Abstract
Purpose
The NSABP (National Surgical Adjuvant Breast and Bowel Project) B-24 study demonstrated significant benefit with adjuvant tamoxifen in patients with ductal carcinoma in situ (DCIS) after lumpectomy and radiation. Patients were enrolled without knowledge of hormone receptor status. The current study retrospectively evaluated the relationship between receptors and response to tamoxifen.
Patients and Methods
Estrogen (ER) and progesterone receptors (PgR) were evaluated in 732 patients with DCIS (41% of original study population). An experienced central laboratory determined receptor status in all patient cases with available paraffin blocks (n = 449) by immunohistochemistry (IHC) using comprehensively validated assays. Results for additional patients (n = 283) determined by various methods (primarily IHC) were available from enrolling institutions. Combined results were evaluated for benefit of tamoxifen by receptor status at 10 years and overall follow-up (median, 14.5 years).
Results
ER was positive in 76% of patients. Patients with ER-positive DCIS treated with tamoxifen (v placebo) showed significant decreases in subsequent breast cancer at 10 years (hazard ratio [HR], 0.49; P < .001) and overall follow-up (HR, 0.60; P = .003), which remained significant in multivariable analysis (overall HR, 0.64; P = .003). Results were similar, but less significant, when subsequent ipsilateral and contralateral, invasive and noninvasive, breast cancers were considered separately. No significant benefit was observed in ER-negative DCIS. PgR and either receptor were positive in 66% and 79% of patients, respectively, and in general, neither was more predictive than ER alone.
Conclusion
Patients in NSABP B-24 with ER-positive DCIS receiving adjuvant tamoxifen after standard therapy showed significant reductions in subsequent breast cancer. The use of adjuvant tamoxifen should be considered for patients with DCIS.
INTRODUCTION
Ductal carcinoma in situ (DCIS) was gradually recognized as a disease distinct from invasive breast cancer (IBC) during the early 20th century.1,2 Between then and the introduction of screening mammography 70 to 80 years later, DCIS accounted for fewer than 5% of newly diagnosed breast cancers.3–6 Since the introduction and widespread use of screening mammography in the United States, the incidence of DCIS has increased dramatically, and today it accounts for 20% to 30% of all breast cancers.3,5,6
In the not too distant past, the standard treatment for DCIS was mastectomy and complete axillary dissection, which eradicated the disease, but at the high cost of disfiguring surgery.4,7,8 Local excision largely replaced this radical approach, and today lumpectomy is the standard of care for the majority of patients with DCIS.9–11 Early experience with lumpectomy alone was disappointing, with local recurrence rates of 30% or more, mainly because of the difficulty of obtaining clear surgical margins.4,12–16 Adjuvant radiotherapy was added to lumpectomy to sterilize occult residual tumor.17–19 The seminal NSABP (National Surgical Adjuvant Breast and Bowel Project) B-17 DCIS trial demonstrated that the addition of radiotherapy reduced the relative risk of local recurrence by 61%, although 13% of patients still eventually developed an ipsilateral recurrence, comparable to patients with IBC treated in a similar manner.20,21 As a result of its success in treating IBCs,22,23 the NSABP conducted its B-24 clinical trial to evaluate adjuvant tamoxifen in DCIS after lumpectomy and radiation. This trial demonstrated an additional 37% reduction in relative risk of local recurrence and a decrease in contralateral breast cancer of comparable magnitude.24 Results were similar in the United Kingdom-ANZ (United Kingdom, Australia, and New Zealand) trial, which also evaluated the efficacy of adjuvant tamoxifen in DCIS.25
We now know that the benefit of tamoxifen in treating IBC is essentially restricted to estrogen receptor (ER) –positive and/or progesterone receptor (PgR) –positive disease, consistent with the biologic mechanism of action of the drug.26 B-24 was initiated before this relationship was firmly established, and receptor status was not involved in patient enrollment. The purpose of the current study was to evaluate retrospectively ER and PgR and their relationships to response to adjuvant tamoxifen in the B-24 trial.
PATIENTS AND METHODS
Study Population
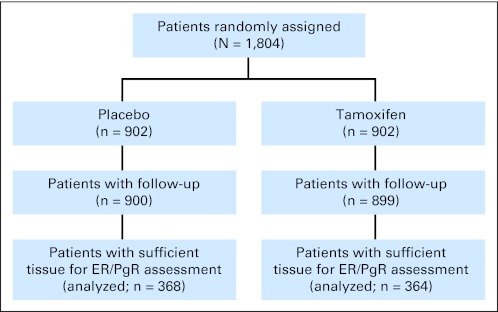
The study samples were derived from patients enrolled onto NSABP Protocol B-24,24 in which 1,804 women with DCIS were randomly assigned to placebo (n = 902) or tamoxifen (n = 902) after lumpectomy and local radiotherapy (Fig 1). Briefly, local radiotherapy (50 Gy) was administered beginning no later than 8 weeks after surgery. Adjuvant placebo or tamoxifen therapy (10 mg twice daily) was initiated within 56 days of surgery and continued for 5 years. Follow-up included physical examination every 6 months and annual mammography. Local recurrences were confirmed histologically. Distant metastases were confirmed clinically and by radiology and/or histology. End point information was based on data through the closure of follow-up to the study on May 1, 2007, obtained and received by the NSABP Biostatistical Center by June 8, 2007. The median time on study for patients in the analysis cohort at this time was 14.5 years. Informed consent was required for all participants. The protocol and consent forms were approved by the National Institutes of Health and the institutional review boards of all participating institutions.
