Abstract
Purpose
To determine whether cardiovascular abnormalities in childhood cancer survivors are restricted to patients exposed to cardiotoxic anthracyclines and cardiac irradiation and how risk factors for atherosclerotic disease and systemic inflammation contribute to global cardiovascular status.
Methods
We assessed echocardiographic characteristics and atherosclerotic disease risk in 201 survivors of childhood cancer with and without exposure to cardiotoxic treatments at a median of 11 years after diagnosis (range, 3 to 32 years) and in 76 sibling controls.
Results
The 156 exposed survivors had below normal left ventricular (LV) mass, wall thickness, contractility, and fractional shortening and above normal LV afterload. The 45 unexposed survivors also had below normal LV mass overall, and females had below normal LV wall thickness. Exposed and unexposed survivors, compared with siblings, had higher levels of N-terminal pro-brain natriuretic peptide (81.7 and 69.0 pg/mL, respectively, v 39.4 pg/mL), higher mean fasting serum levels of non–high-density lipoprotein cholesterol (126.5 and 121.1 mg/dL, respectively, v 109.8 mg/dL), higher insulin levels (10.4 and 10.5 μU/mL, respectively, v 8.2 μU/mL), and higher levels of high-sensitivity C-reactive protein (2.7 and 3.1 mg/L, respectively, v 0.9 mg/L; P < .001 for all comparisons). Age-adjusted, predicted-to-ideal 30-year risk of myocardial infarction, stroke, or coronary death was also higher for exposed and unexposed survivors compared with siblings (2.16 and 2.12, respectively, v 1.70; P < .01 for both comparisons).
Conclusion
Childhood cancer survivors not receiving cardiotoxic treatments nevertheless have cardiovascular abnormalities, systemic inflammation, and an increased risk of atherosclerotic disease. Survivorship guidelines should address cardiovascular concerns, including the risk of atherosclerotic disease and systemic inflammation, in exposed and unexposed survivors.
INTRODUCTION
Currently, more than 325,000 childhood cancer survivors live in the United States.1,2 Survivors are at higher risk for chronic disease and premature death3–5; cardiovascular-related complications are of particular concern.6–11 For 30 years after cancer treatment, survivors are eight times more likely to die from cardiac causes3 and 15 times more likely to experience congestive heart failure (CHF)4 than the general population. Even 45 years after diagnosis, their increasingly higher risk of cardiac death8 is associated with exposure to cardiotoxic treatments (eg, anthracyclines, cardiac irradiation).6–8
In more than half of exposed survivors, cardiotoxic treatments are also associated with subclinical changes in left ventricular (LV) structure and function12–16 that commonly include decreased LV wall thickness and increased LV systolic wall stress (afterload), which can progress to clinically relevant disease.14,17 As a result, cardiac monitoring for early disease is recommended for survivors exposed to cardiotoxic treatments.18 However, these recommendations do not include survivors unexposed to cardiotoxic treatments, who have yet to be systematically studied.
Survivors are also more likely to have traditional risk factors for atherosclerotic disease, including elevated cholesterol, obesity, and insulin resistance,19–22 and regular screening and aggressive management of these risk factors are now recommended.23 Increased systemic inflammation may promote atherosclerotic cardiovascular disease and worsen treatment-related cardiac dysfunction, although these relationships are not well studied in survivors.
The Cardiac Risk Factors in Childhood Cancer Survivors Study, a National Cancer Institute–funded study, evaluated a large, representative cohort of survivors in western New York State.24 We hypothesized that cardiovascular status would differ between survivors exposed and unexposed to cardiotoxic treatments and between both survivor groups and sibling controls.
METHODS
Participants
The protocol for the Cardiac Risk Factors in Childhood Cancer Survivors Study is detailed elsewhere.24 Briefly, survivors were recruited from the Pediatric Long-Term Survivor Clinic at the University of Rochester (Rochester, NY) between 1998 and 2003. The clinic cared for survivors in a defined catchment area consisting of parts of the Finger Lakes region of New York State and northern Pennsylvania. Eligible survivors had received a cancer diagnosis 3 or more years before, were no longer receiving chemotherapy or radiation, and were without active disease. For each survivor, a sibling control (closest in age was preferred), without a history of cancer or serious illness, was invited to participate.
Information on cancer diagnosis and treatment was abstracted from medical records. All other information was collected during a single, daylong study visit that included echocardiography, fasting blood samples, and patient examinations. Values for total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein (HDL) cholesterol, non-HDL cholesterol, insulin, N-terminal pro-brain natriuretic peptide (NT-proBNP), high-sensitivity C-reactive protein (hs-CRP), homocysteine, the ratio of apolipoproteins A1 to B1, and insulin-like growth factor-1 (IGF-1) were determined in a Clinical Laboratory Improvement Amendments–approved laboratory (Strong Memorial Hospital Clinical Laboratory, Rochester, NY), except for hs-CRP, which was determined using the N High-Sensitivity CRP assay (Dade Behring, Newark, DE). Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters.
Traditional risk factors for atherosclerotic disease were aggregated, according to methods appropriate for age, with the modified Pathological Determinants of Atherosclerosis in Youth risk score, which predicts the risk of an advanced atherosclerotic disease lesion in a major coronary artery,25 and by calculating the ratio of predicted-to-ideal risk estimated by the Framingham 30-Year Calculator for myocardial infarction, stroke, or coronary death.26
A cardiologist unaware of the participants' treatment status read two-dimensional and Doppler echocardiograms. The LV fractional shortening and rate-adjusted velocity of fiber shortening were calculated, and LV afterload was measured as meridional end-systolic wall stress.27,28 Contractility was defined as the relationship between end-systolic wall stress and rate-adjusted velocity of fiber shortening with the LV stress-velocity index, a validated index incorporating afterload and independent of preload.26,27 Heart rate and corrected QT interval were measured by electrocardiography.
Statistical Methods
Normal echocardiographic, height, and weight measurements vary in children by age, body-surface area, and sex. To adjust for these influences, z scores were calculated by dividing the difference between a participant's observed and normal predicted value by the standard deviation of normal values. Normal predicted echocardiographic values were calculated with a regression model using data from 296 patients from Children's Hospital Boston whose ages were similar to those of our patients (raw estimates provided in Appendix Table A1, online only).27,28
Wilcoxon rank sum or t tests were used to compare groups on continuous measurements. Fisher's exact test was used to compare proportions. All tests were two-tailed with an α = .05. Because group comparisons were determined a priori, we did not adjust for multiple comparisons, so P values should be interpreted cautiously, given the large number of comparisons. Possible correlations between controls and their exposed and unexposed siblings were accounted for with generalized estimating equations. Additionally, analyses limited to matched survivor-sibling pairs were consistent in direction and magnitude with those reported (except non-HDL cholesterol in unexposed survivors). Correlations among serum biomarkers in survivors were assessed with Spearman's rank correlation coefficient (ρ). Correlations of NT-proBNP and hs-CRP with the echocardiographic characteristics were assessed using the partial Spearman's ρ correlation coefficient to remove the effect of the other biomarker. Data were analyzed with SAS, version 9.2 (SAS Institute, Cary, NC) and STATA, version 11.0 (STATA, College Station, TX).
