Abstract
Numerous studies have demonstrated a link between elevated polyamine biosynthesis and neoplastic growth, but the specific contribution of spermine synthase to epithelial tumor development has never been explored in vivo. Mice with widespread overexpression of spermine synthase (CAG-SpmS) exhibit decreased spermidine levels, increased spermine and a significant rise in tissue spermine:spermidine ratio. We characterized the response of CAG-SpmS mice to two-stage skin chemical carcinogenesis as well as spontaneous intestinal carcinogenesis induced by loss of the Apc tumor suppressor in ApcMin/+ (Min) mice. CAG-SpmS mice maintained the canonical increases in ornithine decarboxylase (ODC) activity, polyamine content and epidermal thickness in response to tumor promoter treatment of the skin. The induction of S-adenosylmethionine decarboxylase (AdoMetDC) activity and its product decarboxylated AdoMet were impaired in CAG-SpmS mice, and the spermine:spermidine ratio was increased 3-fold in both untreated and 12-O-tetradecanoylphorbol-13-acetate (TPA)-treated skin. The susceptibility to 7,12-dimethylbenz[a]anthracene (DMBA)/TPA skin carcinogenesis was not altered in CAG-SpmS mice, and SpmS overexpression did not modify the previously described tumor resistance of mice with targeted antizyme expression or the enhanced tumor response in mice with targeted spermidine/spermine-N1-acetyltransferase expression. CAG-SpmS/Min mice also exhibited elevated spermine:spermidine ratios in the small intestine and colon, yet their tumor multiplicity and size was similar to Min mice. Therefore, studies in two of the most widely used tumorigenesis models demonstrate that increased spermine synthase activity and the resulting elevation of the spermine:spermidine ratio does not alter susceptibility to tumor development initiated by c-Ha-Ras mutation or Apc loss.
Keywords: antizyme, ApcMin/+ mouse, mouse skin carcinogenesis, ornithine decarboxylase, polyamine, S-adenosylmethionine decarboxylase, spermidine/spermine-N1 - acetyltransferase
Introduction
The polyamines spermidine (Spd) and spermine (Spm) and their diamine precursor putrescine consist of simple hydrocarbon backbones with distributed cationic charges. Polyamines associate with essential anionic molecules such as DNA, RNA, proteins and phospholipids, which allows them to modify critical cellular processes and events such as DNA replication and transcription; mRNA processing, stability and translation; signal transduction; oxidative damage; and ion channel function.1-5 Genetic and pharmacological studies demonstrate that polyamines are required for cell proliferation, and elevated levels of polyamine biosynthetic activity and polyamines are associated with neoplastic growth.6,7 Therefore, it is not surprising that extensive regulatory mechanisms have evolved to control cellular polyamine content and their ratios relative to each other.8,9
The enzymes ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (AdoMetDC) produce putrescine and decarboxylated AdoMet (dcAdoMet), respectively. These reactions are rate limiting for polyamine biosynthesis, and early embryonic lethality results when either gene is knocked out in the mouse.10,11 Both enzymes are extremely short-lived and rare in quiescent cells but highly inducible in response to growth promoting stimuli.12,13 Spermidine (Spd) and spermine (Spm) are produced from putrescine and the aminopropyl donor dcAdoMet by sequential action of the independent enzymes spermidine synthase (SpdS) and spermine synthase (SpmS). These aminopropyltransferases are constitutively expressed and in most tissues spermidine synthase is far more abundant.14 Elevated cellular polyamine content induces autoregulatory feedback mechanisms that are mediated by the protein antizyme (AZ).15,16 Efficient translation of AZ mRNA requires polyamine-stimulated ribosomal frameshifting. AZ protein then binds to ODC monomers to inhibit ODC activity and promote ODC degradation. In addition, AZ stimulates polyamine excretion and suppresses the uptake of exogenous polyamines by poorly defined mechanisms. The catabolic enzymes spermine oxidase (SMO) and spermidine/spermine-N1-acetyltransferase (SSAT) also influence polyamine content.9,17 The former directly converts spermine to spermidine while the latter produces acetylated substrates for N1-acetylpolyamine oxidase (APAO)-mediated production of spermidine and putrescine. The contributions of polyamine regulatory proteins to biological processes, including tumorigenesis, can be studied in genetically manipulated mouse models with altered polyamine content.18-20
The two-stage mouse skin chemical carcinogenesis model (reviewed in 21) has been utilized extensively to probe the effects of genetic, pharmacological and dietary manipulations on the initiation, promotion and progression stages of tumorigenesis. In this model, mice are initiated with a single dose of mutagenic 7,12-dimethylbenz[a]anthracene (DMBA), and nearly all tumors harbor a specific activating mutation in codon 61 of c-Ha-Ras. Benign papillomas arise following repeated application of the proliferation-inducing tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA). The role of polyamines in DMBA/TPA carcinogenesis has been studied extensively since the initial report that both ODC and AdoMetDC are induced by TPA application22 and increased in human nonmelanoma skin cancer.23 Studies also found constitutively high levels of ODC and polyamines within DMBA/TPA-induced tumors.24,25 Thus far, it is clear that ODC and SSAT overexpression enhance DMBA/TPA-induced tumor formation whereas AZ overexpression or treatment with the specific ODC inhibitor α-difluoromethylornithine (DFMO) is protective. Taken together, these studies suggest that increased putrescine content is essential for tumor promotion (reviewed in refs. Twenty-six and 27).
One of the most widely used mouse models with spontaneous tumor development is the ApcMin/+ mouse (Min28). Min is an ethylnitrosourea-induced truncation mutation of the murine adenomatous polyposis coli (Apc) gene. In humans, germline mutations in the APC gene lead to familial adenomatous polyposis (FAP) and a dramatic increase in colorectal cancer susceptibility, and the APC gene is also mutated in 80% of spontaneous human colon cancers. FAP patients have elevated ODC activity and polyamine content in the colonic mucosa relative to unaffected family members.29 Min mice develop 50 to 100 adenomas primarily in the small intestine but also in the colon and rarely live beyond 120 d. This model recapitulates many aspects the human FAP syndrome and it has been utilized to identify genes that modulate the Min phenotype, cellular processes involved in tumor progression, and targets for therapeutic intervention.30
The role of polyamines has been extensively studied in this model as well (reviewed in 31–33). Functional APC associates with β–catenin, thereby suppressing c-Myc expression, and ODC is a known transcriptional target of c-Myc. A single nucleotide polymorphism near c-Myc-responsive E-box elements within the ODC gene is correlated with colon adenoma recurrence.32 ODC mRNA and activity can be downregulated in colon carcinoma cells by transfection of functional APC,34 and Min mice exhibit a 6 to 10-fold increase in ODC mRNA levels in small intestine and colon and elevated small intestine polyamine content.35 Dietary putrescine supplementation increases adenoma grade in Min mice,36 while treatment with DFMO decreases tumor multiplicity35 and is especially effective in combination with non-steroidal anti-inflammatory agents.37,38 Similar to the DMBA/TPA model, SSAT overexpression leads to putrescine accumulation and increased tumor multiplicity in Min mice, while SSAT null mice are resistant to tumor development.39
Thus far, numerous studies implicate ODC, AZ and SSAT as polyamine regulatory proteins that strongly modify tumor susceptibility in mouse models of cancer. ODC is a compelling target for chemoprevention7,32 and DFMO is currently in clinical chemoprevention trials for numerous types of cancers with very encouraging results thus far in colorectal and nonmelanoma skin cancer.40,41 However, no studies have examined whether altering Spm levels and the Spm:Spd ratio stimulates or suppresses carcinogenesis. In order to explore the role of SpmS and Spm in epithelial tumor development, we characterized the response of mice with overexpression of SpmS (CAG-SpmS42) to DMBA/TPA skin carcinogenesis as well as spontaneous intestinal carcinogenesis in Min mice.
