Abstract
During post-natal development, tendons undergo a well orchestrated process whereby extensive structural and compositional changes occur in synchrony to produce a normal tissue. Conversely, during the repair response to injury, structural and compositional changes occur, but in this case, a mechanically inferior tendon is produced. As a result, the process of development has been postulated as a potential paradigm through which improved adult tissue healing may occur. In this study we measured the mechanical, compositional, and structural properties in the post-natal mouse Achilles tendon at 4, 7, 10, 14, 21, and 28 days old. Throughout post-natal development, the mechanical properties, collagen content, fibril diameter mean, and fibril diameter standard deviation increased. Biglycan expression decreased and decorin expression and fiber organization were unchanged. This study provides a new mouse model that can be used to quantitatively examine mechanical development, as well as compositional and structural changes and biological mechanisms, during post-natal tendon development. This model is advantageous due to the large number of genetically modified mice and commercially available assays that are not available in other animal models. A mouse model therefore allows future mechanistic studies to build on this work.
Keywords: Collagen, Proteoglycan, Mechanical strength, Fibril, Postnatal, Growth
INTRODUCTION
During post-natal development, tendons undergo a well orchestrated process whereby extensive structural and compositional changes occur in synchrony to produce a normal tissue.20,23 Conversely, during the repair response to injury, structural and compositional changes also occur, but in this case, a mechanically inferior tendon is produced. Tendon injuries affect large numbers of individuals and account for enormous associated costs.29 While the development of new surgical techniques and postoperative rehabilitation protocols have led to improved clinical results, post-surgical scarring, and restoration of normal tendon mechanics following injury remain problematic.2,31 An injured tendon that is mechanically inferior has compromised function and reruptures after treatment are commonly observed clinically. As a result, developmental processes have been postulated as a potential paradigm for elucidation of mechanistic insight required to develop treatment modalities to improve adult tissue healing. Of particular importance is a thorough understanding of the progression of mechanical strength during development. In depth knowledge of the changes and processes during development could lead to new therapeutic treatments that favor regenerative over fibrotic repair and improve functional outcomes in adult tendon healing.
Little quantitative information is available on changes in tendon mechanics, structure, and composition during post-natal growth. It is, however, well established that type I collagen is the most abundant constituent in tendon during development and collagen content increases throughout development into maturity.4,19 The collagen hierarchy is established through fibrillogenesis, a multistep process where each step is independently regulated by specific molecular interactions including fibril-associated macromolecules, two of which include the proteoglycans biglycan and decorin.35 Biglycan is believed to promote fibril diameter growth, whereas decorin is believed to control lateral fusion of the fibrils and increase fibril stability.35 During early development, biglycan is highly expressed while decorin is relatively low; however, as growth progresses, biglycan is down regulated and decorin is up regulated.34 It has been hypothesized that this temporal expression during development plays a crucial role in the mechanical development of tendons.34 In support of this, in vivo studies of decorin and biglycan deficient mice showed unregulated lateral growth, structurally abnormal tendon fibrillogenesis, and altered mechanics.9,34
Similarly, due to the predominance of collagen and its hierarchical organization, it has previously been hypothesized that collagen has a main effect on mechanical strength in tendon.23 Initially during development, collagen fibril diameters are small and have a narrow distribution. As tendons mature, the fibril diameter increases, as does the fibril diameter distribution.20,23 It is during this shift in collagen fibril diameter size and distribution that tendons gain significant mechanical strength.18 Collagen fibril diameter mean has often been used to attempt to determine the extent of mechanical contribution of collagen fibrils, but has not always been a significant predictor for mechanical parameters.10 Therefore, other tendon parameters must also be affecting the mechanical strength.
While there is currently a basic knowledge of compositional and structural changes during post-natal tendon development, there is sparse information on the mechanical development. Previous studies have examined tendon development in the mouse, rat, rabbit, chicken, and horse.18,22–24,34 Each of these animal models presents its own set of benefits and deterrents when choosing an appropriate animal model to study tendon development. One study did mechanically test post-fertilization, and hatchling chicken tendons along with fibril diameter and collagen content.18 However, injury and healing cannot be easily examined in prehatchling chicken tendons. Mice on the other hand, are easy to handle, associated with low husbandry costs, and have similar tendon anatomy to humans. In addition, the wide range of genetically modified mice and commercially available assays allow for rigorous mechanistic studies to be conducted. Most previous studies that have examined mouse post-natal development have not mechanically tested the tendons due to the fragile nature and small size,34 or simply started the study at later stages of development, when the tendons are larger and easier to handle.19,32 However, compositional and structural studies have demonstrated that the most significant changes occur at very early stages of development.19,34 It is during early post-natal growth that compositional and structural changes are most similar to changes observed during healing. Therefore, the establishment of a mouse model of tendon development that includes quantitative measures for composition, structure and mechanics will be a useful tool to determine the specific mechanisms of tendon development. This study will use the post-natal mouse Achilles tendon as a model of tendon development.
The purpose of this study is to develop a quantitative model of post-natal development in the mouse Achilles tendon at 4, 7, 10, 14, 21, and 28 days old. Collagen content, biglycan, and decorin expression, collagen fiber alignment, collagen fibril parameters, and elastic and viscoelastic mechanical properties will be quantified. We hypothesize that tendon mechanical properties, fibril parameters, and collagen content will increase throughout post-natal development. In addition, we hypothesize that decorin and biglycan will be temporally regulated and the collagen fibers will be well aligned throughout development.
MATERIALS AND METHODS
Animals
This study was approved by the University of Pennsylvania IACUC. Post-natal mice in a C57BL/6 (Jackson Laboratory) background were bred in house. All litters were weaned at birth to 6 pups to reduce variance from litter size.14 Mice were weaned at 21 days old and sexes were separated at this time. Both males and females were used in this study. Achilles tendons were harvested from post-natal mice at 4, 7, 10, 14, 21, and 28 days old. Care was taken to ensure that Achilles tendons from the same mouse or from the same litter were not used in the same assay.
