Abstract
Purpose
Mutations in BRCA1/2 dramatically increase the risk of both breast and ovarian cancers. Three mutations in these genes (185delAG, 5382insC, and 6174delT) occur at high frequency in Ashkenazi Jews. We evaluated how these common Jewish mutations (CJMs) affect cancer risks and risk reduction.
Methods
Our cohort comprised 4,649 women with disease-associated BRCA1/2 mutations from 22 centers in the Prevention and Observation of Surgical End Points Consortium. Of these women, 969 were self-identified Jewish women. Cox proportional hazards models were used to estimate breast and ovarian cancer risks, as well as risk reduction from risk-reducing salpingo-oophorectomy (RRSO), by CJM and self-identified Jewish status.
Results
Ninety-one percent of Jewish BRCA1/2-positive women carried a CJM. Jewish women were significantly more likely to undergo RRSO than non-Jewish women (54% v 41%, respectively; odds ratio, 1.87; 95% CI, 1.44 to 2.42). Relative risks of cancer varied by CJM, with the relative risk of breast cancer being significantly lower in 6174delT mutation carriers than in non-CJM BRCA2 carriers (hazard ratio, 0.35; 95% CI, 0.18 to 0.69). No significant difference was seen in cancer risk reduction after RRSO among subgroups.
Conclusion
Consistent with previous results, risks for breast and ovarian cancer varied by CJM in BRCA1/2 carriers. In particular, 6174delT carriers had a lower risk of breast cancer. This finding requires additional confirmation in larger prospective and population-based cohort studies before being integrated into clinical care.
INTRODUCTION
Mutations in BRCA1 and BRCA2 (BRCA1/2) are well-known genetic risk factors for breast and ovarian cancer (BOC). Lifetime breast cancer (BC) risk among BRCA1/2 carriers has been estimated at 56% to 84%.1–3 Ovarian cancer (OC) risk differs by gene, with BRCA1 associated with a 36% to 63% lifetime risk and BRCA2 mutation carriers having a 10% to 27% lifetime risk.3–6 Cancer risks also vary by mutation location,7–9 by mutations in other genes,10,11 and by exposures including oral contraceptive (OCP) use and reproductive history.12–18 Risk-reducing mastectomy (RRM) and risk-reducing salpingo-oophorectomy (RRSO) significantly reduce cancer risk19,20 and mortality in these women.19,21 According to the National Comprehensive Cancer Network guidelines, RRSO is recommended for all BRCA1/2 mutation carriers by age 35 to 40 or once childbearing is complete.22
The following three common mutations have been identified in Ashkenazi Jewish (AJ) BRCA1/2 mutation carriers: c.68_69delAG (185delAG or 187delAG) and c.5266dupC (5382insC or 5385insC) in BRCA1 and c.5946delT (6174delT) in BRCA2.23–25 The prevalence of these mutations is approximately 2.5% in AJs.2,26,27 5382insC is also common in non-Jewish Eastern European populations, as it entered the AJ population in Poland 400 to 500 years ago through population admixture.28 The high frequency of these mutations has led to the development of panel testing for the three common Jewish mutations (CJMs). Unless their specific mutation is known, AJ women generally undergo an initial round of genetic testing using a CJM panel, which is much less expensive than comprehensive sequencing.29 If the CJM panel is negative, further testing can be considered based on family history and high pretest probability of a BRCA1/2 mutation.29 However, these specific panels may not be appropriate for all Jewish populations, because different founder mutations have been observed in Jews of Sephardic descent, as well as those who have immigrated to Israel from Iraq, Yemen, Iran, and Afghanistan.30–32
Heterogeneity in cancer risk in women who carry CJMs may exist,2,33,34 with BC risk seeming to be lower in 6174delT carriers.34 Additionally, it is unclear whether risk-reduction methods, particularly RRSO, differ in effectiveness by mutation. Therefore, we sought to examine BOC risk and risk reduction in CJM carriers and self-identified Jewish women as a whole using data from the Prevention and Observation of Surgical End Points (PROSE) Consortium.20
METHODS
PROSE Study Cohort
Our study data comprised 4,767 women with disease-associated BRCA1/2 mutations, ascertained between 1973 and 2010 from 22 international centers in the PROSE Consortium. The PROSE study protocol, which was approved by the institutional review board at each participating institution, has been previously described.20,21
Participants were excluded from all analyses if they did not have a confirmed disease-associated BRCA1/2 mutation (n = 101) or if they had mutations in both BRCA1 and BRCA2 (n = 17). After these exclusions, there were 4,649 participants available for analysis. Statistical significance was assessed at a two-sided P < .05 level. Analyses were performed using STATA version 11.1 (STATA, College Station, TX).
CJM and Risk Factor Prevalence in Jewish and Non-Jewish BRCA1/2 Carriers
We identified all mutations reported in self-identified Jewish women (n = 969). Classification of participants as Jewish or non-Jewish was determined entirely by self-report. Furthermore, data were not sufficient to accurately distinguish Ashkenazi or Sephardic ancestry, so the ancestral composition of our cohort was not considered, except as a supplementary analysis. We evaluated mutation prevalence both by individual and by family (ie, counting only one mutation carrier out of multiple carriers in a given family to assess bias from overcounting large families with the same mutation). We compared Jewish with non-Jewish women on several potential BOC risk factors, including birth before 1940, OCP use, parity, age at first live birth, education, first-degree relatives with BOC, RRM, RRSO, and age at surgery. Proportions were compared using the normal approximation to the binomial distribution, and means were compared using the t test. Finally, we explored whether Jewish and non-Jewish women differed in terms of RRM and RRSO uptake using logistic regression to control for the previously mentioned potential confounders as well as BRCA1/2 mutation. Surgeries were considered prophylactic if they occurred before OC diagnosis for RRSO or before BC diagnosis for RRM.
Relative Hazard of BOC
We used Cox proportional hazards models to determine the relative hazard for developing BOC by CJM and Jewish status. The primary end point was first cancer diagnosis. In models including both BRCA1 and BRCA2 carriers, we adjusted for BRCA1/2 mutation. All models controlled for age at ascertainment, parity (> v ≤ one pregnancy resulting in live birth), and OCP use (ever v never use). For all models, we censored participants' follow-up time at death or the date of last contact, and we assumed no competing risks that would preclude the primary outcome. Proportional hazards assumptions were tested for all covariates, and a robust variance-covariance estimation method was used to account for familial clustering,35 because some families had multiple BRCA1/2 carriers.