Results
TPA-induced ODC and AdoMetDC activity in CAG-SpmS mice
A composite cytomegalovirus-immediate early gene enhancer/chicken β-actin promoter (CAG) enabled widespread overexpression of SpmS in the mouse.42 The CAG-SpmS animals exhibited up to 2,000-fold increases in tissue SpmS activity and SpdS activity was unchanged. This resulted in a 2 to 4-fold increase in Spm:Spd ratio but there was no appreciable increase in total polyamine levels. An approximately 100-fold increase in SpmS activity was detected in the skin of CAG-SpmS transgenic mice; therefore, we utilized the well characterized mouse skin chemical carcinogenesis model to determine whether SpmS overexpression and the resulting increase in the Spm:Spd ratio alters tumor susceptibility.
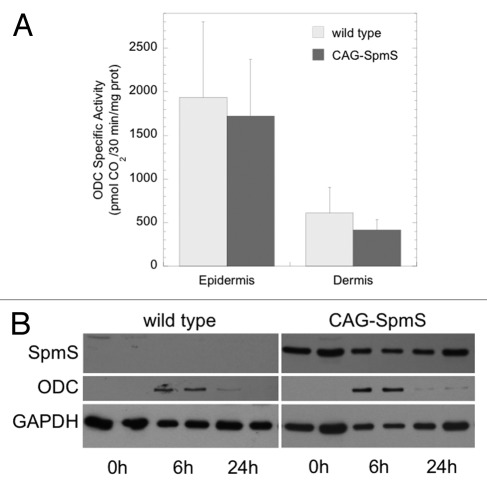
First, CAG-SpmS mice and wild type controls were treated with the tumor promoter TPA (17 nmol) and assayed for skin ODC activity. The induction of ODC activity is a common property of most tumor promoting chemicals.22 SpmS overexpression did not alter the robust increase in epidermal and dermal ODC activity (Fig. 1A) relative to the very low basal levels found in untreated epidermis and dermis (< 20 pmol CO2/30 min/mg protein43). Western blot analysis of epidermal extracts demonstrated obvious SpmS overexpression and the transient nature of the ODC induction (Fig. 1B).
Figure 1. TPA-induced ODC activity and protein in wild type and CAG-SpmS mice. (A) ODC activity in wild type and CAG-SpmS mice treated with TPA (17 nmol). Mice were sacrificed 6 h after TPA application and assayed for epidermal and dermal ODC activity (mean ± S.D.; n = 4). (B) Epidermal extracts (50 µg) from wild type and CAG-SpmS mice were collected at the indicated time after TPA application (17 nmol) and duplicate samples were analyzed by western blotting for SpmS and ODC with GAPDH as a loading control.
Next, AdoMetDC activity was measured in treated skin since TPA is also known to induce this second decarboxylase in the polyamine biosynthetic pathway.22 Epidermal and dermal AdoMetDC activity increased in the 48 h following a single TPA application in wild type and CAG-SpmS mice. The increase in AdoMetDC activity was reduced in epidermal extracts from CAG-SpmS mice (3.8-fold) relative to wild type controls (8.7-fold) but the difference in activity was not statistically significant at 24 or 48 h (Table 1). AdoMet levels increased slightly (1.4 to 2.8-fold) in both wild type and CAG-SpmS mice in response to TPA treatment at both 24 and 48 h. Epidermal dcAdoMet content was increased approximately 4-fold at both 24 and 48 h after TPA treatment of wild type mice. In contrast, TPA weakly induced epidermal dcAdoMet in CAG-SpmS animals and dcAdoMet content was reduced relative to controls at all time points. Dermal dcAdoMet levels were nearly constant in TPA-treated wild type mice but fell significantly in TPA-treated CAG-SpmS animals such that the levels detected at 24 and 48 h were 5-fold less than 0 h. Therefore, the aminopropyl donor dcAdoMet is depleted by SpmS overexpression in the skin of CAG-SpmS mice. Previous studies also detected slightly decreased AdoMetDC activity and dcAdoMet content in tissues of CAG-SpmS mice,44 which is likely related to the negative regulation of AdoMetDC by Spm.13
Table 1. AdoMetDC activity, AdoMet and dcAdoMet content in TPA-treated wild type and CAG-SpmS mice.
| Tissue | Group | Time after TPA (h) | AdoMetDC activity (pmol CO2/30 min/mg protein) |
AdoMet (pmol/mg tissue) |
dcAdoMet (pmol/mg tissue) |
|---|---|---|---|---|---|
| Epidermis |
Wild type |
0 |
49 ± 24 |
7.3 ± 2.0 |
0.081 ± 0.012 |
| |
24 |
202 ± 158e |
18.0 ± 3.9h |
0.321 ± 0.288 |
|
| |
|
48 |
424 ± 237g |
20.6 ± 10.5f |
0.335 ± 0.190g |
| |
CAG-SpmS |
0 |
75 ± 18a |
11.9 ± 3.2a |
0.048 ± 0.036a |
| |
|
24 |
142 ± 65e |
20.5 ± 6.9 |
0.054 ± 0.035a |
| |
|
48 |
281 ± 118g |
18.4 ± 4.9 |
0.087 ± 0.041c |
| |
|
|
|
|
|
| Dermis |
Wild type |
0 |
44 ± 22 |
7.2 ± 2.1 |
0.103 ± 0.033 |
| |
|
24 |
99 ± 18h |
10.2 ± 2.3e |
0.082 ± 0.014 |
| |
|
48 |
129 ± 76e |
11.3 ± 2.9g |
0.087 ± 0.020 |
| |
CAG-SpmS |
0 |
44 ± 12 |
6.7 ± 3.1 |
0.095 ± 0.028 |
| |
|
24 |
76 ± 10b,h |
10.7 ± 1.4 |
0.018 ± 0.004d,g |
| 48 | 90 ± 43e | 9.2 ± 3.2 | 0.017 ± 0.007c,e |
AdoMetDC enzymatic activity as well as AdoMet and dcAdoMet content were determined in 7 to 9-week-old mice. Values represent mean ± SD of 4 to 8 mice. ap < 0.05 vs. wild type at the same time; bp < 0.01 vs. wild type at the same time; cp < 0.005 vs. wild type at the same time; dp < 0.001 vs. wild type at the same time; ep < 0.05 vs. 0h of the same genotype; fp < 0.01 vs. 0h of the same genotype; gp < 0.005 vs. 0h of the same genotype; hp < 0.001 vs. 0h of the same genotype.