Mechanical Analysis
To determine the mechanical properties of the developing post-natal mouse Achilles tendon, one Achilles tendon from each of 48 mice (n = 8/age) was carefully dissected under a dissection microscope. The tendons were cleaned of excess tissue leaving only the calcaneus. Tendon width and thickness were then quantified, and cross-sectional area was measured using a custom built device consisting of LVDTs, a CCD laser, and translation stages.25 Stain lines were placed to measure strain optically. Sand paper was then glued to the calcaneus and myotendinous end of the Achilles tendon. Grip to grip and stain line to stain line gauge lengths were as follows (grip:stain): 4 and 7 days old (2 mm:1 mm), 10 and 14 days old (3 mm:1.5 mm), 21 and 28 days old (4 mm:2 mm). While gauge lengths varied with age due to increased Achilles tendon size, the ratio of grip to stain line gauge length remained constant. Due to the fragile nature of post-natal mouse Achilles tendon, custom fixtures were designed to ensure the Achilles tendon was unharmed during test preparation. Once the tendon was secured in custom grips, a custom grip holder was attached to both grips (Fig. 1). Importantly, this fixture ensured that the tendons remained unloaded during handling and mounting for mechanical testing (Instron 5543, Instron Corp., Canton, MA). Each Achilles tendon underwent the following protocol while immersed in a 37 °C PBS bath—preloaded to a nominal load (0.001 N for 4, 7, and 10 days old, 0.003 N for 14, 21, and 28 days old), preconditioned for 10 cycles (0.005–0.008 N for 4–7 days old, 0.01–0.02 N for 10–17 days old, 0.02–0.04 N for 21–28 days old) at a rate of 0.1%/s, and held for 300 s. Immediately following, a stress relaxation experiment was performed by elongating the tendon to a strain of 5% at a rate of 5%/s, followed by a relaxation for 600 s. The tendon was returned to pre-stress relaxation elongation for 60 s and finally, a ramp to failure was applied at a rate of 0.1%/s.17 A 10 N load cell was used for all tests. Local tissue strain was measured optically as described previously.11 Percent relaxation, toe region stiffness and modulus, the load, stress, and strain at the transition point from toe to linear regions (from a bilinear fit), and linear region stiffness and modulus were determined. The viscoelastic parameter percent relaxation and toe region mechanical parameters were measured to capture functional tendon mechanics throughout development. Due to a percentage of the specimen failing at the grip, failure properties were not reported.
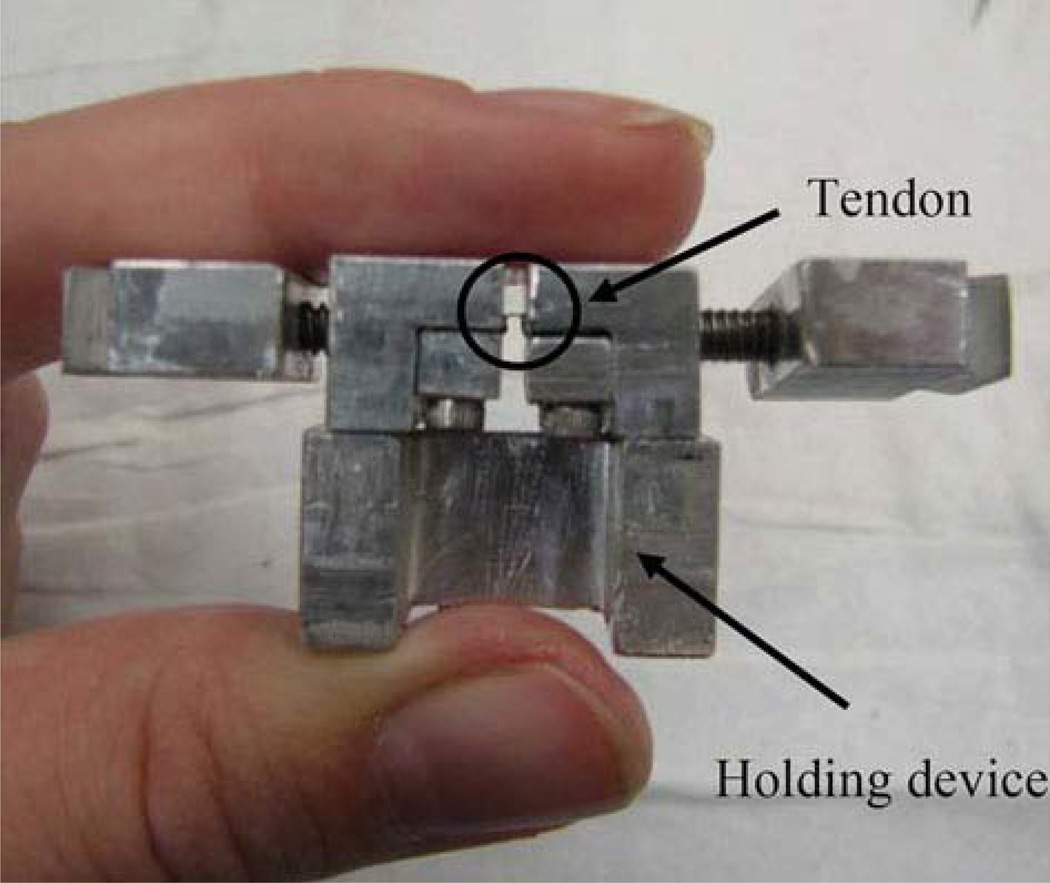
FIGURE 1.
A custom built grip holder was used to ensure that the Achilles tendon was slack between the grips during manipulation which prevented unwanted damage prior to testing.
Hydroxyproline Analysis
For analysis of total collagen content, a total of 24 Achilles tendons (n = 4/age) were cleanly dissected and the length of tendon corresponding to the grip to grip gauge length was isolated. Wet weights were obtained and subsequently Achilles tendons were dried at 65 °C for 24 h. Dry weights were then obtained, followed by tissue digestion in 5 mg/mL proteinase K solution at 65 °C for 18 h. For hydrolysis, samples were sealed in glass vials with 6 N HCl and heated to 110 °C for 16 h. Upon completion of hydrolysis, samples were neutralized by evaporating off HCl in a lyophilizer with NaOH and then resuspended in water. o-Hydroxy-proline (OHP), a measure of collagen content, was determined colorimetrically by reaction of the digest with p-benzaminoaldehyde and chloramine-T. OHP content was converted to total collagen content, assuming hydroxyproline content is 14% of total collagen as previously reported.21
Gene Expression
To determine the mRNA expression of the proteoglycans biglycan and decorin, one Achilles tendon from each of 24 mice (n = 4/age) was used. The Achilles tendon was cleanly dissected and the length of tendon corresponding to the grip to grip gauge length was isolated. Achilles tendons were mechanically homogenized individually with a mortar and pestle in RNase-free conditions. RNA was extracted using the TRIZOL isolation system (Invitrogen, Carlsbad, CA) and RNeasy Mini Kit (Qiagene Inc., Valencia, CA) as described by the manufacturers. cDNA was produced by reverse transcription-polymerase chain reaction (RT-PCR) from 100 ng of total RNA using the Superscript first-strand synthesis system for RT-PCR as described by the manufacturer (Invitrogen). 1 µL aliquots of the cDNA were amplified by real time quantitative polymerase chain reaction (Q-PCR) in a 25 µL reaction volume in a ABI Prism 7300 Sequence Detection System (Perkin-Elmer; Applied Biosystems, Warrington, UK) with a SYBR Green PCR Master Mix (Applied Biosystems). Mouse specific primers were used for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; F-CTC GTC CCG TAG ACA AAA TGG; R-GTG ACC AGG CGC CCA ATA), decorin (DCN; F-GCTGCGGAAATCCGACTTC; R-TTGCCGCCCAGTTCTATGAC), biglycan (BGN; F-CCTTCCGCTGCGTTACTGA; R-GCAACCACTGCCTCTACTTCTTATAA). Each individual Achilles tendon was analyzed in triplicate. The relative quantity of mRNA for each gene of interest was computed using the comparative 2−ΔCT method (ABI; Sequence Detection System User Bulletin #2) relative to GAPDH.
