Trim39 inhibits the ability of APC/CCdh1 to ubiquitylate and promote the degradation of MOAP-1, leading to enhanced apoptosis.
Abstract
Proapoptotic Bcl-2 family members, such as Bax, promote release of cytochrome c from mitochondria, leading to caspase activation and cell death. It was previously reported that modulator of apoptosis protein 1 (MOAP-1), an enhancer of Bax activation induced by DNA damage, is stabilized by Trim39, a protein of unknown function. In this paper, we show that MOAP-1 is a novel substrate of the anaphase-promoting complex (APC/CCdh1) ubiquitin ligase. The influence of Trim39 on MOAP-1 levels stems from the ability of Trim39 (a RING domain E3 ligase) to directly inhibit APC/CCdh1-mediated protein ubiquitylation. Accordingly, small interfering ribonucleic acid–mediated knockdown of Cdh1 stabilized MOAP-1, thereby enhancing etoposide-induced Bax activation and apoptosis. These data identify Trim39 as a novel APC/C regulator and provide an unexpected link between the APC/C and apoptotic regulation via MOAP-1.
Introduction
Apoptosis is critical for proper embryonic development, the response to cellular damage, and overall organismal homeostasis. In the intrinsic apoptotic pathway, cell death stimuli promote release of mitochondrial cytochrome c to the cytoplasm, where it binds Apaf-1 to promote activation of the cell death protease caspase 9 (which in turn activates caspase 3).
Mitochondrial cytochrome c release is governed by Bcl-2 family proteins, including Bax and Bak, that trigger cytochrome c release through oligomerization at the mitochondrial outer membrane (Antonsson and Martinou, 2000; Eskes et al., 2000). Apoptotic signaling induces a conformational change in Bax and translocation to mitochondria, where it can affect outer membrane permeabilization (Antonsson, 2001; Antonsson et al., 2001).
Modulator of apoptosis protein 1 (MOAP-1; originally termed MAP-1) is a Bax-interacting protein whose knockdown inhibits apoptosis triggered by various stimuli (Tan et al., 2001). MOAP-1 association with Bax promotes Bax mitochondrial translocation and activation (Tan et al., 2005; Vos et al., 2006). Under nonapoptotic conditions, MOAP-1 undergoes constitutive degradation by the ubiquitin proteasome system, though the E3 ubiquitin ligase responsible for MOAP-1 ubiquitylation is unknown. After receipt of certain cell death stimuli, MOAP-1 degradation is inhibited by Trim39, a member of the tripartite motif (TRIM) family (Orimo et al., 2000). Therefore, Trim39 overexpression enhances etoposide-induced Bax-mediated apoptosis through stabilization of MOAP-1, whereas Trim39 knockdown dampens cell death after etoposide treatment (Fu et al., 2007; Lee et al., 2009).
Trim39 contains a RING domain, a B-box, and a coiled-coil domain (Deshaies and Joazeiro, 2009). For several TRIM family members, the RING domain confers E3 ubiquitin ligase activity. However, as Trim39 promotes MOAP-1 stabilization rather than degradation, it is clearly not directly responsible for MOAP-1 degradation. Rather, Trim39 must in some way negatively regulate an MOAP-1–directed E3 ligase.
Analysis of Trim39’s primary sequence revealed it to be the closest mammalian homolog of a previously identified E3 ligase from Xenopus laevis known as Xnf7 (Casaletto et al., 2005). Xnf7 has several biological activities, including regulation of mitotic exit through its ability to inhibit the anaphase-promoting complex (APC/C; Casaletto et al., 2005).
The APC/C is a multisubunit E3 ubiquitin ligase that associates with one of two activators, Cdh1 or Cdc20. These activators mediate substrate recognition through consensus motifs present on substrates (e.g., the destruction box RXXLXXXXN/D/E; Pfleger and Kirschner, 2000). Cyclin A and cyclin B1 ubiquitylation in M phase are mediated by the APC/CCdc20; in contrast, Cdh1 modulates the APC/C activity from M phase exit to G1, ubiquitylating Cdc20, cyclin B1, and Geminin. The APC/C also controls degradation of a handful of other substrates involved in DNA replication, glycolysis, and mitochondrial dynamics (Sugimoto et al., 2008; Colombo et al., 2010; Horn et al., 2011).
Here, we identify MOAP-1 as a novel APC/CCdh1 substrate. MOAP-1 is degraded during G1 by APC/CCdh1, and this degradation is inhibited by Trim39 acting on the APC/C. Trim39 E3 ubiquitin ligase activity is required to block MOAP-1 (and other APC/C substrate) destruction. Furthermore, enhanced apoptosis after Cdh1 knockdown depends in part on MOAP-1. Thus, these data link the APC/C and apoptosis and explain the previously reported connection between Trim39 and the Bax activator MOAP-1.