Polyamine accumulation and epidermal hyperproliferation in CAG-SpmS mice after multiple TPA applications
Next, we examined the response of CAG-SpmS mice to repeated tumor promoter application. Wild type and CAG-SpmS mice were treated twice weekly with TPA (17 nmol) or acetone vehicle for a total of four treatments to determine the impact of SpmS overexpression on TPA-induced polyamine content and epidermal thickness (Table 2). Vehicle treated CAG-SpmS mice exhibited an approximately 3-fold increase in the Spm:Spd ratio relative to controls due to elevated Spm and decreased Spd levels, which is in agreement with previous measurements in CAG-SpmS mice on a mixed genetic background.42 TPA treatment increased total polyamine levels 1.5 to 3-fold in wild type as well as CAG-SpmS mice despite the suggestion that dcAdoMet levels may be depleted in the latter group (Table 1). The Spm:Spd ratio is slightly reduced in TPA-treated wild type and CAG-SpmS mice relative to acetone treated animals, but the TPA-treated CAG-SpmS mice maintained a 3-fold increase in Spm:Spd ratio relative to wild type controls. The TPA-induced epidermal hyperproliferative response was observed in CAG-SpmS mice and was equivalent to wild type controls (Table 2). Untreated CAG-SpmS mice also exhibit a very modest but statistically significant increase in epidermal thickness. Therefore, SpmS overexpression did not impair the well-characterized increases in skin polyamines and epidermal thickness in response to TPA treatment.
Table 2. Polyamine content and epidermal thickness in TPA-treated skin from wild type and CAG-SpmS mice.
| Tissue | Treatment | Genotype | Polyamine content (pmol/mg tissue) |
|
Thickness (µm) | ||
|---|---|---|---|---|---|---|---|
| Putrescine | Spermidine | Spermine | Spm:Spd ratio | ||||
| Epidermis |
Acetone |
Wild type |
34 ± 25 |
163 ± 67 |
82 ± 20 |
0.53 ± 0.09 |
8.5 ± 0.4 |
| CAG-SpmS |
14 ± 6 |
72 ± 17 |
116 ± 32 |
1.60 ± 0.09c |
10.4 ± 1.3a |
||
| |
TPA |
Wild type |
48 ± 19 |
392 ± 51 |
110 ± 30 |
0.28 ± 0.06 |
43.4 ± 7.8 |
| CAG-SpmS |
82 ± 35 |
279 ± 27b |
253 ± 73b |
0.90 ± 0.20c |
42.3 ± 9.4 |
||
| |
|
|
|
|
|
|
|
| Dermis |
Acetone |
Wild type |
67 ± 37 |
195 ± 56 |
82 ± 16 |
0.43 ± 0.07 |
|
| |
CAG-SpmS |
26 ± 12 |
87 ± 13a |
99 ± 11 |
1.14 ± 0.07c |
|
|
| TPA |
Wild type |
60 ± 37 |
350 ± 54 |
103 ± 24 |
0.29 ± 0.03 |
|
|
| CAG-SpmS | 81 ± 59 | 187 ± 41c | 162 ± 45a | 0.86 ± 0.11c | |||
Polyamine content was determined in 7-week-old mice treated four times with acetone (n = 3 per genotype) or 17 nmol TPA (n = 5 per genotype). Skin was harvested 24h after the final treatment and processed for polyamine measurement or epidermal thickness (mean ± S.D.). ap < 0.05, bp < 0.005, cp < 0.001 vs. wild type controls.
Effect of SpmS overexpression on mouse skin chemical carcinogenesis
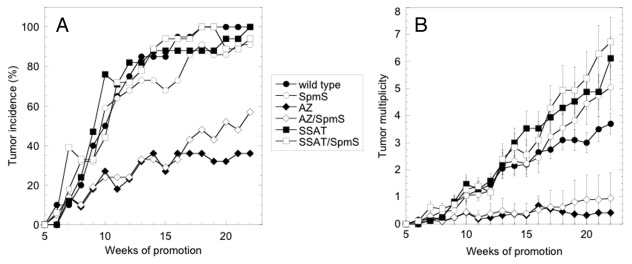
Next, CAG-SpmS mice were subjected to DMBA/TPA skin chemical carcinogenesis to determine if increased SpmS activity altered tumor susceptibility. In addition, SpmS overexpression was also combined with transgenic models of keratin 6 (K6)-driven AZ43 and SSAT overexpression,45 which are known to exhibit reduced or increased susceptibility to DMBA/TPA carcinogenesis, respectively. Over the course of 22 weeks of tumor promotion, CAG-SpmS mice failed to show any alteration in tumor incidence or multiplicity compared with wild type controls (Fig. 2). Tumor responses in K6-AZ and K6-SSAT mice were altered relative to wild type animals as previously reported. The overexpression of SpmS in either of these backgrounds (K6-AZ/CAG-SpmS or K6-SSAT/CAG-SpmS bitransgenic mice) did not alter the tumor incidence or multiplicity phenotypes that result from AZ or SSAT expression alone. In addition, SpmS overexpression did not alter the size of the tumors that were detected in the wild type, K6-AZ or K6-SSAT background (Table 3).
Figure 2. Effect of SpmS, AZ and SSAT on mouse skin carcinogenesis. Mice of the indicated genotype were subjected to DMBA/TPA skin chemical carcinogenesis and tumors were scored weekly. All groups included 17 to 22 animals with an approximately equal number of males and females. Mouse lines are abbreviated as follows: CAG-SpmS, SpmS; K6-AZ, AZ; K6-SSAT, SSAT; and bitransgenic combinations thereof. (A) The percentage of mice in each group with at least one tumor through 22 weeks of promotion. Wild type vs. AZ, p < 0.0001; wild type vs. SpmS, AZ vs. AZ/SpmS, SSAT vs. SSAT/SpmS and wild type vs. SSAT were not significant. (B) The total number of tumors per mouse (mean ± S.E.M.) through 22 weeks of promotion. Wild type vs. AZ, p < 0.0001; wild type vs. SSAT p = 0.011; wild type vs. SpmS, AZ vs. AZ/SpmS and SSAT vs. SSAT/SpmS were not significant.
Table 3. Tumor size in mice initiated with DMBA and promoted with TPA for 22 weeks.
| Tumors per mouse | Wild type (20) | CAG-SpmS (22) |
K6-AZ (22) |
K6-AZ/ CAG-SpmS (21) |
K6-SSAT (17) |
K6-SSAT/ CAG-SpmS (18) |
|---|---|---|---|---|---|---|
| total |
4.25 ± 0.44 |
5.50 ± 0.88 |
0.59 ± 0.16c |
1.00 ± 0.22 |
6.59 ± 0.75b |
6.56 ± 0.92 |
| < 2 mm |
2.05 ± 0.32 |
3.05 ± 0.58 |
0.41 ± 0.14c |
0.62 ± 0.15 |
2.65 ± 0.51 |
2.94 ± 0.53 |
| 2 to 5 mm |
1.70 ± 0.25 |
2.36 ± 0.38 |
0.14 ± 0.07c |
0.29 ± 0.10 |
2.41 ± 0.48 |
2.89 ± 0.54 |
| > 5 mm | 0.50 ± 0.17 | 0.09 ± 0.06a | 0.05 ± 0.05a | 0.10 ± 0.07 | 1.53 ± 0.39a | 0.72 ± 0.21 |
Tumor size (mean ± S.E.M.) was measured 1 week after the final TPA application. The number of mice in each group is shown in parenthesis. There were no significant differences between wild type vs. CAG-SpmS, K6-AZ vs. K6-AZ/CAG-SpmS, or K6-SSAT vs. K6-SSAT/CAG-SpmS. ap < 0.05 vs. wild type; bp < 0.01 vs. wild type; cp < 0.0001 vs. wild type.