Histological Analysis
To determine the organization of the developing Achilles tendon, a total of 31 post-natal mice were euthanized (n = 4–7/age). Achilles tendons were evaluated using a quantitative polarized light microscopy method as described previously.28 Briefly, the hind limb was disarticulated, the skin dissected off, and processed with standard histological techniques. During processing the foot was secured at a 90° angle to the tibia.15 7 µm sagittal sections were cut parallel to the tendon fibers and analyzed using a quantitative polarized light microscopy method. Using circular statistics (Oriana, Kovach Computing Services, Wales, UK), the angular deviation, a measure of collagen fiber orientation, was determined.
Transmission Electron Microscopy
Achilles tendon samples from 24 mice (n = 4/age) were analyzed by transmission electron microscopy.33 Briefly, the Achilles tendon was dissected out with the calcaneus and muscle intact and fixed in 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate, pH 7.4, with 8.0 mM CaCl2, post-fixed with 1% osmium tetroxide. Achilles tendons were dehydrated in an ethanol series, followed by propylene oxide, then infiltrated and embedded in a mixture of EMbed 812, nadic methyl anhydride, dodecenyl succinic anhydride, and DMP-30 (Electron Microscopy Sciences, Hatfield, PA). Thin sections (80 nm) were cut using a Leica ultramicrotome and poststained with 2% aqueous uranyl acetate and 1% phosphotungstic acid, pH 3.2. The AT were examined at 80 kV using a JEOL 1400 transmission electron microscope (JEOL Ltd., Tokyo, Japan) equipped with an Orius widefield side mount CCD camera (Gatan Inc., Pleasanton, CA).
Fibril Diameter Distribution
For each age, four Achilles tendons were analyzed. Micrographs (4/Achilles tendon) from non-overlapping regions of the central portion of the tendon were taken at 60,000×. Micrographs were randomly chosen in a masked manner from each different age and fibril diameters were measured using a RM Biometrics-Bioquant Image Analysis System (Nashville, TN). A region from a single micrograph, containing 27–214 fibrils/field, was measured. Frequency of different diameter fibrils was analyzed in a total of 1514–2995 collagen fibrils/age. All fibrils in the region of interest were measured; multiple regions of interest were used if necessary to collect at least 70 fibril diameter measurements per micrograph. For each age, the total number of regions of interest and minimum and maximum fibril measurements per region are as follows [age/# ROI (min–max)]: 4/16 (146–233), 7/16 (108–168), 10/16 (111–152), 14/20 (52–126), 21/23 (43–127), 28/36 (27–54). For each Achilles tendon the mean and the standard deviation of the fibril diameters were determined and analyzed statistically.
Statistical Analysis
Each parameter was quantitatively measured and the mean ± the standard deviation was reported unless otherwise stated. A one-way ANOVA with Tukey’s post hoc was performed across age for each measured parameter (significance: p < 0.05, trend: p < 0.1).
RESULTS
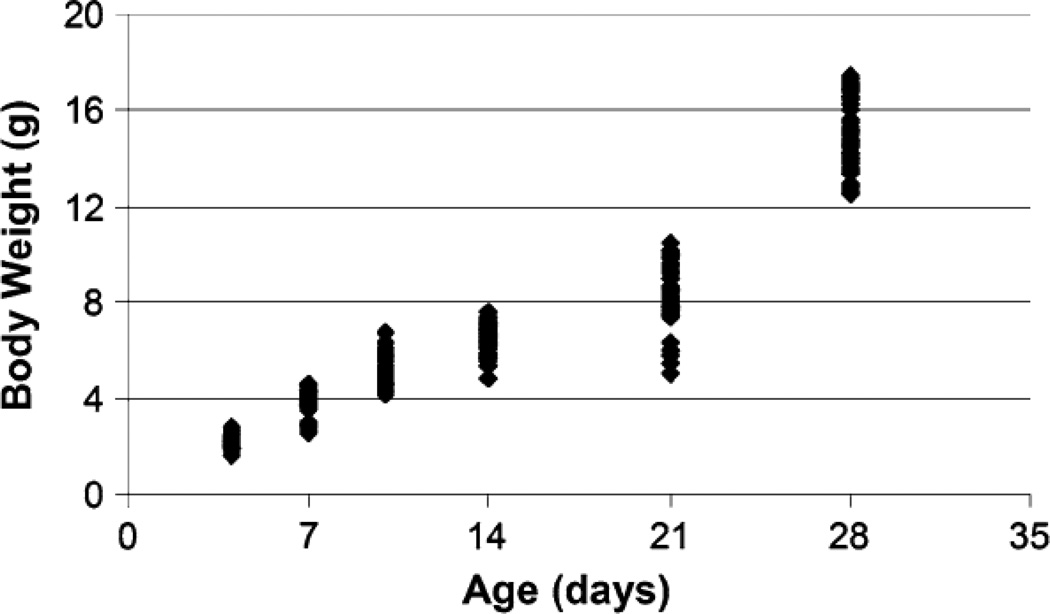
Extensive body weight growth was observed in post-natal mice between the ages of 4 and 28 days old (Fig. 2). To determine if sex had an effect on the mechanical parameters measured in this study, individual t tests were conducted at each age when possible. Due to random assignment of mice, there were not enough female mice at 10 days of age for statistical analysis of a sex effect. Sex did not have an effect on the mechanical parameters measured in this study (data not shown), with one exception of males having a significantly larger transitional stress than females at 21 days of age.
FIGURE 2.
Body weight increased with increasing age in post-natal mice. Each data point represents an individual mouse. All ages were significantly different from each other as demonstrated through a 1-way ANOVA with Tukey’s post hoc (results not shown on graph).