Results and discussion
Trim39 is an Xnf7-related E3 ubiquitin ligase
Previous work from our laboratory identified Xnf7 as a RING domain containing E3 ligase and APC/C inhibitor in Xenopus (Casaletto et al., 2005). In a BLAST search to uncover possible Xnf7 homologs in humans, we identified Trim39, which is 36% identical and 55% similar to Xnf7. These proteins share a similar arrangement of N-terminal RING and B-box domains and contain similar PRY and SPRY domains within their C-terminal regions, suggesting that Trim39 might share functional properties with Xnf7 (Fig. 1 A). Using recombinant E1, E2 (UbcH5a, an E2 that could function with Xnf7), ubiquitin, and Trim39 as the only source of E3 activity, we found that Trim39, like Xnf7, could autoubiquitylate. Several E2s could support Trim39 autoubiquitylation (including UbcH5a), whereas others (most notably UbcH10, an E2 used by the APC/C) could not (Fig. S1 A). Moreover, RING domain mutants expected to disrupt zinc binding (C44A and C52A) were deficient in enzymatic activity (Fig. 1 B).
Figure 1.
Trim39 is an Xnf7-related E3 ubiquitin ligase, and ligase activity is required for APC/C inhibition. (A) Schematic representation of the domains found within Xnf7 and Trim39 proteins. BBC represents the coiled-coil region C terminal to the B-Box domains. (B) In vitro ubiquitylation using recombinant Trim39 WT, RING domain mutant, C44A, and C52A in the absence or presence of ubiquitin and E1/E2 (UbcH5a). (C) Cell lysates were prepared from HeLa cells synchronized after a 1-h release from nocodazole (allowing further release in vitro) and incubated with 1 µM recombinant proteins, as indicated. Samples were collected and immunoblotted with cyclin B1 antibody. (D) Radiolabeled in vitro translated cyclin B1 fragment (1–106) was incubated with ubiquitin, E1 and E2 (UbcH5a, UbcH5c, or UbcH10, as indicated), ATP, His-Cdh1, and APC/C immunoprecipitated from HeLa cell G1 lysates using Cdc27 antibody. Reactions were supplemented with control buffer, MBP, MBP-Trim39 WT, or C44A recombinant protein for 45 min. Samples were detected by SDS-PAGE and autoradiography.
To determine whether Trim39 could inhibit the APC/C, we added either maltose-binding protein (MBP) or MBP-Trim39 protein to lysates prepared from HeLa cells that had been synchronized in nocodazole and then released (by washout of the nocodazole). As shown in Fig. 1 C, cyclin B1 was quickly degraded in the presence of recombinant MBP protein as these lysates exited from the mitotic arrest, whereas degradation of endogenous cyclin B1 was nearly abolished by addition of recombinant MBP-Trim39. This inhibition was not observed using the C44A mutant, suggesting that E3 ubiquitin ligase activity is required for APC/C inhibition (Fig. 1 C). Indeed, cyclin B1 was more rapidly degraded in the presence of the catalytically inactive Trim39 mutant, suggesting that this protein might interfere with the functioning of the endogenous protein. To confirm a direct role for Trim39 in APC/C inhibition, we incubated APC/C immunoprecipitated from HeLa cells with MBP or MBP-Trim39, E1, E2, ubiquitin, and radiolabeled in vitro translated cyclin B1. As shown in Fig. 1 D, cyclin B1 was well ubiquitylated by the isolated APC/C, and this activity was markedly reduced by incubation with MBP-Trim39, as predicted for a direct APC/C regulator. To exclude the possibility that Trim39 inhibited the APC/C by titrating the available E2, we supplemented the in vitro ubiquitylation reaction with UbcH10, which supports APC/C, but not Trim39, E3 ligase activity. Under these conditions, Trim39 still inhibited ubiquitylation of cyclin B1 by the APC/C (Fig. 1 D). We also showed that APC/C inhibition does not result simply from titrating any other limiting factor (e.g., ubiquitin) away from the APC/C by isolating Trim39 truncation mutants that retained the ability to trans-ubiquitylate the inactive C44A RING mutant (Fig. S1 B) but lacked the ability to inhibit cyclin B1 degradation (Fig. S1 C). Furthermore, Trim39 had no effect on an unrelated E3 ubiquitin ligase, DIAP1, which still autoubiquitylated in response to binding of its regulator, Reaper, in MBP-Trim39–supplemented cell lysates released from nocodazole arrest (Fig. S1 D). Thus, Trim39 does not generally suppress ubiquitylation/degradation of proteasomal substrates.
