Abstract
Purpose
The Veterans Health Administration (VHA) provides high-quality preventive chronic care and cancer care, but few studies have documented improved patient outcomes that result from this high-quality care. We compared the survival rates of older patients with cancer in the VHA and fee-for-service (FFS) Medicare and examined whether differences in the stage at diagnosis, receipt of guideline-recommended therapies, and unmeasured characteristics explain survival differences.
Patients and Methods
We used propensity-score methods to compare all-cause and cancer-specific survival rates for men older than age 65 years who were diagnosed or received their first course of treatment for colorectal, lung, lymphoma, or multiple myeloma in VHA hospitals from 2001 to 2004 to similar FFS-Medicare enrollees diagnosed in Surveillance, Epidemiology, and End Results (SEER) areas in the same time frame. We examined the role of unmeasured factors by using sensitivity analyses.
Results
VHA patients versus similar FFS SEER-Medicare patients had higher survival rates of colon cancer (adjusted hazard ratio [HR], 0.87; 95% CI, 0.82 to 0.93) and non–small-cell lung cancer (NSCLC; HR, 0.91; 95% CI, 0.88 to 0.95) and similar survival rates of rectal cancer (HR, 1.05; 95% CI, 0.95 to 1.16), small-cell lung cancer (HR, 0.99; 95% CI, 0.93 to 1.05), diffuse large–B-cell lymphoma (HR, 1.02; 95% CI, 0.89 to 1.18), and multiple myeloma (HR, 0.92; 95% CI, 0.83 to 1.03). The diagnosis of VHA patients at earlier stages explained much of the survival advantages for colon cancer and NSCLC. Sensitivity analyses suggested that additional adjustment for the severity of comorbid disease or performance status could have substantial effects on estimated differences.
Conclusion
The survival rate for older men with cancer in the VHA was better than or equivalent to the survival rate for similar FFS-Medicare beneficiaries. The VHA provision of high-quality care, particularly preventive care, can result in improved patient outcomes.
INTRODUCTION
The Veterans Health Administration (VHA) is the largest integrated health care system in the United States and serves an estimated 6 million veterans yearly. The VHA underwent a major reorganization in the mid-1990s that emphasized improved primary and outpatient care. Since then, studies have demonstrated the provision of high-quality preventive and chronic care in the VHA.1–3 Recent evidence showed that the VHA also provides high-quality care for cancer, which is a complex illness that often requires multiple specialty services.4,5 For example, we recently found higher rates of surgery for colon cancer and recommended chemotherapy for diffuse large–B-cell lymphoma and bisphosphonates for multiple myeloma among older male VHA patients compared with similar men enrolled in FFS Medicare and similar rates of other guideline-recommended therapies for patients with colorectal, prostate, hematologic, and lung cancers.4
However, little is known about whether the improved processes of care translate into improved patient outcomes in the VHA. Studies in the early 1990s to mid-1990s found lower rates of recommended invasive care for cardiac patients in the VHA, with mixed findings about the impact for outcomes.6–8 A recent review of studies published since 1990 found that the survival rate was generally equivalent for VHA patients and patients in other settings,3 which raised the question of why improved processes of care have not resulted in better outcomes. However, most of these studies examined care before the VHA reorganization and could not control for clinical or socioeconomic differences between VHA and non-VHA patients.
We examined survival rates for older veterans with lung, colorectal, or hematologic cancers who were diagnosed or treated in the VHA compared with similar patients with cancer enrolled in FFS Medicare. We also examined whether differences in the stage at diagnosis or cancer treatments were mediators of differences in survival rates and explored the role of unmeasured differences between VHA and non-VHA patients in the explanation of outcome differences.
PATIENTS AND METHODS
Data
VHA.
The Department of Veterans Affairs (VA) Central Cancer Registry collects uniformly reported information on all patients who were diagnosed with or received their first course of treatment for cancer at a VA Medical Center. We linked registry data with VHA encounter data that covered hospitalizations, outpatient visits, and contracted care. Because previous studies have demonstrated that elderly VHA patients often receive care through Medicare9–11 as well as the VHA, we also obtained Medicare claims data for inpatient and outpatient care for Medicare-eligible VHA patients.
FFS Medicare.
We used the Surveillance, Epidemiology, and End Results (SEER) –Medicare data for this analysis.12 SEER registrars collect uniformly reported data from population-based cancer registries that cover approximately 28% of the United States.13 The data are merged with Medicare claims data, which successfully links files for more than 94% of SEER patients age 65 or older.12
The study was approved by the Harvard Medical School Committee on Human Studies.
Cohorts
We studied patients with colon, rectal, non–small-cell lung cancer (NSCLC), small-cell lung cancer, diffuse large–B-cell lymphoma, and multiple myeloma. We created disease-specific cohorts by identifying all male patients age 66 years and older with a first diagnosis of the cancer of interest during 2001 to 2004. We excluded patients with histology that suggested a primary cancer other than the cancer of interest, cancers diagnosed at autopsy or by death certificate only or when the reporting source was unknown, and patients with incomplete data (including no administrative data between 45 days before diagnosis through 195 days after diagnosis because we were concerned data were incomplete). For the FFS-Medicare cohort, we also excluded patients who were not enrolled in both parts A and B of Medicare or enrolled in a Medicare health maintenance organization in the year before diagnosis (to ensure complete data on comorbid illness before diagnosis). The numbers of patients excluded for these reasons are included in the Data Supplement.