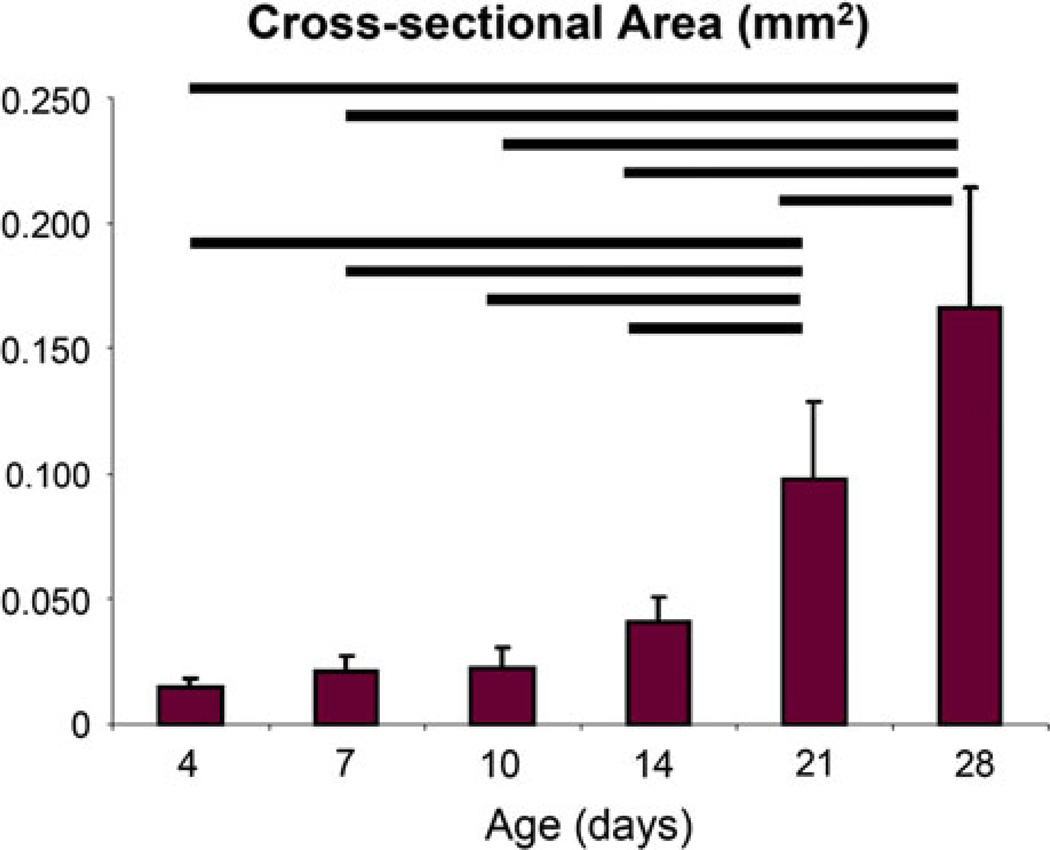
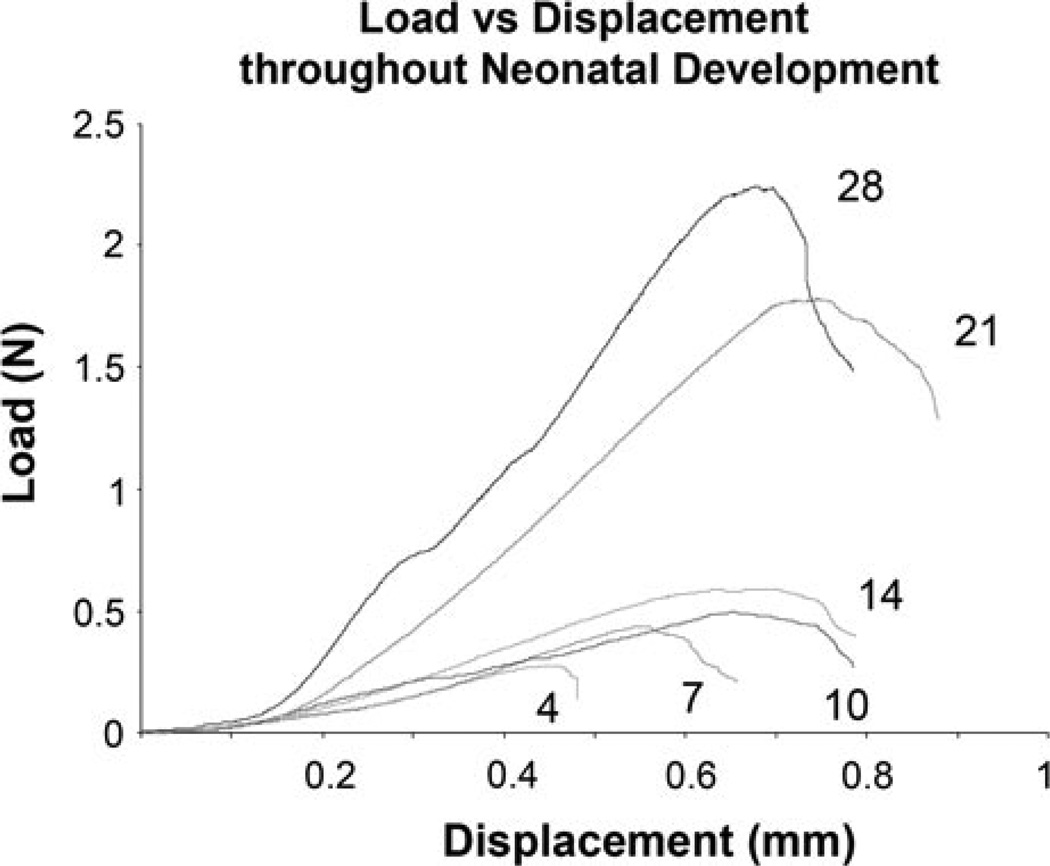
There were obvious changes with age, specifically in size, upon dissection. As expected, the cross-sectional area increased significantly with age (Fig. 3). Achilles tendons at 21 and 28 days old were significantly larger than all other ages studied, and 28 days old Achilles tendons were significantly larger than 21 days old. Most mechanical parameters increased throughout development as demonstrated by load vs. displacement curves of the constant ramp to failure for representative samples at each age (Fig. 4).
FIGURE 3.
The cross-sectional area of the Achilles tendon increased throughout development. Vertical bars represent mean of the parameter and the error bars represent standard deviation. p < 0.05.
FIGURE 4.
Representative load vs. displacement curves of the ramp to failure tensile test of mouse Achilles tendon. Curves demonstrate general trends seen during post-natal development.