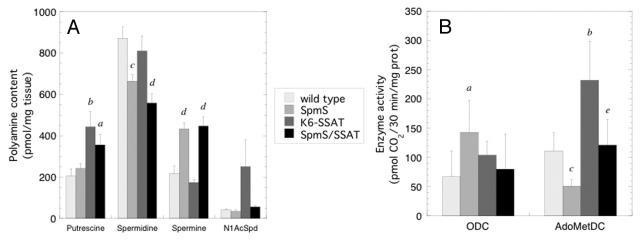
Tumors were harvested one week after the final TPA application to determine polyamine content as well as ODC and AdoMetDC activity in mice with SpmS and/or SSAT overexpression (Fig. 3). The very small size of tumors that developed in the K6-AZ and K6-AZ/CAG-SpmS groups precluded any biochemical measurements. Tumors from CAG-SpmS mice showed significantly decreased spermidine, increased spermine, and a 2.6-fold increase in the Spm:Spd ratio. These tumors also exhibited a significant increase in ODC activity without elevated putrescine content, which suggests that the synthesized putrescine was utilized for production of the higher polyamines. As in Table 1, AdoMetDC activity was again reduced in CAG-SpmS tumors relative to controls, but it did not lead to reduced spermine accumulation in the tumors. As observed previously,46 K6-SSAT mice had slightly reduced spermidine and spermine levels as well as increased putrescine and N1-acetylspermidine (N1-AcSpd) content due to increased polyamine catabolism. These alterations in the polyamine profile are accompanied by concurrent increases in ODC and AdoMetDC activity that are indicative of increased flux through the pathway.39,46 Interestingly, SpmS overexpression in K6-SSAT/CAG-SpmS mice seems to suppress the SSAT-induced changes in spermine and N1-AcSpd content as well as ODC and AdoMetDC activity. It is important to note that previous studies indicate that increased putrescine levels drive the enhanced tumor susceptibility in K6-SSAT mice.26,45 Putrescine levels are elevated in both K6-SSAT and K6-SSAT/CAG-SpmS mice, which is likely to explain the similar tumor response in these two groups.
Figure 3. Polyamine content and ODC and AdoMetDC activities in tumors. (A) Polyamine levels (mean ± S.E.M.) were determined in papillomas (n = 10) from mice with SpmS and/or SSAT overexpression one week after the final TPA application. (B) ODC and SAMDC activity (mean ± S.D.) was determined in papillomas (n = 5) from mice of the indicated genotype one week after the final TPA application. Mouse lines are abbreviated as follows: CAG-SpmS, SpmS; K6-SSAT, SSAT; and bitransgenic combination thereof. a p < 0.05 vs. wild type; b p < 0.01 vs. wild type; c p < 0.005 vs. wild type; d p < 0.001 vs. wild type; e p < 0.05 vs. K6-SSAT.
Effect of SpmS overexpression on polyamine content and tumor burden in ApcMin/+ mice
Next, the CAG-SpmS transgene was crossed into the ApcMin/+ (referred to as Min) background to evaluate the impact of SpmS overexpression on tumor susceptibility driven by a different initiating event in a second epithelial tissue. First, polyamine content was determined in several intestinal regions of CAG-SpmS, Min and Min/CAG-SpmS mice (Table 4). There was no significant difference in polyamine content or Spm:Spd ratio between Min and wild type mice in these young five-week-old animals that are unlikely to harbor neoplastic lesions. As seen in the skin (Table 2) and previous studies,42 SpmS overexpression led to increased spermine and decreased spermidine content. The Spm:Spd ratio was elevated (1.4-fold in small intestine and 2.3-fold in colon) in mice with the CAG-SpmS transgene in both wild type and Min backgrounds.
Table 4. Intestinal polyamine content in wild type, CAG-SpmS, Min and Min/CAG-SpmS mice.
| Region | Genotype | Polyamine content (pmol/mg tissue) |
|
||
|---|---|---|---|---|---|
| Putrescine | Spermidine | Spermine | Spm:Spd ratio | ||
| Proximal SI |
Wild type |
56.8 ± 16.2 |
564 ± 86 |
363 ± 71 |
0.64 ± 0.06 |
| CAG-SpmS |
117.8 ± 29.7a |
527 ± 48 |
515 ± 35b |
0.98 ± 0.10c |
|
|
Min |
36.6 ± 5.5 |
518 ± 70 |
362 ± 32 |
0.70 ± 0.03 |
|
|
Min/CAG-SpmS |
119.8 ± 31.7f |
535 ± 125 |
527 ± 132 |
0.98 ± 0.07g |
|
| |
|
|
|
|
|
| Middle SI |
Wild type |
32.8 ± 3.3 |
578 ± 38 |
356 ± 34 |
0.62 ± 0.02 |
| CAG-SpmS |
60.5 ± 28.5 |
596 ± 56 |
491 ± 33c |
0.83 ± 0.05d |
|
|
Min |
35.1 ± 14.1 |
666 ± 100 |
407 ± 44 |
0.61 ± 0.03 |
|
|
Min/CAG-SpmS |
67.3 ± 21.2 |
552 ± 76 |
477 ± 60 |
0.87 ± 0.09f |
|
| |
|
|
|
|
|
| Distal SI |
Wild type |
48.8 ± 14.5 |
832 ± 91 |
433 ± 15 |
0.52 ± 0.04 |
| CAG-SpmS |
65.8 ± 30.2 |
717 ± 28a |
511 ± 23c |
0.71 ± 0.03d |
|
|
Min |
49.3 ± 12.9 |
748 ± 69 |
399 ± 39 |
0.53 ± 0.01 |
|
|
Min/CAG-SpmS |
77.7 ± 16.7 |
589 ± 41e,j |
460 ± 48 |
0.78 ± 0.07g |
|
| |
|
|
|
|
|
| Colon | Wild type |
18.6 ± 3.2 |
526 ± 43 |
403 ± 26 |
0.77 ± 0.11 |
| CAG-SpmS |
59.1 ± 17.8b |
314 ± 43d |
566 ± 60c |
1.81 ± 0.07d |
|
|
Min |
11.0 ± 2.0 |
409 ± 75 |
315 ± 52 |
0.77 ± 0.07 |
|
| Min/CAG-SpmS | 36.8 ± 5.8h,i | 279 ± 26e | 493 ± 15g,i | 1.78 ± 0.19h | |
Polyamine content (mean ± S.D.) was determined in three small intestine (SI) regions and the colon of five-week-old wild type (n = 3), CAG-SpmS (n = 5), Min (n = 3) and Min/CAG-SpmS mice (n = 4). ap < 0.05 vs. wild type; bp < 0.01 vs. wild type; cp < 0.005 vs.wild type; dp < 0.001 vs. wild type; ep < 0.05 vs. Min; fp < 0.01 vs. Min; gp < 0.005 vs. Min; hp < 0.001 vs. Min; ip < 0.05 vs. CAG-SpmS; jp < 0.001 vs. CAG-SpmS.