Imbalances in age and sex between groups were adjusted using weighted propensity scores. Each participant's data were weighted by the inverse of the probability of being in a specific age and sex group, calculated with generalized logit models.29–31 Sex-specific group values were adjusted for age using this approach. Weights were assessed for balancing and comparing groups.
The institutional review boards at the University of Rochester and University of Miami (Miami, FL) approved the protocol. Written informed consent was obtained from all participants or guardians, and assent was obtained from the participants when appropriate.
RESULTS
Participants
We analyzed 156 survivors exposed to cardiotoxic therapies, 45 survivors unexposed to these therapies, and 76 healthy sibling controls. Both survivor groups were older and more likely than controls to report taking medications, vitamins, or nutritional supplements (Table 1). Although few participants reported health conditions, cardiovascular disease was more common in the exposed survivors (CHF, n = 5; stroke, n = 2; myocardial infarction, n = 1). Unexposed survivors were older and longer from diagnosis than exposed survivors; median age at diagnosis in both groups was close to 6 years, and 90% of survivors received a diagnosis between 1976 and 1996 (Table 2). Exposed survivors were more likely than unexposed survivors to have had leukemia or lymphoma and been treated with a plant alkaloid, antimetabolite, corticosteroids, or asparaginase. Values for the comparisons that follow are age- and sex-adjusted estimates.
Table 1.
Demographic and Medical Characteristics of 201 Long-Term Survivors of Childhood Cancer, by Exposure to Cardiotoxic Cancer Therapy, and 76 Normal Sibling Controls
| Demographic or Medical Characteristic | Exposed Survivors (n = 156) |
Unexposed Survivors (n = 45) |
Sibling Controls (n = 76) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Demographic | ||||||
| Female | 82 | 52.6 | 19 | 42.2 | 35 | 46.1 |
| Race | ||||||
| White | 138 | 88.5 | 40 | 88.9 | 71 | 93.4 |
| African American | 9 | 5.8 | 2 | 4.4 | 2 | 2.6 |
| Other | 9 | 5.8 | 3 | 6.7 | 3 | 3.9 |
| Age at follow-up, years | ||||||
| Median | 17.4* | 23.0† | 15.1*† | |||
| Range | 5.9-39.7 | 8.0-32.8 | 5.3-45.9 | |||
| Health conditions at follow-up | ||||||
| Irregular heartbeat | 7 | 4.5 | 1 | 2.2 | 0 | 0 |
| Congestive heart failure | 5 | 3.2 | 0 | 0 | 0 | 0 |
| Heart attack | 1 | 0.6 | 0 | 0 | 0 | 0 |
| High blood pressure | 5 | 3.2 | 3* | 6.7 | 0* | 0 |
| Stroke | 2 | 1.3 | 0 | 0 | 0 | 0 |
| Pericarditis | 3 | 1.9 | 1 | 2.2 | 0 | |
| Stiff or leaking heart valve | 2 | 1.3 | 0 | 0 | 0 | 0 |
| Genetic syndrome | 0 | 0 | 0 | 0 | 1 | 1.3 |
| Atherosclerosis | 0 | 0 | 0 | 0 | 0 | 0 |
| Structurally abnormal heart at birth | 0 | 0 | 0 | 0 | 0 | 0 |
| Rheumatic heart disease | 0 | 0 | 0 | 0 | 0 | 0 |
| Current smoker‡ | 9 | 12.2 | 3 | 11.1 | 2 | 8.7 |
| Medications at follow-up | ||||||
| Any current medication | 71* | 45.5 | 27† | 60 | 19*† | 25 |
| Neurologic | 11 | 7.1 | 3 | 6.7 | 1 | 1.3 |
| Pain, anti-inflammatory | 12 | 7.7 | 8* | 17.8 | 3* | 3.9 |
| Pain, non-narcotic | 10 | 6.4 | 7* | 15.6 | 1* | 1.3 |
| Pain, narcotic | 3 | 1.9 | 0 | 0 | 0 | 0 |
| Thyroid | 7 | 4.5 | 2 | 4.4 | 1 | 1.3 |
| Sex hormone | 10 | 6.4 | 5* | 11.1 | 1* | 1.3 |
| Growth hormone | 2 | 1.3 | 3* | 6.7 | 0* | 0 |
| Allergy | 9 | 5.8 | 5 | 11.1 | 4 | 5.3 |
| Cardiac medication | 6 | 3.8 | 0 | 0 | 1 | 1.3 |
| GI | 10* | 6.4 | 6† | 13.3 | 0*† | 0 |
| Antibiotic | 4 | 2.6 | 2 | 4.4 | 0 | 0 |
| Vitamins/nutritional supplements | 28* | 17.9 | 10† | 22.2 | 1*† | 1.3 |
Values in the same row differ significantly at P = .05 by the Wilcoxon rank sum test.
Values in the same row differ significantly at P = .05 by the Wilcoxon rank sum test.
Data are from participants 18 years of age or older to control for the effect of laws restricting smoking to this age or older. Sample sizes for this analysis are 74 exposed survivors, 27 unexposed survivors, and 23 sibling controls.
Table 2.
Cancer Diagnosis and Treatment Characteristics of 201 Long-Term Survivors of Childhood Cancer, by Exposure to Cardiotoxic Therapy
| Characteristic | Exposed Survivors (n = 156) |
Unexposed Survivors (n = 45) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Cancer diagnosis | |||||
| Age at diagnosis, years | .22 | ||||
| Median | 6.3 | 5.6 | |||
| Range | 0-24.1 | 0-17.8 | |||
| Time since diagnosis, years | < .001 | ||||
| Median | 10.0 | 15.0 | |||
| Range | 3.3-31.6 | 5.1-25.6 | |||
| Year of diagnosis | .002 | ||||
| Median | 1990 | 1985 | |||
| Range | 1969 to 1998 | 1974 to 1997 | |||
| Cancer type | |||||
| Leukemia | 63 | 40.4 | 6 | 13.3 | < .001 |
| Lymphoma | 35 | 22.4 | 4 | 8.9 | .05 |
| Embryonal | 31 | 19.9 | 11 | 24.4 | .53 |
| Sarcoma | 15 | 9.6 | 10 | 22.2 | .04 |
| Brain | 10 | 6.4 | 9 | 20 | .02 |
| Other | 2* | 1.3 | 5† | 11.1 | .006 |
| Cancer treatment‡ | |||||
| Anthracycline only | 97 | 62.2 | 0 | 0 | |
| Radiation to the heart only | 22 | 14.1 | 0 | 0 | |
| Anthracycline and cardiac radiation | 37 | 23.7 | 0 | 0 | |
| Cranial radiation | 67 | 42.9 | 17 | 37.8 | .61 |
| Plant alkaloid | 139 | 89.1 | 31 | 68.9 | .002 |
| Antibiotic (anthracycline) | 134 | 85.9 | 0 | 0 | |
| Alkylating agent | 9 | 5.8 | 27 | 60 | .86 |
| Antimetabolite | 89 | 57.1 | 11 | 24.4 | < .001 |
| Corticosteroid | 83 | 53.2 | 11 | 24.4 | < .001 |
| Asparaginase | 60 | 38.5 | 6 | 13.3 | .001 |
| Antibiotic (nonanthracycline) | 20 | 12.8 | 3 | 6.7 | .30 |
| Topoisomerase II inhibitor | 3 | 1.9 | 0 | 0 | |
| Immunosuppressant | 1 | 0.6 | 0 | 0 | |
One germ cell cancer (0.7%) and one nonspecific cancer (0.7%).
