Abstract
Purpose
In a randomized trial, early palliative care (EPC) in patients with metastatic non–small-cell lung cancer (NSCLC) was observed to improve survival. In a secondary analysis, we explored the hypothesis that the survival benefit resulted from improving depression.
Patients and Methods
In total, 151 patients with newly diagnosed metastatic NSCLC participated in a randomized trial of EPC integrated with standard oncology care versus standard oncology care alone. Depression was assessed at baseline and at 12 weeks with the Patient Health Questionnaire-9 (PHQ-9) and was scored diagnostically by using Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, criteria for major depression syndrome (MDS). Depression response was considered ≥ 50% reduction in PHQ-9 scores at 12 weeks. Survival differences were tested with log-rank and Cox proportional hazards models.
Results
At baseline, 21 patients (14%) met MDS criteria. MDS significantly predicted worse survival (hazard ratio, 1.82; P = .02). Patients assigned to EPC had greater improvements in PHQ-9 scores at 12 weeks (P < .001); among patients with MDS, those receiving EPC had greater rates of depression response at 12 weeks (P = .04). However, improvement in PHQ-9 scores was not associated with improved survival, except in a sensitivity analysis in which patients who died before 12 weeks were modeled to have worse depression. The group randomly assigned to EPC remained independently associated with survival after adding improvement in PHQ-9 scores to the survival model.
Conclusion
Depression predicted worse survival in patients with newly diagnosed metastatic NSCLC. Although EPC was associated with greater improvement in depression at 12 weeks, the data do not support the hypothesis that treatment of depression mediated the observed survival benefit from EPC.
INTRODUCTION
In a randomized controlled trial of 151 patients with metastatic non–small-cell lung cancer (NSCLC), we observed that integrating palliative care with standard oncology care soon after diagnosis significantly improved survival by approximately 3 months.1 This is particularly important because the median survival of this population is typically 8 to 10 months.2 Although the primary outcome of the trial was quality of life, the observed survival benefit generated great interest in the intervention and the potential underlying mechanisms. Several hypotheses for how early palliative care (EPC) might have extended life include facilitating the receipt of more chemotherapy, improving the management of medical comorbidities, aiding in the discontinuation of futile and possibly detrimental cancer treatments at the end of life, and improving the treatment of depression.
Although previous research has shown depression to be associated with worse survival in individuals with cancer,3,4 whether treating depression in patients with cancer has an impact on survival remains unclear. Interestingly, a recent study5 of patients with metastatic breast cancer demonstrated that improvement in depressive symptoms, not specific to any treatment, was associated with increased survival.
EPC has the potential to treat depression and intervene with patients regarding their health behaviors and their use of health care, any of which may extend survival. In particular, we previously demonstrated that patients receiving EPC had less depressive symptoms at 12 weeks compared with patients receiving standard oncology care alone.1 However, further study is needed to investigate the effect of receiving EPC on changes in depression from baseline to 12 weeks and the possible impact that treatment of depression may have had on survival. We hypothesized that depression is associated with worse survival in patients with newly diagnosed metastatic NSCLC, that receiving EPC is associated with greater improvement in depression at 12 weeks, and that improvement in depression at 12 weeks mediates the observed survival benefit of receiving EPC. This study explores these hypotheses in a secondary analysis of the clinical trial data.
PATIENTS AND METHODS
Sample
Between June 7, 2006, and July 15, 2009, 151 ambulatory patients within 8 weeks of diagnosis of metastatic NSCLC participated in a randomized controlled trial comparing EPC integrated with standard oncology care versus standard oncology care alone at the Massachusetts General Hospital. Full details of the trial are published elsewhere.1 Patients who presented to the outpatient thoracic oncology clinic were eligible to enroll if they had confirmed metastatic NSCLC, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2, and the ability to read and respond to questions in English. Patients who were already receiving care from the palliative care service were not eligible to participate in the study. All medical oncologists in the clinic agreed to approach, recruit, and obtain consent from their patients. Enrolled patients were randomly assigned in a 1:1 fashion without stratification: 77 to EPC integrated with standard care and 74 to standard care alone. Before enrollment began, the Dana-Farber/Partners CancerCare institutional review board approved the study protocol, and all participants provided written informed consent.