Specifically, percent relaxation, a viscoelastic property, was significantly lower in 4 days old Achilles tendon when compared to 14, 21, and 28 days old (Table 1). Significant differences were seen in toe region parameters that were obtained from a bi-linear fit. The transitional strain (strain at the inflection point in the stress–strain curve) was significantly higher in 4 and 7 days old Achilles tendon when compared to 21 and 28 days old. Transitional load (load at the inflection point) was significantly higher at 28 days old than at all other ages except at 21 days old. Interestingly, transitional stress showed no significance at any age. Toe stiffness and toe modulus, parameters obtained from the slope of the toe region, also showed significant increase with age. Toe stiffness was significantly higher at 28 days old than at all other ages. Toe modulus was significantly higher in 28 days old Achilles tendon than at 7 and 14 days old. Similarly, linear stiffness and modulus, parameters obtained from the linear portion of the curve increased with age (Table 1). Achilles tendons were significantly stiffer at 28 days old than at all other ages. Linear modulus was significantly lower in 4 days old Achilles tendons than at 10, 14, 21, and 28 days old Achilles tendon and in 7 days old AT than at 21 and 28 days old.
TABLE 1.
Mechanical, compositional, and organization parameters throughout post-natal development (mean ± st dev).
| Age (days) |
Percent relax (%) |
Trans load (N) |
Trans stress (MPa) |
Trans strain (%) |
Toe stiffness (MPa) |
Toe modulus (MPa) |
Linear stiffness (N/mm) |
Linear modulus (MPa) |
Collagen content (%) |
Ang dev (°) |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 50.03 ± 4.14abc | 0.036 ± 0.023a | 1.43 ± 1.06 | 7.29 ± 4.28ab | 0.55 ± 019a | 24.6 ± 17.2 | 2.50 ± 1.42a | 87.1 ± 37.3abcd | 2.88 ± 1.04abc | 9.0 ± 2.4 |
| 7 | 56.86 ± 6.05 | 0.038 ± 0.025a | 1.47 ± 1.14 | 7.73 ± 4.78ab | 0.57 ± 0.24a | 19.4 ± 9.7a | 5.87 ± 3.06a | 192.2 ± 93.4ab | 6.38 ± 0.8abc | 6.7 ± 3.5 |
| 10 | 56.26 ± 9.06 | 0.047 ± 0.026a | 1.71 ± 0.72 | 3.36 ± 1.45 | 1.26 ± 1.14a | 62.0 ± 40.0 | 6.67 ± 2.33a | 345.8 ± 150.4 | 10.85 ± 1.81ab | 6.0 ± 1.0 |
| 14 | 60.51 ± 4.92 | 0.035 ± 0.026a | 0.80 ± 0.43 | 4.32 ± 1.95 | 0.61 ± 0.20a | 21.7 ± 10.1a | 9.99 ± 2.97a | 386.8 ± 205.4 | 18.74 ± 2.92a | 6.8 ± 1.5 |
| 21 | 62.58 ± 4.22 | 0.096 ± 0.065 | 1.12 ± 087 | 2.25 ± 1.39 | 2.03 ± 1.27a | 45.7 ± 35.9 | 21.15 ± 9.97a | 431.4 ± 152.7 | 27.17 ± 7.09 | 6.2 ± 3.3 |
| 28 | 60.85 ± 5.82 | 0.237 ± 0.199 | 1.45 ± 1.32 | 1.91 ± 0.93 | 6.39 ± 4.01 | 72.3 ± 48.7 | 50.35 ± 30.58 | 544.0 ± 240.8 | 36.20 ± 9.76 | 6.2 ± 1.0 |
Trans transitional, Ang Dev angular deviation.
Significantly different from 28 days.
Significantly different from 21 days.
Significantly different from 14 day.
Signifïcantly different from 10 days. Significance set at p ≤ 0.05.
Throughout post-natal development collagen content increased as expected (Table 1). Specifically, the percent of collagen (per dry weight) in Achilles tendon was significantly larger 28 days old than at 4, 7, 10, and 14 days old. 21 days old Achilles tendon also had significantly higher collagen content than at 4, 7, and 10 days old. Lastly, 14 days old Achilles tendon had significantly more collagen than at 4 and 7 days old.
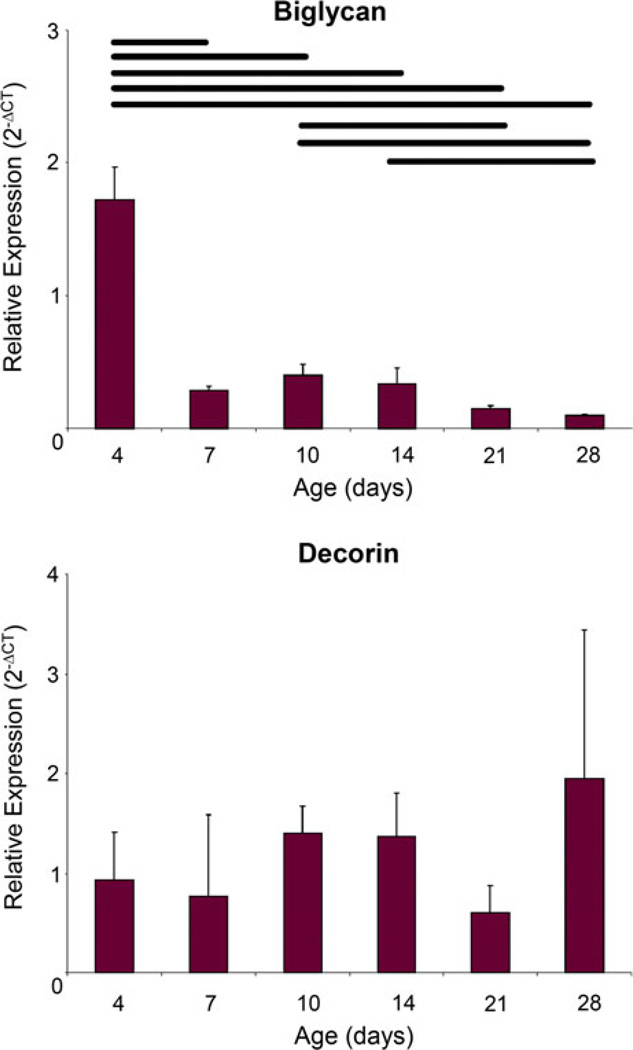
The relative expression of biglycan decreased with age (Fig. 5). Achilles tendon at 4 days old had significantly higher biglycan expression than all other ages. Achilles tendon at 10 days old had significantly higher biglycan than at 21 and 28 days old and 14 days old Achilles tendon had significantly higher biglycan than at 28 days old. No significant differences were seen in decorin expression between any of the ages studied.
FIGURE 5.
Biglycan expression was initially high in 4 days old Achilles tendon but then rapidly decreased while decorin expression remained constant throughout development. Vertical bars represent mean of the parameter and the error bars represent standard deviation. p < 0.05.