Trim39 inhibits MOAP-1 degradation during interphase
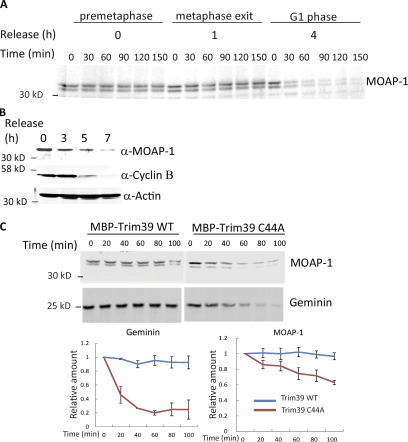
As Trim39 had been reported to modulate the ubiquitylation and degradation of the MOAP-1 and we had now found that Trim39 could inhibit the APC/C, we hypothesized that MOAP-1 might be an APC/C substrate. To see whether temporal degradation of MOAP-1 in the cell cycle was consistent with a role for the APC/C, we prepared HeLa cell extracts arrested at either prometaphase by nocodazole or further released for 0, 1, or 4 h (representing prometaphase, metaphase, or G1 extracts, respectively) and supplemented these extracts with radiolabeled in vitro translated MOAP-1. MOAP-1 was not degraded in prometaphase or metaphase extracts but was quickly degraded in G1 extracts, suggesting a role for Cdh1-activated APC/C in MOAP-1 degradation (Fig. 2 A). Indeed, endogenous MOAP-1 levels were reduced in cells released into G1 phase from nocodazole arrest, slightly delayed relative to endogenous cyclin B1 degradation (Fig. 2 B). This cell cycle–regulated degradation was similar to that seen for other APC/C substrates.
Figure 2.
MOAP-1 protein is degraded in a cell cycle–specific manner, and Trim39 attenuates the degradation. (A) HeLa cells were synchronized at prometaphase with nocodazole and collected at the indicated times after release. Radiolabeled in vitro translated cyclin B1 and MOAP-1 proteins were incubated in each lysate. Samples were taken at each time point, and reactions were stopped by addition of SDS loading buffer. (B) HeLa cells were synchronized with nocodazole and collected after release at the indicated times. Lysates were analyzed by immunoblotting with the indicated antibodies. (C) 1 µM MBP-Trim39 WT and C44A recombinant proteins were incubated in nocodazole-synchronized cell lysates for 3 h and then supplemented with in vitro translated, radiolabeled MOAP-1 or Geminin. Samples were taken at each time point, and reactions were stopped by addition of SDS loading buffer. The line graphs below represent quantitation of three independent experiments. Error bars are SD.
As Trim39 inhibited the APC/C (Fig. 1, C and D), we asked whether this MOAP-1 degradation could be inhibited by Trim39. Therefore, we added radiolabeled MOAP-1 or Geminin (a control APC/C substrate) into cell lysates prepared from nocodazole-arrested cells and then preincubated with MBP-Trim39 wild-type (WT) or MBP-Trim39 C44A proteins during the release into G1 phase. Although MOAP-1 degradation was not as robust as Geminin degradation, both proteins were degraded more rapidly in lysate containing MBP-Trim39 C44A than in lysate containing MBP-Trim39 (Fig. 2 C). These data are consistent with the idea that APC/C promotes degradation of MOAP-1 and that this degradation can be inhibited by Trim39.
MOAP-1 is an APC/CCdh1 substrate
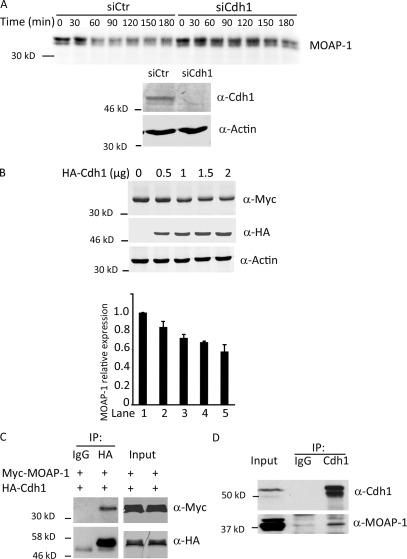
As MOAP-1 was degraded in G1 cell lysates, we reasoned that the APC/CCdh1 might ubiquitylate MOAP-1 to promote its proteasomal degradation. To assess this, we performed degradation assays using radiolabeled in vitro translated MOAP-1 as a substrate and found MOAP-1 degradation to be significantly slower in lysates in which Cdh1 had been knocked down using siRNA (Fig. 3 A). Moreover, overexpression of HA-Cdh1 in conjunction with Myc–MOAP-1 in 293T cells led to a dose-dependent decrease in MOAP-1 levels, even in unsynchronized cells (Fig. 3 B). Whereas Trim39 knockdown led to loss of MOAP-1 protein, MOAP-1 levels were restored by coordinate knockdown of both Trim39 and Cdh1 (Fig. S2 A). Furthermore, Trim39 WT, but not C44A, protein substantially inhibited ubiquitylation of MOAP-1 by the APC/C in vitro (Fig. S2 B). Consistent with these findings, MOAP-1 and Cdh1 could coimmunoprecipitate (Fig. 3, C and D).
