstein PL, Briner K, Richardson TI, Arnold MB, Backer RT, Buckmaster JL, Canada EJ, Doecke CW, Hertel LW, Honigschmidt N, Hsiung HM, Husain S, Kuklish SL, Martinelli MJ, Mullaney JT, O'Brien TP, Reinhard MR, Rothhaar R, Shah J, Wu Z, Xie C, Zgombick JM, Fisher MJ. Synthesis and Structure–activity Relationships of Novel Dipeptides and Reduced Dipeptides as Ligands for Melanocortin Subtype-4 Receptor. Bioorg. Med. Chem. Lett. 2006;16:2341–2346. doi: 10.1016/j.bmcl.2005.10.103. [DOI] [PubMed] [Google Scholar]
- 7.(a) Tomita F, Takahashi K, Shimizu K-I. a Novel Antitumor Antibiotic 1. Taxonomy, Fermentation and Biological Activity. J. Antibiot. 1983;36:463–467. doi: 10.7164/antibiotics.36.463. [DOI] [PubMed] [Google Scholar]; (b) Takahashi K, Tomita F. DC-52, a Novel Antitumor Antibiotic 2. Isolation, Physico-chemical Characteristics and Structure Determination. J. Antibiot. 1983;36:468–470. doi: 10.7164/antibiotics.36.468. [DOI] [PubMed] [Google Scholar]
- 8.Miyazawa M, Yoshio K, Ishikawa Y, Kameoka H. Insecticidal Alkaloids from Corydalis bulbosa against Drosophila melanogaster. J. Agric. Food Chem. 1998;46:1914–1919. [Google Scholar]
- 9.Ito C, Itoigawa M, Tokuda H, Kuchide M, Nishino H, Furukawa H. Chemopreventive Activity of Isoquinoline Alkaloids from Corydalis Plants. Planta Med. 2001;67:473–475. doi: 10.1055/s-2001-15815. [DOI] [PubMed] [Google Scholar]
- 10.(a) Fodale V, Santamaria LB. Laudanosine, an Atracurium and Cisatracurium Metabolite. Eur. J. Anaesthesiol. 2002;19:466–473. doi: 10.1017/s0265021502000777. [DOI] [PubMed] [Google Scholar]; (b) Katz Y, Weizman A, Pick CG, Pasternak GW, Liu L, Fonia O, Gavish M. Interactions between laudanosine, GABA, and opioid subtype receptors: implication for laudanosine seizure activity. Brain Res. 1994;646:235–241. doi: 10.1016/0006-8993(94)90084-1. [DOI] [PubMed] [Google Scholar]; (c) Exley R, Iturriaga-Vásquez P, Lukas RJ, Sher E, Cassels BK, Bermudez I. Evaluation of Benzyltetrahydroisoquinolines as Ligands for Neuronal Nicotinic Acetylcholine Receptors. Br. J. Pharmacol. 2005;146:15–24. doi: 10.1038/sj.bjp.0706307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiaang W-T, Chen Y-S, Hsu T, Wu S-H, Chien C-H, Chang C-N, Chang S-P, Lee S-J, Chen X. Novel Isoindoline Compounds for Potent and Selective Inhibition of Prolyl Dipeptidase DPP8. Bioorg. Med. Chem. Lett. 2005;15:687–691. doi: 10.1016/j.bmcl.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Jesudason CD, Beavers LS, Cramer JW, Dill J, Finley DR, Lindsley CW, Stevens FC, Gadski RA, Oldham SW, Pickard RT, Siedem CS, Sindelar DK, Singh A, Watson BM, Hipskind PA. Synthesis and SAR of Novel Histamine H3 Receptor Antagonists. Bioorg. Med. Chem. Lett. 2006;16:3415–3418. doi: 10.1016/j.bmcl.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 13.For some recent reports of isoindoline synthesis: Clary KN, Parvez M, Back TG. Rearrangements and Intramolecular Diels–Alder Reactions of Normal and Vinylogous Aza-Morita–Baylis–Hillman Products Leading to Isoindoline Derivatives. J. Org. Chem. 2010;75:3751–3760. doi: 10.1021/jo1005087. Solé D, Serrano O. Selective Synthesis of Either Isoindole- or Isoindoline-1-carboxylic Acid Esters by Pd(0)-Catalyzed Enolate Arylation. J. Org. Chem. 2010;75:6267–6270. doi: 10.1021/jo101054j. Bauer RA, DiBlasi CM, Tan DS. The tert-Butylsulfinamide Lynchpin in Transition-Metal-Mediated Multiscaffold Library Synthesis. Org. Lett. 2010;12:2084–2087. doi: 10.1021/ol100574y. Smith K, El-Hiti GA, Hegazy AS, Fekri A. A Simple and Convenient High Yielding Synthesis of Substituted Isoindolines. Heterocycles. 