Fig 1.
CONSORT diagram summarizing the random assignment of patients in the clinical trial and distribution of patient cases available for this study. ER, estrogen receptor; PgR, progesterone receptor.
ER and PgR results for this study were available in a subset of patients (41%; n = 732) enrolled onto the original clinical trial. This subset was statistically similar to the remaining study population with regard to the distribution of treatment (placebo v tamoxifen) and other available factors including age, race, eligibility status, tumor size, and surgical margin status (data not shown).
Evaluation of ER and PgR
Formalin-fixed paraffin-embedded tissue (FFPET) blocks of DCIS were available for 449 patents and were used to determine ER and PgR status by immunohistochemistry (IHC) in an experienced central laboratory. The IHC assays used mouse monoclonal antibodies 6F11 for ER (Novacastra, Burlingame, CA) and 1294 for PgR (Dako, Carpenteria, CA) and were comprehensively validated in several previous studies of IBCs.27–30 The Allred score was used to estimate the proportion and intensity of positive cells (range, 0 to 8), and scores of 3 or greater were defined as clinically positive.28,30 Representative examples of positive and negative IHC results for ER in DCIS are shown in Figure 2.
Fig 2.
Representative examples of estrogen receptor (ER) expression in ductal carcinoma in situ (DCIS) determined by immunohistochemistry. (A) Low-grade DCIS, strongly ER positive (Allred score, 5 + 3 = 8/8); (B) high-grade DCIS, ER negative (Allred score, 0/8).
ER and PgR results from laboratories used by enrolling institutions were available for an additional 283 patients, although FFPET blocks were not available for central laboratory testing for those patients. These results were determined by a variety of methods reflecting daily practice at each institution (IHC, 62%; radio-labeled ligand binding assay, 24%; enzyme immunoassay, 5%; unknown, 9%). Local interpretations of results, which also varied methodologically, were relied on to define positive and negative receptor status. There were no significant differences in the proportions of patient cases evaluated in central versus outside laboratories regarding treatment (placebo v tamoxifen), age, race, tumor size, surgical margin status, or the presence of comedo necrosis (data not shown).
Statistical Analyses
The primary end point of analyses in this study was time to the occurrence of any breast cancer as a first event subsequent to the original diagnosis of DCIS. Events included all local, regional, or distant recurrences and contralateral breast cancers. Other primary cancers or deaths without breast cancer were censored for the analysis. Secondary analyses included time to ipsilateral and contralateral breast cancers (IBC and/or DCIS) as first events. Events used in the analyses were medically reviewed and classified as being either invasive or noninvasive (ie, DCIS).
Cox proportional hazards models were employed to make formal inferences about group comparisons, and Kaplan-Meier curves were used to quantify the percentage of patients who were free of recurrence over time. Both unadjusted and adjusted Cox regression analyses were employed to quantify hazard ratios (HRs) comparing the two treatment groups.31 Adjustments were made for stratification of age at entry. Formal tests of treatment by ER status interactions were also conducted. To further explore the variability of treatment effects within ER subgroups, age-adjusted models were fitted. P values associated with HRs were based on large sample approximations, except in settings where expected events were quite rare (defined as < five in any treatment group). For the latter cases, Fisher's exact tests were employed by assuming that the numbers of failures were governed by Poisson processes.31 Analyses were also performed to determine if significant treatment by stratification variable interactions existed with respect to the end points.32 The incidences of site-specific failures were calculated using cumulative incidence curves.33–35 P values for treatment comparisons of cumulative incidence curves were obtained using cause-specific hazard rates that adjusted for the stratification variables.35
RESULTS
Distribution of Receptor Status and Other Clinicopathologic Features
ER and PgR were positive in 76% and 66% of patients, respectively, based on results from central and institutional results combined (n = 732). In patient cases with both ER and PgR results (n = 714), 64% were ER positive/PgR positive, 13% were ER positive/PgR negative, 2% were ER negative/PgR positive, and 21% were ER negative/PgR negative. Patients were almost evenly distributed between the placebo and tamoxifen arms of the study (78% v 82%; P = .30). There were no significant differences in receptor status or other available and potentially confounding factors between the two treatment groups, including tumor size, margin status, comedo necrosis, age, race, and method of detection (Table 1).