We then assessed tumor burden in 100-d-old Min and Min/CAG-SpmS animals to evaluate the impact of increased Spm:Spd ratio on spontaneous intestinal tumor development (Table 5). A very modest increase in tumor incidence, multiplicity and size was correlated with SpmS overexpression in the small intestine and colon but none of the differences were statistically significant. Interestingly, proximal small intestine and colon exhibited the greatest increase in putrescine levels in Min/CAG-SpmS mice relative to Min (3.3-fold, Table 4) as well as the largest increase in tumor multiplicity (1.5 and 1.9-fold, respectively, Table 5). Tumor quantification in 60-d-old Min (n = 13) and Min/CAG-SpmS (n = 5) animals showed similar results (data not shown). Overall, there was no clear indication that SpmS overexpression and the resulting increase in the Spm:Spd ratio alters intestinal tumor susceptibility in the Min model. Discussion
Table 5. Intestinal tumor burden in Min and Min/CAG-SpmS mice.
| Region | Genotype | Tumor incidence (%) | Tumor multiplicity | Tumor diameter (mm) |
|---|---|---|---|---|
| Proximal SI |
Min |
79 |
4.1 ± 3.2 |
1.7 ± 0.3 |
|
Min/CAG-SpmS |
100 |
6.0 ± 3.2 |
1.9 ± 0.6 |
|
| Middle SI |
Min |
93 |
9.6 ± 8.0 |
1.2 ± 0.2 |
|
Min/CAG-SpmS |
100 |
8.4 ± 4.7 |
1.4 ± 0.4 |
|
| Distal SI |
Min |
86 |
7.0 ± 8.2 |
1.1 ± 0.1 |
|
Min/CAG-SpmS |
100 |
9.8 ± 11.8 |
1.4 ± 0.4 |
|
| Colon |
Min |
64 |
1.2 ± 1.8 |
2.0 ± 1.0 |
| Min/CAG-SpmS | 75 | 2.3 ± 2.0 | 2.4 ± 0.7 |
Tumor incidence, number and size (mean ± S.D.) was scored in three small intestine (SI) regions and the colon of Min (n = 14) and Min/CAG-SpmS (n = 8) mice at 100 d of age.
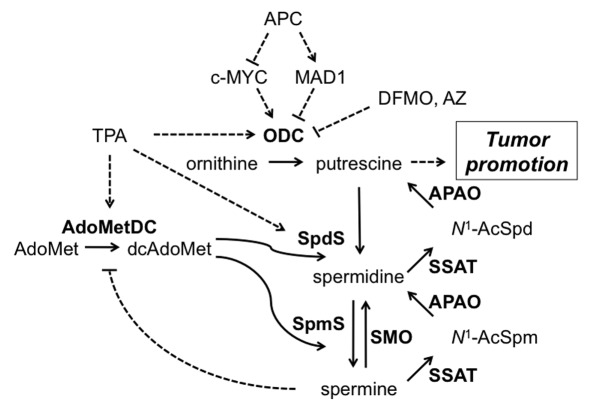
Many experimental systems are utilized to investigate the molecular mechanisms whereby polyamines modulate neoplastic growth. In vitro reconstitution assays allow the specific biochemical functions of a single polyamine to be assayed, but these systems are inadequate models of the complex cellular interactions and metabolic events associated with tumorigenesis. The specific biochemical functions of the individual polyamines are difficult to assess in vivo due to: (a) the extensive regulatory controls on biosynthesis, catabolism and transport, (b) interconversion between putrescine, Spd and Spm and (c) the inability to distinguish between bound and free intracellular polyamines. However, polyamine levels have been successfully manipulated in genetically engineered mouse models, and theses studies demonstrate that even modest changes in tissue polyamine content in response to Odc heterozygosity47,48 or targeted expression of AZ26,43 or SSAT39,46 can lead to dramatic changes in tumor susceptibility (Fig. 4). The effects of SSAT overexpression were tissue dependent in that the enhanced polyamine catabolism led to a surprising and unpredicted increase in susceptibility in some tissues39,46 but decreased tumor development in others.49
Figure 4. Regulation of cellular polyamine content and tumor promotion. Details of polyamine biosynthesis and catabolism are provided in the Introduction. TPA treatment and APC loss are both known to elevate polyamine biosynthesis and increase putrescine levels. DFMO treatment and AZ expression reduce putrescine content by inhibiting ODC, whereas SSAT expression increases polyamine backconversion and elevates putrescine. SpmS overexpression leads to reduced spermidine, increased spermine, an elevated spermine:spermidine ratio and reduced AdoMetDC activity and dcAdoMet but minimal alteration in putrescine levels. The potential mechanisms whereby putrescine modifies signaling and tumorigenesis are described in the Discussion. Solid lines represent enzyme-catalyzed reactions and dotted lines indicate positive (arrow) or negative (line) regulation. Enzymes are in bold and all abbreviations are defined in the text.
Therefore, there was a strong rationale for examining the role of SpmS overexpression in carcinogenesis and to do so in multiple tissues. No in vivo models enable complete elimination of cellular putrescine and/or spermidine, but male Gyro (Gy) mice have a nearly complete absence of tissue spermine due to a large deletion on the X chromosome that includes most of the SpmS gene. Gy mice exhibit profound abnormalities such as deafness, inner ear abnormalities, circling behavior, sterility and a greatly reduced lifespan.50,51 The viability of Gy mice indicates that there is no essential role for Spm in mammalian growth and development, but the short lifespan greatly complicates their usage in long-term carcinogenesis studies. Therefore, we examined the impact of a transgene-driven increase in SpmS activity on tumor development in the skin and intestine.
Since elevated polyamine levels are associated with neoplastic growth, one might intuitively predict that increased Spm content would promote tumor development. The tetraamine Spm is the most highly charged polyamine, is clearly the most difficult to deplete through pharmacological inhibition of polyamine biosynthesis, and it exhibits the strongest “activity” in many in vitro assays of polyamine biochemical effects.3,4,52 CAG-SpmS mice maintained the canonical increases in ODC activity, polyamine content and epidermal thickness in response to tumor promoter application, but also exhibited reduced AdoMetDC activity and dcAdoMet content. Skin and intestinal tissues from CAG-SpmS mice clearly demonstrated a very consistent increase in Spm and the Spm:Spd ratio. However, this change in polyamine profile failed to significantly alter tumor incidence, multiplicity or size in either DMBA/TPA skin chemical carcinogenesis or spontaneous intestinal carcinogenesis in Min mice. Skin tumor histopathology also was not altered by SpmS expression and intestinal tumors were not examined histologically.