Survival Rates
For the FFS-Medicare cohorts, dates of death were included in Medicare enrollment and death-certificate data, including the cause of death, from a National Death Index match. For the VA cohort, we obtained vital status data from Medicare enrollment data, the National Death Index (including the cause of death), and VA administrative sources. We computed the time to death as a result of all causes and the time to death as a result of cancer. We censored patients alive as of December 31, 2005 (the last date with complete vital status data available from all sources). In analyses of the time to death as a result of cancer, we censored patients who died as a result of other causes when they died.
Mediating Factors
We examined whether the stage at diagnosis and tumor size, which were obtained from registry data, explained observed survival differences. For diagnoses made in 2001 to 2003, we used the modified American Joint Committee on Cancer stage. Starting in 2004, both VHA and SEER registries used collaborative stage groupings.14 The stage at diagnosis was collected for patients with lymphoma only in 2004 and was not available for multiple myeloma patients in all years. For small-cell lung cancer patients, we categorized patients with stage I to III cancer as having limited-stage cancer and patients with stage IV cancer as having extensive-stage cancer.
We also examined whether differences in the use of guideline-recommended therapies15–20 explained survival differences. Specifically, we examined curative surgery for stage I to III colon cancer, stage I to III rectal cancer, and stage I and II NSCLC, adjuvant chemotherapy for stage III colon cancer, adjuvant chemotherapy and radiation therapy for stage II and III rectal cancer, chemotherapy and radiation therapy for limited-stage small-cell lung cancer, and cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy for diffuse large–B-cell lymphoma as described elsewhere.4
Patient Characteristics
We obtained information about age, race/ethnicity, marital status, and history of previous cancer from registry data. We characterized comorbid illnesses on the basis of inpatient and outpatient encounters during the year before diagnosis by using the Klabunde modification of the Charlson score.21,22 Information on sociodemographic indicators was obtained from 2000 Census data for the zip code of each patient.
Analyses
Veterans were eligible for care through the VHA primarily because of service-related disabilities or economic disadvantage, and thus, older patients with cancer treated in the VHA differed from FFS-Medicare patients with respect to many important sociodemographic and clinical characteristics. We used a propensity-score23,24 approach to account for differences in characteristics of patients at the time of their cancer diagnosis to estimate the effect of receiving care through the VHA compared with what would have been obtained had they decided to receive non-VHA care through FFS Medicare.
To conduct the propensity-score adjustment, we first used a logistic regression model to calculate the propensity of being treated in the VHA on the basis of age, race, marital status, Charlson score, previous cancer, census region, quarter-year of diagnosis, and census variables that described socioeconomic conditions in the zip code of the residence of the patient. We used regression coefficients and observed covariates to estimate the propensity for each man to be treated in the VHA (p). We applied a standardized mortality ratio propensity-score weight that equaled 1 for VHA patients and the propensity odds [p ÷ (1 − p)] for FFS-Medicare patients.25,26 This application gave additional weight to FFS-Medicare patients who most resemble VHA patients so that the weighted distribution of characteristics in the two cohorts was well balanced and equaled that of the original VHA cohort (Data Supplement). Thus, the standardized mortality ratio–weighted effects estimated the survival rate in a typical VHA patient had they received care under FFS Medicare.
We compared all-cause and cancer-specific survival rates by plotting weighted Kaplan-Meier survival curves for VHA and Medicare patients and tested for differences by using a weighted Cox proportional hazard model with VHA or FFS-Medicare status as the only covariate. CIs were computed by using a robust variance estimator that accounts for unequal weighting of observations and the correlation among patients within treatment settings. We used the hospital that reported the cancer diagnosis as the clustering unit for VHA patients because outpatient care in the VHA tends to occur at clinics associated with inpatient facilities. For FFS-Medicare patients, we used the hospital service area as a proxy for the local practice setting.
Mediating Effects
To investigate the ability of differences in stage at diagnosis or receipt of recommended therapy to mediate the relationship between the treatment setting and survival rates, we replicated the analyses and included these variables in the propensity score analysis. We compared adjusted hazard ratios (HRs) estimated by using the new propensity-score weights to those estimated by using original propensity-score weights to determine whether HRs were attenuated or exacerbated when differences in stage or therapies was equalized between VHA and FFS-Medicare patients.
Sensitivity Analyses
Because propensity-score analyses can control only for observed characteristics, we examined the robustness of estimated treatment effects to unobserved confounders.27 To do this, we considered an unobserved variable, such as poor performance status, associated with both care in the VHA and worse survival rates. We updated estimates of the HR by comparing survival rates between VHA and FFS-Medicare patients after adjustment for this confounder under specific assumptions regarding differences between VHA and FFS-Medicare patients in the prevalence of the confounders and the relationship of confounders with survival rates.
We considered the following four potential unmeasured confounders: poor Eastern Cooperative Oncology Group performance status (≥ 2), lack of college education, more severe comorbid illness, and smoking status. We obtained estimates of the relationship between these factors and survival rates from previously published literature.28–31 We estimated performance-status differences between VHA and non-VHA patients with cancer from clinical trial data for patients with lung cancer.32 We estimated the prevalence of severe comorbidity in VHA patients from medical record abstraction of a subset of patients. The prevalence of severe comorbidity in FFS-Medicare patients was estimated on the basis of analyses of the Cancer Care Outcomes Research and Surveillance (CanCORS) patients with lung and colorectal cancer.33,34 We obtained estimates of differences in rates of college education and smoking status between VHA and non-VHA patients with cancer from an analysis of the National Health Interview Survey for population-based cohorts of veterans and nonveterans.35
RESULTS
VHA patients were younger, more likely to be African American, more likely to live in areas with lower levels of education and income, and more likely to live in the South compared with FFS-Medicare patients (Table 1). After propensity weighting, the cohorts appeared well balanced (Data Supplement). Median follow-up was 3 years.