Table 1.
Clinicopathologic Features of Patient Tumors With Known ER Status
| Clinicopathologic Variable | Patients (%) |
P* | |
|---|---|---|---|
| Placebo (n = 368) | Tamoxifen (n = 364) | ||
| ER | .30 | ||
| Negative | 26 | 22 | |
| Positive | 74 | 78 | |
| PgR | .11 | ||
| Negative | 36 | 31 | |
| Positive | 61 | 68 | |
| Tumor size, cm | .20 | ||
| Unknown | 3 | 2 | |
| < 1 | 80 | 83 | |
| 1-2 | 15 | 10 | |
| > 2 | 4 | 5 | |
| Surgical margin | .22 | ||
| Unknown | 2 | 1 | |
| Negative | 71 | 70 | |
| Positive | 16 | 13 | |
| Comedo necrosis | .13 | ||
| Unknown | 13 | 17 | |
| Absent | 43 | 50 | |
| Present | 55 | 49 | |
| Age at entry, years | .72 | ||
| Unknown | 2 | 1 | |
| < 50 | 34 | 36 | |
| 50-59 | 29 | 27 | |
| > 60 | 37 | 37 | |
| Race | .92 | ||
| White | 87 | 85 | |
| Black | 6 | 7 | |
| Other | 5 | 6 | |
| Unknown | 2 | 2 | |
| Method of detection | .054 | ||
| Mammography | 83 | 77 | |
| Clinical | 17 | 23 | |
Abbreviations: ER, estrogen receptor; PgR, progesterone receptor.
Comparing treatment groups.
Receptor Status and Response to Tamoxifen
Table 2 shows the risk of subsequent breast cancer in all patients with DCIS stratified by treatment arm and ER status at overall follow-up. Patients with ER-positive DCIS who received adjuvant tamoxifen versus placebo showed significant reductions in any breast cancer event (HR, 0.58; P = .001), any IBC (HR, 0.53; P = .005), and any contralateral breast cancer (HR, 0.50; P = .02). Reductions were also observed for any DCIS, any ipsilateral cancer, ipsilateral IBC, ipsilateral DCIS, contralateral IBC, and contralateral DCIS, but none were statistically significant. Results were similar in analyses of patients stratified by PgR and receptor (ER and/or PgR) status (Appendix Table A1, online only) although overall, they were not more predictive than when ER status was considered alone. Analyses at 10 years were similar to those of overall follow-up (Appendix Table A1). No significant reductions were associated with ER-negative DCIS in any setting.
Table 2.
BC Development by ER Status and Type of DCIS Treatment in Patients From NSABP B-24
| Type of BC | Placebo (n = 368) |
Tamoxifen (n = 364) |
HR* | 95% CI | P† | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| ER positive | |||||||
| Any | |||||||
| BC | 84 | 31 | 58 | 20 | 0.58 | 0.415 to 0.81 | .0015 |
| IBC | 52 | 19 | 33 | 12 | 0.53 | 0.34 to 0.82 | .005 |
| DCIS | 32 | 12 | 25 | 9 | 0.66 | 0.39 to 1.12 | .12 |
| Ipsilateral | |||||||
| BC | 47 | 17 | 39 | 14 | 0.68 | 0.44 to 1.03 | .07 |
| IBC | 26 | 9 | 20 | 7 | 0.61 | 0.34 to 1.09 | .10 |
| DCIS | 21 | 8 | 19 | 7 | 0.76 | 0.41 to 1.42 | .39 |
| Contralateral | |||||||
| BC | 32 | 11 | 18 | 6 | 0.50 | 0.28 to 0.88 | .02 |
| IBC | 21 | 8 | 12 | 4 | 0.51 | 0.25 to 1.03 | .06 |
| DCIS | 11 | 4 | 6 | 2 | 0.47 | 0.17 to 1.27 | .14 |
| ER negative | |||||||
| Any | |||||||
| BC | 25 | 27 | 20 | 25 | 0.88 | 0.49 to 1.59 | .68 |
| IBC | 14 | 15 | 9 | 11 | 0.69 | 0.30 to 1.59 | .38 |
| DCIS | 11 | 12 | 11 | 14 | 1.15 | 0.50 to 2.65 | .75 |
| Ipsilateral | |||||||
| BC | 16 | 17 | 17 | 21 | 1.18 | 0.60 to 2.34 | .63 |
| IBC | 6 | 6 | 7 | 9 | 1.24 | 0.42 to 3.70 | .70 |
| DCIS | 10 | 11 | 10 | 13 | 1.15 | 0.48 to 2.75 | .76 |
| Contralateral | |||||||
| BC | 7 | 7 | 3 | 4 | 0.46 | 0.12 to 1.80 | .35 |
| IBC | 6 | 6 | 2 | 3 | 0.36 | 0.07 to 1.77 | .29 |
| DCIS | 1 | 1 | 1 | 1 | 1.15 | 0.07 to 18.44 | 1.00 |
Abbreviations: BC, breast cancer; IBC, invasive breast cancer; DCIS, ductal carcinoma in situ; ER, estrogen receptor; HR, hazard ratio; NSABP, National Surgical Adjuvant Breast and Bowel Project.