2010;80:941–956. Yang D, Micalizio GC. A Convergent Stereoselective Synthesis of Quinolizidines and Indolizidines: Chemoselective Coupling of 2-Hydroxymethyl-Substituted Allylic Silanes with Imines. J. Am. Chem. Soc. 2009;131:17548–17549. doi: 10.1021/ja908504z. Garcia L, Pla-Quintana A, Roglans A. Synthesis of non-proteinogenic phenylalanine derivatives by rhodium-catalyzed [2+2+2] cycloaddition reactions. Org. Biomol. Chem. 2009;7:5020–5027. doi: 10.1039/b910961g. Ben-Othman R, Othman M, Ciamala K, Knorr M, Strohmann C, Decroix B. Synthesis of Diversely Functionalized Pyrrolizidines and Indolizidines Using Olefin Ring-closing Metathesis. Tetrahedron. 2009;65:4846–4854. Satyanarayana G, Maichle-Mössmer C, Maier ME. Formation of Pentacyclic Structures By a Domino Sequence on Cyclic Enamides. Chem. Commun. 2009;12:1571–1573. doi: 10.1039/b820636h. Suwa T, Shibata I, Nishino K, Baba A. Synthesis of Nitrogen Heterocycles by Intramolecular Michael Type of Amination via Reduction of Imines with Di-n-butyliodotin Hydride (n-Bu2SnIH) Org. Lett. 1999;1:1579–1581.
- 14.For the synthesis and bioactivities of isoquinolines: Chrzanowska M, Rozwadowska MD. Asymmetric Synthesis of Isoquinoline Alkaloids. Chem. Rev. 2004;104:3341–3370. doi: 10.1021/cr030692k. and references cited therein.
- 15.(a) Priebbenow DL, Pfeffer FM, Stewart SG. A One-Pot, Three-Component Approach to Functionalised Tetrahydroisoquinolines Using Domino Heck–aza-Michael Reactions. Eur. J. Org. Chem. 2011:1632–1635. [Google Scholar]; (b) Priebbenow DL, Stewart SG, Pfeffer FM. A General Approach to N-heterocyclic Scaffolds using Domino Heck–aza-Michael Reactions. Org. Biomol. Chem. 2011;9:1508–1515. doi: 10.1039/c0ob00835d. [DOI] [PubMed] [Google Scholar]; (c) Ca' ND, Motti E, Mega A, Catellani M. One-Pot Palladium-Catalyzed Synthesis of Selectively Substituted Phenanthridines by Sequential Aryl-Aryl and Heck Couplings, Aza-Michael and Retro-Mannich Reactions. Adv. Synth. Catal. 2010;352:1451–1454. [Google Scholar]; (d) Priebbenow DL, Henderson LC, Pfeffer FM, Stewart SG. Domino Heck–Aza-Michael Reactions: Efficient Access to 1-Substituted Tetrahydro-β-carbolines. J. Org. Chem. 2010;75:1787–1790. doi: 10.1021/jo902652h. [DOI] [PubMed] [Google Scholar]; (e) Ferraccioli R, Carenzi D, Catellani M. Synthesis of 1,2,3,4-Tetrahydroisoquinolines and 2,3,4,5-Tetrahydro-1H-2-benzazepines Combining Sequential Palladium-catalysed ortho Alkylation/vinylation with aza-Michael Addition Reactions. Tetrahedron Lett. 2004;45:6903–6907. [Google Scholar]
- 16.Khan Md. W, Reza AFGM. Palladium Mediated Synthesis of Isoindolinones and Isoquinolinones. Tetrahedron. 2005;61:11204–11210. [Google Scholar]