EPC
Patients assigned to EPC met with a member of the palliative care team, which consisted of board-certified palliative care physicians and advanced practice nurses, within 3 weeks of enrollment and at least monthly thereafter in the ambulatory setting until death. Members of the care team or patients could schedule additional visits with the palliative care service at their discretion. The study protocol included general guidelines for the ambulatory palliative care visits, which we adapted from the National Consensus Project for Quality Palliative Care.6 Participants randomly assigned to the standard care only group met with the palliative care service on request from the patient, family, or oncologist. These participants did not cross over to the EPC group or follow the specified EPC protocol. Regardless of group assignment, all participants continued to receive routine oncology care throughout the study period.
Depression
Depression was assessed at baseline and 12 weeks with the Patient Health Questionnaire-9 (PHQ-9).7 The PHQ-9 is a nine-item self-report instrument developed to screen for major depressive disorder in primary care settings by using Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria. It can be scored continuously for a measure of symptom levels and categorically for presumed diagnoses of major depression syndrome (MDS). A diagnosis of MDS is given according to DSM-IV criteria if an individual endorses at least one of the first two items as occurring at least half the days and at least four of the other seven items as occurring at least half the days. The PHQ-9 has been validated in samples of patients with cancer and used in clinical trials with this population.8,9 Patients were considered depression responders if they had MDS at baseline and displayed a ≥ 50% reduction in PHQ-9 scores at 12 weeks, which is the standard criterion for response in depression treatment trials.10 By using an intent-to-treat analysis, participants who met criteria for depression at baseline but did not survive to 12 weeks were considered nonresponders. Finally, we reviewed participants' electronic medical records to collect data on new prescriptions for antidepressant medications and any mental health visits (with social workers, psychologists, or psychiatrists) from baseline until 12 weeks.
Statistical Methods
We performed statistical analyses by using SPSS, version 16.0 (SPSS, Chicago, IL). Descriptive summaries of demographic and clinical variables were compiled. We examined differences in categorical variables with χ2 and Fisher's exact tests and differences in continuous variables with independent samples t tests. Survival was calculated from time of enrollment until death or to the time of censoring data on September 20, 2010. We analyzed associations between baseline depression and survival by using both categorical and continuous scoring of the PHQ-9. In an unadjusted analysis, we used a log-rank test to examine the difference in survival between participants who met criteria for MDS and those who did not. All other tests of associations between depression and survival were conducted as adjusted analyses by using Cox proportional hazards models and controlling for potential confounding (PS, age, sex, race, marital status, and smoking history). We considered all P values at the two-sided α level of .05 to be statistically significant.
We explored the hypothesis that improvement in depression mediated the observed survival benefit from receiving EPC by conducting a series of analyses that used the method described by Baron and Kenny.11 An association between receiving EPC and survival in this trial had already been reported in the primary study.1 We examined the association of receiving EPC with changes in depression from baseline to 12 weeks (continuous PHQ-9 change scores) by using linear regression and controlling for baseline PHQ-9 score. To illustrate the clinical significance of changes in depression, we also examined differences in depression response rates at 12 weeks among patients who met criteria for MDS at baseline by using Fisher's exact test. We then tested mediation by adding PHQ-9 change scores to a Cox proportional hazards model that included EPC group randomization, baseline PHQ-9 score, PS, age, sex, race, marital status, and smoking history. If EPC group randomization and survival were no longer independently associated in this model, there would be evidence that change in depression mediates the survival benefit from receiving EPC.
Finally, to account for missing data in PHQ-9 scores for those who died before 12 weeks, we conducted sensitivity analyses of the survival models. Specifically, we tested three additional models that assigned different PHQ-9 change scores for the deceased: change score = 0 (last end point carried forward), average change score, and average change score among those who worsened from baseline to 12 weeks.