HRs comparing tamoxifen with placebo adjusted by age at entry (≤ 49, ≥ 50 years) over all follow-up time.
P values for age-adjusted HRs determined based on large sample Wald statistic from Cox proportional hazards models for all categories except ER-negative contralateral breast cancer, which was determined by Fisher's exact test.
Figure 3 shows Kaplan-Meier curves comparing treatment groups for ER-positive and -negative patients, demonstrating that the reduction of subsequent breast cancer was restricted to patients with receptor-positive DCIS treated with tamoxifen. In multivariable analysis (Table 3) of patients with available ER results, the only independently significant predictors of subsequent breast cancer were treatment status (tamoxifen v placebo; HR, 0.64; P = .003) and age at entry (≤ 49 v ≥ 50 years; HR, 0.61; P < .001). Results were similar for all patients in B-24.
Fig 3.
Kaplan-Meier curves showing probability of any subsequent breast cancer in patients with (A) estrogen receptor (ER) –negative and (B) ER-positive ductal carcinoma in situ (DCIS) treated with adjuvant placebo versus tamoxifen. Tamoxifen benefit (42% reduction in relative risk; P = .001) was restricted to ER-positive DCIS.
Table 3.
Multivariate Analyses* of Patients With DCIS in NSABP B-24
| Model Variable† | Time to Any Breast Cancer As First Event |
||
|---|---|---|---|
| HR | 95% CI | P | |
| Patients with known ER status (n = 732) | |||
| Treatment (placebo‡v tamoxifen) | 0.643 | 0.481 to 0.861 | .003 |
| Age at entry, years (≤ 49‡ v ≥ 50) | 0.609 | 0.457 to 0.812 | < .001 |
| All patients with follow-up (n = 1,799) | |||
| Treatment (placebo‡v tamoxifen) | 0.687 | 0.563 to 0.837 | < .001 |
| Age at entry, years (≤ 49‡ v ≥ 50) | 0.621 | 0.510 to 0.756 | < .001 |
Abbreviations: DCIS, ductal carcinoma in situ; ER, estrogen receptor; HR, hazard ratio; NSABP, National Surgical Adjuvant Breast and Bowel Project; PgR, progesterone receptor.
End point, any subsequent breast cancer.
The following variables were not significantly related to time to any breast cancer in the multivariate analysis: clinical tumor size, ER status, PgR status, comedo necrosis status, and margin status.
Baseline value used for comparison.
DISCUSSION
The NSABP B-24 clinical trial demonstrated that adjuvant tamoxifen reduces subsequent ipsilateral and contralateral breast cancers in women with DCIS after lumpectomy and radiation.24 United Kingdom-ANZ, the only other major randomized trial evaluating tamoxifen in DCIS, failed to show a benefit in the lumpectomy plus radiation arm of the study (comparable to B-24), although significant benefit was observed in the lumpectomy plus tamoxifen alone arm.25 Tamoxifen has now been shown to significantly reduce subsequent breast cancer in randomized clinical trials of patients with IBC (eg, NSABP B-14),23,36 patients with DCIS (eg, NSABP B-24 and United Kingdom-ANZ),24,25,37 and women who are at high risk for developing breast cancer (eg, NSABP P-1).38
Numerous studies and clinical trials over the past 25 years have shown that the benefit of adjuvant tamoxifen in IBC is restricted to patients with receptor-positive disease.36 The B-24 trial was initiated before hormone receptor status was routinely evaluated in DCIS, and patients were enrolled without this information. The current study was undertaken to evaluate, retrospectively, the relationship between adjuvant tamoxifen and receptor status in DCIS, with the expectation that the results would be similar to those in IBC. Indeed, with prolonged follow-up, adjuvant tamoxifen was shown to significantly reduce subsequent ipsilateral breast cancer only in patients with ER-positive DCIS. Tamoxifen reduced contralateral breast cancer in patients with ER-positive and -negative DCIS, as expected (ie, prevention). No ipsilateral benefit was observed in ER-negative disease, emphasizing that tamoxifen must bind to functional ER to exert its beneficial effect on pre-existing residual tumor cells.26,39
The current study was based on results from 732 patients with DCIS and known receptor status. Receptors in a majority (61%) were evaluated by IHC in an experienced central laboratory using comprehensively validated assays for ER and PgR.27–30,40 The remainder (39%) were evaluated by various methods (the majority by IHC) in institutional laboratories (FFPET blocks were not available for central confirmation). All results were combined to increase the statistical power of the study overall. However, a subset of patient cases (n = 102) was evaluated for ER by IHC in both central and institutional laboratories, and agreement (positive v negative) was only 74.5%. There was also a significant difference in the incidence of ER-negative patient cases when the results from central and outside laboratories were compared (20% v 30%; P = .002). Assuming the central results are correct, this suggests a substantial rate of false negatives from institutions. To investigate this issue, response to tamoxifen was compared in patients with ER-negative DCIS determined in central versus institutional laboratories (Table 4). Although it was not statistically significant, there was an apparent benefit in ER-negative patient cases determined by institutional laboratories (eg, HR, 0.58; P = .28 for any subsequent cancer), whereas no benefit was observed in patients with central results (HR, 1.09; P = .85 for any cancer). These results are consistent with a false-negative rate of 30% to 40% from institutional laboratories, which may be responsible for the small apparent benefit observed in ER-negative patient cases overall. Similar errors in receptor testing by IHC in IBCs have been observed in laboratories around the world.41–44 In response, the National Cancer Comprehensive Network, College of American Pathologists, and American Society of Clinical Oncologists published guidelines to help improve the quality IHC testing of hormone receptors in breast cancer.40,45
Table 4.