Table 1.
Patient Demographics and Clinical Characteristics by Cohort
| Demographic or Clinical Characteristic | Colon Cancer |
Rectal Cancer |
NSCLC |
Small-Cell Lung Cancer |
Non-Hodgkin's Lymphoma |
Multiple Myeloma |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VHA | SEER-Medicare | VHA | SEER-Medicare | VHA | SEER-Medicare | VHA | SEER-Medicare | VHA | SEER-Medicare | VHA | SEER-Medicare | |
| N | 7,003 | 20,734 | 1,757 | 4,562 | 13,434 | 31,868 | 2,111 | 4,669 | 613 | 3,192 | 900 | 3,170 |
| Age, % | ||||||||||||
| 66-69 years | 17.8 | 15.0 | 18.1 | 17.8 | 21.9 | 18.3 | 24.4 | 21.2 | 16.0 | 14.4 | 18.6 | 16.9 |
| 70-74 years | 28.0 | 23.3 | 30.4 | 25.6 | 30.5 | 27.3 | 32.6 | 28.7 | 24.6 | 22.6 | 25.0 | 24.8 |
| 75-79 years | 28.5 | 25.2 | 28.1 | 25.3 | 29.2 | 26.9 | 27.6 | 28.0 | 29.2 | 25.9 | 28.4 | 25.5 |
| 80-84 years | 19.2 | 20.9 | 18.5 | 18.5 | 15.0 | 18.0 | 12.4 | 15.1 | 23.0 | 22.7 | 23.1 | 19.6 |
| ≥ 85 years | 6.5 | 15.7 | 5.0 | 12.8 | 3.4 | 9.6 | 3.0 | 7.1 | 7.2 | 14.4 | 4.9 | 13.2 |
| Race, % | ||||||||||||
| White | 78.3 | 90.3 | 80.8 | 92.0 | 80.7 | 89.4 | 85.1 | 91.7 | 88.8 | 94.4 | 64.7 | 82.9 |
| African American | 16.3 | 8.1 | 13.7 | 6.0 | 17.2 | 9.1 | 12.6 | 6.9 | 6.2 | 3.3 | 27.8 | 14.6 |
| Hispanic | 5.4 | 1.5 | 5.5 | 2.0 | 2.2 | 1.5 | 2.3 | 1.4 | 5.0 | 2.3 | 6.5 | 2.4 |
| Missing | 2.1 | 4.9 | 2.3 | 5.6 | 1.5 | 4.7 | 1.5 | 4.0 | 2.4 | 5.0 | 2.1 | 3.2 |
| Marital status, % | ||||||||||||
| Single | 44.0 | 29.4 | 44.8 | 29.4 | 47.7 | 31.9 | 46.9 | 30.8 | 43.4 | 24.9 | 39.0 | 26.5 |
| Married | 56.0 | 70.6 | 55.2 | 70.6 | 52.3 | 68.1 | 53.1 | 69.3 | 56.6 | 75.2 | 61.0 | 73.5 |
| Missing | 2.6 | 3.1 | 2.5 | 3.4 | 2.3 | 2.6 | 1.8 | 2.3 | 1.1 | 3.1 | 1.9 | 4.9 |
| Census region, % | ||||||||||||
| Northeast | 17.1 | 25.1 | 15.9 | 24.6 | 14.6 | 22.1 | 13.8 | 20.5 | 13.5 | 23.5 | 14.6 | 22.6 |
| Midwest | 20.0 | 15.8 | 21.1 | 15.4 | 21.3 | 15.8 | 24.8 | 17.3 | 21.5 | 15.7 | 21.8 | 17.6 |
| South | 46.2 | 19.2 | 45.7 | 18.8 | 46.9 | 23.5 | 43.9 | 25.7 | 42.6 | 16.5 | 43.7 | 19.2 |
| West | 16.7 | 39.9 | 17.3 | 41.3 | 17.2 | 38.6 | 17.5 | 36.5 | 22.4 | 44.2 | 20.0 | 40.7 |
| Socioeconomic variables* | ||||||||||||
| With college degree in zip code of residence, % | 25.6 | 31.7 | 24.6 | 30.8 | 24.6 | 29.7 | 24.6 | 28.8 | 27.6 | 34.2 | 26.8 | 32.4 |
| Professionals in zip code of residence, % | 29.5 | 34.3 | 28.8 | 33.6 | 28.9 | 32.9 | 28.8 | 32.1 | 31.0 | 36.1 | 30.4 | 34.9 |
| Median household income in zip code of residence, $ | 44,800 | 56,500 | 44,400 | 55,500 | 44,500 | 54,000 | 45,200 | 53,000 | 47,300 | 59,600 | 45,300 | 57,300 |
| Age ≥ 65 years with income < poverty level in zip code of residence, % | 12.5 | 9.2 | 12.3 | 9.2 | 12.2 | 9.7 | 11.5 | 9.8 | 11.2 | 8.4 | 13.0 | 9.4 |
| Hispanic in zip code of residence, % | 12.2 | 11.7 | 12.9 | 12.5 | 10.3 | 11.6 | 10.0 | 11.5 | 11.7 | 11.5 | 13.0 | 11.7 |
| African American in zip code of residence, % | 16.1 | 10.1 | 14.0 | 9.1 | 16.8 | 10.7 | 14.0 | 9.8 | 10.6 | 7.3 | 20.2 | 12.3 |
| Missing census data, % | 5.7 | 2.9 | 4.7 | 2.9 | 4.6 | 2.9 | 4.7 | 2.6 | 4.6 | 2.7 | 6.7 | 2.8 |
| Charlson comorbidity score, % | ||||||||||||
| 0 | 44.4 | 49.0 | 51.0 | 57.1 | 54.1 | 56.9 | 53.0 | 55.2 | 41.3 | 50.0 | 40.1 | 47.5 |