Figure 3.
Cdh1 regulates MOAP-1 stability. (A, top) HeLa cells were treated with control or Cdh1 siRNA, arrested in nocodazole, released for 4 h, and lysed, and lysates were supplemented with radiolabeled in vitro translated MOAP-1 protein. Samples were withdrawn into SDS loading buffer at the indicated times and resolved by SDS-PAGE and autoradiography. (bottom) Immunoblot of Cdh1 from the Cdh1 siRNA–treated (siCdh1) cells above. siCtr, siRNA control. (B) 293T cells were transfected with 0.5 µg Myc–MOAP-1 plasmid and increasing amounts of HA-Cdh1, as indicated. Cell lysates were collected and probed with anti-Myc, -HA, or -actin antibody. Relative Myc-MOAP1 levels (normalized to levels seen in the absence of exogenous Cdh1 in lane 1) are quantitated below based on three repetitions of the experiment. Error bars are SD. (C) 293T cells were transfected with 0.5 µg HA-Cdh1 and 1.5 µg Myc–MOAP-1. Cell lysates were prepared and immunoprecipitated (IP) by normal IgG or anti-HA antibody and probed with anti-HA or -Myc antibody. (D) Anti-Cdh1 or control IgG immunoprecipitates from 20 µM MG132-treated 293T cells were immunoblotted with anti–MOAP-1 or anti-Cdh1 antibody.
Our identification of Trim39 as a candidate APC/C inhibitor was based on its sequence homology with Xnf7 (Casaletto et al., 2005). To determine whether Xnf7 and Trim39 were functionally similar, we knocked down Trim39, resulting in nearly undetectable levels of MOAP-1; when WT, but not RING mutant, Xnf7 was transfected into these cells, MOAP-1 was stabilized, suggesting that Xnf7 can, like Trim39, inhibit APC/C-mediated MOAP-1 degradation (Fig. S2 C).
In scanning the MOAP-1 sequence, we identified four potential D-box sequences that might confer recognition by Cdh1 (Fig. 4 A). As shown in Fig. 4 A, we mutated the underlined D-box consensus amino acids to Ala and transfected MOAP-1 WT or D-box mutant constructs into 293T cells. MOAP-1 WT protein could be coimmunoprecipitated with HA-Cdh1, whereas MOAP-1 mutated at all four putative D-boxes (MOAP-1 MT) could not (Fig. 4 B; we saw slight reduction of binding with each single D-box mutant; not depicted). Mutation of the D-box sequences caused a similar reduction in Cdh1–MOAP-1 interactions when recombinant His-Cdh1 was pulled down using MBP–MOAP-1 (WT or MT; Fig. 4 C). Moreover, Myc–MOAP-1 MT protein expressed in 293T cells was notably stabilized when compared with MOAP-1 WT after cycloheximide treatment (Fig. 4 D). MOAP-1 WT was more robustly ubiquitylated than MOAP-1 MT both in cells overexpressing Cdh1 and treated with the proteasome inhibitor MG132 and by the purified APC/CCdh1 in vitro (Fig. 4, E and F). These data strongly suggest that MOAP-1 degradation results from APC/CCdh1-mediated D-box–dependent ubiquitylation.
Figure 4.
MOAP-1 is an APC/CCdh1 substrate with D-box motifs. (A) Sequences of putative D-boxes on MOAP-1. D-box consensus amino acids mutated to Ala and transfected MOAP-1 WT or D-box mutant constructs into 293T cells are underlined. (B) 293T cells were transfected with HA-Cdh1 and Myc–MOAP-1 WT or MOAP-1 MT. Cells were collected and immunoprecipitated (IP) by anti-HA antibody. Samples resolved by SDS-PAGE were immunoblotted using anti-Myc or -HA antibodies. (C) MBP or MBP–MOAP-1 WT or MBP–MOAP-1 MT was incubated with His-Cdh1 for 2 h at 4°C, and proteins were retrieved from the mixture using amylose beads. After SDS-PAGE, samples were immunoblotted with anti-MBP or -Cdh1 antibodies. (D) 293T cells were transfected with Myc–MOAP-1 WT or Myc–MOAP-1 MT and treated with 100 µM cycloheximide (CHX). Cell lysates were prepared at the indicated times after cycloheximide treatment and immunoblotted with anti-Myc or -actin antibodies. (E) 293T cells were transfected with Myc–MOAP-1 WT or MT in the presence or absence of HA-Cdh1. After 48 h, cells were treated with 20 µM MG132 for 16 h, and lysates were collected for immunoblotting with anti-Myc, -HA, or -actin antibodies. (F) Recombinant MBP–MOAP-1 WT or MT proteins were incubated with ubiquitin, E1 and E2 (UbcH10 and Ube2S), ATP, and APC/C precipitated from HeLa G1 cell lysates using anti-Cdc27 antibody. Samples were immunoblotted with anti–MOAP-1 antibody.