Participants were observed from date of ascertainment until cancer diagnosis or censoring. For BC analyses, RRM, RRSO, and OC were treated as time-dependent covariates to avoid bias from the change in BC risk after mastectomy or oophorectomy.19,20,36,37 To perform this analysis, we divided participants' follow-up time into separate exposure windows for each time-dependent covariate that occurred during the study follow-up period. We then conditioned our Cox models on the individual to account for multiple exposure windows per participant. Participants were excluded if they had BC (n = 2,030) or were censored (n = 248) before ascertainment or if they were missing necessary data to determine follow-up (n = 9), leaving 2,362 participants available for analysis. These participants underwent a total of 12,070 years of follow-up, with mean and median follow-up times of 5.1 and 3.7 years, respectively. For OC analyses, RRSO was treated as a time-dependent covariate, and participants were excluded if they had OC (n = 437) or were censored (n = 397) before ascertainment or if they were missing necessary data to determine follow-up (n = 28), leaving 3,787 participants available for analysis. These participants underwent a total of 20,638 years of follow-up, with mean and median follow-up times of 5.4 and 4.2 years, respectively.
Because RRSO was treated as a time-dependent covariate, we could examine its effect on BOC risk. To see whether cancer risk reduction from RRSO varied by specific BRCA1/2 mutation, we added an interaction term between RRSO and mutation type to the previously mentioned models. The significance of interactions was tested via a joint Wald test for all levels of the interaction terms.
Absolute Risk of BOC
We estimated absolute risk as the age-specific cumulative incidence of BC and OC for CJM carriers, adapting the method of Antoniou et al.38 Age-specific cumulative incidence rates were calculated using the following formula:
where F′(t) is the CJM-specific cumulative incidence at age t, F(t) is the baseline cumulative incidence at age t, and HR is the mutation-specific hazard ratio (HR). To estimate a baseline cumulative incidence, we calculated the adjusted Kaplan-Meier failure function in our cohort for BC and OC separately. For BC, we adjusted for RRSO, RRM, and mutation type; for OC, we adjusted for RRSO and mutation type. Thus, the baseline risk assumes that individuals do not have a CJM and have not had prophylactic surgery. We used HR 95% CIs to calculate the absolute risk CIs. If there were no observed events in a particular age interval, upper confidence limits were calculated according to the Wilson's score method.39
RESULTS
We observed 39 unique BRCA1 mutations and 31 unique BRCA2 mutations in 969 self-identified Jewish women (Table 1). Ninety-one percent of Jewish women (n = 885) and 10% of non-Jewish women (n = 372) had a CJM. Among Jewish women, two unique non-CJM BRCA1 mutations were seen in more than one family (332-11T>G and del exon 1). All other non-CJM BRCA1/2 mutations among Jewish women were observed in a single family. Data on the usage of various genetic testing procedures to determine mutation status, although incomplete, are summarized in Appendix Table A1 (online only). Data on ethnic background and ancestry were also incomplete; however, Jewish non-CJM carriers did not seem to be substantially different from Jewish CJM carriers in this regard (Appendix Table A2, online only). Mutation prevalence estimates in our cohort were not substantially altered when calculated based on the number of distinct families with a given mutation, rather than based on the number of individuals with a given mutation (data not shown).
Table 1.
Common BRCA1/2 Mutations Overall and by Self-Identified Jewish Status (N = 4,649)
| Group | Total Mutation Carriers |
Mutation Carriers Who Self-Identify As Jewish |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| BRCA1 | ||||
| Total sample | 2,943 | 649 | ||
| 185delAG | 599 | 20 | 454 | 70 |
| 5382insC | 297 | 10 | 149 | 23 |
| 332-11T>G | 6 | 0.2 | 5 | 0.8 |
| del Exon 1 | 2 | 0.07 | 2 | 0.3 |
| Any CJM | 896 | 30 | 603 | 93 |
| Non-CJM* | 2,047 | 70 | 46 | 7.1 |
| BRCA2 | ||||
| Total sample | 1,706 | 320 | ||
| 6174delT | 361 | 21 | 282 | 88 |
| Any CJM | 361 | 21 | 282 | 88 |
| Non-CJM† | 1,345 | 79 | 38 | 12 |
NOTE. Mutations were considered common if they were present in more than one family in our cohort.
Abbreviation: CJM, common Jewish mutation.
Unique non-CJM BRCA1mutations among self-identified Jewish women were 1240delC, 1675delA, 185insA, 187delT, 188del11, 1VS4-1G>T, 2072del4, 2294delG, 2594delC, 2606delT, 2800delAA, 2838del4, 360C>T, 3875del4, 3878delTA, 4184del4, 5055delG, 5060delC, 5221delTG, A1708E, C2457T, C4731T, E1250X (3867G>T), E84X, exon 18 G5255A, IVS12 + 1G>T, IVS23-2A>G, IVS5 + 3A>G, Q541X(174OC>T), R1443X, S713X, S955X, 332-11T>G, Y978X, c.1505_1509delTAAAG, del exon 1, and del exons 3-8.
Unique non-CJM BRCA2 mutations among self-identified Jewish women were 1815delTinsCA, 2041insA, 279delAC, 3034del4, 3773delTT, 4075delGT, 5164del4, 5301insA, 5358del4, 5466insT, 6048del14, 6056delC, 6252insG, 6357insA, 6659delA, 6672insT, 8061del10, 8504del4, 9610C>T, C2256X, E2918E, G1639T, I2627F (8107A>T), IVS9 + 1G>T, K944X, Q1994X, Q2042X, R2336P, S1121X, and Y1894X.
Jewish women were less likely to have used OCPs, were older at first live birth, were more likely to have higher education, and were older at ascertainment (Table 2). Jewish women, compared with non-Jewish women, were also less likely to have first-degree relatives with either BC (53% v 63%, respectively) or OC (24% v 28%, respectively). However, Jewish women had significantly smaller families on average and were not different in percentage of first-degree relatives with BOC. Among Jewish women, 54% underwent RRSO, whereas 41% of non-Jewish women underwent RRSO (adjusted odds ratio, 1.87; 95% CI, 1.44 to 2.42). However, Jewish women were significantly older when they did undergo RRSO. Jewish women were not more likely to have undergone RRM (adjusted odds ratio, 1.02; 95% CI, 0.69 to 1.52).
Table 2.