Three germ cell cancers (6.7%) and two nonspecific cancers (4.4%).
Drugs used in cancer treatment included the following: plant alkaloids: vincristine, vinblastine, and etoposide; antibiotics (anthracycline): doxorubicin, daunorubicin, idarubicin, and mitoxantrone; alkylating agents: dacarbazine, carmustine, melphalan, busulfan, carboplatin, cyclophosphamide, ifosfamide, mustard/nitrogen mustard/mechlorethamine, cisplatin, procarbazine, and thiotepa; antimetabolites: methotrexate, mercaptopurine, thioguanine, hydroxyurea, azacitidine, fluorouracil, and cytarabine; corticosteroids: prednisone, hydrocortisone, and dexamethasone; asparaginases: l-asparaginase, Escherichia coli asparaginase, Erwinia asparaginase, and pegaspargase; antibiotics (nonanthracycline): dactinomycin and bleomycin; topoisomerase II inhibitor: teniposide; and immunosuppressant: antithymocyte globulin.
LV Structure and Function
Exposed survivors had below normal LV mass and wall thickness (Table 3) and had above normal LV afterload, which was also higher than in unexposed survivors. Compared with sibling controls and unexposed survivors, exposed survivors had impaired LV load– independent contractility and LV fractional shortening and lower systolic blood pressure. Exposed survivors also had a faster heart rate, a longer corrected QT interval, and higher serum levels of NT-proBNP than the other groups. Female exposed survivors had a longer corrected QT interval and higher serum levels of NT-proBNP; the mean (104.2 pg/mL) is consistent with cardiomyopathy.
Table 3.
Selected Echocardiographic and Electrocardiographic Characteristics of 201 Long-Term Survivors of Childhood Cancer, With and Without Exposure to Cardiotoxic Cancer Treatments, by Study Group and Sex
| Characteristic | Total (Males and Females) |
Males |
Females |
Male v Female, P |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed Survivors (n = 156) |
Unexposed Survivors (n = 45) |
Sibling Controls (n = 76) |
Exposed Survivors (n = 74) |
Unexposed Survivors (n = 26) |
Sibling Controls (n = 41) |
Exposed Survivors (n = 82) |
Unexposed Survivors (n = 19) |
Sibling Controls (n = 35) |
|||||||||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Exposed Survivors | Unexposed Survivors | Sibling Controls | |
| Echocardiographic characteristic, z score* | |||||||||||||||||||||
| LV mass | −1.10† | 0.10 | −1.16† | 0.18 | −0.65‡ | 0.10 | −1.06† | 0.15 | −0.87† | 0.21 | −0.38‡ | 0.11 | −1.12 | 0.13 | −1.60 | 0.30 | −1.00‡ | 0.17 | .77 | .04 | .002 |
| LV end-diastolic posterior wall thickness | −1.15† | 0.10 | −1.02 | 0.18 | −0.61‡ | 0.12 | −1.11†§ | 0.15 | −0.63 | 0.19 | −0.55‡ | 0.14 | −1.19† | 0.13 | −1.55† | 0.30 | −0.55‡ | 0.21 | .68 | .01 | .99 |
| LV end-diastolic dimension | −0.74 | 0.09 | −0.93 | 0.17 | −0.58‡ | 0.12 | −0.68 | 0.13 | −0.67 | 0.21 | −0.30‡ | 0.15 | −0.80 | 0.13 | −1.29 | 0.24 | −0.89‡ | 0.13 | .52 | .06 | .003 |
| LV thickness-to-dimension ratio | −0.31 | 0.11 | −0.11 | 0.16 | 0.02 | 0.16 | −0.21 | 0.16 | 0.08 | 0.21 | −0.04 | 0.18 | −0.40† | 0.14 | −0.39 | 0.27 | 0.26 | 0.26 | .37 | .17 | .35 |
| LV afterload (end-systolic wall stress) | −0.05†§ | 0.16 | −0.72 | 0.28 | −1.19‡ | 0.24 | −0.07 | 0.22 | −0.71 | 0.37 | −0.77‡ | 0.35 | −0.05† | 0.24 | −0.69 | 0.42 | −1.40‡ | 0.29 | .95 | .96 | .16 |
| LV fractional shortening | 0.55†§ | 0.14 | 1.60 | 0.36 | 1.73‡ | 0.24 | 0.62† | 0.20 | 1.35 | 0.35 | 1.54‡ | 0.36 | 0.53†§ | 0.21 | 1.79 | 0.52 | 1.76‡ | 0.26 | .75 | .48 | .62 |
| LV load–independent contractility (stress-velocity index) | 0.39†§ | 0.11 | 1.03 | 0.28 | 0.97‡ | 0.18 | 0.42 | 0.15 | 0.78 | 0.30 | 0.97‡ | 0.29 | 0.37§ | 0.16 | 1.32 | 0.41 | 0.95‡ | 0.23 | .81 | .29 | .95 |
| Blood pressure, z score | |||||||||||||||||||||
| Systolic | 0.06†§ | 0.09 | 0.48 | 0.16 | 0.35 | 0.11 | 0.25 | 0.14 | 0.54 | 0.23 | 0.57 | 0.15 | −0.16 | 0.11 | 0.31 | 0.23 | 0.08 | 0.16 | .02 | .49 | .02 |
| Diastolic | 1.12 | 0.08 | 1.21 | 0.13 | 1.23 | 0.11 | 1.24 | 0.12 | 1.11 | 0.17 | 1.37 | 0.12 | 0.98 | 0.10 | 1.23 | 0.21 | 1.04 | 0.16 | .10 | .64 | .10 |
| Electrocardiographic characteristics | |||||||||||||||||||||
| Heart rate, beats per minute | 82.53† | 1.45 | 77.26 | 2.30 | 77.67 | 1.80 | 81.88† | 2.16 | 74.32 | 3.59 | 75.63 | 2.13 | 83.31 | 1.87 | 80.84 | 2.41 | 81.00 | 2.62 | .62 | .13 | .11 |
| Corrected QT interval, milliseconds | 407.01†§ | 1.80 | 398.66 | 3.50 | 400.75 | 2.37 | 403.96§ | 2.72 | 388.33 | 4.64 | 395.73 | 3.07 | 409.55 | 2.29 | 411.05 | 3.72 | 405.84 | 2.71 | .12 | < .001 | .01 |
| NT-proBNP, pg/mL | 81.08† | 7.90 | 69.25† | 9.86 | 37.12 | 3.56 | 58.01† | 7.41 | 37.79 | 6.81 | 35.32 | 4.36 | 104.32† | 13.16 | 97.49† | 15.17 | 40.92 | 6.29 | .002 | < .001 | .46 |
Abbreviations: LV, left ventricular; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Total values were adjusted using a weighted propensity score technique for age and sex. Sex-specific values were adjusted using a weighted propensity score technique for age.