| 1 | 30.8 | 26.6 | 29.7 | 25.0 | 25.8 | 24.4 | 25.3 | 24.9 | 31.5 | 25.8 | 25.4 | 23.2 |
| 2 | 14.1 | 13.4 | 11.0 | 10.0 | 12.2 | 10.8 | 12.4 | 10.8 | 14.7 | 13.0 | 17.2 | 14.2 |
| ≥ 3 | 10.7 | 11.0 | 8.3 | 8.0 | 7.9 | 8.0 | 9.3 | 9.1 | 12.6 | 11.2 | 17.2 | 15.1 |
| COPD, % | 45.0 | 41.8 | 44.5 | 42.9 | ||||||||
| Prior cancer, % | 16.7 | 23.5 | 16.1 | 23.3 | 21.3 | 25.9 | 19.2 | 21.7 | 19.3 | 25.9 | 20.0 | 24.3 |
| Tumor grade, % | ||||||||||||
| Well differentiated | 11.9 | 10.4 | 10.4 | 9.3 | 6.6 | 6.7 | ||||||
| Moderately differentiated | 72.9 | 70.5 | 75.5 | 73.7 | 33.4 | 30.4 | ||||||
| Undifferentiated | 15.3 | 19.2 | 14.1 | 17.1 | 60.1 | 63.0 | ||||||
| Tumor grade missing, % | 13.9 | 10.1 | 18.2 | 13.3 | 50.8 | 48.7 | ||||||
| Stage at diagnosis, % | ||||||||||||
| I | 31.7 | 26.4 | 38.3 | 39.7 | 28.0 | 23.4 | 23.8§ | 33.1§ | ||||
| II | 28.1 | 31.2 | 26.9 | 23.3 | 7.1 | 5.6 | 39.7† | 37.7† | 12.2§ | 17.7§ | ||
| III | 22.7 | 24.7 | 19.9 | 22.1 | 26.5 | 29.5 | 23.2§ | 15.9§ | ||||
| IV | 17.5 | 17.6 | 14.9 | 14.9 | 38.4 | 41.6 | 60.3‡ | 62.3‡ | 40.9§ | 33.3§ | ||
| Stage missing, % | 7.7 | 5.0 | 11.6 | 9.4 | 5.2 | 9.0 | 4.6 | 6.8 | 9.9§ | 8.5§ | ||
| Tumor size, %¶ | ||||||||||||
| T1 | 22.3 | 17.6 | 24.8 | 23.9 | 25.1 | 21.8 | ||||||
| T2 | 20.1 | 17.2 | 23.7 | 25.3 | 38.4 | 35.4 | ||||||
| T3 | 51.2 | 54.7 | 45.7 | 45.5 | 12.1 | 9.2 | ||||||
| T4 | 6.5 | 10.5 | 5.8 | 5.3 | 24.5 | 33.7 | ||||||
| Tumor size missing, %¶ | 1.2 | 1.9 | 2.4 | 5.5 | 12.3 | 10.1 | ||||||
Abbreviations: COPD, chronic obstructive pulmonary disease; NSCLC, non–small-cell lung cancer; SEER, Surveillance, Epidemiology, and End Results; VHA, Veterans Health Administration.
Obtained from 2000 Census by linking to the zip code of the residence of the patient.
Limited stage.
Extensive stage.
Stage collected in 2004 only.
Among stage I/II/III cancers.
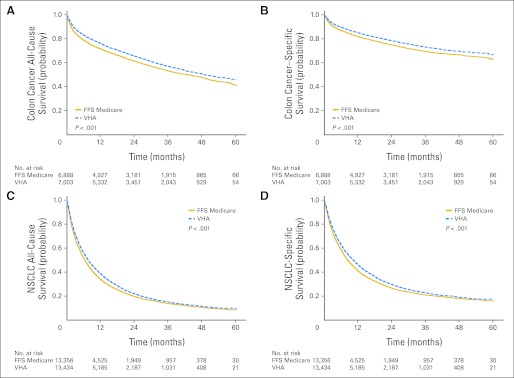
VHA patients diagnosed with colon cancer had better all-cause and cancer-specific survival rates (Figs 1A and 1B; all-cause adjusted HR, 0.87; 95% CI, 0.82 to 0.93) as did patients with NSCLC (Figs 1C and 1D; all-cause adjusted HR, 0.91; 95% CI, 0.88 to 0.95). All-cause and cancer-specific survival rates were similar after diagnosis with rectal cancer (HR, 1.05; 95% CI, 0.95 to 1.16), small-cell lung cancer (HR, 0.99; 95% CI, 0.93 to 1.05), diffuse large B-cell lymphoma (HR, 1.02; 95% CI, 0.89 to 1.18), and multiple myeloma (HR, 0.92; 95% CI, 0.83 to 1.03).
Fig 1.
All-cause and cancer-specific mortality. Kaplan-Meier survival curves in Veterans Health Administration (VHA) and fee-for-service (FFS) –Medicare patients are shown. Curves were adjusted by using standardized mortality ratio (SMR) propensity weights. SMR-weighted effects estimate survival rates that a typical VHA patient would experience under FFS Medicare. (A) All-cause survival rates in patients with colon cancer. Adjusted median survival was 49 months in VHA patients versus 43 months in FFS-Medicare patients. (B) Cancer-specific survival rates in patients with colon cancer. (C) All-cause survival rates in patients with non–small-cell lung cancer (NSCLC). The adjusted median survival was 8 months in VHA patients versus 6 months in FFS-Medicare patients. (D) Cancer-specific survival rates in patients with NSCLC.