Clinical Characteristics and Surgical Uptake by Self-Identified Jewish Status (N = 4,649)
| Clinical Characteristic | Overall | Non-Jewish | Jewish | P* |
|---|---|---|---|---|
| Age at ascertainment, years | < .001 | |||
| Mean | 43.5 | 42.7 | 46.6 | |
| SD | 12.7 | 12.6 | 12.4 | |
| Born before 1940 | .11 | |||
| No. | 486 | 371 | 115 | |
| % | 10.5 | 10.1 | 11.9 | |
| Ever used oral contraceptive pills | .02 | |||
| No. | 3,105 | 2,418 | 687 | |
| % | 77.5 | 78.3 | 74.6 | |
| High parity (> 1 term birth) | .31 | |||
| No. | 2,858 | 2,245 | 613 | |
| % | 63.0 | 62.6 | 64.4 | |
| Age at first term birth, years | < .001 | |||
| Mean | 25.9 | 25.2 | 28.2 | |
| SD | 5.3 | 5.2 | 5.0 | |
| More than high school education | < .001 | |||
| No. | 2,049 | 1,555 | 494 | |
| % | 81.0 | 77.6 | 93.9 | |
| No. of first-degree family relatives | < .001 | |||
| Mean | 3.8 | 4.1 | 2.6 | |
| SD | 2.7 | 2.9 | 1.6 | |
| Known first-degree family relative with breast cancer† | < .001 | |||
| No. | 2,845 | 2,332 | 513 | |
| % | 61.2 | 63.4 | 52.9 | |
| No. of first-degree family relatives with breast cancer | < .001 | |||
| Mean | 1.0 | 1.1 | 0.7 | |
| SD | 1.0 | 1.1 | 0.9 | |
| % of first-degree family relatives with breast cancer | .82 | |||
| Mean | 30.6 | 30.6 | 30.4 | |
| SD | 31.4 | 30.5 | 34.5 | |
| Known first-degree family relative with ovarian cancer† | .005 | |||
| No. | 1,269 | 1,039 | 230 | |
| % | 27.3 | 28.2 | 23.7 | |
| No. of first-degree family relatives with ovarian cancer | < .001 | |||
| Mean | 0.4 | 0.4 | 0.3 | |
| SD | 0.6 | 0.7 | 0.6 | |
| % of first-degree family relatives with ovarian cancer | .09 | |||
| Mean | 11.2 | 10.9 | 12.4 | |
| SD | 22.2 | 21.3 | 25.4 | |
| Known first-degree family relative with breast or ovarian cancer | < .001 | |||
| No. | 3,387 | 2,746 | 641 | |
| % | 72.9 | 74.6 | 66.2 | |
| Known first- or second-degree family relative with breast or ovarian cancer | .001 | |||
| No. | 4,025 | 3,217 | 808 | |
| % | 86.6 | 87.4 | 83.4 | |
| RRSO | < .001 | |||
| No. | 2,024 | 1,502 | 522 | |
| % | 43.5 | 40.8 | 53.9 | |
| Age at RRSO, years | < .001 | |||
| Mean | 45.9 | 45.4 | 47.3 | |
| SD | 8.5 | 8.4 | 8.8 | |
| RRM | .48 | |||
| No. | 461 | 358 | 103 | |
| % | 11.8 | 11.6 | 12.5 | |
| Age at RRM, years | .16 | |||
| Mean | 40.9 | 40.6 | 42.0 | |
| SD | 8.7 | 8.6 | 9.0 |
Abbreviations: RRM, risk-reducing mastectomy; RRSO, risk-reducing salpingo-oophorectomy; SD, standard deviation.
P values based on the t test for continuous variables and on the normal approximation to the binomial distribution for categorical variables.
First-degree relatives with cancer were determined by self-report. We were unable to assess whether individuals either chose not to report or were unaware of family cancer history as a result of social, cultural, or other factors, such as a cultural stigma associated with cancer diagnosis or genetic abnormalities.
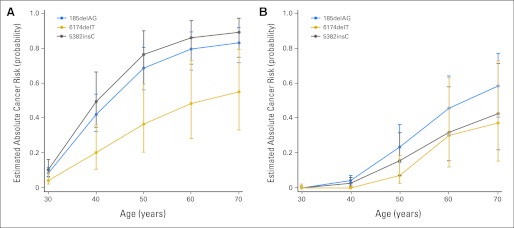
BC relative hazard was significantly lower in 6174delT carriers than in non-CJM BRCA2 carriers (Table 3; HR, 0.35; 95% CI, 0.18 to 0.69). Other BOC relative hazards in specific CJMs and in self-identified Jewish women were not significantly different from their respective reference groups (Tables 3 and 4). In a sensitivity analysis, relative hazard estimates were similar when OC and death were treated as competing risks, rather than time-dependent and censoring events, respectively (data not shown). Estimated cumulative incidence of BOC by CJM is shown in Figure 1 and Table 5. Overall, RRSO significantly reduced the relative hazard of BC (HR, 0.62; 95% CI, 0.47 to 0.83) and OC (HR, 0.08; 95% CI, 0.04 to 0.16). No significant difference in BC hazard reduction from RRSO was observed among specific CJM carriers (joint Wald test, P = .61). In addition, no apparent difference in OC hazard reduction from RRSO was observed among specific CJM carriers, although there were not enough events in our cohort to formally conduct a test of interaction.
Table 3.
Breast Cancer Hazard Ratios in Specific Groups Among Prospective Study Participants (n = 2,362)
| Group | No. of Participants | Age of Ascertainment (years) |
Follow-Up (years) |
No. of Participants Diagnosed With Cancer | Age at Diagnosis (years) |
Hazard Ratio | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | |||||
| BRCA1 | ||||||||||
| Non-CJM | 1,087 | 37.9 | 2.2-88.6 | 5.7 | 0.0-33.3 | 171 | 42.3 | 22.2-72.7 | Ref | |
| 185delAG | 312 | 41.3 | 10.2-90.4 | 4.7 | 0.0-33.1 | 51 | 43.5 | 27.6-73.2 | 1.23 | 0.87 to 1.73 |
| 5382insC | 124 | 41.4 | 12.3-87.6 | 4.6 | 0.0-18.1 | 27 | 43.2 | 24.9-62.7 | 1.53 | 0.96 to 2.45 |
| BRCA2 | ||||||||||
| Non-CJM | 663 | 40.5 | 2.0-89.3 | 4.6 | 0.0-28.3 | 134 | 45.9 | 19.1-80.1 | Ref | |
| 6174delT | 176 | 45.4 | 17.7-79.1 | 4.1 | 0.0-16.2 | 13 | 48.1 | 32.5-70.5 | 0.35 | 0.18 to 0.69 |
| Non-Jewish | 1,874 | 39.1 | 2.0-89.3 | 5.2 | 0.0-33.3 | 334 | 43.7 | 19.1-80.1 | Ref | |
| Jewish | 488 | 42.7 | 10.2-90.4 | 4.7 | 0.0-33.1 | 62 | 45.2 | 27.6-73.2 | 0.76 | 0.56 to 1.01 |
| RRSO | ||||||||||
| No | 1,599 | 38.2 | 2.0-90.4 | 4.5 | 0.0-28.3 | 317 | 42.9 | 19.1-80.1 | Ref | |
| Yes | 763 | 43.2 | 15.4-83.7 | 6.5 | 0.0-33.3 | 79 | 47.9 | 33.3-72.7 | 0.62 | 0.47 to 0.83 |
Abbreviations: CJM, common Jewish mutation; Ref, reference; RRSO, risk-reducing salpingo-oophorectomy.