Cumulative Incidence of Developing Subsequent Breast Cancer After Treatment at 10 Years in Patients With ER-Negative DCIS in NSABP B-24*
| Event | Placebo (%) | Tamoxifen (%) | HR | 95% CI | P† |
|---|---|---|---|---|---|
| Any breast cancer | |||||
| Total | 25 | 21 | 0.84 | 0.45 to 1.58 | .59 |
| Central | 23 | 27 | 1.09 | 0.47 to 2.51 | .85 |
| Institutional | 28 | 15 | 0.58 | 0.22 to 1.56 | .28 |
| Ipsilateral breast cancer | |||||
| Total | 18 | 21 | 1.06 | 0.51 to 2.20 | .87 |
| Central | 19 | 24 | 1.23 | 0.48 to 3.20 | .67 |
| Institutional | 16 | 18 | 0.84 | 0.27 to 2.66 | .77 |
Abbreviations: DCIS, ductal carcinoma in situ; ER, estrogen receptor; HR, hazard ratio; NSABP, National Surgical Adjuvant Breast and Bowel Project.
In patients determined to be ER negative (central laboratory testing: n = 89 [placebo, 48; tamoxifen, 41]; institutional laboratory testing: n = 85 [placebo, 46; tamoxifen, 39]).
P values determined based on large sample Wald statistic from Cox proportional hazards models.
In summary, retrospective analyses of hormone receptors of patients enrolled onto the NSABP B-24 clinical trial showed a significant benefit for adjuvant tamoxifen in patients with ER-positive DCIS after standard therapy. This offers an additional therapeutic option for patients and physicians to consider.
Supplementary Material
Acknowledgment
We thank Edwin R. Fisher, MD (deceased), for his contributions to this study. D.C.A. also thanks Barbara C. Good, PhD, for editorial assistance.
Appendix
Table A1.
Cumulative Percentage of Breast Cancer Development at 10 Years and Overall Follow-Up by Receptor Status and Adjuvant Therapy in Patients With DCIS After Lumpectomy and Local Radiotherapy (n = 732)
| Event by Receptor Status | 10 Years |
Overall |
||||||
|---|---|---|---|---|---|---|---|---|
| Placebo* |
Tamoxifen* |
Hazard Ratio | P† | |||||
| No. | % | No. | % | Hazard Ratio | P† | |||
| Any breast cancer | ||||||||
| Any | ||||||||
| ER negative | 23 | 25 | 17 | 21 | 0.84 | .59 | 0.88 | .68 |
| ER positive | 69 | 26 | 38 | 14 | 0.49 | < .001 | 0.60 | .003 |
| PgR negative | 32 | 25 | 22 | 20 | 0.79 | .39 | 0.86 | .57 |
| PgR positive | 58 | 27 | 33 | 14 | 0.47 | < .001 | 0.55 | .001 |
| HR negative | 21 | 25 | 15 | 21 | 0.82 | .56 | 0.87 | .66 |
| HR positive | 71 | 26 | 40 | 14 | 0.51 | < .001 | 0.61 | .003 |
| Ipsilateral | ||||||||
| ER negative | 15 | 17 | 14 | 18 | 1.06 | .87 | 1.18 | .64 |
| ER positive | 42 | 16 | 26 | 10 | 0.55 | .02 | 0.72 | .14 |
| PgR negative | 21 | 17 | 18 | 16 | 0.98 | .96 | 1.18 | .59 |
| PgR positive | 35 | 16 | 22 | 9 | 0.52 | .02 | 0.65 | .07 |
| HR negative | 14 | 17 | 13 | 18 | 1.07 | .87 | 1.19 | .63 |
| HR positive | 43 | 16 | 27 | 10 | 0.57 | .02 | 0.73 | .14 |
| Contralateral | ||||||||
| ER negative | 6 | 6 | 3 | 5 | 0.57 | .51‡ | 0.46 | .35‡ |