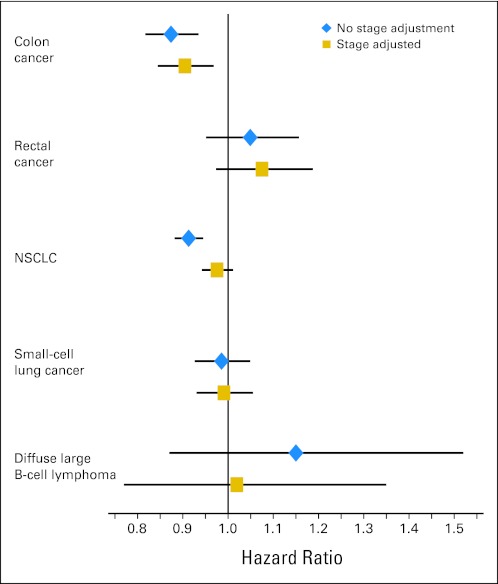
VHA patients with colon and NSCLC were diagnosed at earlier stages and with smaller tumors than FFS-Medicare patients (Table 1). An earlier stage at diagnosis explained almost all of the survival advantage in NSCLC patients (Fig 2). Among patients diagnosed at equivalent stages and with similar tumor sizes, the hazard of death was 2% lower (adjusted HR, 0.98; 95% CI, 0.94 to 1.01) in VHA versus FFS-Medicare patients versus 9% lower (adjusted HR, 0.91; 95% CI, 0.88 to 0.94) in cohorts with similar sociodemographic characteristics but without adjustment for stage and tumor size. Accounting for an earlier stage of diagnosis also decreased the survival differences among patients with colon cancer, but even among patients with a similar stage and tumor size, VHA patients had significantly better all-cause (adjusted HR, 0.90; 95% CI, 0.85 to 0.97) and cancer-specific survival rates. Differences in the stage at diagnosis and tumor size shifted the estimated HR among patients with rectal cancer, but larger CIs associated with the smaller number of patients with rectal cancer resulted in a substantial overlap in estimates with and without adjustment for stage. In addition, although in 2004 (the only year in which stage data were available), patients with diffuse large B-cell lymphoma in the VHA were diagnosed at later stages than Medicare FFS, small sample sizes precluded our ability to understand whether such differences affected survival rates in the two settings.
Fig 2.
Adjusted hazard ratios (95% CIs) of death in Veterans Health Administration (VHA) versus fee-for-service (FFS)–Medicare patients with cancer with and without adjustment for the stage at diagnosis. Values were adjusted by using a Cox proportional hazard model with standardized mortality ratio (SMR) propensity weights. SMR-weighted effects estimated the survival rate that a typical VHA patient would have experienced in FFS Medicare. Blue diamonds depict hazard ratios that were adjusted only for sociodemographic characteristics and comorbidity. Gold squares depict hazard ratios when the stage and tumor size were also included in the propensity score model. NSCLC, non–small-cell lung cancer.
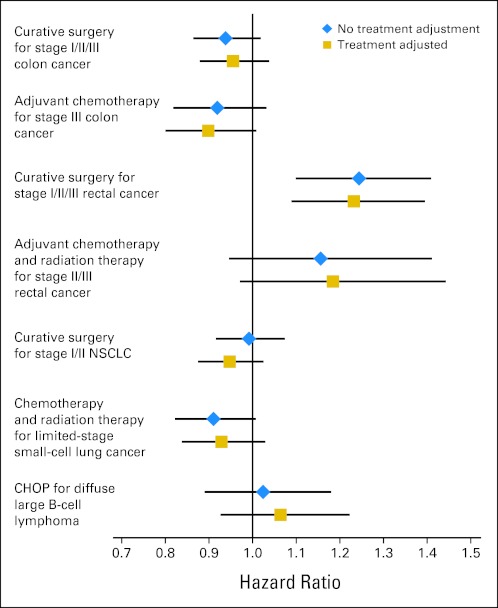
We observed few differences in survival rates between VHA and FFS-Medicare patients among stage-specific cohorts eligible for receipt of specific therapies, with the exception of stages I to III rectal cancer, in which we observed worse survival rates in VHA relative to FFS-Medicare patients (Fig 3). We previously observed similar rates of guideline-recommended therapies in VHA patients compared with similar FFS-Medicare patients for most treatments and higher rates for some treatments.4 When differences in the use of effective therapies between VHA and FFS-Medicare patients were controlled for, there were small impacts on survival differences (Fig 3).
Fig 3.
Adjusted hazard ratios (95% CIs) of death in Veterans Health Administration (VHA) versus fee-for-service (FFS)–Medicare patients with cancer with and without adjustment for receipt of guideline-recommended therapy. Values were adjusted by using a Cox proportional hazard model with standardized mortality ratio (SMR) propensity weights. SMR-weighted effects estimated the survival rate that a typical VHA patient would have experienced in FFS Medicare. Blue diamonds depict hazard ratios that were adjusted for sociodemographic characteristics, comorbidity, tumor size, and stage at diagnosis. Gold squares depict hazard ratios when receipt of therapy was also included in the propensity score model. CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; NSCLC, non–small-cell lung cancer.