Depletion of Cdh1 enhances susceptibility to apoptosis in response to DNA damage
MOAP-1 has been reported to enhance apoptosis by activating Bax, and knockdown of Trim39 has been reported to decrease etoposide-dependent apoptosis. We predicted that loss of Cdh1 would lead to elevation of MOAP-1 (as this would disable the APC/C in much the same way as Trim39 expression) and enhancement of etoposide-induced Bax activation. In agreement with this, in 293T cells treated with etoposide, the 6a7 antibody that recognizes conformationally active Bax immunoprecipitated greater amounts of Bax in cells knocked down for Cdh1 than in cells treated with control siRNA. Cells treated with siRNA for MOAP-1 activated less Bax than control cells. Importantly, the increased Bax activation seen after knockdown of Cdh1 was significantly dampened after MOAP-1 knockdown (Fig. 5 A; see Fig. 5 B for protein levels after knockdown).
Figure 5.
Higher Bax activation in Cdh1 knockdown cells as a result of MOAP-1. (A) 293T cells treated with Cdh1 (siCdh1), MOAP-1 (siMOAP-1), or control siRNA (siCtr) were treated with control or 100 µM etoposide for 48 h. Bax activation was monitored by IP with 6a7 antibody from 293T cells followed by immunoblotting of total Bax (N20). Input lysates were immunoblotted with anti-actin and -Bax (N20) antibodies. (B) Cell lysates from A were immunoblotted with anti-Cdh1 or anti–MOAP-1 antibodies. (C) Control, Cdh1, and MOAP-1 knockdown PC3 cells were treated with 100 µM etoposide for 48 h. The percentage of cells with sub-G1 DNA content was measured by PI staining and flow cytometry. (D) PC3 cells were processed as in C but treated with 20 µM cisplatin. (C and D) The asterisks indicate that the difference between the two experiments is significant (P < 0.05), using an unpaired t test. The data shown represent three independent experiments. Error bars are SEM. (E) Cdh1 or MOAP-1 was knocked down using siRNA in PC3 cells, and samples were immunoblotted for Cdh1, MOAP-1, or actin. (F) Cells treated as in D were lysed, and lysates were immunoblotted to detect caspase 3 processing.
To evaluate the effect of Cdh1/MOAP-1 knockdown on chemotherapy-induced apoptosis, we treated PC3 prostate cancer cells with siRNA directed against Cdh1, MOAP1, or both and monitored cell death by propidium iodide (PI) staining and flow cytometry. As shown in Fig. 5 (C and D), apoptosis was increased in Cdh1 knockdown cells in response to both etoposide and cisplatin treatment, but this increase was partially lost in cells that were ablated for both Cdh1 and MOAP-1 (see Fig. 5 E for knockdown efficiency). This effect was even more marked with cisplatin than etoposide, and the observed changes in cell death in response to cisplatin correlated well with caspase 3 cleavage (Fig. 5 F). Thus, although there are likely additional APC/CCdh1 substrates that contribute to the increased apoptotic response after loss of Cdh1, MOAP-1 degradation by APC/C contributes to setting the apoptotic threshold.
The data presented here identify Trim39 as a new human E3 ligase able to regulate the APC/C and explain the previously reported link between Trim39 and MOAP-1 abundance (Lee et al., 2009). Moreover, they demonstrate a role for the APC/CCdh1 in controlling apoptosis through degradation of the Bax activator MOAP-1. Currently, it is not clear how Trim39 inhibits the APC/C. Like Xenopus Xnf7, the E3 ligase activity of Trim39 is required for this inhibition (Casaletto et al., 2005). Trim39 can coimmunoprecipitate with the core APC/C (unpublished data), and it is possible that it can ubiquitylate an APC/C subunit or an associated regulator. Alternatively, Trim39 may require autoubiquitylation to act as an APC/C inhibitor.
Our results indicate that the APC/CCdh1 can ubiquitylate MOAP-1, providing a link between the APC/CCdh1 and regulation of apoptosis. Indeed, MOAP-1 has been reported to be a short half-life protein (∼25 min; Fu et al., 2007), and its stability is decreased once cells enter G1, the point at which APC/CCdh1 is known to act (Fig. 2). It is not yet clear whether MOAP-1 has apoptotic effects that are cell cycle specific or how cell cycle–specific degradation of MOAP-1 would help coordinate cell cycle status and apoptosis. Interestingly, etoposide treatment can stabilize MOAP-1 (Fu et al., 2007), suggesting that there are pathways linking the status of DNA to either Trim39 or APC/CCdh1.