The difference in the characteristic for either exposed or unexposed survivors versus sibling controls for either sex is significant at P < .05.
The difference in the characteristic for the sibling control group versus the Boston cohort, upon which the formulas to calculate the z scores for echocardiographic parameters were derived, is significant at P < .05.
The difference in the characteristic for exposed versus unexposed survivors for either sex is significant at P < .05.
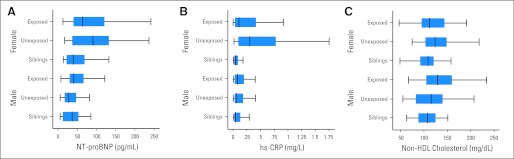
Unexposed survivors had below normal LV mass overall and below normal wall thickness (P = .07), which was statistically significant in female survivors (Table 3). Unexposed survivors had normal LV load–independent contractility, fractional shortening, and systolic blood pressure but had higher levels of NT-proBNP than did sibling controls. Unexposed female survivors had lower LV wall thickness and higher serum levels of NT-proBNP; the mean (102.4 pg/mL) is consistent with cardiomyopathy (Fig 1).
Fig 1.
(A-C) Serum levels of cardiac biomarkers by cancer treatment history and sex. Box plots show the minimum, maximum, interquartile range (box), and median values for survivors of childhood cancer exposed or unexposed to known cardiotoxic treatments and for sibling controls, by sex. HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Risk Factors for Atherosclerotic Disease and Related Measures
Both survivor groups were shorter than controls (Table 4). Mean BMI was higher in unexposed survivors than in exposed survivors and controls. Serum levels of IGF-1 were lower in all survivors than in controls.
Table 4.
Cardiac Risk Factors and Measures of Inflammation for Long-Term Survivors of Childhood Cancer, With and Without Exposure to Cardiotoxic Cancer Treatments, and Normal Sibling Controls, by Study Group and Sex
| Characteristic | Total (Males and Females) |
Males |
Females |
Male v Female, P |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed Survivors (n = 156) |
Unexposed Survivors (n = 45) |
Sibling Controls (n = 76) |
Exposed Survivors (n = 74) |
Unexposed Survivors (n = 26) |
Sibling Controls (n = 41) |
Exposed Survivors (n = 82) |
Unexposed Survivors (n = 19) |
Sibling Controls (n = 35) |
|||||||||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Exposed Survivors | Unexposed Survivors | Sibling Controls | |
| Anthropometric measurements* | |||||||||||||||||||||
| Body mass index, kg/m2 | 23.1† | 0.17 | 24.9‡ | 0.44 | 22.6 | 0.21 | 22.8‡ | 0.23 | 22.7 | 0.40 | 22.0 | 0.30 | 23.3† | 0.26 | 27.3‡ | 0.82 | 23.0 | 0.28 | .20 | < .001 | .02 |
| Weight, z score | 0.27‡ | 0.06 | 0.08‡ | 0.14 | 0.56 | 0.06 | 0.27‡ | 0.06 | 0.08‡ | 0.14 | 0.56 | 0.06 | 0.19‡ | 0.10 | 0.70 | 0.34 | 0.56 | 0.08 | .49 | .09 | .99 |
| Height, z score | −0.45‡ | 0.05 | −0.44‡ | 0.1 | 0.18 | 0.05 | −0.39†‡ | 0.07 | −0.71‡ | 0.12 | 0.35 | 0.07 | −0.53†‡ | 0.06 | −0.02 | 0.17 | −0.04 | 0.08 | .13 | < .001 | < .001 |
| Blood characteristics* | |||||||||||||||||||||
| Non-HDL cholesterol, mg/dL | 126.5†‡ | 1.17 | 121.1‡ | 2.15 | 109.8 | 1.41 | 135.6†‡ | 1.88 | 114.0 | 2.56 | 108.4 | 1.65 | 117.0†‡ | 1.25 | 130.5‡ | 3.52 | 111.8 | 2.33 | < .001 | < .001 | .23 |
| LDL cholesterol, mg/dL | 103.4‡ | 0.97 | 100.8‡ | 1.92 | 92.9 | 1.20 | 108.8†‡ | 1.5 | 93.5 | 2.34 | 92.7 | 1.46 | 97.9†‡ | 1.18 | 110.1‡ | 3.08 | 93.2 | 1.90 | < .001 | < .001 | .82 |
| HDL cholesterol, mg/dL | 51.3 | 0.37 | 50.0 | 0.80 | 50.5 | 0.46 | 47.8‡ | 0.45 | 46.8‡ | 0.84 | 50.1 | 0.52 | 54.6‡ | 0.57 | 53.3 | 1.43 | 51.0 | 0.85 | < .001 | < .001 | .35 |
| Insulin, μU/mL | 10.4‡ | 0.27 | 10.5‡ | 0.65 | 8.2 | 0.21 | 10.9†‡ | 0.42 | 8.9‡ | 0.44 | 7.5 | 0.23 | 10.0‡ | 0.37 | 12.3‡ | 1.30 | 9.0 | 0.35 | .10 | .01 | < .001 |
| Apolipoprotein A1/B1 ratio | 0.60‡ | 0.01 | 0.61‡ | 0.01 | 0.52 | 0.01 | 0.67†‡ | 0.01 | 0.57‡ | 0.01 | 0.53 | 0.01 | 0.55‡ | 0.01 | 0.67‡ | 0.02 | 0.51 | 0.01 | < .001 | < .001 | .16 |
| Homocysteine, μmol/L | 7.2 | 0.07 | 7.3 | 0.14 | 6.9 | 0.14 | 7.4 | 0.11 | 7.5 | 0.20 | 7.1 | 0.17 | 6.9‡ | 0.09 | 6.9 | 0.18 | 6.5 | 0.15 | < .001 | .03 | .02 |
| IGF-1, ng/mL | 213.7†‡ | 3.32 | 172.9‡ | 4.61 | 257.9 | 5.31 | 209.4‡ | 5.06 | 193.5‡ | 6.52 | 241.8 | 6.90 | 217.4†‡ | 4.43 | 150.3‡ | 6.17 | 277.7 | 8.18 | .23 | < .001 | < .001 |
| T4, ng/dL | 1.05†‡ | 0.006 | 1.12 | 0.13 | 1.18 | 0.03 | 1.04†‡ | 0.008 | 1.13‡ | 0.012 | 1.26 | 0.05 | 1.07 | 0.008 | 1.12‡ | 0.02 | 1.06 | 0.13 | .008 | .67 | .15 |
| TSH, μU/mL | 2.24‡ | 0.05 | 2.41‡ | 0.20 | 1.94 | 0.06 | 2.14‡ | 0.06 | 1.93 | 0.09 | 1.81 | 0.06 | 2.33 | 0.08 | 2.86 | 0.41 | 2.22 | 0.14 | .06 | .03 | .007 |
| hs-CRP, mg/L | 2.7‡ | 0.2 | 3.1‡ | 0.3 | 0.9 | 0.1 | 1.8‡ | 0.1 | 1.6‡ | 0.1 | 1.1 | 0.1 | 3.5†‡ | 0.3 | 4.9‡ | 0.5 | 0.7 | 0.01 | < .001 | < .001 | .005 |
| Atherosclerotic risk aggregation tools | |||||||||||||||||||||
| Modified Pathological Determinants of Atherosclerosis in Youth risk score§ | 2.95‡ | 0.17 | 3.59†‡ | 0.26 | 2.07 | 0.20 | 3.81†‡ | 0.19 | 2.98‡ | 0.29 | 1.77 | 0.18 | 1.20† | 0.10 | 2.90‡ | 0.27 | 0.89 | 0.14 | < .001 | .88 | < .001 |
| Ratio of Framingham calculator predicted-to-ideal 30-year hard cardiovascular disease risk∥ | 2.16‡ | 0.10 | 2.12‡ | 0.11 | 1.70 | 0.07 | 2.75†‡ | 0.17 | 2.05 | 0.10 | 2.12 | 0.06 | 1.70†‡ | 0.10 | 2.17 | 0.17† | 1.02 | 0.05 | < .001 | .55 | < .001 |
Abbreviations: HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; IGF-1, insulin-like growth factor-1; LDL, low-density lipoprotein; NT-proBNP, N-terminal pro-brain natriuretic peptide; T4, free thyroxine; TSH, thyroid-stimulating hormone.