RESULTS
Table 1 includes a summary of the study sample characteristics. Of the 151 participants, 150 completed the baseline PHQ-9. Twenty-one (14%) met diagnostic criteria for MDS at baseline. Baseline rates of MDS were not significantly different between the EPC and standard oncology care alone groups, and we previously reported that there were no significant differences between these groups with respect to PS, age, sex, marital status, and smoking history.1 We also observed no statistically significant differences in baseline characteristics between those with and without MDS.
Table 1.
Patient Characteristics
| Characteristic | All Patients (N = 151) |
No MDS (n = 129) |
MDS (n = 21) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age, years | .27 | ||||||
| Mean | 64.4 | 64.0 | 66.5 | ||||
| SD | 9.6 | 9.6 | 9.4 | ||||
| Male | 73 | 48.3 | 60 | 46.5 | 12 | 57.1 | .37 |
| ECOG PS | .16 | ||||||
| 0 | 56 | 37.1 | 51 | 39.5 | 5 | 23.8 | |
| 1 | 81 | 53.6 | 68 | 52.7 | 12 | 57.1 | |
| 2 | 14 | 9.3 | 10 | 7.8 | 4 | 19.0 | |
| White | 141 | 93.4 | 126 | 97.8 | 20 | 95.3 | .46 |
| Married | 93 | 61.6 | 82 | 63.6 | 10 | 47.6 | .16 |
| Smoker | 124 of 149* | 83.2 | 106 of 127* | 83.5 | 17 of 21* | 81.0 | .76 |
| Smoking pack years | 37.1 | 33.1 | 35.4 | 31.1 | 41.1 | 32.7 | .43 |
| EGFR mutation | 16 | 10.6 | 16 | 12.4 | 0 | 0 | .23 |
| Brain metastases | 43 | 28.5 | 40 | 31.0 | 3 | 14.3 | .12 |
| Platinum-doublet first-line therapy | 70 | 46.4 | 60 | 46.5 | 10 | 47.6 | .93 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; MDS, major depression syndrome; PS, performance status.
Smoking status was assessed in 149 patients. One hundred forty-eight patients had both smoking status and baseline PHQ-9.
At 12 weeks, 27 participants (18%) had died: 17 assigned to standard oncology care alone and 10 receiving EPC. Of those alive at 12 weeks, 104 (85%) completed the PHQ-9 for the second time. Failure to complete the PHQ-9 again at 12 weeks was not associated with EPC randomization group, baseline MDS, or baseline PHQ-9 score.
Depression and Survival
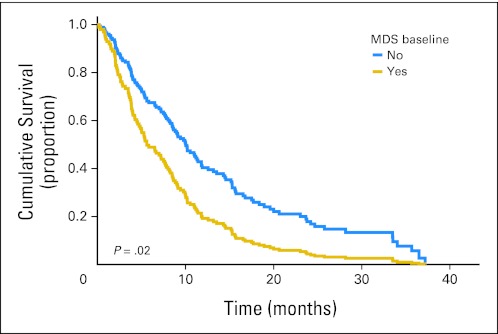
In the entire sample, median survival was shorter for patients with depression at baseline. Patients with MDS had a median survival of 5.4 months versus 10 months for those without MDS (log-rank P = .001). After controlling for PS, sex, age, race, smoking history, and marital status, MDS remained associated with worse survival (hazard ratio [HR], 1.82; 95% CI, 1.10 to 3.01; P = .02). Adjusted survival curves are displayed in Figure 1. Continuous PHQ-9 scores were also associated with survival, controlling for PS, sex, age, race, smoking hisory, and marital status (HR, 1.04; 95% CI, 1.01 to 1.08; P = .02).
Fig 1.
Adjusted survival curves by major depression syndrome (MDS) status. Cox proportional hazards model adjusted for performance status, age, sex, race, marital status, and smoking history.