| ER positive | 22 | 8 | 12 | 3 | 0.49 | .04 | 0.49 | .01 |
| PgR negative | 9 | 7 | 4 | 4 | 0.51 | .26 | 0.40 | .12 |
| PgR positive | 19 | 9 | 11 | 5 | 0.48 | .053 | 0.49 | .02 |
| HR negative | 5 | 6 | 2 | 3 | 0.46 | .36‡ | 0.36 | .29‡ |
| HR positive | 23 | 8 | 13 | 5 | 0.51 | .049 | 0.50 | .02 |
| Invasive breast cancer only | ||||||||
| Any | ||||||||
| ER negative | 12 | 13 | 6 | 8 | 0.81 | .45 | 0.69 | .38 |
| ER positive | 39 | 15 | 21 | 8 | 0.60 | .002 | 0.55 | .007 |
| PgR negative | 16 | 13 | 9 | 8 | 0.83 | .43 | 0.80 | .53 |
| PgR positive | 34 | 16 | 18 | 8 | 0.57 | .001 | 0.47 | .002 |
| HR negative | 11 | 13 | 6 | 8 | 0.80 | .43 | 0.75 | .49 |
| HR positive | 40 | 15 | 21 | 8 | 0.61 | .002 | 0.54 | .005 |
| Lpsilateral | ||||||||
| ER negative | 5 | 6 | 4 | 5 | 0.90 | 1.00‡ | 1.23 | .71 |
| ER positive | 21 | 8 | 12 | 4 | 0.50 | .06 | 0.65 | .15 |
| PgR negative | 7 | 6 | 5 | 5 | 0.81 | .72 | 1.23 | .67 |
| PgR positive | 19 | 9 | 11 | 5 | 0.48 | .0498 | 0.59 | .09 |
| HR negative | 5 | 6 | 4 | 6 | 0.91 | 1.00‡ | 1.24 | .69 |
| HR positive | 21 | 8 | 12 | 4 | 0.51 | .06 | 0.66 | .16 |
| Contralateral | ||||||||
| ER negative | 5 | 6 | 2 | 3 | 0.46 | .45‡ | 0.36 | 0.29‡ |
| ER positive | 13 | 5 | 9 | 3 | 0.62 | .27 | 0.50 | .054 |
| PgR negative | 7 | 5 | 4 | 4 | 0.65 | .49 | 0.55 | .33 |
| PgR positive | 11 | 5 | 7 | 3 | 0.53 | .19 | 0.42 | .03 |
| HR negative | 4 | 5 | 2 | 3 | 0.58 | .69‡ | 0.43 | 0.46‡ |
| HR positive | 14 | 5 | 9 | 3 | 0.58 | .20 | 0.48 | .04 |
| Noninvasive breast cancer (DCIS) only | ||||||||
| Any | ||||||||
| ER negative | 11 | 12 | 11 | 14 | 1.14 | .75 | 1.14 | .75 |
| ER positive | 30 | 11 | 17 | 6 | 0.51 | .03 | 0.69 | .17 |
| PgR negative | 16 | 13 | 13 | 12 | 0.94 | .86 | 0.94 | .86 |
| PgR positive | 24 | 11 | 15 | 6 | 0.53 | .051 | 0.69 | .22 |
| HR negative | 10 | 12 | 9 | 13 | 1.04 | .93 | 1.04 | .93 |
| HR positive | 31 | 11 | 19 | 7 | 0.55 | .04 | 0.73 | .23 |
| Ipsilateral | ||||||||
| ER negative | 10 | 11 | 10 | 13 | 1.14 | .76 | 1.14 | .76 |
| ER positive | 21 | 8 | 14 | 5 | 0.60 | .14 | 0.81 | .51 |
| PgR negative | 14 | 11 | 13 | 12 | 1.07 | .86 | 1.14 | .72 |
| PgR positive | 16 | 7 | 11 | 5 | 0.58 | .17 | 0.74 | .40 |
| HR negative | 9 | 11 | 9 | 13 | 1.15 | .76 | 1.15 | .76 |
| HR positive | 22 | 8 | 15 | 5 | 0.62 | .15 | 0.82 | .52 |
| Contralateral | ||||||||
| ER negative | 1 | 1 | 1 | 1 | 1.13 | 1.00‡ | 1.13 | 1.00‡ |
| ER positive | 9 | 3 | 3 | 1 | 0.29 | .07 | 0.47 | .14 |
| PgR negative | 2 | 2 | 0 | 0 | — | .50‡ | — | .25‡ |
| PgR positive | 8 | 4 | 4 | 2 | 0.41 | .15 | 0.62 | .35 |
| HR negative | 1 | 1 | 0 | 0 | — | 1.00‡ | — | 1.00‡ |
| HR positive | 9 | 3 | 4 | 1 | 0.39 | .12 | 0.55 | .22 |
Abbreviations: ER, estrogen receptor; DCIS, ductal carcinoma in situ; HR, hormone receptor; PgR, progesterone receptor.