Conversely, there are several reasons that one may predict tumor resistance in SpmS-overexpressing mice. Studies in spermine-deficient cells have shown that polyamines protect against damage induced by the chloroethylating agent 1,3-bis(2-chloroethyl)-N-nitrosourea (BCNU) and reactive oxygen species.2,53 Our studies suggest that Spm does not provide protection from the direct-acting carcinogen DMBA, or DNA is already saturated when Spm is present at normal levels and there is no increased protection with supraphysiological spermine. Increased oxidative stress may contribute to the enhanced tumor susceptibility of K6-SSAT mice,45 but we found no evidence that an elevated Spm:Spd ratio provides any protection in this background. There was no indication that the increased skin Spm content stimulated AZ translation and synthesis based on ODC activity (Fig. 1) and AZ western blotting (data not shown), and SpmS expression failed to enhance the tumor protective phenotype of K6-AZ mice. The tumor incidence of K6-AZ/CAG-SpmS mice appears to be increased relative to K6-AZ mice at later time points during DMBA/TPA carcinogenesis (Fig. 2A). However, the difference failed to achieve statistical significance by the log-rank test of the Kaplan-Meier tumor-free survival curves or by comparison of the week 22 incidence data by chi-square or Fisher’s test. Based on this trend it is possible that SpmS-expressing mice may show divergent responses with additional weeks of TPA treatment or during the process of tumor progression, but our study was designed to detect an effect of SpmS expression on the tumor promotion phase of carcinogenesis.
One could also argue that Spd levels are more critical to cell proliferation and the reduced Spd content and/or increased Spm:Spd ratio could suppress tumor development. Previous studies indicate that spermine conversion to spermidine is required to rescue cells from DFMO-mediated growth arrest.52 While total polyamine levels increase substantially following TPA application, it is Spd rather than Spm that is elevated and the Spm:Spd ratio is reduced in TPA-treated skin and in DMBA/TPA-induced tumors.25,54 TPA-induced epidermal hyperplasia and DMBA/TPA-induced tumor development are unchanged in CAG-SpmS mice even though the SpmS expression prevented these reductions in Spm:Spd ratio (Table 2, Figure 3). In addition, the tumor protective effects of AZ expression55,56 and DFMO treatment57 are both associated with increased Spm:Spd levels. The Spm:Spd ratio is consistently elevated in the skin (2.6 to 3.2-fold) and intestine (1.4 to 2.3-fold) of CAG-SpmS mice relative to control animals. Importantly, this increase in Spm:Spd ratio is equal to or greater than the 1.4 to 2-fold changes observed with AZ expression or DFMO treatment.55-57 Therefore, we can conclude that the Spm:Spd ratio is not the primary effector of polyamine-modulated tumor susceptibility because SpmS overexpression altered this ratio but failed to change tumor responses in the skin and intestine.
The approximately 100-fold increase in SpmS activity might be predicted to lead to a far greater accumulation of tissue Spm at the expense of putrescine and Spd. However, studies in wild type mice demonstrate little correlation between tissue SpmS activity and Spm levels because the aminopropyltransferases are regulated by substrate availability.42 Numerous studies have demonstrated that Spm accumulation is strongly dependent upon dcAdoMet levels, which may be sequestered by binding to the more abundant SpdS protein.42,44,58 Depletion of dcAdoMet is apparent in CAG-SpmS skin and tumors (Table 1 and Fig. 3) and is likely explained by Spm-mediated feedback suppression of AdoMetDC.13 Although dcAdoMet levels are reduced in CAG-SpmS mice, they are clearly sufficient to support polyamine accumulation and hyperproliferation following TPA treatment and papilloma outgrowth following the DMBA/TPA protocol. Mouse models with increased AdoMetDC activity targeted to the skin have been generated and exhibit a 8-fold increase in dcAdoMet content (C. Shi and D. Feith, manuscript in preparation). The combination of these animals with CAG-SpmS mice will remove any restrictions imposed by dcAdoMet depletion in order to enable further studies on the consequences of greater Spm accumulation within the initiated cell population. The combined overexpression of SpmS and AdoMetDC in the murine heart resulted in embryonic lethality.42
The lack of a more massive accumulation of tissue Spm could also be explained by increased polyamine catabolism and backconversion. The inability to detect N1-acetylpolyamines in any of the tissue samples from CAG-SpmS mice argues against an induction of SSAT activity. This result also suggests that SpmS overexpression is unable to recapitulate the increased polyamine flux that is induced by SSAT overexpression. Flux through the polyamine metabolic pathway, and the associated changes in substrate and product levels, has been proposed as a critical modifier of carcinogenesis.20,31 In fact, tumor polyamine measurements and enzyme activities (Fig. 3) suggest that increased SpmS activity may slightly suppress SSAT-mediated polyamine flux. Further studies will be required to investigate the potential role of SMO, but its activity was not altered in initial studies of CAG-SpmS mice.42 Increased expression of the SMO gene (Smox) has been reported in TPA treated skin along with the genes for ODC and SpdS.59
TPA-induction of ODC activity and putrescine levels is not affected in a statistically significant manner by SpmS overexpression (Fig. One and Table 2). This is an important result that allows us to evaluate the effects of Spm and the Spm:Spd ratio in the absence of alterations in putrescine, which is a well-known regulator of tumor promotion.26 Papilloma ODC activity was elevated in CAG-SpmS mice relative to wild type controls, which may indicate a requirement for increased putrescine synthesis to support tumor promotion in these transgenic animals. Slightly increased tumor multiplicity was associated with a concurrent increase in tissue putrescine in the proximal small intestine and colon of Min/CAG-SpmS mice, which is in agreement with previous studies that link elevated putrescine to enhanced tumorigenesis in Min mice.36,39 However, the modest enhancement of tumor multiplicity was not statistically significant and was not pursued in further studies with much larger cohorts of animals. There was no detectable change in the small intestine and colon polyamine content of 35-d-old Min mice (Table 4). The previous report of elevated polyamine content, primarily putrescine, in the proximal small intestine of Min mice35 utilized older (65 and 114-d-old) animals that are more likely to harbor pre-neoplastic and neoplastic lesions.
As mentioned above, there is abundant evidence that tumor susceptibility or resistance is associated with increased or decreased cellular putrescine levels, respectively (reviewed in 26, 27). Previous studies in mouse skin and keratinocytes demonstrate a role for putrescine in proliferation, differentiation, neovascularization, chromatin remodeling, inflammation and immune suppression.27,61,62 Polyamines associate with numerous intracellular anions but specific downstream events can be differentially responsive to changes in the levels of a particular polyamine, such as putrescine, rather than the total polyamine content. The molecular mechanisms whereby putrescine modulates tumorigenesis remain poorly defined and there are many examples of potential downstream mediators (reviewed in 1, 3–6, 9). Polyamine depletion and accumulation are known to impact DNA and chromatin structure as well as interactions between cis-elements in DNA and trans-acting factors that regulate transcription. Specific subsets of genes are differentially transcribed in response to changes in polyamine content, and such genes include c-Myc and the AP-1 transcription factors c-Jun and c-Fos. Altered polyamine content also impacts the stability and translation of mRNAs for numerous growth regulatory proteins via the RNA-binding protein HuR and miRNA interactions.60 Polyamines directly impact the translation of specific mRNAs, including the polyamine regulatory proteins AZ, AdoMetDC and SSAT. As a result of one or more of these mechanisms, polyamine content can modify many growth regulatory proteins such as p53, p21 and p27 as well as cell signaling events including the MAPK/ERK, TGFβ, EGFR and NF-κB pathways. Intensive study is needed to elucidate and unravel the vast number of potential putrescine-responsive cellular events. Even greater complexity is introduced when one considers that there may be tissue-specific responses and that polyamine depletion and accumulation may modify different and independent proteins and signaling pathways.