Changes in Depression
Participants receiving EPC had greater improvement in their PHQ-9 scores from baseline to 12 weeks compared with those receiving standard oncology care alone (mean change, −0.96 [standard deviation, 4.65] v 0.06 [standard deviation, 4.07]), respectively, and this difference was significant controlling for baseline PHQ-9 scores (P < .001).
Among patients with baseline MDS, those assigned to EPC had greater rates of depression response at 12 weeks compared with those in the standard oncology care alone group (42.9% v 0%; P = .04). Despite the greater improvement in patients receiving EPC, rates of new antidepressant prescriptions and mental health visits did not differ significantly between the two groups (Table 2).
Table 2.
Interventions for Depression by Group
| Population Studied | No. of Patients | Treatment Group | No. of Patients | Antidepressants |
P | Mental Health Visits |
P | ||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||||
| Entire sample | 151 | Standard* | 74 | 13 | 18 | .92 | 26 | 35 | .16 |
| EPC† | 77 | 14 | 18 | 19 | 25 | ||||
| Patients with MDS | 21 | Standard* | 12 | 3 | 25 | 1.0 | 7 | 58 | .67 |
| EPC† | 9 | 3 | 33 | 4 | 44 | ||||
Abbreviations: EPC, early palliative care; MDS, major depression syndrome.
Standard, standard oncology care alone.
EPC, standard oncology care integrated with EPC.
Effect of Changes in Depression on Survival
Associations between changes in PHQ-9 scores from baseline to 12 weeks and survival are summarized in Table 3 for the complete case analysis (ie, analysis of only patients with PHQ-9 data at 12 weeks) and the three sensitivity analysis models to account for patients who had died by 12 weeks. We observed no significant association in the complete case analysis as well as in two sensitivity analysis models (ie, assigning those who had died by 12 weeks change scores of 0 and the average change). However, in the model in which we assigned those who had died by 12 weeks the average change score of those who had higher depression scores at 12 weeks, we did find a significant association between change in depression and survival.
Table 3.
Adjusted HRs for Improvement in PHQ-9 Scores at 12 Weeks
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Complete case analysis | 0.99 | 0.93 to 1.06 | .88 |
| Sensitivity analysis models to account for patients who had died by 12 weeks | |||
| Last end point carried forward (improvement = 0) | 0.98 | 0.93 to 1.04 | .45 |
| Assign average change in sample (improvement = 0.5) | 0.99 | 0.93 to 1.04 | .60 |
| Assign average change in portion of sample that worsened at 12 weeks (improvement = −3.24) | 0.93 | 0.88 to 0.98 | .004 |
Abbreviations: HR, hazard ratio; PHQ-9, Patient Health Questionnaire-9.
Depression Mediating Survival Benefit From EPC
Table 4 displays the HRs for the survival models that include EPC randomization group, baseline PHQ-9 score, and change in PHQ-9 score from baseline to 12 weeks. In the complete case analysis and all three sensitivity analysis models, EPC randomization group remained independently associated with survival with the inclusion of change in PHQ-9 scores. Baseline PHQ-9 score remained independently associated with survival only in the sensitivity analysis model of worsening depression scores for those who had died by 12 weeks.
Table 4.