Table 4.
Ovarian Cancer Hazard Ratios in Specific Groups Among Prospective Study Participants (n = 3,787)
| Group | No. of Participants | Age of Ascertainment (years) |
Follow-Up (years) |
No. of Participants Diagnosed With Cancer | Age at Diagnosis (years) |
Hazard Ratio | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | |||||
| BRCA1 | ||||||||||
| Non-CJM | 1,668 | 40.2 | 2.2-88.6 | 6.0 | 0.0-33.3 | 96 | 50.6 | 30.1-89.3 | Ref | |
| 185delAG | 472 | 43.5 | 10.2-90.4 | 5.1 | 0.0-33.1 | 20 | 53.2 | 36.8-74.0 | 0.97 | 0.58 to 1.63 |
| 5382insC | 228 | 43.4 | 12.3-87.6 | 5.6 | 0.0-25.4 | 6 | 53.6 | 44.8-63.3 | 0.61 | 0.27 to 1.38 |
| BRCA2 | ||||||||||
| Non-CJM | 1,132 | 43.1 | 2.0-89.3 | 5.1 | 0.0-28.3 | 24 | 55.8 | 44.0-74.9 | Ref | |
| 6174delT | 287 | 47.5 | 17.7-76.6 | 4.1 | 0.0-16.2 | 5 | 59.8 | 49.4-76.4 | 1.34 | 0.48 to 3.73 |
| Non-Jewish | 3,034 | 41.5 | 2.0-89.3 | 5.6 | 0.0-33.3 | 127 | 51.7 | 30.1-89.3 | Ref | |
| Jewish | 753 | 45.1 | 10.2-90.4 | 5.0 | 0.0-33.1 | 24 | 54.4 | 36.8-76.4 | 0.93 | 0.59 to 1.46 |
| RRSO | ||||||||||
| No | 2,086 | 39.7 | 2.0-90.4 | 4.5 | 0.0-28.3 | 139 | 52.4 | 30.1-89.3 | Ref | |
| Yes | 1,701 | 45.3 | 15.4-83.7 | 6.6 | 0.0-33.3 | 12 | 49.7 | 37.8-61.6 | 0.08 | 0.04 to 0.16 |
Abbreviations: CJM, common Jewish mutation; Ref, reference; RRSO, risk-reducing salpingo-oophorectomy.
Fig 1.
Estimated age-specific cumulative risk of (A) breast and (B) ovarian cancers by common Jewish mutations (CJMs). The average proportion of specific CJM carriers developing cancer by a given age was calculated based on the hazard ratios shown in Table 3, with baseline cancer risks adjusted for no CJM and no prophylactic surgery. Error bars represent 95% CIs. Numerical values for these risks are listed in Table 4.
Table 5.
Estimated Cumulative Incidence of Breast and Ovarian Cancer by CJM
| Cancer | Estimated Mutation-Specific Cumulative Incidence of Cancer by Age |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30 Years |
40 Years |
50 Years |
60 Years |
70 Years |
||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Breast cancer | ||||||||||
| BRCA1, no CJM* | 7.0 | 36 | 61 | 72 | 76 | |||||
| 185delAG | 8.5 | 6.1 to 12 | 42 | 32 to 54 | 69 | 56 to 80 | 79 | 68 to 89 | 83 | 72 to 92 |
| 5382insC | 11 | 6.7 to 16 | 49 | 35 to 66 | 76 | 59 to 90 | 86 | 71 to 96 | 89 | 75 to 97 |
| BRCA2, no CJM* | 12 | 48 | 73 | 85 | 90 | |||||
| 6174delT | 4.3 | 2.2 to 8.4 | 20 | 11 to 36 | 37 | 21 to 59 | 48 | 28 to 73 | 55 | 33 to 79 |
| Ovarian cancer | ||||||||||
| BRCA1, no CJM* | 0.0 | 4.4 | 24 | 47 | 60 | |||||
| 185delAG | 0.0 | 0.0 to 1.0† | 4.2 | 2.5 to 7.0 | 23 | 15 to 36 | 46 | 30 to 64 | 58 | 41 to 77 |
| 5382insC | 0.0 | 0.0 to 1.0† | 2.7 | 1.2 to 6.0 | 16 | 7.2 to 32 | 32 | 16 to 58 | 42 | 22 to 71 |
| BRCA2, no CJM* | 0.0 | 0.0 | 5.3 | 23 | 29 | |||||
| 6174delT | 0.0 | 0.0 to 2.2† | 0.0 | 0.0 to 0.7† | 7.0 | 2.6 to 18 | 30 | 12 to 63 | 37 | 15 to 73 |
Abbreviation: CJM, common Jewish mutation.
Because baseline risks were adjusted for no CJM or prophylactic surgery, we could not estimate 95% CIs.
Upper confidence limits for cells with no observed events were calculated according to Wilson's score method.
DISCUSSION
Previous reports in retrospective cohorts and cross-sectional samples have estimated BOC risks conferred by CJMs. Using a cohort with 120 CJM carriers, Struewing et al2 estimated that AJ CJM carriers had a 56% risk of BC and a 16% risk of OC by age 70 years. Antoniou et al34 conducted a meta-analysis containing pedigree data from 196 CJM carriers, showing that 185delAG conferred a 64% and 14% risk, 5382insC conferred a 67% and 33% risk, and 6174delT conferred a 43% and 20% risk of BC and OC by age 70 years, respectively. Our cohort was larger than these, comprising 4,649 BRCA1/2 carriers, of whom 1,257 had a CJM. Additionally, 2,382 participants (including 608 CJM carriers) in our cohort were ascertained prospectively (ie, they did not have BC or OC before ascertainment), which ensures that we were theoretically able to observe cancer cases for all individuals who were at risk for developing cancer in our analyses.
