Abstract
Fibroblasts migrate on two-dimensional (2D) surfaces by forming lamellipodia—actin-rich extensions at the leading edge of the cell that have been well characterized. In this issue, Petrie et al. (2012. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201201124) show that in some 3D environments, including tissue explants, fibroblasts project different structures, termed lobopodia, at the leading edge. Lobopodia still assemble focal adhesions; however, similar to membrane blebs, they are driven by actomyosin contraction and do not accumulate active Rac, Cdc42, and phosphatidylinositol 3-kinases.
In 1970, Abercrombie et al. published a series of papers on “The locomotion of fibroblasts in culture” (Abercrombie et al., 1970a,b,c; Kardash et al., 2010). By filming fibroblasts migrating on serum-coated coverslips, they described the multistep model of lamellipodia-based cell migration. This work has shaped the field of cell migration for the last 40 yr, and despite many open questions, we now have a solid conceptual framework for how the lamellipodium drives cell motility: Actin filaments polymerizing below the leading plasma membrane generate the pushing force required for protrusion. As the tension of the plasma membrane opposes the free anterograde expansion of the actin network, the filaments are pushed back into the cell body, which is visible as retrograde actin flow. Through integrin-mediated adhesion complexes that couple the cytoskeleton to the substrate, these retrograde-directed forces, which are enforced by actomyosin contraction, are translated into forward locomotion of the cell body (Vicente-Manzanares et al., 2009).
A central mechanical concept of the lamellipodium is that actin polymerization drives the protrusion of the membrane. This principle also underlies other protrusions, such as filopodia, which are more explorative than force generating and invadosomes, which are responsible for invasion of tissue barriers (Ridley, 2011). There is only one known alternative to actin-driven protrusion: membrane blebs. These are anterior cellular extensions free of actin filaments. Here, intracellular hydrostatic pressure generated by actomyosin contraction causes rupture of either the actin cortex itself (Tinevez et al., 2009) or the linkage between actin and plasma membrane (Charras et al., 2006). Once the membrane loses its support, intracellular pressure inflates a membrane bleb that grows until a new actin cortex is reassembled, which eventually contracts and allows the cycle to restart (Charras and Paluch, 2008).
Studies of lamellipodial-based migration have dominated the literature, and many epithelial and mesenchymal cell types use these structures to migrate in vivo. However, blebbing is also a physiologically relevant locomotion strategy, notably in germ cells, which migrate efficiently by a directed and persistent blebbing motion (Blaser et al., 2006). Naturally, experimentally selected model systems for each migration mode tend to represent the “cleanest” and most prototypic examples. In reality, many cells are able to switch between blebbing and polymerization-driven motility depending on the environmental conditions or in response to genetic or pharmacological manipulation (Lämmermann and Sixt, 2009; Diz-Muñoz et al., 2010). In particular, malignantly transformed cells are able to adopt several poorly characterized crossover strategies in which blebs occur as leading extensions or epiphenomena at the trailing edge (Poincloux et al., 2011).
Three issues have been the focus of interest when studying blebbing versus lamellipodial locomotion strategies: (1) the role of substrate adhesion, (2) the role of substrate geometry, and (3) the signaling modules required for front–back polarization. Stabilized cell–cell or cell matrix adhesions can be involved in both blebbing and lamellipodial motility, although both types might also be functional without them (Renkawitz and Sixt, 2010). In general, the stability and physiological importance of adhesions appear to be decreased in fast and flexibly migrating amoeboid cells, which can either use the blebbing or the lamellipodial mode (Lämmermann and Sixt, 2009). Slow mesenchymal movement, which relies completely on focalized substrate adhesions, was generally considered lamellipodial and has not yet been associated with blebbing. Regarding the dimensionality of the environment, there is good evidence that all modes of movement can occur in 2D as well as in 3D environments, with the general notion that 3D but not 2D environments also allow motility under minimal adhesion forces (Friedl and Wolf, 2010). How polarity is established and maintained appears to be tightly coupled to the type of protrusion the cell employs. The key regulators are the Rho GTPases Rac, Cdc42, and RhoA. Rac is essential for lamellipodial expansion by activating the WAVE complex that in turn triggers actin nucleation by the Arp2/3 complex (Steffen et al., 2006; Wu et al., 2012). Cdc42 contributes by activating formins and the Arp2/3 complex (via the actin nucleation-promoting factor Wiskott–Aldrich syndrome protein) that stimulate actin polymerization during formation of filopodia and invadosomes. RhoA switches on actomyosin contractility by regulating myosin II and formins (Ridley, 2006). Thus, the Rho GTPases are the central signaling hubs through which diverse input signals are funneled. Depending on cell type and physiological context, completely different internally amplified or externally triggered pathways influence Rho GTPase activation levels and thereby cytoskeletal polarity. At the same time, numerous feedback loops have been identified that stabilize polarity. Best established are the phosphatidylinositol 3-kinases (PI3 kinases) that act both upstream and downstream of Rac and modulate the leading edge in many cell types (Cain and Ridley, 2009). The fact that actin polymerization (Millius et al., 2009) as well as actomyosin contractility and adhesion coupling (Vicente-Manzanares et al., 2009) can feed back on Rho GTPases further allows the cell to sense its environment and possibly to adapt the migration mode to geometry, chemical composition, and mechanical properties of its surroundings.