Total values were adjusted using a weighted propensity score technique for age and sex. Sex-specific values were adjusted using a weighted propensity score technique for age.
The difference in the characteristic for exposed versus unexposed survivors for either sex is significant at P < .05.
The difference in the characteristic for either exposed or unexposed survivors versus sibling controls for either sex is significant at P < .05.
The modified Pathological Determinants of Atherosclerosis in Youth risk score measures the increased risk of having an advanced atherosclerotic disease lesion in a major coronary artery so that a 1-unit increase in the score is equivalent to a multiplicative change in the odds of having a lesion as a result of a 1-year increase in age.24
The ratio of Framingham calculator predicted-to-ideal 30-year risk of hard cardiovascular disease represents the increasing risk of having a myocardial infarction, stroke, or coronary death in the next 30 years, with the ratio representing the increased risk relative to the ideal.25
Fasting serum levels of non-HDL cholesterol were higher in all survivors than in controls and were even higher in exposed survivors than in unexposed survivors (Table 4). Both survivor groups had higher fasting serum insulin levels than did controls. Unexposed female survivors had higher fasting serum levels of non-HDL cholesterol and insulin than did unexposed males. Exposed male survivors had higher fasting serum levels of non-HDL cholesterol than did exposed females (Fig 1). When these traditional risk factors for atherosclerotic disease were considered in the aggregate, both survivor groups seemed to be at increased risk for cardiovascular disease (Table 4).
Systemic Inflammation
Serum levels of hs-CRP were similar in the survivor groups and higher in both survivor groups than in controls (Table 4). Exposed and unexposed female survivors had higher serum levels of hs-CRP than exposed and unexposed male survivors (Table 4).
Correlation Among Biomarkers for Cardiac Domains
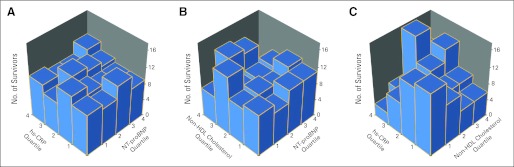
Levels of NT-proBNP were not correlated with either hs-CRP or non-HDL cholesterol (Fig 2). Non-HDL cholesterol was moderately correlated with hs-CRP (ρ = 0.35, P < .001).
Fig 2.
(A-C) Correlations between serum levels of cardiac biomarkers in survivors of childhood cancer. Bivariate histograms show the number of survivors of childhood cancer on the vertical axes in each quartile-quartile bin for the biomarker quartiles listed on the diagonal axes. Each bar represents the number of survivors who were in a specific quartile-quartile pair for each of the biomarkers listed on the two diagonal axes. The bars in any one row should add to the total in that quartile for the biomarker listed on the referent axis. Spearman's rank correlation coefficient and associated P value are also reported for the association between these biomarkers. HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Correlation of Biomarkers for Cardiac Domains With LV Structure and Function
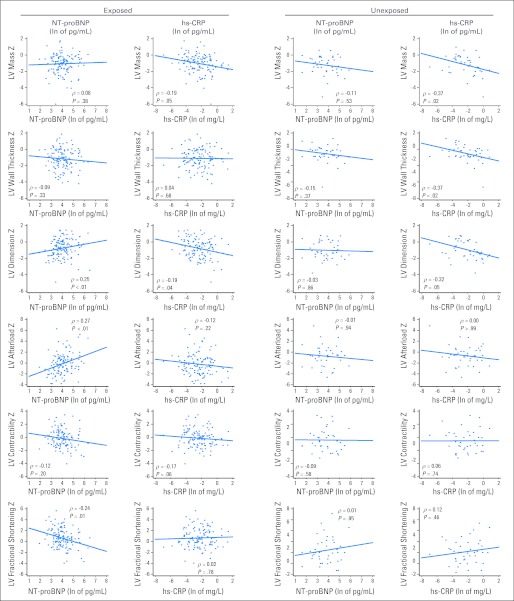
In exposed survivors, NT-proBNP was moderately correlated with LV dimension (ρ = 0.25, P < .01), afterload (ρ = 0.27, P < .001), and fractional shortening (ρ = −0.24, P = .01; Appendix Fig A1, online only). In unexposed survivors, hs-CRP was moderately correlated with LV mass (ρ = −0.37, P = .02), wall thickness (ρ = −0.37, P = .02), and dimension (ρ = −0.32, P = .05).
DISCUSSION
Both exposed and unexposed survivors had abnormalities in LV structure and function, traditional risk factors for atherosclerotic disease, and systemic inflammation. Although several large observational studies have documented an increased risk of cardiovascular disease in survivors, they have focused on self-reported disease or death certificate data and have provided little information on the clinical course underlying these outcomes.3–8 The cardiovascular-related abnormalities presented here support these findings and identify the processes likely underlying excess cardiovascular disease risk. Further, these findings suggest that this cardiovascular-related health burden will increase as this expanding population ages.
Even without exposure to cardiotoxic treatments, survivors had abnormal LV structure and function. Unexposed survivors had decreased LV mass in both sexes and decreased wall thickness in females, which are probably reflected by increased serum levels of NT-proBNP that accompany increased stress on the remaining LV cardiomyocytes. In addition, unexposed survivors had a higher mean BMI and higher fasting serum non-HDL cholesterol and insulin levels than controls. Aggregated, these risk factors increase the risk of atherosclerotic disease in unexposed survivors. Systemic inflammation, measured as hs-CRP, was also present and may exacerbate these abnormalities and the risk of atherosclerosis.
As expected,12–16 exposed survivors had decreased LV mass and wall thickness, increased LV afterload, decreased LV load–independent contractility, and decreased LV fractional shortening. These changes likely reflect treatment-induced cardiovascular damage that kills cardiomyocytes and damages the remaining cardiomyocytes and progenitor cells. The normal LV end-diastolic dimensions accompanying these cardiovascular abnormalities and elevated serum levels of NT-proBNP indicate the restrictive nature of anthracycline- and radiation-induced cardiomyopathies. Longer corrected QT intervals in exposed survivors probably reflect cardiovascular damage and may worsen prognosis.32,33 Exposed survivors also had reduced systolic blood pressure and increased heart rate, which may be early signs of reduced cardiac output and future CHF.