It was reported that APC/CCdc20 ubiquitylates the antiapoptotic Bcl-2 family protein Mcl-1 after prolonged M phase arrest (Harley et al., 2010). In that MOAP-1 is stable during M phase and is not degraded until G1, it may participate in relaying the apoptotic signal after Mcl-1 is degraded in the event of prolonged M phase arrest. Normally, at M phase exit, APC/CCdc20 is degraded by APC/CCdh1 followed by MOAP-1 degradation. At the same time, Mcl-1 can reaccumulate to prevent cell death. This handoff of apoptotic control between various short half-life proteins throughout the cell cycle may allow maximum flexibility/rapid cellular changes in response to cell cycle cues or cellular damage.
Recently, it was reported that knockdown of Cdh1 in primary cortical neurons leads to apoptotic cell death (Almeida et al., 2005). It has been hypothesized that the APC/C helps to maintain the differentiated state by preventing accumulation of cyclin B1 and aberrant cell cycle reentry. According to this scenario, death from loss of Cdh1 would follow a failure to suppress cell cycle progression (Aulia and Tang, 2006). These effects may more directly stem from stabilization of apoptotic regulators that are APC/C substrates. As MOAP-1 belongs to the human paraneoplastic Ma antigen family and this family of proteins is particularly highly expressed in the brain, it would be interesting to determine whether MOAP-1 accumulation also contributes to apoptosis in neurons ablated for Cdh1 (Schüller et al., 2005). Similarly, it has been suggested that Cdh1 degradation after UV irradiation may promote cell death as a result of aberrant accumulation of cyclin B1 (Liu et al., 2008). Our results raise the possibility that Cdh1 degradation after irradiation might also lead to increases in MOAP-1 abundance to sensitize cells to UV-induced apoptosis.
Materials and methods
Plasmids and protein preparation
HA-Cdh1 and Myc–MOAP-1 plasmids were gifts from M. Pagano (New York University, New York, NY) and V.C. Yu (Institute of Molecular and Cell Biology, Singapore). Recombinant MBP fused to MOAP-1 was cloned from the pXJ40-Myc MOAP-1 plasmid into BamHI and SalI sites in the pMal vector and expressed in BL21 bacteria. MBP-Trim39 was prepared from a construct purchased from American Type Culture Collection. MOAP-1 mutants were prepared using QuikChange site-directed mutagenesis kits (Agilent Technologies). In vitro translated 35S-labeled MOAP-1, cyclin B1, and Geminin proteins were generated by using the TNT quick-coupled transcription/translation system (Promega).
Cell culture and synchronization
HeLa and HEK 293T cells were grown in DME with 10% FBS medium at 37°C. PC3 cells were grown in F12K with 10% FBS medium. For nocodazole synchronization experiments, HeLa cells were double thymidine blocked using 2.5 mM thymidine and released into medium containing 100 ng/ml nocodazole after PBS washing for 16 h. To prepare cell extracts at different cell cycle stages, prometaphase cells were collected by shakeoff followed by washing with PBS and releasing into DME media for 0, 1, or 4 h. The cells were lysed in hypotonic buffer (20 mM Hepes, pH 7.7, 5 mM KCl, 1.5 mM MgCl2, and 1 mM DTT) on ice and released into the cell cycle at room temperature.
Transfection and siRNA
FuGENE 6 (Roche) was used to transfect cells with plasmids according to the manufacturer’s instructions. Lipofectamine RNAiMAX (Invitrogen) was used to perform siRNA transfections. siRNA ON-TARGETplus SMARTpool-targeted MOAP-1 and Trim39 were purchased from Thermo Fisher Scientific. Cdh1 siRNA (5′-GGAACACGCUGACAGGACA-3′) was based on a previously reported effective sequence and purchased from Thermo Fisher Scientific (Song et al., 2011). siRNA directed against a nonmammalian protein, firefly luciferase (5′-CGUACGCGGAAUACUUCGA-3′), was used as the control siRNA.
Antibodies and immunoprecipitation (IP)
The following antibodies were used in this study: anti–cyclin B1, anti-HA, anti-Myc, anti-actin, and anti-Cdc27 (APC3; Santa Cruz Biotechnology, Inc.); anti-MBP (Cell Signaling Technology); anti–MOAP-1 (Sigma-Aldrich); anti-Bax (N20 and 6a7; BD); anti-Cdh1 (Abcam); and anti–caspase 3 (Cell Signaling Technology).
Cells were lysed with EB buffer (20 mM Hepes, pH 7.4, 150 mM NaCl, 12.5 mM β-glycophosphate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, 1 mM PMSF, 20 µM aprotinin, and 0.5% Triton X-100), and extracts containing equal amounts of proteins were incubated with 1 µg HA antibody and protein A Sepharose beads overnight at 4°C. After washing three times in IP buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, and 0.1% NP-40) and two times with IP buffer plus 150 mM NaCl and 0.02% Triton X-100, samples were resolved by SDS-PAGE for immunoblotting.