Our finding of heterogeneity in BOC risk among CJM carriers is consistent with previous results.2,33,34 Of note, 6174delT conferred a lower risk of BC compared with non-CJM BRCA2 mutations (Table 3). By contrast, OC risk in this group was not significantly different from the risk in those with non-CJM BRCA2 mutations. These findings are consistent with the location of 6174delT within the OC cluster region in BRCA2, a region in exon 11 of BRCA2 that has been associated with an increased risk of OC relative to BC.40 Notably, the prospectively ascertained 6174delT carriers in our cohort were older at ascertainment than carriers of other mutations (Table 3); however, it is unclear what is causing this discrepancy and whether it could have substantially impacted our results. Conceivably, we may have failed to ascertain certain 6174delT carriers who would have developed BC at a younger age. Alternatively, later ascertainment may simply reflect lower rates of BOC among first-degree relatives of 6174delT carriers than non-CJM BRCA2 carriers (69% v 78%, respectively; P = .005).
Our estimates of absolute risk are consistent with some of the original studies to report BOC risk estimates in BRCA1/2 carriers overall that were based on multiple-case families (Table 5).4,41–43 However, our risk estimates are substantially higher than more recent studies that derived their estimates of BOC risk in CJM carriers and BRCA1/2 carriers overall from population-based cohorts.2,5,44–46
One possible explanation for the observed elevated BOC risk estimates in our cohort is ascertainment bias, resulting from individuals at a higher risk for cancer being more likely to seek out and enroll onto studies than those at lower risk for cancer. This effect has been documented in several previous studies, especially those that were based on familial aggregation of cancer in high-risk families.47 Although our study is a large cohort study rather than a familial aggregation study, it is still quite likely that individuals from high-risk families preferentially enrolled onto our study. In our cohort, 73% of BRCA1/2 carriers had a known first-degree relative with BOC, making them more likely to be from a high-risk family. However, in a sensitivity analysis, having a first-degree relative with BOC was not significantly associated with an increased hazard of either BC (HR, 0.93; 95% CI, 0.70 to 1.23) or OC (HR, 1.21; 95% CI, 0.78 to 1.89). As a result, it is difficult to determine from our own data the extent to which ascertainment bias may be impacting our results.
Additionally, our risk estimates were based only on prospectively ascertained participants, and these individuals, in theory, may have systematically higher baseline cancer risk than retrospectively ascertained individuals for reasons similar to those described earlier. In a sensitivity analysis, however, prospectively ascertained individuals in our cohort had a significantly lower hazard of both BC (HR, 0.76; 95% CI, 0.68 to 0.85) and OC (HR, 0.79; 95% CI, 0.64 to 0.98). Thus, the exclusion of retrospectively ascertained individuals seems unlikely to account for the elevated BOC risks observed in our study.
Furthermore, as has been previously argued,38 the vast majority of individuals who seek BRCA1/2 genetic testing come from high-risk families with multiple cases, the same types of individuals who would be most likely to enroll onto our study. As a result, our estimates may not be especially high in this target population, as opposed to the general population of CJM carriers. However, because of uncertainty in the accuracy of our estimates, more studies involving prospective and population-based cohorts will be essential before mutation-specific BOC risk estimates can be used as a basis for clinical risk management decisions. Our results should prompt others to update and re-evaluate their data, so the most accurate possible penetrance estimates can be made available to women with BRCA1/2 mutations.
In our cohort, Jewish women were significantly more likely than non-Jewish women to undergo RRSO (54% v 41%, respectively). This discrepancy may indicate increased awareness, acceptance, or access to RRSO among Jewish women or their physicians. There was no difference between Jewish and non-Jewish women in use of RRM.
Overall, RRSO significantly reduced the hazard of BOC (Tables 3 and 4), consistent with previous studies in BRCA1/2 mutation carriers.19,20 HRs were not significantly different for each of the specific CJMs, suggesting that RRSO is equally effective regardless of BRCA1/2 mutation. The efficacy of RRSO is especially impressive given that 35% of CJM carriers and 33% of non-CJM BRCA1/2 carriers receiving RRSO were postmenopausal at the time of surgery in our cohort. In a sensitivity analysis, however, RRSO was not found to be significantly less effective at reducing BC risk if performed after menopause (test for interaction, P = .70), although we were likely underpowered for such an analysis. We did not have enough events to determine whether RRSO was less effective at reducing OC risk if performed after menopause.
Our data indicate the potential for using mutation-specific information to determine individual cancer risk, although additional validation may be required before counseling based on mutation-specific risks is ready for clinical practice. BOC risks seem to vary by CJM, with 6174delT conferring a lower risk of BC than other mutations. However, our data are limited to average cancer risks among a population. Individual cancer risk may still vary substantially around this average, with some individuals having a low risk and some having a high risk of BOC. Until accurate individualized risk prediction can be accomplished, perhaps including knowledge of genetic and environmental risk modifiers, we do not suggest any alteration from current management approaches in BRCA1/2 mutation carriers as a whole.
In our cohort, 72% of Jewish BRCA1/2 carriers with no first- or second-degree relatives with BOC were ascertained after being diagnosed with cancer themselves. Furthermore, among these women, 84% were CJM carriers. Because effective interventions exist that reduce mortality in BRCA1/2 carriers,19 there may be a benefit to case-based screening and, possibly, to wider, population-based screening for CJMs. Furthermore, cost-utility analyses have predicted that implementing a CJM screening program in the US Jewish population would lead to 2,800 fewer cases of OC and cost about $8,300 per quality-adjusted life-year gained,48 compared with $10,000 to $25,000 per quality-adjusted life-year gained for mammographic screening in the general population.49 Thus, a discussion among key constituencies on wider screening for CJMs in the AJ population seems warranted. Such a screening program would have complex social, cultural, and policy implications,48,50 and clinicians and genetic counselors would need to be alert to the many unique aspects of genetic testing in the Jewish population.51–55
Our findings help fill a gap in knowledge of BOC risk and risk reduction in Jewish women who carry BRCA1/2 mutations. There seems to be variability in BOC risks conferred by the different CJMs, with 6174delT carriers in particular seeming to have a lower BC risk. Thus, a patient's specific mutation could one day provide clinically relevant information about individual cancer risk. RRSO seemed equally effective at reducing both BC and OC risks in all subgroups, suggesting that this is an appropriate risk-reduction strategy regardless of specific mutation. These results may ultimately help improve individualized risk assessment and management strategies in the Jewish population.