Patients experiencing treatment failure (values in columns represent No. of cumulative incidence percentage).
P values determined based on large sample Wald statistic from Cox proportional hazards models unless otherwise indicated.
P value determined by Fisher's exact test.
Footnotes
See accompanying editorial on page 1249 and article on page 1384; listen to the podcast by Dr Baum at www.jco.org/podcasts
Supported by Public Health Service Grants No. U10-CA-37377, U10-CA-69974, U10-CA-12027, and U10-CA-69651 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and by the Breast Cancer Research Foundation and AstraZeneca.
Presented in part at the 26th Annual San Antonio Breast Cancer Symposium, December 3-6, 2003, San Antonio, TX.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: PDQ: NSABP-B-24.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: D. Lawrence Wickerham, AstraZeneca Research Funding: Joseph P. Costantino, National Cancer Institute Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: D. Craig Allred, Stewart J. Anderson, Soonmyung Paik, Sandra M. Swain, Eleftherios P. Mamounas, Joseph P. Costantino, Norman Wolmark
Administrative support: Soonmyung Paik, Charles E. Geyer Jr, Joseph P. Costantino, Norman Wolmark
Provision of study materials or patients: Soonmyung Paik
Collection and assembly of data: Stewart J. Anderson, Soonmyung Paik, D. Lawrence Wickerham, Eleftherios P. Mamounas, Joseph P. Costantino, Stephanie R. Land
Data analysis and interpretation: Stewart J. Anderson, Soonmyung Paik, Iris D. Nagtegaal, Eleftherios P. Mamounas, Thomas B. Julian, Charles E. Geyer Jr, Joseph P. Costantino, Stephanie R. Land
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Bloodgood JC. Comedo carcinoma or comedo-adenoma of the female breast. Am J Cancer. 1934;22:842–853. [Google Scholar]
- 2.Cheatle GL, Cutler M. Malignant epithelial neoplasia: Carcinoma—The precancerous or potentially carcinomatous state. In: Cheatle GL, Cutler M, editors. Tumours of the Breast. Philadelphia, PA: Lippincott; 1926. pp. 161–332. [Google Scholar]
- 3.Ernster VL, Barclay J. Increases in ductal carcinoma in situ (DCIS) of the breast in relation to mammography: A dilemma. Natl Cancer Inst Monogr. 1997;22:151–156. doi: 10.1093/jncimono/1997.22.151. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein MJ. Ductal carcinoma in situ of the breast. Annu Rev Med. 2000;51:17–32. doi: 10.1146/annurev.med.51.1.17. [DOI] [PubMed] [Google Scholar]
- 5.Weaver DL, Rosenberg RD, Barlow WE, et al. Pathologic findings from the Breast Cancer Surveillance Consortium: Population-based outcomes in women undergoing biopsy after screening mammography. Cancer. 2006;106:732–742. doi: 10.1002/cncr.21652. [DOI] [PubMed] [Google Scholar]
- 6.Kerlikowske K. Epidemiology of ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;41:139–141. doi: 10.1093/jncimonographs/lgq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutuli B, Cohen-Solal-Le Nir C, De Lafontan B, et al. Ductal carcinoma in situ of the breast results of conservative and radical treatments in 716 patients. Eur J Cancer. 2001;37:2365–2372. doi: 10.1016/s0959-8049(01)00303-3. [DOI] [PubMed] [Google Scholar]
- 8.Nakhlis F, Morrow M. Ductal carcinoma in situ. Surg Clin North Am. 2003;83:821–839. doi: 10.1016/S0039-6109(03)00072-0. [DOI] [PubMed] [Google Scholar]
- 9.Burstein HJ, Polyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430–1441. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 10.Silverstein MJ, Lagios MD, Recht A, et al. Image-detected breast cancer: State of the art diagnosis and treatment. J Am Coll Surg. 2005;201:586–597. doi: 10.1016/j.jamcollsurg.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Virnig BA, Tuttle TM, Shamliyan T, et al. Ductal carcinoma in situ of the breast: A systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 12.Hetelekidis S, Collins L, Silver B, et al. Predictors of local recurrence following excision alone for ductal carcinoma in situ. Cancer. 1999;85:427–431. [PubMed] [Google Scholar]
- 13.Horst KC, Smitt MC, Goffinet DR, et al. Predictors of local recurrence after breast-conservation therapy. Clin Breast Cancer. 2005;5:425–438. doi: 10.3816/cbc.2005.n.001. [DOI] [PubMed] [Google Scholar]
- 14.Millar EK, Leong AS. Significance and assessment of margin status in ductal carcinoma in situ of the breast. Adv Anat Pathol. 2001;8:338–344. doi: 10.1097/00125480-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Meijnen P, Gilhuijs KG, Rutgers EJ. The effect of margins on the clinical management of ductal carcinoma in situ of the breast. J Surg Oncol. 2008;98:579–584. doi: 10.1002/jso.21041. [DOI] [PubMed] [Google Scholar]