Adjusted HR in Mediation Model of Survival
| Variable | EPC Randomization |
Baseline PHQ-9 |
Change in PHQ-9 Score at 3 Months |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Without change in depression in model | 0.66 | 0.45 to .95 | .03 | 1.04 | 1.00 to 1.08 | .05 | |||
| Complete case analysis | 0.59 | 0.36 to .97 | .04 | 1.04 | 0.99 to 1.10 | .12 | 1.01 | 0.94 to 1.08 | .77 |
| Sensitivity analysis models to account for patients who had died by 12 weeks | |||||||||
| Last end point carried forward (improvement = 0) | 0.60 | 0.40 to .910 | .02 | 1.05 | 0.99 to 1.09 | .06 | 0.99 | 0.94 to 1.05 | .82 |
| Assign average change in sample (improvement = 0.5) | 0.60 | 0.40 to .90 | .02 | 1.04 | 0.99 to 1.09 | .07 | 1.0 | 0.94 to 1.06 | .99 |
| Assign average change in portion of sample that worsened at 12 weeks (improvement = −3.24) | 0.66 | 0.44 to 1.00 | .05 | 1.07 | 1.02 to 1.11 | .002 | 0.94 | 0.89 to .99 | .02 |
Abbreviations: EPC, early palliative care; HR, hazard ratio; PHQ-9, Patient Health Questionnaire-9.
DISCUSSION
Depression in patients with newly diagnosed metastatic NSCLC appears to be associated with worse survival. Although a relationship between depression and survival has not been consistently found in other studies of advanced lung cancer, this study is one of the largest, and it replicates our previous findings in patients with newly diagnosed metastatic NSCLC in a single-arm feasibility study of EPC.12–17 Depression, which is treatable, may represent a target to improve both quality of life and survival in this population with a heavy symptom burden and poor prognosis.
Although previous trials of psychotherapy have shown a reduction in depressive symptoms in individuals with cancer, few intervention studies have specifically targeted major depression.18,19 Randomized trials of antidepressants have yet to show significant benefit over placebo in individuals with depression and cancer, although antidepressants have been shown to be superior to placebo in trials in individuals with medical illnesses.20–22 EPC appears to have efficacy for the treatment of depression in patients with metastatic NSCLC. The greater depression response rate in patients receiving EPC is not accounted for by differences in antidepressant prescriptions and mental health visits. Nonetheless, researchers have shown that collaborative care models, which involve sharing care between mental health and medical clinicians, appear to be effective for treating depression in samples of patients with cancer.8,23 Such approaches are similar to integrated EPC in which oncologists and palliative care clinicians share in the care of patients with newly diagnosed metastatic NSCLC. In fact, the palliative care clinicians in the trial spent a substantial portion of their visits providing counseling on coping.24
It remains unclear whether improvements in depression result in improved survival in patients with metastatic lung cancer. In our complete case analysis and sensitivity analysis of three different models for changes in depression in patients who died before the second depression assessment, only one model showed a significant association between changes in depression and survival: the model in which depression worsened in those who died by 12 weeks. However, a longitudinal study of depression in patients with advanced lung cancer demonstrated worsening depression as death approaches, which makes this model the most likely.25
We did not find evidence to support the hypothesis that the survival benefit from receiving EPC is due to the treatment of depression. This negative finding, however, does not necessarily mean that improvement in depression had no impact on the observed survival benefit. There could be other contributing factors, and the better treatment of depression alone might not be sufficient to explain the association between receiving EPC and improved survival. These data suggest that EPC is likely having an impact on survival in other ways besides the treatment of depression. Several hypotheses for the effect of EPC on survival remain, such as facilitating the receipt of more chemotherapy, improving the management of medical comorbidities, and aiding in the discontinuation of futile and possibly detrimental cancer treatments at the end of life.
Another interesting finding is that in the sensitivity analysis model in which improvement in depression is associated with longer survival, baseline depression still appears to be associated with worse survival. In other words, even if treating depression improves survival in patients with newly diagnosed metastatic NSCLC, there may still be a survival decrement from having depression at baseline. One potential explanation for this is the possibility that depressed patients might have more aggressive or treatment-resistant tumors or be more likely to have EGFR wild-type tumors,26 which could have an impact on their prognosis regardless of the treatment of depression. A more optimistic alternative could be that whatever effect depression may be having on survival is occurring early and that the treatment of depression may have occurred too late. In that case, patients with newly diagnosed metastatic NSCLC should be assessed for depression and treated as early as possible, such as at the first oncology visit, to minimize the effects of baseline depression.