Trim39 in vitro ubiquitylation
0.25 µM recombinant MBP-Trim39 WT, C44A, and C52A were separately incubated with 12.5 nM E1 and 250 nM E2 (UbcH5c or other E2s, as indicated) and 10 µM ATP at 30°C for 1 h. For Trim39-truncated mutant E3 ligase activity assay, 0.25 µM recombinant MBP, MBP-Trim39 WT, C44A, and 1–105 and 1–336 were individually incubated with in vitro translated 35S-labeled Trim39 C44A, 12.5 nM E1, and 250 nM E2 (UbcH5c) and 10 µM ATP at 30°C for 1 h.
APC/C assay
The APC/C assay was performed as previously described (Tang et al., 2010). APC/C was precipitated using Cdc27 (APC3) antibody from M or G1 phase HeLa cells and then incubated with in vitro translated 35S-labeled proteins, 240 µM ubiquitin, 12.5 nM E1, and 250 nM E2 (UbcH5a, UbcH5c, UbcH10, or Ube2S, as indicated) and 10 µM ATP and 2.25 µM His-Cdh1 at 30°C for 1 h. In some experiments, more than two E2s are added (each concentration is 250 nM); therefore, the total E2 concentration is increased.
Pull-down assay
2 µg MBP (0.25 µM), MBP–MOAP-1 WT, or 0.1 µM D-box MT was incubated with 0.75 µg His-Cdh1 (68 nM) in a total volume of 200 µl of IP buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, and 0.1% NP-40) for 2 h at 4°C. Amylose beads were added for another 1 h to pull down His-Cdh1 and washed five times with IP buffer. Samples were analyzed by immunoblotting.
Flow cytometry assay
Cells were trypsinized and washed with PBS. For sub-G1 analysis, cells were fixed with 70% ethanol in PBS at −20°C overnight and stained with PI for flow cytometry analysis.
Online supplemental material
Fig. S1 demonstrates that Trim39 can function with several E2s but not with UbcH10, an E2 that functions in conjunction with the APC/C. Fig. S2 shows the effects on MOAP-1 levels of knocking down Trim39 alone or in conjunction with Cdh1 and shows that Trim39 can inhibit ubiquitylation of MOAP-1 in vitro. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201111141/DC1.
Acknowledgments
This work was supported by National Institutes of Health grants RO1 GM088175 and CA102707 to S. Kornbluth and the National Research Service Award grant T32 CA059365 to L. Zhang.
The authors declare no conflict of interest.
Footnotes
Abbreviations used in this paper:
- IP
- immunoprecipitation
- MBP
- maltose-binding protein
- PI
- propidium iodide
- WT
- wild type
References
- Almeida A., Bolaños J.P., Moreno S. 2005. Cdh1/Hct1-APC is essential for the survival of postmitotic neurons. J. Neurosci. 25:8115–8121 10.1523/JNEUROSCI.1143-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson B. 2001. Bax and other pro-apoptotic Bcl-2 family “killer-proteins” and their victim the mitochondrion. Cell Tissue Res. 306:347–361 10.1007/s00441-001-0472-0 [DOI] [PubMed] [Google Scholar]
- Antonsson B., Martinou J.C. 2000. The Bcl-2 protein family. Exp. Cell Res. 256:50–57 10.1006/excr.2000.4839 [DOI] [PubMed] [Google Scholar]
- Antonsson B., Montessuit S., Sanchez B., Martinou J.C. 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276:11615–11623 10.1074/jbc.M010810200 [DOI] [PubMed] [Google Scholar]
- Aulia S., Tang B.L. 2006. Cdh1-APC/C, cyclin B-Cdc2, and Alzheimer’s disease pathology. Biochem. Biophys. Res. Commun. 339:1–6 10.1016/j.bbrc.2005.10.059 [DOI] [PubMed] [Google Scholar]
- Casaletto J.B., Nutt L.K., Wu Q., Moore J.D., Etkin L.D., Jackson P.K., Hunt T., Kornbluth S. 2005. Inhibition of the anaphase-promoting complex by the Xnf7 ubiquitin ligase. J. Cell Biol. 169:61–71 10.1083/jcb.200411056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo S.L., Palacios-Callender M., Frakich N., De Leon J., Schmitt C.A., Boorn L., Davis N., Moncada S. 2010. Anaphase-promoting complex/cyclosome-Cdh1 coordinates glycolysis and glutaminolysis with transition to S phase in human T lymphocytes. Proc. Natl. Acad. Sci. USA. 107:18868–18873 10.1073/pnas.1012362107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R.J., Joazeiro C.A. 2009. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 78:399–434 10.1146/annurev.biochem.78.101807.093809 [DOI] [PubMed] [Google Scholar]