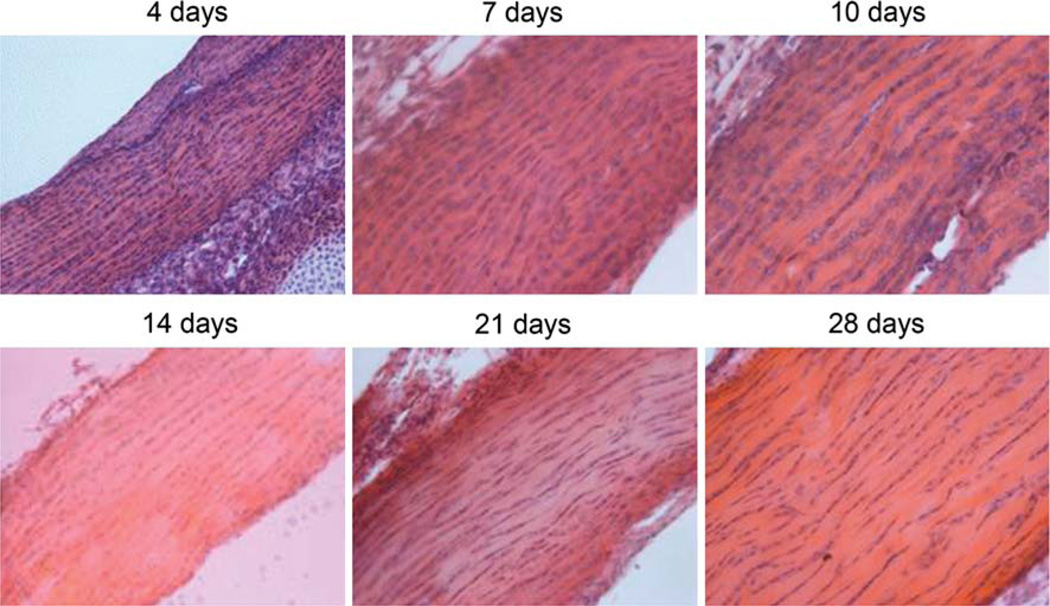
No differences were shown in angular deviation between any of the post-natal ages studied (Table 1). Qualitative observation of the hematoxylin and eosin stained sections of the Achilles tendon at each age confirmed that the fibers are well aligned throughout development (Fig. 6).
FIGURE 6.
Representative H&E stained slides from each age examined demonstrating little change in fiber organization throughout post-natal development in the Achilles tendon. All images were taken at ×20.
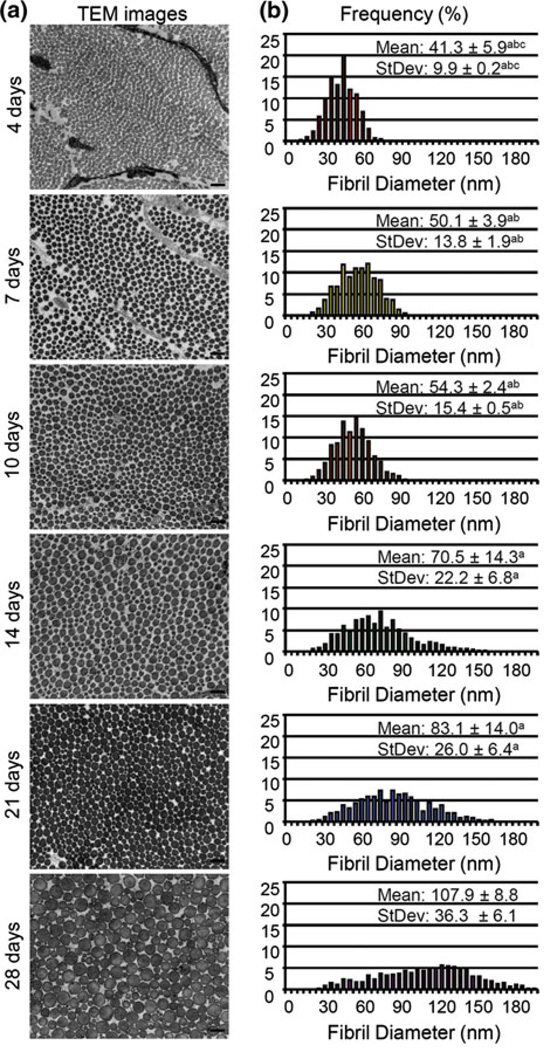
Ultrastructural analyses demonstrated circular fibril profiles. Fibril diameters were initially small and uniform as demonstrated in the 4 days old Achilles tendon (Fig. 7). As the mice developed, fibril diameters increased and the distribution of fibril diameter sizes increased. By 28 days old the fibril distribution ranged from 15 to 210 nm, the largest range in any group and no sub population of fibrils dominated the whole population. These changes are demonstrated quantitatively by examining the mean, and standard deviation (StDev) of the fibril diameters throughout development (Fig. 7). All measured parameters demonstrated a significant increase with age during development. Specifically, both the mean and standard deviation of the fibril diameters were significantly larger in 28 days old Achilles tendon when compared to all other ages, 21 days old Achilles tendon were significantly larger than 4, 7, and 10 days old and were significantly larger in 14 days old Achilles tendon than in 4 and 7 days old.
FIGURE 7.
(a) Representative samples of collagen fibrils throughout post-natal development. Scale bar = 200 µm. (b) Histogram of fibril distribution demonstrating increased fibril diameter mean and spread throughout post-natal Achilles tendon development. a: significantly different from 28 days; b: significantly different from 21 days; c: significantly different from 14 day; d: significantly different from 10 days. Significance set at p ≤ 0.05.
DISCUSSION
The goal of this study was to quantify compositional, structural, and mechanical changes during post-natal development in a mouse Achilles tendon. The results demonstrated an increase in mechanical strength throughout development. These changes were accompanied by increased collagen content, decreased biglycan expression, increased fibril diameter mean, and standard deviation and no changes to decorin expression or fiber angle. Previously known alterations in tendon composition and structure during development include increased fibril diameter means, area fraction and distributions, increased collagen content, temporally regulated proteoglycan content9,34 and other minor constituents,1,4 and increased mechanical strength.18,19 Previous studies have focused on the chicken, rabbit, rat, and horse due to either accessibility of embryonic tendons or the larger size of fetal and post-natal tendons. This study provides additional fundamental, quantitative knowledge of the development of post-natal tendons. This new post-natal mouse Achilles tendon model allows for future studies to be conducted on the mechanisms behind not only compositional and structural parameters but also mechanical development during post-natal growth due to the availability of genetically modified mice and commercially available assays for mice.