Acknowledgment
We acknowledge an anonymous reviewer for valuable comments regarding our analyses. We would also like to acknowledge Niecee Schonberger, who participated in reading and comments on behalf of Sharsheret, as well as the following people from The Institute of Cancer Research and Royal Marsden National Health Service Trust for their help in patient recruitment: Elizabeth Page, Susan Shanley, Astrid Stormorken, Jennifer Wiggins, Kelly Kohut, Audrey Ardern-Jones, and Elena Castro.
Appendix
Table A1.
Type of BRCA1/2 Mutation Testing (N = 4,649)
| Testing Type |
BRCA1 |
BRCA2 |
||||||
|---|---|---|---|---|---|---|---|---|
| Total |
Self-Reported Jewish |
Total |
Self-Reported Jewish |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Test not performed* | 461 | 9.9 | 24 | 2.5 | 875 | 18.8 | 97 | 10 |
| SSCP | 17 | 0.4 | 3 | 0.3 | 3 | 0.1 | 0 | 0.0 |
| SSCP + heteroduplex analysis | 118 | 2.5 | 22 | 2.3 | 101 | 2.2 | 18 | 1.9 |
| CSGE | 29 | 0.6 | 3 | 0.3 | 24 | 0.5 | 3 | 0.3 |
| Denaturing high-performance liquid chromatography | 364 | 7.8 | 1 | 0.1 | 195 | 4.2 | 1 | 0.1 |
| Southern blot | 4 | 0.1 | 0 | 0.0 | 41 | 0.9 | 8 | 0.8 |
| Protein truncation assay | 42 | 0.9 | 2 | 0.2 | 38 | 0.8 | 1 | 0.1 |
| Full gene sequencing or CSGE | 868 | 18.7 | 141 | 14.6 | 756 | 16.3 | 110 | 11.4 |
| Partial gene sequencing | 17 | 0.4 | 3 | 0.3 | 66 | 1.4 | 30 | 3.1 |
| Jewish panel | 427 | 9.2 | 360 | 37.2 | 400 | 8.6 | 342 | 35.3 |
| Other† | 87 | 1.9 | 27 | 2.8 | 12 | 0.3 | 0 | 0.0 |
| Single family mutation | 498 | 10.7 | 31 | 3.2 | 370 | 8.0 | 21 | 2.2 |
| Missing | 1,717 | 36.9 | 352 | 36.3 | 1,768 | 38.0 | 338 | 34.9 |
NOTE. Data represent first reported testing procedure. Data on multiple testing procedures in a single participant, if performed, are unavailable.
Abbreviations: CSGE, conformation-sensitive gel electrophoresis; SSCP, single-strand conformation polymorphism.
Some participants did not receive testing for mutations in both BRCA1and BRCA2; however, all participants received testing for mutations in at least one of the two genes.
Other mutation testing procedures include multiplex ligation-dependent probe amplification, heteroduplex analysis, and quantitative polymerase chain reaction, as well as unspecified procedures.
Table A2.
Reported Ethnic Composition by CJM Status in Self-Identified Jewish Individuals (n = 783)
| Ethnicity | Non-CJM Mutation (n = 68) |
CJM Mutation (n = 715) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Jewish, unspecified | 2 | 2.9 | 41 | 5.7 | .33 |
| Jewish, Ashkenazi | 64 | 94 | 649 | 91 | .36 |
| Jewish, Sephardic | 3 | 4.4 | 5 | 0.7 | .004 |
| White, unknown country of origin | 39 | 57 | 343 | 48 | .14 |
| English | 2 | 2.9 | 23 | 3.2 | .90 |
| German | 4 | 5.9 | 29 | 4.1 | .47 |
| Polish | 3 | 4.4 | 45 | 6.3 | .54 |
| Russian | 4 | 5.9 | 76 | 11 | .22 |
NOTE. The most common ethnicities plus Sephardic ancestry are listed. Not all participants reported ethnicity. Individuals could report up to four ethnicities, so percentages add up to greater than 100%.
Abbreviation: CJM, common Jewish mutation.
P values are based on the normal approximation to the binomial distribution.
Footnotes
Written on behalf of the Prevention and Observation of Surgical End Points Consortium.
Supported by National Institutes of Health (NIH) Grants No. R01-CA083855 and R01-CA102776 (T.R.R.) and by Medical Scientist Training Program Grant No. T32-GM07170 from the NIH, as well as institutional funds from the University of Pennsylvania School of Medicine (B.S.F.). C.I. is supported by the Cancer Genetics Network and by National Cancer Institute Grant No. P30-CA051008-17. R.E. also receives support from the National Institute for Health Research to The Biomedical Research Center at The Institute of Cancer Research and Royal Marsden National Health Service (NHS) Foundation Trust. Part of the Carrier Clinic at The Institute of Cancer Research and Royal Marsden NHS Foundation Trust receives support from Cancer Research United Kingdom Grant No. C5047/A8385.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Rosalind Eeles, Tepnel (Gen-Probe), Vista Diagnostics, Illumina Expert Testimony: None Other Remuneration: Fergus J. Couch, Myriad Genetics and Laboratories
AUTHOR CONTRIBUTIONS
Conception and design: Brian S. Finkelman, Wendy S. Rubinstein, Sue Friedman, Tara M. Friebel, Judy E. Garber, Mary B. Daly, Susan L. Neuhausen, D. Gareth Evans, Steven A. Narod, Timothy R. Rebbeck
Financial support: Timothy R. Rebbeck
Administrative support: Timothy R. Rebbeck
Provision of study materials or patients: Olufunmilayo I. Olopade, Henry T. Lynch, Claudine Isaacs, Rosalind Eeles, D. Gareth Evans, Timothy R. Rebbeck
Collection and assembly of data: Brian S. Finkelman, Wendy S. Rubinstein, Tara M. Friebel, Christian F. Singer, Joanne L. Blum, Nadine Tung, Olufunmilayo I. Olopade, Jeffrey N. Weitzel, Henry T. Lynch, Carrie Snyder, Judy E. Garber, Joellen Schildkraut, Mary B. Daly, Claudine Isaacs, Gabrielle Pichert, Susan L. Neuhausen, Fergus J. Couch, Laura van't Veer, Rosalind Eeles, Elizabeth Bancroft, D. Gareth Evans, Patricia A. Ganz, Gail E. Tomlinson, Steven A. Narod, Ellen Matloff, Susan Domchek, Timothy R. Rebbeck
Data analysis and interpretation: Brian S. Finkelman, Wendy S. Rubinstein, Sue Friedman, Tara M. Friebel, Shera Dubitsky, Niecee Schonberger, Rochelle Shoretz, Christian F. Singer, Joanne L. Blum, Nadine Tung, Jeffrey N. Weitzel, Henry T. Lynch, Carrie Snyder, Judy E. Garber, Mary B. Daly, Claudine Isaacs, Susan L. Neuhausen, Laura van't Veer, D. Gareth Evans, Patricia A. Ganz, Steven A. Narod, Susan Domchek, Timothy R. Rebbeck
Manuscript writing: All authors
Final approval of manuscript: All authors
Affiliations