- 17.(a) Zang Q, Javed S, Ullah F, Zhou A, Knudtson CA, Bi D, Basha FZ, Organ MG, Hanson PR. Application of a Double aza-Michael Reaction in a 'Click, Click, Cy-Click' Strategy: From Bench to Flow. Synthesis. 2011:2743–2750. doi: 10.1055/s-0030-1260112. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fenster E, Long T, Zang Q, Hill D, Neunswander B, Lushington G, Zhao A, Santini C, Hanson PR. Automated Synthesis of a 184-Member Library of Thiadiazepan-1,1-dioxide-4-ones. ACS Combi. Sci. 2011;13:244–250. doi: 10.1021/co100060x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jeon KO, Rayabarapu D, Rolfe A, Volp K, Omar I, Hanson PR. Metathesis Cascade Strategies (ROM-RCM-CM): A DOS Approach to Skeletally Diverse Sultams. Tetrahedron. 2009;65:4992–5000. doi: 10.1016/j.tet.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhou A, Rayabarapu D, Hanson PR. #x00022;Click, Click, Cyclize”: A DOS Approach to Sultams Utilizing Vinyl Sulfonamide Linchpins. Org. Lett. 2009;11:531–534. doi: 10.1021/ol802467f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhou A, Hanson PR. Synthesis of Sultam Scaffolds via Intramolecular Oxa-Michael and Diastereoselective Baylis–Hillman Reactions. Org. Lett. 2008;10:2951–2954. doi: 10.1021/ol8009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Rolfe A, Young K, Hanson PR. Domino Heck-Aza-Michael Reactions: A One-pot, Multi-Component Approach to 1,2-Benzisothiazoline-3-acetic acid 1,1-dioxides. Eur. J. Org. Chem. 2008:5254–5262. doi: 10.1002/ejoc.200800651. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rolfe A, Young K, Volp K, Schoenen F, Neuenswander B, Lushington GH, Hanson PR. A One-Pot, 3-Component, Domino Heck-aza-Michael Approach to Libraries of Functionalized 1,1-Dioxido-1,2-benzisothiazoline-3-acetic Acids. J. Comb. Chem. 2009;11:732–738. doi: 10.1021/cc900025e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akella LB, Marcaurelle LA. Application of a Sparse Matrix Design Strategy to the Synthesis of DOS Libraries. ACS Comb. Sci. 2011;13:357–364. doi: 10.1021/co200020j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Full in silico data and detailed calculation information is provided in the Supporting Information.
- 21.Mazik M, Kuschel M. Amide Amino Hydroxy and Aminopyridine Groups as Building Blocks for Carbohydrate Receptors. Eur. J. Org. Chem. 2008:1517–1526. [Google Scholar]
- 22.Moro WB, Yang Z, Kane TH, Zhou Q, Harville S, Brouillette CG, Brouillette WJ. SAR Studies for A New Class of Antibacterial NAD Biosynthesis Inhibitors. J. Comb. Chem. 2009;11:617–625. doi: 10.1021/cc9000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Chudasama V, Wilden JD. A Versatile Synthesis of 2,4-Substituted Oxazoles. Chem. Comm. 2008;32:3768–3770. doi: 10.1039/b805430d. [DOI] [PubMed] [Google Scholar]; (b) Qiao C, Wilson DJ, Bennett EM, Aldrich CC. A Mechanism-Based Aryl Carrier Protein/Thiolation Domain Affinity Probe. J. Am. Chem. Soc. 2007;129:6350–6351. doi: 10.1021/ja069201e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zeng B-B, King SB. Palladium-Catalyzed Synthesis of Water-Soluble Symmetric 9,10-Disubstituted Anthracenes. Synthesis. 2002;16:2335–2337. [Google Scholar]; (d) Harada H, Kazami J-I, Watanuki S, Tsuzuki R, Sudoh K, Fujimori A, Tokunaga T, Tanaka A, Tsukamoto S-I, Yanagisawa I. Synthesis and Structure-activity Relationships in a Series of Ethenesulfonamide Derivatives, a Novel Class of Endothelin Receptor Antagonists. Chem. Pharm. Bull. 2001;49:1593–1603. doi: 10.1248/cpb.49.1593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.