In summary, results obtained in two of the most widely used mouse carcinogenesis models demonstrate that increased SpmS activity and the resulting elevation of the Spm:Spd ratio neither stimulates nor suppresses carcinogenesis driven by c-Ha-Ras activation or Apc loss. The downstream molecular targets that are selectively modified in the presence of altered polyamine content and mediate tumor susceptibility remain poorly characterized and are a critical area of research. However, our data provide further evidence that putrescine, rather than spermine, spermidine, N1-AcSpd or the Spm:Spd ratio, is the most likely mediator that modifies tumor susceptibility. Our studies also suggest that SpmS is not as effective as SSAT at inducing increased metabolic flux through the polyamine pathway, perhaps due to feedback repression of AdoMetDC and the associated depletion of dcAdoMet. Future studies utilizing increasingly more sophisticated transgenic and knockout mouse models and combinations thereof will enable more refined spatial and temporal manipulations of cellular polyamine metabolism in order to further elucidate the role of polyamines in neoplastic growth.
Materials and Methods
Materials
All chemicals, unless noted, were purchased from Sigma Chemical Company (St. Louis, MO, USA). Oligonucleotides used as primers were synthesized and purified in the Macromolecular Core Facility of the Pennsylvania State University College of Medicine. [35S]-dcAdoMet was synthesized from L-[35S]methionine (PerkinElmer Life Sciences, Boston, MA, USA) as described previously.63 S-adenosyl-L-[carboxyl-14C]methionine (~57 mCi/mmol) was purchased from Amersham Biosciences (Piscataway, NJ, USA), [1-14C]Ornithine (47.7 mCi/mmol) from NEN Radiochemicals (Waltham, MA, USA), DMBA from Kodak Laboratory Chemicals (Rochester, NY, USA), and TPA and protease inhibitor cocktail were purchased from Calbiochem (San Diego, CA, USA).
Breeding and PCR identification of transgenic mice
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine. Genomic DNA was extracted from tail biopsies from potential transgenic mice and subjected to PCR analysis to detect the transgene DNA using the REDExtract-N-Amp Tissue PCR Kit (Sigma). PCR genotyping was performed as described previously for CAG-SpmS line 8,42 K6-AZ line 5243 and K6-SSAT line 8245 mice. To generate bitransgenic animals, heterozygous mice from two different transgenic lines were bred and offspring were genotyped for both transgenes by PCR. All experimental groups included both male and female mice and no sex-dependent differences were observed.
Short-term TPA treatment and biochemical assays
A region of posterior dorsal skin (approximately 2 cm x 2 cm) was shaved and only mice in the resting phase (telogen) of the hair cycle were utilized for the experiments. TPA (17 nmol) or acetone vehicle was applied to shaved skin and mice were euthanized at the indicated time after treatment. Treated skin was excised and processed into epidermal and dermal fractions for biochemical measurements as described previously.56 Dermal and epidermal samples were processed in the appropriate harvest buffer and activity was assayed in duplicate for AdoMetDC64 and ODC.43 Dermis was minced and homogenized on ice using a Polytron for 30 sec with 15 sec on/15 sec off and centrifuged at 20,000 xg for 30 min at 4°C. Epidermis was processed on ice by sonication for 30 sec with 10 sec on/10 sec off and centrifuged in the same manner. Cytosolic proteins (50 µg) were fractionated by SDS-PAGE and transferred to PVDF membrane for western blotting using antibodies to SpdS,65 ODC and GAPDH43 as described previously. Signals were visualized with a chemiluminescence detection system (Cell Signaling Technology, Beverly, MA, USA).
To quantify epidermal thickness a region of treated skin was fixed in 10% neutral buffered formalin, embedded in paraffin and sectioned. Thickness was measured in hematoxylin and eosin stained sections using a Nikon DS-5M camera and control unit DS-L1. For each mouse, four measurements were taken in each of 12 regions of the section, and the 12 regions were averaged to derive an epidermal thickness for that mouse.
HPLC analysis of polyamine and AdoMet/dcAdoMet content
For polyamine quantification, tissue samples were homogenized as described above in 10% trichloroacetic acid (Fisher Scientific, Pittsburgh, PA, USA) and analyzed by HPLC using an ion-pair reverse-phase separation method with post-column derivatization using o-phthaldialdehyde as described previously43 and normalized to tissue wet weight. N1Ac-Spd levels were calculated from the spermidine standard curve. N1Ac-Spm was not detected. For AdoMet and dcAdoMet quantification, tissue extracts were reacted with chloroacetaldehyde to convert AdoMet and dcAdoMet to fluorescent 1,N6-etheno derivatives, which were then separated and quantified by HPLC as described previously44 and normalized to tissue wet weight.
Skin chemical carcinogenesis
Two-stage skin carcinogenesis was conducted as described previously43 with DMBA initiation (400 nmol) followed by twice weekly TPA promotion (17 nmol). For skin carcinogenesis studies, all transgenic lines had been backcrossed to the C57BL/6J inbred strain (The Jackson Laboratory, Bar Harbor, ME, USA) for more than 10 generations. All genotypes were confirmed using a second tail clip biopsy at the conclusion of the study. Papillomas were harvested one week after the final TPA application and processed for biochemical measurements as described above.
Intestinal carcinogenesis
Heterozygous CAG-SpmS line 842 mice were bred with heterozygous ApcMin/+ (referred to as Min) mice (The Jackson Laboratory) to produce wild type, CAG-SpmS, Min and Min/CAG-SpmS animals. The offspring were genotyped for the CAG-SpmS transgene as described above and for the Min mutation as described previously.66 Min mice were maintained on the C57BL/6J inbred strain and CAG-SpmS were backcrossed to this strain from the original mixed B6D2 background. The percentage of the genome derived from the inbred C57BL/6J was 93.4% for animals used for polyamine measurements and 87.5% for those used to monitor tumor development. Animals were maintained on the Teklad Global 2019 diet (Harlan Teklad, Madison, WI, USA) with a minimum 9% crude fat to promote tumor development. For polyamine measurements, 8 cm sections of proximal, middle and distal small intestine as well as the entire colon were harvested from five-week-old mice and processed as described above. For tumor studies, small intestine and colon tissues were removed from Min and Min/CAG-SpmS mice, fixed overnight in neutral buffered formalin and then transferred to 70% ethanol. The number and size of tumors was quantified in 8 cm sections of the proximal, middle and distal small intestine as well as the entire colon using a dissecting microscope with eyepiece micrometer essentially as described previously.66 A single investigator (P.A.W.) scored tumors without prior knowledge of mouse genotype.
Statistical analysis
Comparisons between groups for the skin carcinogenesis study utilized a log-rank test of the Kaplan-Meier tumor-free survival curves for tumor incidence and the Mann Whitney U test for tumor multiplicity (Prism 4.0, Graphpad Software, San Diego, CA, USA).21 All other statistical comparisons utilized a two-tailed unpaired Student’s t-test.
Disclosure of Potential Conflicts of Interest
The authors declare that there are no potential conflicts of interest.
Acknowledgments
The authors wish to thank Dr. Anthony Pegg for helpful discussions during the completion of these studies, Dr. Timothy Cooper for histopathological analysis of tumor sections, Kerry Keefer for excellent technical support, Dr. Xiaojing Wang for CAG-SpmS transgenic mice and SpmS antibody, the Penn State University College of Medicine Macromolecular Synthesis Core and Department of Comparative Medicine technicians for expert animal care. Supported by National Institutes of Health grant CA-018138 (DJF).