Brian S. Finkelman, Tara M. Friebel, Susan Domchek, and Timothy R. Rebbeck, Perelman School of Medicine, University of Pennsylvania; Mary B. Daly, Fox Chase Cancer Centre, Philadelphia, PA; Wendy S. Rubinstein, NorthShore University HealthSystem, Evanston; Olufunmilayo I. Olopade, University of Chicago, Chicago, IL; Sue Friedman, FORCE: Facing Our Risk of Cancer Empowered, Tampa, FL; Shera Dubitsky, Neicee Singer Schonberger, Rochelle Shoretz, Sharsheret, Teaneck, NJ; Joanne L. Blum, Baylor-Charles A. Sammons Cancer Center; Gail E. Tomlinson, University of Texas Southwestern Medical Center, Dallas; Gail E. Tomlinson, University of Texas Health Science Center at San Antonio, San Antonio, TX; Nadine Tung, Beth Israel Deaconess Medical Center; Judy E. Garber, Dana-Farber Cancer Institute, Boston, MA; Jeffrey N. Weitzel and Susan L. Neuhausen, City of Hope Comprehensive Cancer Center and Beckman Research Institute, City of Hope, Duarte; Patricia A. Ganz, Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, CA; Henry T. Lynch and Carrie Snyder, Creighton University, Omaha, NE; Joellen Schildkraut, Duke University Medical Center, Durham, NC; Claudine Isaacs, Lombardi Cancer Center, Georgetown University, Washington, DC; Fergus J. Couch, Mayo Clinic College of Medicine, Rochester, MN; Ellen Matloff, Yale Cancer Center, New Haven, CT; Christian F. Singer, Medical University of Vienna, Vienna, Austria; Gabrielle Pichert, Guy's Hospital, London; Rosalind Eeles, Elizabeth Bancroft, The Institute of Cancer Research and Royal Marsden National Health Service Trust, Sutton, Surrey; D. Gareth Evans, St Mary's Hospital, Manchester, United Kingdom; Laura van't Veer, Netherlands Cancer Institute, Amsterdam, the Netherlands; and Steven A. Narod, Women's College Hospital, Toronto, Ontario, Canada.
REFERENCES
- 1.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 2.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families: The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anglian Breast Cancer Study Group. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer. 2000;83:1301–1308. doi: 10.1054/bjoc.2000.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satagopan JM, Boyd J, Kauff ND, et al. Ovarian cancer risk in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Clin Cancer Res. 2002;8:3776–3781. [PubMed] [Google Scholar]
- 7.Gayther SA, Warren W, Mazoyer S, et al. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995;11:428–433. doi: 10.1038/ng1295-428. [DOI] [PubMed] [Google Scholar]
- 8.Thompson D, Easton D. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68:410–419. doi: 10.1086/318181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lubinski J, Phelan CM, Ghadirian P, et al. Cancer variation associated with the position of the mutation in the BRCA2 gene. Fam Cancer. 2004;3:1–10. doi: 10.1023/B:FAME.0000026816.32400.45. [DOI] [PubMed] [Google Scholar]
- 10.Milne RL, Antoniou AC. Genetic modifiers of cancer risk for BRCA1 and BRCA2 mutation carriers. Ann Oncol. 2011;22(suppl 1):i11–i17. doi: 10.1093/annonc/mdq660. [DOI] [PubMed] [Google Scholar]
- 11.Gaudet MM, Kirchhoff T, Green T, et al. Common genetic variants and modification of penetrance of BRCA2-associated breast cancer. PLoS Genet. 2010;6:e1001183. doi: 10.1371/journal.pgen.1001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: A meta-analysis. Eur J Cancer. 46:2275–2284. doi: 10.1016/j.ejca.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Milne RL, Osorio A, Ramon y, Cajal T, et al. Parity and the risk of breast and ovarian cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 119:221–232. doi: 10.1007/s10549-009-0394-1. [DOI] [PubMed] [Google Scholar]
- 14.Narod SA, Dube MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773–1779. doi: 10.1093/jnci/94.23.1773. [DOI] [PubMed] [Google Scholar]
- 15.Newcomb PA, Trentham-Dietz A, Hampton JM, et al. Late age at first full term birth is strongly associated with lobular breast cancer. Cancer. 2011;117:1946–1956. doi: 10.1002/cncr.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antoniou AC, Shenton A, Maher ER, et al. Parity and breast cancer risk among BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2006;8:R72. doi: 10.1186/bcr1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brohet RM, Goldgar DE, Easton DF, et al. Oral contraceptives and breast cancer risk in the international BRCA1/2 carrier cohort study: A report from EMBRACE, GENEPSO, GEO-HEBON, and the IBCCS Collaborating Group. J Clin Oncol. 2007;25:3831–3836. doi: 10.1200/JCO.2007.11.1179. [DOI] [PubMed] [Google Scholar]
- 18.Chang-Claude J, Andrieu N, Rookus M, et al. Age at menarche and menopause and breast cancer risk in the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol Biomarkers Prev. 2007;16:740–746. doi: 10.1158/1055-9965.EPI-06-0829. [DOI] [PubMed] [Google Scholar]
- 19.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 21.Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: A prospective cohort study. Lancet Oncol. 2006;7:223–229. doi: 10.1016/S1470-2045(06)70585-X. [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network: Genetic/Familial High-Risk Assessment. Breast and Ovarian, NCCN Clinical Practice Guidelines in Oncology (ed v. 1.2011) http://www.nccn.org/professionals/physician_ gls/f_guidelines.asp. [DOI] [PubMed]
- 23.Neuhausen S, Gilewski T, Norton L, et al. Recurrent BRCA2 6174delT mutations in Ashkenazi Jewish women affected by breast cancer. Nat Genet. 1996;13:126–128. doi: 10.1038/ng0596-126. [DOI] [PubMed] [Google Scholar]
- 24.Oddoux C, Struewing JP, Clayton CM, et al. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1% Nat Genet. 1996;14:188–190. doi: 10.1038/ng1096-188. [DOI] [PubMed] [Google Scholar]