In another population of childhood survivors, increased LV afterload was significantly related to LV fractional shortening.13 This relationship is confirmed here and extended to show that cardiomyopathy (higher NT-proBNP levels) is related to lower LV fractional shortening, as expected. In unexposed survivors, higher levels of generalized inflammation are associated with a smaller LV (lower mass, wall thickness, and dimension), suggesting that chronic inflammation may predispose survivors to develop restrictive cardiomyopathy.
Exposed survivors had higher fasting serum levels of non-HDL cholesterol and insulin. Systemic inflammation, as measured by hs-CRP, was also present and may exacerbate these cardiovascular abnormalities and the risk of atherosclerotic disease. Although increased cardiovascular mortality in childhood cancer survivors is consistent across studies, screening recommendations have not focused on concurrently identifying multiple causes of excess cardiovascular mortality. Instead, the focus has been on assessing LV function, not abnormal LV structure and cardiometabolic abnormalities. Our results support the need for comprehensive assessments of global risk for cardiovascular disease in all childhood cancer survivors. This recommendation is supported by the small correlations between serum levels of biomarkers across cardiac domains. Survivors with and without cardiac dysfunction, as reflected by being in a higher NT-proBNP quartile, were equally likely to be in a lower or higher quartile for non-HDL cholesterol and hs-CRP. Screening across a single cardiac domain will not identify all survivors at increased risk for cardiac disease.
The sex-related differences we found are consistent with reports indicating that female survivors are more vulnerable to cardiotoxic treatments.13,34,35 Our study adds three findings to this relationship. First, sex is associated with several altered risk factors for atherosclerotic disease. Exposed male survivors had higher fasting serum levels of non-HDL cholesterol than did exposed females. However, among unexposed survivors, females had higher fasting serum levels of non-HDL cholesterol and insulin. Second, both exposed and unexposed female survivors had higher levels of hs-CRP. Third, unexposed female survivors were more likely to have altered LV structure and function, as indicated by decreased LV wall thickness and increased NT-proBNP levels, both indicating increased LV stress.
These sex-related differences raise the possibility that although overall long-term cardiovascular mortality rates in survivors of either sex may be similar, the mechanisms underlying their increased risk, and thus their specific needs for intervention, may differ. Female survivors may be at increased risk of CHF, whereas male survivors may be at increased risk of atherosclerotic cardiovascular disease. This hypothesis is supported by Childhood Cancer Survivors Study data showing that during the first 30 years after cancer diagnosis, female survivors were 40% more likely to experience CHF but 40% less likely to experience myocardial infarction.6 These mortalities may manifest at different times, with females having higher early cardiovascular-related mortality, as seen in this same cohort where females had a 40% increased cardiovascular-related mortality.3
Although studies of increased systemic inflammation in cancer survivors are limited,36,37 increased values may help evaluate cardiovascular status. Systemic inflammation may worsen the cardiovascular status of many survivors who already have impaired LV structure and function.38,39 Levels of NT-proBNP and hs-CRP may provide valuable information on the cardiovascular status of survivors and identify survivors in greatest need of further cardiovascular screening. Emerging evidence indicates that NT-proBNP levels within the range seen in both exposed and unexposed survivors are abnormal and associated with impaired cardiac function.40–43 As in pediatric CHF, monitoring such markers may provide valuable information on worsening cardiovascular status.44 Biomarker monitoring could possibly be incorporated into existing protocols for survivor follow-up.
Growth hormone deficiency may underlie many of these cardiovascular abnormalities and increases in risk factors for atherosclerotic disease.45 Both survivor groups had lower serum levels of IGF-1 and lower height. Unexposed female survivors had lower serum levels of IGF-1 than did unexposed males and, as mentioned earlier, had higher fasting serum levels of non-HDL cholesterol and insulin, as well as thinner LV walls. These characteristics are consistent with the hypothesis that cranial radiation damages the hypothalamic-pituitary axis, with growth hormone deficiency occurring before other endocrinopathies and at lower radiation exposures.46 A link between such damage and metabolic derangements has also been suggested.19,37,47,48 Patients with growth hormone deficiency from other causes have altered cardiac status and lipid profiles.49,50
The psychosocial impact of survivorship may also lead to lifestyle behaviors, such as physical inactivity, that mediate the risk factor profiles reported here.51,52 Systemic inflammation may be caused by these factors or may be related to novel pathophysiologic pathways. Cancer treatments may also have a lasting effect on the immune system, leaving survivors with low-grade inflammation.
Current guidelines do not address cardiovascular screening in unexposed survivors,18 but our results, in conjunction with recent studies,6,7 suggest that screening for cardiac disease may be warranted. Screening may be less frequent in unexposed survivors, although the optimum frequency is unknown even for exposed survivors. Guidelines should also consider the possible effects of increased systemic inflammation and atherosclerotic disease risk in unexposed survivors.
Study outcomes, such as changes in LV structure and function and increased risk for atherosclerotic disease and systemic inflammation, reflect risk factors in the general population that have yet to be validated in survivors. As with other childhood cancer survivor studies where patients have not been observed throughout their entire life, the true clinical meaning and importance of statistically significant, subclinical changes detected by surrogate markers that are generally within the normal range can be questioned. For example, the specific cardiovascular consequences of a 10 to 15 mg/dL, statistically significant increase in serum non-HDL cholesterol in survivors are unknown. However, given the young age of the survivor population and the rarity of cardiovascular complications in younger patients, these intermediate outcomes are the most likely to provide information on their cardiovascular risks and may predict clinically important outcomes.53 In the general population, even minor variations in serum cholesterol are strongly associated with cardiovascular disease mortality.54
The size of the differences in echocardiographic z scores in this study might seem small relative to those that guide daily clinical decisions by cardiologists, but they are consistent with those that independently predict mortality in children.55,56 The sibling control group, against which comparisons are made, did display statistically significant differences in cardiac measurements compared with the Boston cohort. Although this might suggest that the sibling controls were abnormal relative to the Boston cohort against which z scores were calculated, we believe such differences reflect variability in echocardiographic practice rather than differences in cardiac status and underscore the importance of having had the survivor and sibling echocardiograms read by a single reader blinded to their cancer histories.57
Survivors of childhood cancer, regardless of exposure to cardiotoxic treatments, have cardiovascular abnormalities related not only to abnormal LV structure and function but also to increased traditional risk factors for atherosclerotic disease and systemic inflammation. Our findings suggest that all survivors have a higher long-term risk of cardiovascular diseases and may benefit from screening across several cardiovascular domains. Markers, such as serum cholesterol, NT-proBNP, and hs-CRP, may help identify patients in greatest need of more detailed cardiovascular assessment. Screening guidelines should consider including specific recommendations for survivors who did not receive cardiotoxic treatments, and future investigations should consider to what extent atherosclerotic disease and systemic inflammation exacerbate treatment-related cardiotoxicity. Evaluations limited to assessing LV fractional shortening are unlikely to identify all survivors at risk for premature cardiovascular disease from all causes.
Appendix
Table A1.