Although our findings may be provocative, they are the result of unplanned and exploratory analyses of a post hoc observation. Several limitations prevent us from drawing definitive conclusions, such as the modest sample size of depressed participants as well as the lack of diagnostic interviews for major depressive disorder, infrequent measures of depression, and failure to evaluate coping styles. The fact that this study was conducted at a single institution may also limit its generalizability.
However, the findings do raise several important questions that require further investigation. Whether treating depression improves survival in patients with newly diagnosed metastatic NSCLC remains unclear, but possible. Future studies need to include repeated assessments of depression within the first 12 weeks. Because 18% of these newly diagnosed patients will not be alive by 12 weeks, determining the trajectory of their depressive symptoms is critical.
With accumulating evidence for an association between depression and worse survival in patients with cancer, research needs to move beyond the descriptive and focus on potential underlying mechanisms. Our data suggest that the negative impact of depression might be occurring early, and potential mechanisms for the association between depression and survival should be explored in future research at the time of diagnosis and shortly afterward. If biologic factors are having an impact on the survival of depressed patients with metastatic NSCLC, then these patients may require different cancer treatments, such as more aggressive therapy or more specific care for their tumors. Identifying early coping patterns, behaviors, and decision making that might be maladaptive could also lead to new interventions to improve survival in depressed patients with metastatic NSCLC. Importantly, until we know the mechanism of the association between depression and survival, there is the question of a potential need for stratification by depression status in clinical trials for metastatic NSCLC.
Nonetheless, a key finding in this analysis is that depression is clearly treatable in patients with metastatic NSCLC. Although survival might be considered the ultimate end point in oncology, depression is a disabling illness associated with suffering and poor quality of life that warrants intervention regardless of the potential impact on survival. EPC might represent another option for its treatment.
Supplementary Material
Footnotes
Supported by American Society of Clinical Oncology Career Development Award (J.S.T.) and Grant No. K23 CA115908 from the National Cancer Institute, National Institutes of Health (W.F.P.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT01038271.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: William F. Pirl, Forest Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: William F. Pirl, Joseph A. Greer, Vicki Jackson, Inga T. Lennes, Rebecca S. Heist, Jennifer S. Temel
Provision of study materials or patients: Jennifer S. Temel
Collection and assembly of data: Emily R. Gallagher, Pedro Perez-Cruz, Jennifer S. Temel
Data analysis and interpretation: William F. Pirl, Joseph A. Greer, Lara Traeger, Jennifer S. Temel
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 2.Stinchcombe TE, Lee CB, Socinski MA. Current approaches to advanced-stage non-small-cell lung cancer: First-line therapy in patients with a good functional status. Clin Lung Cancer. 2006;7(suppl 4):S111–S117. doi: 10.3816/clc.2006.s.002. [DOI] [PubMed] [Google Scholar]
- 3.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 4.Pinquart M, Duberstein PR. Depression and cancer mortality: A meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giese-Davis J, Collie K, Rancourt KM, et al. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: A secondary analysis. J Clin Oncol. 2011;29:413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Consensus Project for Quality Palliative Care. Clinical practice guidelines for quality palliative care. ed 2. Pittsburgh, PA: National Consensus Project for Quality Palliative Care; 2009. [Google Scholar]