- 25.Neuhausen SL, Mazoyer S, Friedman L, et al. Haplotype and phenotype analysis of six recurrent BRCA1 mutations in 61 families: Results of an international study. Am J Hum Genet. 1996;58:271–280. [PMC free article] [PubMed] [Google Scholar]
- 26.Hartge P, Struewing JP, Wacholder S, et al. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet. 1999;64:963–970. doi: 10.1086/302320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roa BB, Boyd AA, Volcik K, et al. Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet. 1996;14:185–187. doi: 10.1038/ng1096-185. [DOI] [PubMed] [Google Scholar]
- 28.Hamel N, Feng BJ, Foretova L, et al. On the origin and diffusion of BRCA1 c.5266dupC (5382insC) in European populations. Eur J Hum Genet. 2011;19:300–306. doi: 10.1038/ejhg.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berliner JL, Fay AM. Risk assessment and genetic counseling for hereditary breast and ovarian cancer: Recommendations of the National Society of Genetic Counselors. J Genet Couns. 2007;16:241–260. doi: 10.1007/s10897-007-9090-7. [DOI] [PubMed] [Google Scholar]
- 30.Sagi M, Eilat A, Ben Avi L, et al. Two BRCA1/2 founder mutations in Jews of Sephardic origin. Fam Cancer. 2011;10:59–63. doi: 10.1007/s10689-010-9395-9. [DOI] [PubMed] [Google Scholar]
- 31.Lerer I, Wang T, Peretz T, et al. The 8765delAG mutation in BRCA2 is common among Jews of Yemenite extraction. Am J Hum Genet. 1998;63:272–274. doi: 10.1086/301924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiri-Sverdlov R, Gershoni-Baruch R, Ichezkel-Hirsch G, et al. The Tyr978X BRCA1 mutation in non-Ashkenazi Jews: Occurrence in high-risk families, general population and unselected ovarian cancer patients. Community Genet. 2001;4:50–55. doi: 10.1159/000051156. [DOI] [PubMed] [Google Scholar]
- 33.Levy-Lahad E, Catane R, Eisenberg S, et al. Founder BRCA1 and BRCA2 mutations in Ashkenazi Jews in Israel: Frequency and differential penetrance in ovarian cancer and in breast-ovarian cancer families. Am J Hum Genet. 1997;60:1059–1067. [PMC free article] [PubMed] [Google Scholar]
- 34.Antoniou AC, Pharoah PD, Narod S, et al. Breast and ovarian cancer risks to carriers of the BRCA1 5382insC and 185delAG and BRCA2 6174delT mutations: A combined analysis of 22 population based studies. J Med Genet. 2005;42:602–603. doi: 10.1136/jmg.2004.024133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin D. Robust inferences for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 36.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meijers-Heijboer H, van Geel B, van Putten WL, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:159–164. doi: 10.1056/NEJM200107193450301. [DOI] [PubMed] [Google Scholar]
- 38.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. [Google Scholar]
- 40.Gayther SA, Mangion J, Russell P, et al. Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet. 1997;15:103–105. doi: 10.1038/ng0197-103. [DOI] [PubMed] [Google Scholar]
- 41.Ford D, Easton DF, Bishop DT, et al. Risks of cancer in BRCA1-mutation carriers: Breast Cancer Linkage Consortium. Lancet. 1994;343:692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 42.Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers: Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265–271. [PMC free article] [PubMed] [Google Scholar]
- 43.Antoniou AC, Gayther SA, Stratton JF, et al. Risk models for familial ovarian and breast cancer. Genet Epidemiol. 2000;18:173–190. doi: 10.1002/(SICI)1098-2272(200002)18:2<173::AID-GEPI6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 44.Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hopper JL, Southey MC, Dite GS, et al. Population-based estimate of the average age-specific cumulative risk of breast cancer for a defined set of protein-truncating mutations in BRCA1 and BRCA2: Australian Breast Cancer Family Study. Cancer Epidemiol Biomarkers Prev. 1999;8:741–747. [PubMed] [Google Scholar]
- 46.Thorlacius S, Struewing JP, Hartge P, et al. Population-based study of risk of breast cancer in carriers of BRCA2 mutation. Lancet. 1998;352:1337–1339. doi: 10.1016/s0140-6736(98)03300-5. [DOI] [PubMed] [Google Scholar]
- 47.Begg CB. On the use of familial aggregation in population-based case probands for calculating penetrance. J Natl Cancer Inst. 2002;94:1221–1226. doi: 10.1093/jnci/94.16.1221. [DOI] [PubMed] [Google Scholar]
- 48.Rubinstein WS, Jiang H, Dellefave L, et al. Cost-effectiveness of population-based BRCA1/2 testing and ovarian cancer prevention for Ashkenazi Jews: A call for dialogue. Genet Med. 2009;11:629–639. doi: 10.1097/GIM.0b013e3181afd322. [DOI] [PubMed] [Google Scholar]
- 49.Neumann PJ, Rosen AB, Weinstein MC. Medicare and cost-effectiveness analysis. N Engl J Med. 2005;353:1516–1522. doi: 10.1056/NEJMsb050564. [DOI] [PubMed] [Google Scholar]
- 50.Rubinstein WS. Hereditary breast cancer in Jews. Fam Cancer. 2004;3:249–257. doi: 10.1007/s10689-004-9550-2. [DOI] [PubMed] [Google Scholar]
- 51.Liede A, Metcalfe K, Offit K, et al. A family with three germline mutations in BRCA1 and BRCA2. Clin Genet. 1998;54:215–218. doi: 10.1111/j.1399-0004.1998.tb04287.x. [DOI] [PubMed] [Google Scholar]
- 52.Mor P, Oberle K. Ethical issues related to BRCA gene testing in orthodox Jewish women. Nurs Ethics. 2008;15:512–522. doi: 10.1177/09697330080150041201. [DOI] [PubMed] [Google Scholar]
- 53.Rosner F. Judaism, genetic screening and genetic therapy. Mt Sinai J Med. 1998;65:406–413. [PubMed] [Google Scholar]
- 54.Rubinstein WS. Roles and responsibilities of a medical geneticist. Fam Cancer. 2008;7:5–14. doi: 10.1007/s10689-007-9148-6. [DOI] [PubMed] [Google Scholar]
- 55.Weitzel JN, Lagos VI, Cullinane CA, et al. Limited family structure and BRCA gene mutation status in single cases of breast cancer. JAMA. 2007;297:2587–2595. doi: 10.1001/jama.297.23.2587. [DOI] [PubMed] [Google Scholar]