Selected Echocardiographic Characteristics of 201 Long-Term Survivors of Childhood Cancer, With and Without Exposure to Cardiotoxic Cancer Treatments, by Study Group and Sex
| Echocardiography Characteristic* | Total (Males and Females) |
Males |
Females |
Male v Female, P |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed Survivors (n = 156) |
Unexposed Survivors (n = 45) |
Sibling Controls (n = 76) |
Exposed Survivors (n = 74) |
Unexposed Survivors (n = 26) |
Sibling Controls (n = 41) |
Exposed Survivors (n = 82) |
Unexposed Survivors (n = 19) |
Sibling Controls (n = 35) |
|||||||||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Exposed Survivors | Unexposed Survivors | Sibling Controls | |
| LV mass, g | 118.5† | 3.63 | 121.6 | 7.14 | 135.2 | 5.83 | 125.9† | 5.96 | 126.9 | 10.39 | 145.9 | 8.87 | 108.8 | 3.92 | 111.2 | 9.63 | 121.1 | 6.41 | .02 | .27 | .02 |
| LV end-diastolic posterior wall thickness, cm | 0.76* | 0.01 | 0.78* | 0.02 | 0.83 | 0.02 | 0.78 | 0.02 | 0.82 | 0.03 | 0.83 | 0.02 | 0.73† | 0.02 | 0.73† | 0.02 | 0.83 | 0.03 | .06 | .01 | .99 |
| LV end-diastolic dimension, cm | 4.44† | 0.04 | 4.48 | 0.10 | 4.60 | 0.08 | 4.50 | 0.07 | 4.54 | 0.15 | 4.73 | 0.11 | 4.34 | 0.05 | 4.39 | 0.14 | 4.46 | 0.08 | .06 | .46 | .04 |
| LV thickness-to-dimension ratio | 0.17 | 0.003 | 0.18 | 0.004 | 0.18 | 0.004 | 0.18 | 0.004 | 0.18 | 0.005 | 0.18 | 0.005 | 0.17† | 0.003 | 0.17 | 0.007 | 0.19 | 0.006 | .37 | .17 | .35 |
| LV afterload (end-systolic wall stress), g/cm2 | 49.42† | 1.12 | 45.89 | 2.17 | 40.77 | 1.73 | 48.61 | 1.46 | 45.70 | 2.73 | 43.69 | 2.37 | 50.20† | 1.70 | 46.51 | 3.55 | 38.73 | 2.14 | .48 | .86 | .12 |
| LV fractional shortening, % | 34.43†‡ | 0.36 | 36.85 | 0.91 | 37.27 | 0.58 | 34.62† | 0.50 | 36.34 | 0.84 | 36.86 | 0.87 | 34.37† | 0.52 | 37.17 | 1.35 | 37.31 | 0.66 | .43 | .60 | .68 |
Abbreviation: LV, left ventricular.
Total values were adjusted using a weighted propensity score technique for age and sex. Sex-specific values were adjusted using a weighted propensity score technique for age.
The difference in the characteristic for either exposed or unexposed survivors versus sibling controls for either sex is significant at P < .05.
The difference in the characteristic for exposed versus unexposed survivors for either sex is significant at P < .05.
Fig A1.
Scatter plots of left ventricular (LV) structure and function by N-terminal pro-brain natriuretic peptide (NT-proBNP) and high-sensitivity C-reactive protein (hs-CRP) in survivors of childhood cancer by exposure to known cardiotoxic treatments. Scatter plots show the paired values for specific echocardiographic characteristics and serum biomarkers with a linear regression line overlaid. Partial Spearman's rank correlation coefficients (ρ), with the effect of the other biomarker removed, and associated P values are also reported for the associations between these biomarkers and echocardiographic characteristics. Ln, natural logarithm.
Footnotes
Supported by the National Institutes of Health (Grants No. HL072705, HL078522, HL053392, CA127642, CA068484, HD052104, AI50274, CA068484, HD052102, HL087708, HL079233, HL004537, HL087000, HL007188, HL094100, HL095127, and HD80002), the American Heart Association (Grant No. 11PRE 790000), the Children's Cardiomyopathy Foundation, the University of Miami Women's Cancer Association, and the Lance Armstrong Foundation.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Steven E. Lipshultz, Stuart R. Lipsitz, Andrea S. Hinkle, Louis S. Constine, Tracie L. Miller
Provision of study materials or patients: Steven E. Lipshultz, Andrea S. Hinkle, Louis S. Constine, Cindy Proukou, Tracie L. Miller
Collection and assembly of data: Steven E. Lipshultz, Gabriela Lopez-Mitnik, Stuart R. Lipsitz, Andrea S. Hinkle, Louis S. Constine, Carol A. French, Amy M. Rovitelli, Cindy Proukou, Tracie L. Miller
Data analysis and interpretation: David C. Landy, Gabriela Lopez-Mitnik, Stuart R. Lipsitz
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 2.Silverman LB, Stevenson KE, O'Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000) Leukemia. 2010;24:320–334. doi: 10.1038/leu.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivors Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 5.Möller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: A population-based study in the Nordic countries. J Clin Oncol. 2001;19:3173–3181. doi: 10.1200/JCO.2001.19.13.3173. [DOI] [PubMed] [Google Scholar]
- 6.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28:1308–1315. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 8.Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–179. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 9.Levitt G, Anazodo A, Burch M, et al. Cardiac or cardiopulmonary transplantation in childhood cancer survivors: An increasing need? Eur J Cancer. 2009;45:3027–3034. doi: 10.1016/j.ejca.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94:525–533. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 11.Krischer JP, Epstein S, Cuthbertson DD, et al. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: The Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544–1552. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 12.Lipshultz SE, Colan SD, Gelber RD, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 13.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 14.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 15.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 16.Sorensen K, Levitt GA, Bull C, et al. Late anthracycline cardiotoxicity after childhood cancer: A prospective longitudinal study. Cancer. 2003;97:1991–1998. doi: 10.1002/cncr.11274. [DOI] [PubMed] [Google Scholar]
- 17.Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: Beginning with the end in mind. J Clin Oncol. 2010;28:1276–1281. doi: 10.1200/JCO.2009.26.5751. [DOI] [PubMed] [Google Scholar]
- 18.Shankar SM, Marina N, Hudson MM, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: Report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121:e387–e396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 19.Talvensaari KK, Lanning M, Tapanainen P, et al. Long-term survivors of childhood cancer have an increased risk of manifesting the metabolic syndrome. J Clin Endocrinol Metab. 1996;81:3051–3055. doi: 10.1210/jcem.81.8.8768873. [DOI] [PubMed] [Google Scholar]
- 20.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer: Increased risk associated with radiation therapy—A report for the Childhood Cancer Survivor Study. Arch Intern Med. 2009;169:1381–1388. doi: 10.1001/archinternmed.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: A report of the Childhood Cancer Survivor Study. Cancer. 2005;103:1730–1739. doi: 10.1002/cncr.20960. [DOI] [PubMed] [Google Scholar]
- 22.Miller TL, Lipsitz SR, Lopez-Mitnik G, et al. Characteristics and determinants of adiposity in pediatric cancer survivors. Cancer Epidemiol Biomarkers Prev. 2010;19:2013–2022. doi: 10.1158/1055-9965.EPI-10-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: A scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research—Endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 24.Hinkle AS, Proukou C, French CA, et al. A clinic-based, comprehensive care model for studying late effects in long-term survivors of pediatric illnesses. Pediatrics. 2004;113(suppl 4):1141–1145. [PubMed] [Google Scholar]