Previous studies have examined tendon development extensively in the chicken and the mouse, including one study that examined mechanical development in a chicken18 and defined specific stages of fibrillogenesis during development in each of these animal models. Briefly, until post-fertilization day 14 in chicks and in post-natal mice at 4 days old immature fibril intermediates/protofibrils that have small diameters with a homogeneous distribution and short lengths are assembled.5,35 Linear and lateral growth of protofibrils into mature fibrils begins at post-fertilization day 17 in chicks and at 10 days old in post-natal mice. Rapid increases in diameter due to lateral growth begins in hatchlings and sometime between 10 days and 1 month old in mice leading to mature collagen fibrils and tendon.18 Increased mechanical strength was demonstrated throughout all stages of both chicken (previously demonstrated) and mouse development (current study). In this study all structural parameters increased with age, as expected considering the large increase in cross-sectional area during post-natal development. Similarly, most material parameters increased with age in both the chick and the mouse.18 However, in this study, the transitional stress showed no increase with post-natal age even though changes were seen in transitional load. It appears that the quality of tissue initially present during post-natal development is sufficient for stresses experienced within the toe region throughout development in the mouse.
Interestingly, transitional strain in the post-natal mouse decreased after 7 days of age and remained low whereas no change was demonstrated in the chick.18 Previous studies have shown that by post-fertilization day 17 in chicks, almost no protofibrils/fibril intermediates could be identified in the chicken tendon,3,5 only mature fibrils. Full length fibrils would translate forces initially to uncrimp the collagen (toe region) and then along the length of the fibrils (linear region). In the mouse, intermediate fibrils are still forming until about 10 days of age. Therefore, the toe region in 4 and 7 days old mouse Achilles tendon may not be an effect of collagen uncrimping alone, but also collagen fibril sliding and shearing. Further mechanical and histological studies need to be done to confirm this hypothesis. In addition, it should be noted that significant differences in mechanical testing were employed in each study. Most notably, strain was measure optically in this study vs. grip to grip strain in the chick tendon study. Optical strain is a better measure of the local strains experienced by the tissue; therefore, the grip to grip strains reported in the previous study may have masked changes in transitional strain.
The Achilles tendon was chosen for this study due to the relatively large size during post-natal development and ease of accessibility. In addition, the Achilles tendon is surgically accessible allowing for the introduction of a developmental injury model in the future. While some of the parameters studied were previously defined during development, it is imperative that these parameters were defined in the proposed model due to differences in species, applied experimental strains,26 and tendons examined.13 For instance, extensive fibril diameter measures have been examined in mouse flexor tendons,13 and while general trends in this study are similar, the mouse flexor tendon has a larger fibril diameter mean and spread at all comparable ages. In addition, decorin expression has been previously shown to decrease during post-natal development in the flexor digitorum longus tendon and remain at a moderate level34; however, in this study a moderate level of decorin was expressed throughout development. Differences in structure and composition have also been demonstrated in the same tendon in mice with different genetic backgrounds.26 Therefore, it is imperative to clearly define quantitative parameters in a new model, and also to take into account which tendon, species, and strain rate were used when comparing and contrasting with previous studies.
Adult tendons heal through a reparative process and undergo a set of coordinated responses that include inflammation, extracellular matrix production, and remodeling of the tissue.30 Subsequent to fibroblast proliferation, increased extracellular matrix production alters the composition and structure of the tendon during healing from its uninjured state. Some of these changes are mirrored during development while some are unique to healing. Specifically, collagen production increases during healing27 similar to increases seen during development. During healing, biglycan levels increase and remain elevated8 and decorin levels remain unaltered or decrease8 whereas during development, biglycan expression is initially high then quickly decrease and decorin levels remain moderate. When examining the structure of the healing tendon, the fibril diameter size distribution is narrowed and consists mainly of small diameter fibrils.12 This distribution is similar to what is seen during the early stages of post-natal development. However, the healing fibril population does not mature into large fibrils with a wide fibril diameter spread as occurs during development. Instead healing fibrils remain fairly small and unimodal for an extended time post-injury. The fibers of healing tendon are also altered during healing, losing the parallel alignment that is present in uninjured tendons.16 The parallel alignment does return to an organized state throughout remodeling; this is in stark contrast to development where fibers are aligned throughout. It is important to note that in this study, the oldest tendons, 28 days old, had a collagen content of 36%, whereas mature tendons typically have a collagen content of approximately 80%, indicating that these mice have not yet fully matured. The post-natal development model presented in this study not only provides compositional and structural changes similar to those seen during adult healing, but also provides a model of increased mechanical strength and function. Parameters that show a differential effect during development and healing can potentially be implemented in new tissue engineered constructs. For instance, adding an element to a construct that decreases the expression of biglycan may improve healing. Additionally, a treatment post-injury that promotes the lateral fusion of the collagen fibrils during healing may help reestablish mechanical strength thereby improving the functional outcome of adult tendon healing.
This study is not without limitations. For instance, the increase in collagen content observed during healing and development usually has a larger percentage of type III collagen than is observed in normal mature tendon. A relationship between type III collagen and small diameter fibrils has previously been reported in the literature.4 Type III collagen is present during early development but disappears from the mid-substance of the tendon when the fibril diameters start to increase; however, these changes occur before the youngest age examined in this study.6 Therefore, it can be assumed that the contribution of type III collagen to the total collagen content measured in this study is insignificant.
While it is currently unknown if there are sex differences at the ages examined, previous studies showed little to no effect of sex on tendon mechanics and composition in 1 month old mice or rats.7,19 It is important to note that effects due to sex were seen at older ages, after the onset of puberty in some studies.19 While there is still a possibility of sex differences even in pre-pubescent mice, we were unable to demonstrate any in the mechanical parameters in this study. Compositional and structural parameters were not evaluated for sex differences due to the low number of total specimens per group; however, based on previous studies it is assumed that there is no effect due to sex.
In summary, quantitative mechanical, compositional, and structural changes during post-natal development of the mouse Achilles tendon were demonstrated. Through this mouse model, the mechanisms governing the development of mechanical strength can be rigorously studied with genetically modified mice and commercially available assays. In addition, by comparing and contrasting development and healing new therapies for adult tendon healing can be ascertained.
ACKNOWLEDGMENTS
This work was supported, in whole or in part, by National Institutes of Health Grant AR050950 from NIAMS, supporting the Penn Center for Musculoskeletal Disorders and by National Institutes of Health Grant AR44745. The authors would like to thank David Beason for his expertise in helping develop the mechanical testing fixtures.