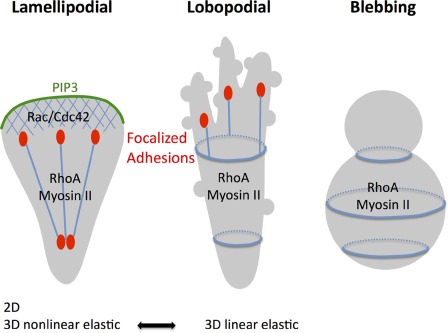
In their new study, Petrie et al. (in this issue) find cylindrical-shaped lobopodia, which have features of both blebs and lamellipods to be the predominant protrusion type of mesenchymal cells migrating in physiological 3D environments. Interestingly, like Abercrombie et al. (1970a,b,c) 40 yr ago, they used fibroblasts as a model. However, instead of growing the cells on coverslips they placed them in or on top of different types of 3D extracellular matrix scaffolds, including skin explants. In the tissue as well as in the cell-derived extracellular matrix the cells migrated with blunt-ended protrusions that developed multiple small lateral blebs. Unlike lamellipodia, these lobopodia accumulated neither active Rac and Cdc42 nor PI3 kinases, but the cells still formed focalized adhesions (Fig. 1). Similar to blebbing cells, lobopodia were very sensitive to perturbations of actomyosin contractility. Decreased contractility caused an instantaneous switch to the classical lamellipodial migration mode—notably without significant alterations in cell velocity. It was not only the geometry that induced lobopodia. Although the cells consistently developed lamellipodia in 2D when placed on top of different matrices, they also used lamellipodia when incorporated into gels made of noncross-linked bovine collagen. A combination of protocols to manipulate collagen fiber cross-linking and biophysical measurements led the authors to the conclusion that, once incorporated into a 3D matrix, the cells “read out” the elastic properties of the scaffold. Intuitively, one might have thought that lobopodia develop in environments of low stiffness, where actomyosin contraction might automatically squeeze the cells as the fibers to which the cells bind to give in. However, it was not simply the stiffness of the surroundings that caused a switch in protrusions but rather the shape of its stress strain curve: lobopodia only formed in linearly elastic environments, such as skin, and the cell-derived matrix but not in noncross-linked collagen gels that show strain stiffening. In the latter, lamellipodia predominated.
Figure 1.
Lobopodial migration combines aspects of lamellipodial and blebbing locomotion. Depending on the dimensionality and the elastic properties of the extracellular environment, fibroblasts can instantaneously switch between a classical lamellipodia-driven migration mode and lobopodia-mediated locomotion. Lamellipodia are found in all 2D environments and are characterized by a dense anterior actin meshwork (blue), polarized PIP3, Cdc42, and Rac activation, and focalized integrin-mediated adhesions with associated RhoA-driven stress fibers. In 3D environments with nonlinear elastic features, lamellipodia dominate, whereas in a regimen showing linear elasticity, fibroblasts rather use lobopodia. These blunt-ended protrusions display many small membrane blebs that are driven by RhoA-dependent myosin II activity but no polarized Rac, Cdc42, and PIP3. Interestingly, focalized adhesions and stress fibers can still be found in lobopodial migration, distinguishing it from blebbing motility that also depends on actomyosin contractility.
There is certainly still much to learn about the lobopodial migration mode. It is quite puzzling that the cells show stable functional polarity, i.e., they persistently undergo directional migration. How is this achieved despite delocalization of the main polarity modules? How can the cells reliably distinguish linear elasticity from strain stiffening over such a wide range of elasticity? What features that feed back on the extracellular environment are associated with lobopodial migration: do the cells use extracellular proteolysis, what kind of extracellular matrix do they secrete, and how do they remodel it? Finally, it will be interesting to see in which physiological context fibroblasts use lobopodial migration and whether other cells can adopt similar types of locomotion.