- 25.McMahan CA, Gidding SS, Fayad ZA, et al. Risk scores predict atherosclerotic lesions in young people. Arch Intern Med. 2005;165:883–890. doi: 10.1001/archinte.165.8.883. [DOI] [PubMed] [Google Scholar]
- 26.Pencina MJ, D'Agostino RB, Sr, Larson MG, et al. Predicting the 30-year risk of cardiovascular disease: The Framingham Heart Study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colan SD, Parness IA, Spevak PJ, et al. Developmental modulation of myocardial mechanics: Age- and growth-related alterations in afterload and contractility. J Am Coll Cardiol. 1992;19:619–629. doi: 10.1016/s0735-1097(10)80282-7. [DOI] [PubMed] [Google Scholar]
- 28.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 29.Rosenbaum PR. Model-based direct adjustment. J Am Stat Assoc. 1987;82:387–394. [Google Scholar]
- 30.Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making. 2009;29:661–677. doi: 10.1177/0272989X09341755. [DOI] [PubMed] [Google Scholar]
- 31.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: A comparative study. Stat Med. 2004;23:2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz CL, Hobbie WL, Truesdell S, et al. Corrected QT interval prolongation in anthracycline-treated survivors of childhood cancer. J Clin Oncol. 1993;11:1906–1910. doi: 10.1200/JCO.1993.11.10.1906. [DOI] [PubMed] [Google Scholar]
- 33.Gupta M, Thaler HT, Friedman D, et al. Presence of prolonged dispersion of QT intervals in late survivors of childhood anthracycline therapy. Pediatr Hematol Oncol. 2002;19:533–542. doi: 10.1080/08880010290097387. [DOI] [PubMed] [Google Scholar]
- 34.Lipshultz SE, Shearer WT, Thompson B, et al. Cardiac effects of antiretroviral therapy in HIV-negative infants born to HIV-positive mothers: NHLBI CHAART-1 (National Heart, Lung, and Blood Institute Cardiovascular Status of HAART Therapy in HIV-Exposed Infants and Children Cohort Study) J Am Coll Cardiol. 2011;57:76–85. doi: 10.1016/j.jacc.2010.08.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: Long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11:950–961. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oeffinger KC, Adams-Huet B, Victor RG, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:3698–3704. doi: 10.1200/JCO.2008.19.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Link K, Moëll C, Garwicz S, et al. Growth hormone deficiency predicts cardiovascular risk in young adults treated for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab. 2004;89:5003–5012. doi: 10.1210/jc.2004-0126. [DOI] [PubMed] [Google Scholar]
- 38.Lipshultz SE, Miller TL, Scully RE, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: Associations with long-term echocardiographic outcomes. J Clin Oncol. doi: 10.1200/JCO.2010.30.3404. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratnasamy C, Kinnamon DD, Lipshultz SE, et al. Associations between neurohormonal and inflammatory activation and heart failure in children. Am Heart J. 2008;155:527–533. doi: 10.1016/j.ahj.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Salem M, El Behery S, Adly A, et al. Early predictors of myocardial disease in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2009;10:513–521. doi: 10.1111/j.1399-5448.2009.00517.x. [DOI] [PubMed] [Google Scholar]
- 41.van den Berg J, Strengers JL, Wielopolski PA, et al. Assessment of biventricular functional reserve and NT-proBNP levels in patients with RV volume overload after repair of tetralogy of Fallot at young age. Int J Cardiol. 2009;133:364–370. doi: 10.1016/j.ijcard.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Kremastinos DT, Hamodraka E, Parissis J, et al. Predictive value of B-type natriuretic peptides in detecting latent left ventricular diastolic dysfunction in beta-thalassemia major. Am Heart J. 2010;159:68–74. doi: 10.1016/j.ahj.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 43.Germanakis I, Kalmanti M, Parthenakis F, et al. Correlation of plasma N-terminal pro-brain natriuretic peptide levels with left ventricle mass in children treated with anthracyclines. Int J Cardiol. 2006;108:212–215. doi: 10.1016/j.ijcard.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Rusconi PG, Ludwig DA, Ratnasamy C, et al. Serial measurements of serum NT-proBNP as markers of left ventricular systolic function and remodeling in children with heart failure. Am Heart J. 2010;160:776–783. doi: 10.1016/j.ahj.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipshultz SE, Vlach SA, Lipsitz SR, et al. Cardiac changes associated with growth hormone therapy among children treated with anthracyclines. Pediatrics. 2005;115:1613–1622. doi: 10.1542/peds.2004-1004. [DOI] [PubMed] [Google Scholar]
- 46.Rutter MM, Rose SR. Long-term endocrine sequelae of childhood cancer. Curr Opin Pediatr. 2009;19:480–487. doi: 10.1097/MOP.0b013e3282058b56. [DOI] [PubMed] [Google Scholar]
- 47.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 48.Trimis G, Moschovi M, Papassotiriou I, et al. Early indicators of dysmetabolic syndrome in young survivors of acute lymphoblastic leukemia in childhood as a target for preventing disease. J Pediatr Hematol Oncol. 2007;29:309–314. doi: 10.1097/MPH.0b013e318059c249. [DOI] [PubMed] [Google Scholar]
- 49.Colao A. The GH-IGF-1 axis and the cardiovascular system: Clinical implications. Clin Endocrinol. 2008;69:347–358. doi: 10.1111/j.1365-2265.2008.03292.x. [DOI] [PubMed] [Google Scholar]
- 50.Verhelst J, Abs R. Cardiovascular risk factors in hypopituitary GH-deficient adults. Eur J Endocrinol. 2009;161(suppl 1):S41–S49. doi: 10.1530/EJE-09-0291. [DOI] [PubMed] [Google Scholar]
- 51.van Brussel M, Takken T, Lucia A, et al. Is physical fitness decreased in survivors of childhood leukemia? A systematic review. Leukemia. 2005;19:13–17. doi: 10.1038/sj.leu.2403547. [DOI] [PubMed] [Google Scholar]
- 52.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2009;115:1984–1994. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipshultz SE, Colan SD. Cardiovascular trials in long-term survivors of childhood cancer. J Clin Oncol. 2004;22:769–773. doi: 10.1200/JCO.2004.12.937. [DOI] [PubMed] [Google Scholar]
- 54.Prospective Studies Collaboration. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 55.Lipshultz SE, Easley KA, Orav EJ, et al. Cardiac dysfunction and mortality in HIV-infected children: The prospective P2C2 HIV multicenter study—Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group. Circulation. 2000;102:1542–1548. doi: 10.1161/01.cir.102.13.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisher SD, Easley KA, Orav EJ, et al. Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: The prospective P2C2 HIV multicenter study. Am Heart J. 2005;150:439–447. doi: 10.1016/j.ahj.2005.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lipshultz SE, Easley KA, Orav EJ, et al. Reliability of multicenter pediatric echocardiographic measurements of left ventricular structure and function. Circulation. 2001;104:310–316. doi: 10.1161/01.cir.104.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]