- 7.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study—Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 8.Ell K, Xie B, Quon B, et al. Randomized controlled trial of collaborative care management of depression among low-income patients with cancer. J Clin Oncol. 2008;26:4488–4496. doi: 10.1200/JCO.2008.16.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fann JR, Berry DL, Wolpin S, et al. Depression screening using the Patient Health Questionnaire-9 administered on a touch screen computer. Psycooncology. 2009;18:14–22. doi: 10.1002/pon.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khin NA, Chen YF, Yang Y, et al. Exploratory analyses of efficacy data from major depressive disorder trials submitted to the US Food and Drug Administration in support of new drug applications. J Clin Psychiatry. 2011;72:464–472. doi: 10.4088/JCP.10m06191. [DOI] [PubMed] [Google Scholar]
- 11.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 12.Pirl WF, Temel JS, Billings A, et al. Depression after diagnosis of non-small cell lung cancer and survival: A pilot study. Psychosomatics. 2008;49:218–224. doi: 10.1176/appi.psy.49.3.218. [DOI] [PubMed] [Google Scholar]
- 13.Buccheri G. Depressive reactions to lung cancer are common and often followed by poor outcome. Eur Respir J. 1998;11:173–178. doi: 10.1183/09031936.98.11010173. [DOI] [PubMed] [Google Scholar]
- 14.Faller H, Bülzebruck H, Drings P, et al. Coping, distress, and survival among patients with lung cancer. Arch Gen Psychiatry. 1999;56:756–762. doi: 10.1001/archpsyc.56.8.756. [DOI] [PubMed] [Google Scholar]
- 15.Faller H, Schmidt M. Prognostic value of depressive coping and depression in survival of lung cancer patients. Psychooncology. 2004;13:359–363. doi: 10.1002/pon.783. [DOI] [PubMed] [Google Scholar]
- 16.Nakaya N, Saito-Nakaya K, Akechi T, et al. Negative psychological aspects and survival in lung cancer patients. Psychooncology. 2008;17:466–473. doi: 10.1002/pon.1259. [DOI] [PubMed] [Google Scholar]
- 17.Akechi T, Okamura H, Okuyama T, et al. Psychosocial factors and survival after diagnosis of inoperable non-small cell lung cancer. Psychooncology. 2009;18:23–29. doi: 10.1002/pon.1364. [DOI] [PubMed] [Google Scholar]
- 18.Pirl WF. Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. J Natl Cancer Inst Monogr. 2004;32:32–39. doi: 10.1093/jncimonographs/lgh026. [DOI] [PubMed] [Google Scholar]
- 19.Akechi T, Okuyama T, Onishi J, et al. Psychotherapy for depression among incurable cancer patients. Cochrane Database Syst Rev. 2008;2:CD005537. doi: 10.1002/14651858.CD005537.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill D, Hatcher S. Antidepressants for depression in people with physical illness. Cochrane Database Syst Rev. 2000;2:CD001312. doi: 10.1002/14651858.CD001312. [DOI] [PubMed] [Google Scholar]
- 21.Razavi D, Allilaire JF, Smith M, et al. The effect of fluoxetine on anxiety and depression symptoms in cancer patients. Acta Psychiatr Scand. 1996;94:205–210. doi: 10.1111/j.1600-0447.1996.tb09850.x. [DOI] [PubMed] [Google Scholar]
- 22.Musselman DL, Somerset WI, Guo Y, et al. A double-blind, multicenter, parallel-group study of paroxetine, desipramine, or placebo in breast cancer patients (stages I, II, III, and IV) with major depression. J Clin Psychiatry. 2006;67:288–296. doi: 10.4088/jcp.v67n0217. [DOI] [PubMed] [Google Scholar]
- 23.Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): A randomised trial. Lancet. 2008;372:40–48. doi: 10.1016/S0140-6736(08)60991-5. [DOI] [PubMed] [Google Scholar]
- 24.Jacobsen J, Jackson V, Dahlin C, et al. Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med. 2011;14:459–464. doi: 10.1089/jpm.2010.0382. [DOI] [PubMed] [Google Scholar]
- 25.Lo C, Zimmermann C, Rydall A, et al. Longitudinal study of depressive symptoms in patients with metastatic gastrointestinal and lung cancer. J Clin Oncol. 2010;28:3084–3089. doi: 10.1200/JCO.2009.26.9712. [DOI] [PubMed] [Google Scholar]
- 26.Pirl WF, Traeger L, Greer JA, et al. Tumor epidermal growth factor receptor genotype and depression in stage IV non-small cell lung cancer. Oncologist. 2011;16:1299–1306. doi: 10.1634/theoncologist.2011-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.