References
- Abercrombie M., Heaysman J.E., Pegrum S.M. 1970a. The locomotion of fibroblasts in culture. 3. Movements of particles on the dorsal surface of the leading lamella. Exp. Cell Res. 62:389–398 10.1016/0014-4827(70)90570-7 [DOI] [PubMed] [Google Scholar]
- Abercrombie M., Heaysman J.E., Pegrum S.M. 1970b. The locomotion of fibroblasts in culture. I. Movements of the leading edge. Exp. Cell Res. 59:393–398 10.1016/0014-4827(70)90646-4 [DOI] [PubMed] [Google Scholar]
- Abercrombie M., Heaysman J.E., Pegrum S.M. 1970c. The locomotion of fibroblasts in culture. II. “Ruffling”. Exp. Cell Res. 60:437–444 10.1016/0014-4827(70)90537-9 [DOI] [PubMed] [Google Scholar]
- Blaser H., Reichman-Fried M., Castanon I., Dumstrei K., Marlow F.L., Kawakami K., Solnica-Krezel L., Heisenberg C.P., Raz E. 2006. Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev. Cell. 11:613–627 10.1016/j.devcel.2006.09.023 [DOI] [PubMed] [Google Scholar]
- Cain R.J., Ridley A.J. 2009. Phosphoinositide 3-kinases in cell migration. Biol. Cell. 101:13–29 10.1042/BC20080079 [DOI] [PubMed] [Google Scholar]
- Charras G., Paluch E. 2008. Blebs lead the way: how to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 9:730–736 10.1038/nrm2453 [DOI] [PubMed] [Google Scholar]
- Charras G.T., Hu C.K., Coughlin M., Mitchison T.J. 2006. Reassembly of contractile actin cortex in cell blebs. J. Cell Biol. 175:477–490 10.1083/jcb.200602085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Muñoz A., Krieg M., Bergert M., Ibarlucea-Benitez I., Muller D.J., Paluch E., Heisenberg C.P. 2010. Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 8:e1000544 10.1371/journal.pbio.1000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Wolf K. 2010. Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 188:11–19 10.1083/jcb.200909003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardash E., Reichman-Fried M., Maître J.L., Boldajipour B., Papusheva E., Messerschmidt E.M., Heisenberg C.P., Raz E. 2010. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat. Cell Biol. 12:47–53 10.1038/ncb2003 [DOI] [PubMed] [Google Scholar]
- Lämmermann T., Sixt M. 2009. Mechanical modes of ‘amoeboid’ cell migration. Curr. Opin. Cell Biol. 21:636–644 10.1016/j.ceb.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Millius A., Dandekar S.N., Houk A.R., Weiner O.D. 2009. Neutrophils establish rapid and robust WAVE complex polarity in an actin-dependent fashion. Curr. Biol. 19:253–259 10.1016/j.cub.2008.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie R.J., Gavara N., Chadwick R.S., Yamada K.M. 2012. Nonpolarized signaling reveals two distinct modes of 3D cell migration. J. Cell Biol. 197:439–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincloux R., Collin O., Lizárraga F., Romao M., Debray M., Piel M., Chavrier P. 2011. Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proc. Natl. Acad. Sci. USA. 108:1943–1948 10.1073/pnas.1010396108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawitz J., Sixt M. 2010. Mechanisms of force generation and force transmission during interstitial leukocyte migration. EMBO Rep. 11:744–750 10.1038/embor.2010.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A.J. 2006. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16:522–529 10.1016/j.tcb.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Ridley A.J. 2011. Life at the leading edge. Cell. 145:1012–1022 10.1016/j.cell.2011.06.010 [DOI] [PubMed] [Google Scholar]
- Steffen A., Faix J., Resch G.P., Linkner J., Wehland J., Small J.V., Rottner K., Stradal T.E. 2006. Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes. Mol. Biol. Cell. 17:2581–2591 10.1091/mbc.E05-11-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinevez J.Y., Schulze U., Salbreux G., Roensch J., Joanny J.F., Paluch E. 2009. Role of cortical tension in bleb growth. Proc. Natl. Acad. Sci. USA. 106:18581–18586 10.1073/pnas.0903353106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M., Choi C.K., Horwitz A.R. 2009. Integrins in cell migration—the actin connection. J. Cell Sci. 122:199–206 10.1242/jcs.018564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Asokan S.B., Berginski M.E., Haynes E.M., Sharpless N.E., Griffith J.D., Gomez S.M., Bear J.E. 2012. Arp2/3 is critical for lamellipodia and response to extracellular matrix cues but is dispensable for chemotaxis. Cell. 148:973–987 10.1016/j.cell.2011.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]