- Eskes R., Desagher S., Antonsson B., Martinou J.C. 2000. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20:929–935 10.1128/MCB.20.3.929-935.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu N.Y., Sukumaran S.K., Yu V.C. 2007. Inhibition of ubiquitin-mediated degradation of MOAP-1 by apoptotic stimuli promotes Bax function in mitochondria. Proc. Natl. Acad. Sci. USA. 104:10051–10056 10.1073/pnas.0700007104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley M.E., Allan L.A., Sanderson H.S., Clarke P.R. 2010. Phosphorylation of Mcl-1 by CDK1-cyclin B1 initiates its Cdc20-dependent destruction during mitotic arrest. EMBO J. 29:2407–2420 10.1038/emboj.2010.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn S.R., Thomenius M.J., Johnson E.S., Freel C.D., Wu J.Q., Coloff J.L., Yang C.S., Tang W., An J., Ilkayeva O.R., et al. 2011. Regulation of mitochondrial morphology by APC/CCdh1-mediated control of Drp1 stability. Mol. Biol. Cell. 22:1207–1216 10.1091/mbc.E10-07-0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.S., Fu N.Y., Sukumaran S.K., Wan K.F., Wan Q., Yu V.C. 2009. TRIM39 is a MOAP-1-binding protein that stabilizes MOAP-1 through inhibition of its poly-ubiquitination process. Exp. Cell Res. 315:1313–1325 10.1016/j.yexcr.2008.11.021 [DOI] [PubMed] [Google Scholar]
- Liu W., Li W., Fujita T., Yang Q., Wan Y. 2008. Proteolysis of CDH1 enhances susceptibility to UV radiation-induced apoptosis. Carcinogenesis. 29:263–272 10.1093/carcin/bgm251 [DOI] [PubMed] [Google Scholar]
- Orimo A., Yamagishi T., Tominaga N., Yamauchi Y., Hishinuma T., Okada K., Suzuki M., Sato M., Nogi Y., Suzuki H., et al. 2000. Molecular cloning of testis-abundant finger Protein/Ring finger protein 23 (RNF23), a novel RING-B box-coiled coil-B30.2 protein on the class I region of the human MHC. Biochem. Biophys. Res. Commun. 276:45–51 10.1006/bbrc.2000.3380 [DOI] [PubMed] [Google Scholar]
- Pfleger C.M., Kirschner M.W. 2000. The KEN box: An APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14:655–665 [PMC free article] [PubMed] [Google Scholar]
- Schüller M., Jenne D., Voltz R. 2005. The human PNMA family: Novel neuronal proteins implicated in paraneoplastic neurological disease. J. Neuroimmunol. 169:172–176 10.1016/j.jneuroim.2005.08.019 [DOI] [PubMed] [Google Scholar]
- Song M.S., Carracedo A., Salmena L., Song S.J., Egia A., Malumbres M., Pandolfi P.P. 2011. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 144:187–199 10.1016/j.cell.2010.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N., Kitabayashi I., Osano S., Tatsumi Y., Yugawa T., Narisawa-Saito M., Matsukage A., Kiyono T., Fujita M. 2008. Identification of novel human Cdt1-binding proteins by a proteomics approach: Proteolytic regulation by APC/CCdh1. Mol. Biol. Cell. 19:1007–1021 10.1091/mbc.E07-09-0859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K.O., Tan K.M., Chan S.L., Yee K.S., Bevort M., Ang K.C., Yu V.C. 2001. MAP-1, a novel proapoptotic protein containing a BH3-like motif that associates with Bax through its Bcl-2 homology domains. J. Biol. Chem. 276:2802–2807 10.1074/jbc.M008955200 [DOI] [PubMed] [Google Scholar]
- Tan K.O., Fu N.Y., Sukumaran S.K., Chan S.L., Kang J.H., Poon K.L., Chen B.S., Yu V.C. 2005. MAP-1 is a mitochondrial effector of Bax. Proc. Natl. Acad. Sci. USA. 102:14623–14628 10.1073/pnas.0503524102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Wu J.Q., Chen C., Yang C.S., Guo J.Y., Freel C.D., Kornbluth S. 2010. Emi2-mediated inhibition of E2-substrate ubiquitin transfer by the anaphase-promoting complex/cyclosome through a D-box-independent mechanism. Mol. Biol. Cell. 21:2589–2597 10.1091/mbc.E09-08-0708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos M.D., Dallol A., Eckfeld K., Allen N.P., Donninger H., Hesson L.B., Calvisi D., Latif F., Clark G.J. 2006. The RASSF1A tumor suppressor activates Bax via MOAP-1. J. Biol. Chem. 281:4557–4563 10.1074/jbc.M512128200 [DOI] [PubMed] [Google Scholar]