In sensitivity analyses to evaluate whether unmeasured variables might confound survival differences between VHA and FFS-Medicare patients, we found that adjustment for performance status and severe comorbidity could alter conclusions about survival differences in the two systems (Table 2). For example, when observed characteristics were controlled for, we estimated that patients with small-cell lung cancer in the VHA had similar survival rates compared with FFS-Medicare patients. If we could have also controlled for the severity of comorbidity, we estimated that we would have observed a 16% lower hazard of death among VHA patients. Adjustment for differences in college education and smoking status had smaller effects.
Table 2.
Hazard Ratios After Propensity Score Adjustment and Additional Adjustment for Potential Unobserved Variables
| ECOG Performance Status ≥ 2 | Severe Comorbidity* | College Education | Current Smoker | |
|---|---|---|---|---|
| Prevalence of unobserved confounder in VHA, % | 1732 | 45 for lung and 30 for hematology and colorectal† | 1735 | 1935 |
| Prevalence of unobserved confounder in FFS Medicare, % | 932 | 28 for lung and 18 for hematology and colorectal33,34 | 2335 | 1635 |
| Effect on survival, hazard ratio | 2.028,32 | 2.529 | 0.7530 | 1.2531 |
| Death in FFS Medicare Relative to VHA With Adjustment for Observed Covariates |
Accounting for Differences in Performance Status |
Accounting for Differences in Severe Comorbidity |
Accounting for Differences in Education |
Accounting for Differences in Smoking Status |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted Hazard Ratio | 95% CI | Adjusted Hazard Ratio | 95% CI | Adjusted Hazard Ratio | 95% CI | Adjusted Hazard Ratio | 95% CI | Adjusted Hazard Ratio | 95% CI | |
| Colon cancer | 0.87 | 0.82 to 0.93 | 0.82 | 0.77 to 0.88 | 0.77 | 0.72 to 0.82 | 0.86 | 0.81 to 0.92 | 0.87 | 0.81 to 0.93 |
| Rectal cancer | 1.05 | 0.95 to 1.16 | 0.98 | 0.89 to 1.08 | 0.92 | 0.83 to 1.01 | 1.03 | 0.94 to 1.14 | 1.04 | 0.94 to 1.15 |
| NSCLC | 0.91 | 0.88 to 0.95 | 0.86 | 0.83 to 0.89 | 0.77 | 0.75 to 0.80 | 0.90 | 0.87 to 0.93 | 0.91 | 0.87 to 0.94 |
| Small-cell lung cancer | 0.99 | 0.93 to 1.05 | 0.92 | 0.87 to 0.98 | 0.84 | 0.79 to 0.89 | 0.97 | 0.91 to 1.03 | 0.98 | 0.92 to 1.04 |
| Diffuse large B-cell lymphoma | 1.02 | 0.89 to 1.18 | 0.96 | 0.84 to 1.10 | 0.90 | 0.78 to 1.03 | 1.01 | 0.88 to 1.16 | 1.02 | 0.88 to 1.17 |
| Multiple myeloma | 0.92 | 0.83 to 1.03 | 0.87 | 0.78 to 0.97 | 0.81 | 0.73 to 0.90 | 0.91 | 0.82 to 1.02 | 0.92 | 0.82 to 1.02 |
NOTE. Values < 1 reflect better survival in VHA. Values > 1 reflect better survival in FFS Medicare. Bold values were statistically significant at P < .05.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FFS, fee-for-service; NSCLC, non–small-cell lung cancer; VHA, Veterans Health Administration.
Measured on the basis of the Adult Comorbidity Evaluation–27 from medical record abstraction or presence of severe chronic obstructive pulmonary disease (forced expiratory volume < 0.75) for patients with lung cancer.
On the basis of medical record abstraction for a subset of VHA patients with colorectal cancer or NSCLC.
DISCUSSION
In this large study of survival rates in veterans diagnosed with or treated for cancer in the VHA versus FFS-Medicare patients, we found similar or better survival rates in all six cohorts studied. Diagnosis at earlier stages explained much of the survival advantage in patients with colon cancer and NSCLC.
Although there is growing evidence that the VHA provides excellent preventive and chronic care, few studies have demonstrated improved patient outcomes associated with such care. Our finding of improved colon cancer outcomes in the VHA suggest that the success of the VHA with cancer screening3 and an earlier stage at diagnosis4 is associated with improved colon cancer outcomes. Although we expected similar benefits from cancer screening in rectal cancer, we did not observe improved cancer outcomes in patients with rectal cancer. With smaller cohorts and lower survival rates for rectal versus colon cancer, we may have had a low statistical power to detect benefits associated with earlier detection. However, we also observed worse survival rates in patients with early-stage rectal cancer in the VHA versus FFS Medicare. Treatment for rectal cancer is more complex than for colon cancer and requires more careful integration of radiation, surgery, and chemotherapy, and the volume-outcome relationship is stronger for the more technically demanding rectal versus colon surgery.36 With the more complex treatment, there may be more opportunity for a delay and interruption of treatment in VHA patients with other comorbidity than there is in colon cancer.
We also observed better survival rates after diagnosis with NSCLC in VHA compared with FFS-Medicare patients that was largely explained by the earlier stage at diagnosis. Better follow-up and coordination of care for patients with lung disease in the VHA may have led to an increased detection of early-stage lung cancer. However, although recent evidence on screening with computed tomography is promising,37 no screening modality has previously been shown to reduce lung cancer mortality. The survival advantage we observed among patients diagnosed with lung cancer in our study may have resulted from an overdiagnosis bias associated with an incidental detection of indolent disease or a lead time bias from an earlier detection of cancers that would have eventually been diagnosed clinically. Our sensitivity analyses shed light on why previous work has not consistently demonstrated a link between an improved quality of care and better patient outcomes in the VHA. VHA patients are economically disadvantaged and have high levels of comorbidity.38–40 Differences between VHA and non-VHA patients are difficult to adjust for in observational studies because information is typically lacking in available databases. Our sensitivity analyses suggested that these factors can have substantial effects on outcome differences.