REFERENCES
- 1.Ansorge HL, Meng X, Zhang G, Veit G, Sun M, Klement JF, Beason DP, Soslowsky LJ, Koch M, Birk DE. Type XIV collagen regulates fibrillogenesis: premature collagen fibril growth and tissue dysfunction in null mice. J. Biol. Chem. 2009;284:8427–8438. doi: 10.1074/jbc.M805582200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beredjiklian PK. Biologic aspects of flexor tendon laceration and repair. J. Bone Joint Surg. Am. 2003;85-A:539–550. doi: 10.2106/00004623-200303000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Birk DE, Hahn RA, Linsenmayer CY, Zycband EI. Characterization of collagen fibril segments from chicken embryo cornea, dermis and tendon. Matrix Biol. 1996;15:111–118. doi: 10.1016/s0945-053x(96)90152-3. [DOI] [PubMed] [Google Scholar]
- 4.Birk DE, Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol. 1997;72:352–361. [PubMed] [Google Scholar]
- 5.Birk DE, Zycband EI, Winkelmann DA, Trelstad RL. Collagen fibrillogenesis in situ: fibril segments are intermediates in matrix assembly. Proc. Natl Acad. Sci. USA. 1989;86:4549–4553. doi: 10.1073/pnas.86.12.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bland YS, Ashhurst DE. Fetal and postnatal development of the patella, patellar tendon and suprapatella in the rabbit; changes in the distribution of the fibrillar collagens. J. Anat. 1997;190(Pt 3):327–342. doi: 10.1046/j.1469-7580.1997.19030327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth FW, Tipton CM. Ligamentous strength measurements in pre-pubescent and pubescent rats. Growth. 1970;34:177–185. [PubMed] [Google Scholar]
- 8.Boykiw R, Sciore P, Reno C, Marchuk L, Frank CB, Hart DA. Altered levels of extracellular matrix molecule mRNA in healing rabbit ligaments. Matrix Biol. 1998;17:371–378. doi: 10.1016/s0945-053x(98)90089-0. [DOI] [PubMed] [Google Scholar]
- 9.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derwin KA, Soslowsky LJ. A quantitative investigation of structure–function relationships in a tendon fascicle model. J. Biomech. Eng. 1999;121:598–604. doi: 10.1115/1.2800859. [DOI] [PubMed] [Google Scholar]
- 11.Derwin KA, Soslowsky LJ, Green WD, Elder SH. A new optical system for the determination of deformations and strains: calibration characteristics and experimental results. J. Biomech. 1994;27:1277–1285. doi: 10.1016/0021-9290(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich HP, Lambert PA, Saggers GC, Myers RL, Hauck RM. Dynamic changes appearing in collagen fibers during intrinsic tendon repair. Ann. Plast. Surg. 2005;54:201–206. doi: 10.1097/01.sap.0000141380.52782.db. [DOI] [PubMed] [Google Scholar]
- 13.Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Festing MF. Design and statistical methods in studies using animal models of development. ILAR J. 2006;47:5–14. doi: 10.1093/ilar.47.1.5. [DOI] [PubMed] [Google Scholar]
- 15.Franchi M, Fini M, Quaranta M, De Pasquale V, Raspanti M, Giavaresi G, Ottani V, Ruggeri A. Crimp morphology in relaxed and stretched rat Achilles tendon. J. Anat. 2007;210:1–7. doi: 10.1111/j.1469-7580.2006.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J. Biomech. 2004;37:739–749. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Lin TW, Cardenas L, Soslowsky LJ. Tendon properties in interleukin-4 and interleukin-6 knockout mice. J. Biomech. 2005;38:99–105. doi: 10.1016/j.jbiomech.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 18.McBride DJ, Trelstad RL, Silver FH. Structural and mechanical assessment of developing chick tendon. Int. J. Biol. Macromol. 1988;10:194–200. [Google Scholar]
- 19.Mikic B, Amadei E, Rossmeier K, Bierwert L. Sex matters in the establishment of murine tendon composition and material properties during growth. J. Orthop. Res. 2010;28:631–638. doi: 10.1002/jor.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore MJ, De Beaux A. A quantitative ultrastructural study of rat tendon from birth to maturity. J. Anat. 1987;153:163–169. [PMC free article] [PubMed] [Google Scholar]
- 21.Neuman RE, Logan MA. The determination of hydroxyproline. J. Biol. Chem. 1950;184:299–306. [PubMed] [Google Scholar]
- 22.Oryan A, Shoushtari AH. Histology and ultrastructure of the developing superficial digital flexor tendon in rabbits. Anat. Histol. Embryol. 2008;37:134–140. doi: 10.1111/j.1439-0264.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- 23.Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc. R. Soc. Lond. B Biol. Sci. 1978;203:305–321. doi: 10.1098/rspb.1978.0107. [DOI] [PubMed] [Google Scholar]
- 24.Parry DA, Craig AS, Barnes GR. Tendon and ligament from the horse: an ultrastructural study of collagen fibrils and elastic fibres as a function of age. Proc. R. Soc. Lond. B Biol. Sci. 1978;203:293–303. doi: 10.1098/rspb.1978.0106. [DOI] [PubMed] [Google Scholar]
- 25.Peltz CD, Perry SM, Getz CL, Soslowsky LJ. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J. Orthop. Res. 2009;27:416–420. doi: 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rigozzi S, Muller R, Snedeker JG. Collagen fibril morphology and mechanical properties of the Achilles tendon in two inbred mouse strains. J. Anat. 2010;216:724–731. doi: 10.1111/j.1469-7580.2010.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomopoulos S, Hattersley G, Rosen V, Mertens M, Galatz L, Williams GR, Soslowsky LJ. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J. Orthop. Res. 2002;20:454–463. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 28.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J. Orthop. Res. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 29.Webster T. Putting a strain on workers’ health. Compens. Work. Cond. 1999 Spring;:29–31. http://www.bls.gov/opub/cwc/archive/spring1999brief2.pdf.
- 30.Woo SL, Debski RE, Zeminski J, Abramowitch SD, Saw SS, Fenwick JA. Injury and repair of ligaments and tendons. Annu. Rev. Biomed. Eng. 2000;2:83–118. doi: 10.1146/annurev.bioeng.2.1.83. [DOI] [PubMed] [Google Scholar]
- 31.Woo SL, Gelberman RH, Cobb NG, Amiel D, Lothringer K, Akeson WH. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop. Scand. 1981;52:615–622. doi: 10.3109/17453678108992156. [DOI] [PubMed] [Google Scholar]
- 32.Woo SL, Orlando CA, Gomez MA, Frank CB, Akeson WH. Tensile properties of the medial collateral ligament as a function of age. J. Orthop. Res. 1986;4:133–141. doi: 10.1002/jor.1100040201. [DOI] [PubMed] [Google Scholar]
- 33.Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J. Biol. Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 35.Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: regulation of collagen fibrillogenesis. J. Musculoskelet. Neuronal Interact. 2005;5:5–21. [PubMed] [Google Scholar]