- 16.Schnitt SJ. Local outcomes in ductal carcinoma in situ based on patient and tumor characteristics. J Natl Cancer Inst Monogr. 2010;2010:158–161. doi: 10.1093/jncimonographs/lgq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kane RL, Virnig BA, Shamliyan T, et al. The impact of surgery, radiation, and systemic treatment on outcomes in patients with ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010:130–133. doi: 10.1093/jncimonographs/lgq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverstein MJ, Barth A, Poller DN, et al. Ten-year results comparing mastectomy to excision and radiation therapy for ductal carcinoma in situ of the breast. Eur J Cancer. 1995;31A:1425–1427. doi: 10.1016/0959-8049(95)00283-o. [DOI] [PubMed] [Google Scholar]
- 19.Solin LJ. The impact of adding radiation treatment after breast conservation surgery for ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:187–192. doi: 10.1093/jncimonographs/lgq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher B, Costantino J, Redmond C, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328:1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 21.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 22.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 23.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: Updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–690. doi: 10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 25.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: Long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan VC. Third Annual William L. McGuire Memorial Lecture: “Studies on the estrogen receptor in breast cancer”—20 years as a target for the treatment and prevention of cancer. Breast Cancer Res Treat. 1995;36:267–285. doi: 10.1007/BF00713399. [DOI] [PubMed] [Google Scholar]
- 27.Elledge RM, Green S, Pugh R, et al. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: A Southwest Oncology Group Study. Int J Cancer. 2000;89:111–117. [PubMed] [Google Scholar]
- 28.Harvey JM, Clark GM, Osborne CK, et al. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 29.Love RR, Duc NB, Allred DC, et al. Oophorectomy and tamoxifen adjuvant therapy in premenopausal Vietnamese and Chinese women with operable breast cancer. J Clin Oncol. 2002;20:2559–2566. doi: 10.1200/JCO.2002.08.169. [DOI] [PubMed] [Google Scholar]
- 30.Mohsin SK, Weiss H, Havighurst T, et al. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: A validation study. Mod Pathol. 2004;17:1545–1554. doi: 10.1038/modpathol.3800229. [DOI] [PubMed] [Google Scholar]
- 31.Feller W. An Introduction to Probability Theory and Its Applications. ed 2. Volume I. New York, NY: John Wiley and Sons; 1964. [Google Scholar]
- 32.Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41:361–372. [PubMed] [Google Scholar]
- 33.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: Examples from clinical oncology data. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 34.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 35.Kalbfleisch JP, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley and Sons; 1980. [Google Scholar]
- 36.Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1727. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 37.Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: Randomised controlled trial. Lancet. 2003;362:95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 38.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 39.Fuqua SAW, Schiff S. The biology of estrogen receptors. In: Harris JR, Lippman ME, Morrow M, et al., editors. Diseases of the Breast. Philadelphia, PA: Lippincott Williams and Wilkins; 2004. pp. 585–602. [Google Scholar]
- 40.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134:e48–e72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 41.Allred DC. Commentary: Hormone receptor testing in breast cancer—A distress signal from Canada. Oncologist. 2008;13:1134–1136. doi: 10.1634/theoncologist.2008-0184. [DOI] [PubMed] [Google Scholar]
- 42.Gown AM. Current issues in ER and HER2 testing by IHC in breast cancer. Mod Pathol. 2008;21(suppl 2):S8–S15. doi: 10.1038/modpathol.2008.34. [DOI] [PubMed] [Google Scholar]
- 43.Hede K. Breast cancer testing scandal shines spotlight on black box of clinical laboratory testing. J Natl Cancer Inst. 2008;100:836–837. 844. doi: 10.1093/jnci/djn200. [DOI] [PubMed] [Google Scholar]
- 44.Mathews AW. Bad cancer tests drawing scrutiny. The Wall Street Journal. 2008 Jan 4;:B1–B2. [Google Scholar]
- 45.Allred DC, Carlson RW, Berry DA, et al. NCCN Task Force report: Estrogen receptor and progesterone receptor testing in breast cancer by immunohistochemistry. J Natl Compr Canc Netw. 2009;7(suppl 6):S1–S21. doi: 10.6004/jnccn.2009.0079. quiz S22-S23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.