We found that differential rates of guideline-recommended therapies had little impact on survival differences. We previously observed higher rates of cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with diffuse large–B-cell lymphoma4 that would be expected to led to improved survival rates.20 However, patients with diffuse large–B-cell lymphoma were diagnosed at later stages in the VHA. We were not able to adjust for these differences because stage data were only collected in the final year of our study.
The strengths of our study included large samples of patients with six cancers and the use of statistical analyses to identify FFS-Medicare patients most similar to VHA patients. However, our study had some limitations. First, FFS-Medicare patients are typically cared for in heterogeneous settings rather than in an integrated delivery system like the VHA. Quality and outcomes may be better in Medicare Advantage patients or in other more integrated systems with quality monitoring. Second, we could not control for many potential confounders, although we performed extensive sensitivity analyses to address this limitation. Third, we matched patients on the basis of characteristics observed at the time of diagnosis, including comorbidity. If care before cancer diagnosis in the VHA system led to better (or worse) noncancer health, this analysis may have understated (or overstated) the impact of VHA care on survival rates after a cancer diagnosis. Fourth, our analyses that tested the sensitivity of our findings to unobserved confounders were based on data from other populations and should be considered exploratory. Fifth, we estimated the impact of the receipt VHA care in a population of typical VHA patients. Other weighting schemes that estimate the impact of VHA treatment on the general population could lead to other conclusions, particularly if VHA treatment is tailored to the VHA population. However, the ability of the VHA to tailor to this unique population has important policy relevance. Finally, with 1 to 5 years of follow-up, we were limited in our ability to assess long-term outcomes. Nevertheless, mortality rates were high for most of the diseases studied.
In conclusion, we found that survival rates for VHA patients with cancer are equivalent to or better than the survival rates of similar patients treated under FFS Medicare. Importantly, improved survival rates in colon cancer appeared to be mediated by earlier stages at cancer diagnosis, which is a finding that was likely related to improved preventive care in the VHA compared with FFS Medicare. Because these findings may reflect the positive effects of an integrated, coordinated system of care on outcomes for a complex patient population, the VHA system might serve as a model for care delivery as health care reform is implemented.41 Our sensitivity analyses highlight the importance of factors that are not typically available in administrative data. Future studies that compared outcomes between VHA and non-VHA patients should collect data on disease severity, performance status, health behaviors, and socioeconomic status.
Supplementary Material
Acknowledgment
We thank the late Rose Zummo, MS, and Elena Kouri, PhD, for project assistance, Jeffrey Souza, Stephanie Segers, and Larry Zaborski for expert programming assistance, and Garrett Kirk for research and administrative assistance. The analysis would not have been possible without the invaluable feedback we received from the Veterans Affairs (VA) Oncology Program Evaluation Team, especially members with extensive clinical oncology experience within the VA system, including, Albert Muhleman, MD (Cincinnati VA Medical Center [VAMC]), Nirmala Bhoopalam, MD (Hines, IL VAMC), Paulette Mehta, MD (Veterans Health Administration [VHA]), Dawn Provenzale, MD (VA Health Services Research & Development, Durham, NC), Michael Kelley, MD (VHA), Robert Kerns, MD (VHA), and Raye Anne Dorn (VHA–DC VAMC). We also acknowledge Marshall Amesquita and Barbara Stephens, Contracting Officers' Technical Representatives, and the rest of our VA Oncology Program Evaluation Team as follows: Stanlie Daniels (VHA), Heidi Martin (VHA), Diana Ordin (VHA), Karen Pane (VA), Archna Sharma, MD (VHA), Anecia Thibodeau (VHA), and Patricia Vandenberg (VHA Office of Policy and Planning). This study used the linked Surveillance, Epidemiology, and End Results–Medicare database. We acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services; and the SEER Program tumor registries in the creation of the SEER-Medicare database. MBL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
See accompanying editorial on page 1027
Supported by the Department of Veterans Affairs through the Office of Policy and Planning.
The views reflected in this article are those of the authors and not the Department of Veterans Affairs. The interpretation and reporting of data were the sole responsibilities of the authors.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Steven H. Krasnow, Veterans Affairs Medical Center (C) Consultant or Advisory Role: Jennifer R. Brown, Calistoga Pharmaceuticals (C), Celgene (C), Pharmacyclics (C) Stock Ownership: None Honoraria: None Research Funding: Steven H. Krasnow, Department of Veterans Affairs; Jennifer R. Brown, Celgene, Genzyme Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Barbara J. McNeil
Collection and assembly of data: Mary Beth Landrum, Nancy L. Keating, Samuel R. Bozeman, Barbara J. McNeil
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med. 2004;141:938–945. doi: 10.7326/0003-4819-141-12-200412210-00010. [DOI] [PubMed] [Google Scholar]
- 2.Jha AK, Perlin JB, Kizer KW, et al. Effect of the transformation of the Veterans Affairs Health Care System on the quality of care. N Engl J Med. 2003;348:2218–2227. doi: 10.1056/NEJMsa021899. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi AN, Matula S, Miake-Lye I, et al. Systematic review: Comparison of the quality of medical care in Veterans Affairs and non-Veterans Affairs settings. Med Care. 2011;49:76–88. doi: 10.1097/MLR.0b013e3181f53575. [DOI] [PubMed] [Google Scholar]
- 4.Keating NL, Landrum MB, Lamont EB, et al. Quality of cancer care for older cancer patients in the Veterans Health Administration versus the private sector: A cohort study. Ann Intern Med. 2011;154:727–736. doi: 10.7326/0003-4819-154-11-201106070-00004. [DOI] [PubMed] [Google Scholar]
- 5.Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28:3176–3181. doi: 10.1200/JCO.2009.26.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landrum MB, Guadagnoli E, Zummo R, et al. Care following acute myocardial infarction in the Veterans Health Administration: A comparison with Medicare. Health Serv Res. 2004;39:1773–1792. doi: 10.1111/j.1475-6773.2004.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen LA, Normand SL, Daley J, et al. Outcome of myocardial infarction in Veterans Health Administration patients as compared with Medicare patients. N Engl J Med. 2000;343:1934–1941. doi: 10.1056/NEJM200012283432606. [DOI] [PubMed] [Google Scholar]
- 8.Petersen LA, Normand SL, Leape LL, et al. Regionalization and the underuse of angiography in the Veterans Affairs Health Care System as compared with a fee-for-service system. N Engl J Med. 2003;348:2209–2217. doi: 10.1056/NEJMsa021725. [DOI] [PubMed] [Google Scholar]
- 9.Ashton CM, Souchek J, Petersen NJ, et al. Hospital use and survival among Veterans Affairs beneficiaries. N Engl J Med. 2003;349:1637–1646. doi: 10.1056/NEJMsa003299. [DOI] [PubMed] [Google Scholar]
- 10.Hynes DM, Koelling K, Stroupe K, et al. Veterans' access to and use of Medicare and Veterans Affairs health care. Med Care. 2007;45:214–223. doi: 10.1097/01.mlr.0000244657.90074.b7. [DOI] [PubMed] [Google Scholar]
- 11.Fleming C, Fisher ES, Chang CH, et al. Studying outcomes and hospital utilization in the elderly: The advantages of a merged data base for Medicare and Veterans Affairs hospitals. Med Care. 1992;30:377–391. doi: 10.1097/00005650-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 13.Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000;88:2398–2424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.American Joint Committee on Cancer. Collaborative stage data collection system. http://www.cancerstaging.org/cstage/
- 15.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Colon cancer. 2001 Version 1.2001. [Google Scholar]
- 16.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Rectal cancer. 2001 Version 1.2001. [Google Scholar]
- 17.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Lung cancer. 2001 Version 1.2001. [Google Scholar]
- 18.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Multiple myeloma cancer. 2001 Version 1.2001. [Google Scholar]
- 19.Laurie SA, Logan D, Markman BR, et al. Practice guideline for the role of combination chemotherapy in the initial management of limited-stage small-cell lung cancer. Lung Cancer. 2004;43:223–240. doi: 10.1016/j.lungcan.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 23.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J American Stat Assoc. 1984;79:516–524. [Google Scholar]
- 25.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14:680–686. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 27.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54:948–963. [PubMed] [Google Scholar]
- 28.Lamont EB, Hayreh D, Pickett KE, et al. Is patient travel distance associated with survival on phase II clinical trials in oncology? J Natl Cancer Inst. 2003;95:1370–1375. doi: 10.1093/jnci/djg035. [DOI] [PubMed] [Google Scholar]
- 29.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 30.Stavraky KM, Skillings JR, Stitt LW, et al. The effect of socioeconomic status on the long-term outcome of cancer. J Clin Epidemiol. 1996;49:1155–1160. doi: 10.1016/0895-4356(96)00179-5. [DOI] [PubMed] [Google Scholar]
- 31.Jackson NA, Fuchs CS, Niedzwiecki D, et al. The impact of smoking on cancer recurrence and survival in patients with stage III colon cancer: Findings from intergroup trial CALGB 89803. J Clin Oncol. 2008;26(suppl):187s. abstr 4039. [Google Scholar]
- 32.Lamont EB, Landrum MB, Keating NL, et al. Differences in clinical trial patient attributes and outcomes according to enrollment setting. J Clin Oncol. 2010;28:215–221. doi: 10.1200/JCO.2008.21.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA. 2010;303:1037–1045. doi: 10.1001/jama.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chrischilles EA, Pendergast JF, Kahn KL, et al. Adverse events among the elderly receiving chemotherapy for advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:620–627. doi: 10.1200/JCO.2009.23.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klevens RM, Giovino GA, Peddicord JP, et al. The association between veteran status and cigarette-smoking behaviors. Am J Prev Med. 1995;11:245–250. [PubMed] [Google Scholar]
- 36.Rabeneck L, Davila JA, Thompson M, et al. Surgical volume and long-term survival following surgery for colorectal cancer in the Veterans Affairs Health-Care System. Am J Gastroenterol. 2004;99:668–675. doi: 10.1111/j.1572-0241.2004.04135.x. [DOI] [PubMed] [Google Scholar]
- 37.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- 39.Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: Results from the Veterans Health Study. Arch Intern Med. 1998;158:626–632. doi: 10.1001/archinte.158.6.626. [DOI] [PubMed] [Google Scholar]
- 40.Selim AJ, Berlowitz DR, Fincke G, et al. The health status of elderly veteran enrollees in the Veterans Health Administration. J Am Geriatr Soc. 2004;52:1271–1276. doi: 10.1111/j.1532-5415.2004.52355.x. [DOI] [PubMed] [Google Scholar]
- 41.Ryoo JJ, Malin JL. Reconsidering the Veterans Health Administration: A model and a moment for publicly funded health care delivery. Ann Intern Med. 2011;154:772–773. doi: 10.7326/0003-4819-154-11-201106070-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.