Glossary
Abbreviations:
- AdoMet
S-adenosylmethionine
- AdoMetDC
S-adenosylmethionine decarboxylase
- Apc
adenomatous polyposis coli gene
- AZ
ornithine decarboxylase antizyme
- CAG
composite cytomegalovirus immediate early gene enhancer-chicken b-actin promoter
- dcAdoMet
decarboxylated S-adenosylmethionine
- DFMO
α-difluoromethylornithine
- DMBA
7,12-dimethylbenz[a]anthracene
- K6
keratin 6
- Min
ApcMin/+ mice
- N1AcSpd
N1-acetylspermidine
- ODC
ornithine decarboxylase
- Spd
spermidine
- SpdS
spermidine synthase
- Spm
spermine
- SpmS
spermine synthase
- SSAT
spermidine/spermine-N1-acetyltransferase
- TPA
12-O-tetradecanoylphorbol-13-acetate
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/19241
References
- 1.Thomas T, Thomas TJ. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell Mol Life Sci. 2001;58:244–58. doi: 10.1007/PL00000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rider JE, Hacker A, Mackintosh CA, Pegg AE, Woster PM, Casero RA., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids. 2007;33:231–40. doi: 10.1007/s00726-007-0513-4. [DOI] [PubMed] [Google Scholar]
- 3.Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39–51. doi: 10.1016/j.biocel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Childs AC, Mehta DJ, Gerner EW. Polyamine-dependent gene expression. Cell Mol Life Sci. 2003;60:1394–406. doi: 10.1007/s00018-003-2332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang JY. Polyamines and mRNA stability in regulation of intestinal mucosal growth. Amino Acids. 2007;33:241–52. doi: 10.1007/s00726-007-0518-z. [DOI] [PubMed] [Google Scholar]
- 6.Gerner EW, Meyskens FL., Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–92. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 7.Casero RA, Jr., Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6:373–90. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 8.Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–94. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura K, Nakatsu F, Kashiwagi K, Ohno H, Saito T, Igarashi K. Essential role of S-adenosylmethionine decarboxylase in mouse embryonic development. Genes Cells. 2002;7:41–7. doi: 10.1046/j.1356-9597.2001.00494.x. [DOI] [PubMed] [Google Scholar]
- 11.Pendeville H, Carpino N, Marine JC, Takahashi Y, Muller M, Martial JA, et al. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol Cell Biol. 2001;21:6549–58. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pegg AE. Regulation of ornithine decarboxylase. J Biol Chem. 2006;281:14529–32. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- 13.Pegg AE. S-Adenosylmethionine decarboxylase. Essays Biochem. 2009;46:25–45. doi: 10.1042/bse0460003. [DOI] [PubMed] [Google Scholar]
- 14.Ikeguchi Y, Bewley MC, Pegg AE. Aminopropyltransferases: function, structure and genetics. J Biochem. 2006;139:1–9. doi: 10.1093/jb/mvj019. [DOI] [PubMed] [Google Scholar]
- 15.Coffino P. Regulation of cellular polyamines by antizyme. Nat Rev Mol Cell Biol. 2001;2:188–94. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- 16.Kahana C. Regulation of cellular polyamine levels and cellular proliferation by antizyme and antizyme inhibitor. Essays Biochem. 2009;46:47–61. doi: 10.1042/bse0460004. [DOI] [PubMed] [Google Scholar]
- 17.Casero RA, Pegg AE. Polyamine catabolism and disease. Biochem J. 2009;421:323–38. doi: 10.1042/BJ20090598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feith DJ. Carcinogenesis studies in mice with genetically engineered alterations in polyamine metabolism. Methods Mol Biol. 2011;720:129–41. doi: 10.1007/978-1-61779-034-8_7. [DOI] [PubMed] [Google Scholar]
- 19.Jänne J, Alhonen L, Pietilä M, Keinänen TA. Genetic approaches to the cellular functions of polyamines in mammals. Eur J Biochem. 2004;271:877–94. doi: 10.1111/j.1432-1033.2004.04009.x. [DOI] [PubMed] [Google Scholar]
- 20.Jänne J, Alhonen L, Pietilä M, Keinänen TA, Uimari A, Hyvönen MT, et al. Genetic manipulation of polyamine catabolism in rodents. J Biochem. 2006;139:155–60. doi: 10.1093/jb/mvj035. [DOI] [PubMed] [Google Scholar]
- 21.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4:1350–62. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien TG. The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis. Cancer Res. 1976;36:2644–53. [PubMed] [Google Scholar]
- 23.Scalabrino G, Ferioli ME. Degree of enhancement of polyamine biosynthetic decarboxylase activities in human tumors: a useful new index of degree of malignancy. Cancer Detect Prev. 1985;8:11–6. [PubMed] [Google Scholar]
- 24.Gilmour SK, Verma AK, Madara T, O’Brien TG. Regulation of ornithine decarboxylase gene expression in mouse epidermis and epidermal tumors during two-stage tumorigenesis. Cancer Res. 1987;47:1221–5. [PubMed] [Google Scholar]
- 25.Koza RA, Megosh LC, Palmieri M, O’Brien TG. Constitutively elevated levels of ornithine and polyamines in mouse epidermal papillomas. Carcinogenesis. 1991;12:1619–25. doi: 10.1093/carcin/12.9.1619. [DOI] [PubMed] [Google Scholar]
- 26.Pegg AE, Feith DJ. Polyamines and neoplastic growth. Biochem Soc Trans. 2007;35:295–9. doi: 10.1042/BST0350295. [DOI] [PubMed] [Google Scholar]
- 27.Gilmour SK. Polyamines and nonmelanoma skin cancer. Toxicol Appl Pharmacol. 2007;224:249–56. doi: 10.1016/j.taap.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 29.Giardiello FM, Hamilton SR, Hylind LM, Yang VW, Tamez P, Casero RA., Jr. Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res. 1997;57:199–201. [PubMed] [Google Scholar]
- 30.Shoemaker AR, Gould KA, Luongo C, Moser AR, Dove WF. Studies of neoplasia in the Min mouse. Biochim Biophys Acta. 1997;1332:F25–48. doi: 10.1016/s0304-419x(96)00041-8. [DOI] [PubMed] [Google Scholar]
- 31.Berger FG, Kramer DL, Porter CW. Polyamine metabolism and tumorigenesis in the Apc(Min/+) mouse. Biochem Soc Trans. 2007;35:336–9. doi: 10.1042/BST0350336. [DOI] [PubMed] [Google Scholar]
- 32.Gerner EW, Meyskens FL., Jr. Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin Cancer Res. 2009;15:758–61. doi: 10.1158/1078-0432.CCR-08-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ignatenko NA, Gerner EW, Besselsen DG. Defining the role of polyamines in colon carcinogenesis using mouse models. J Carcinog. 2011;10:10. doi: 10.4103/1477-3163.79673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fultz KE, Gerner EW. APC-dependent regulation of ornithine decarboxylase in human colon tumor cells. Mol Carcinog. 2002;34:10–8. doi: 10.1002/mc.10043. [DOI] [PubMed] [Google Scholar]
- 35.Erdman SH, Ignatenko NA, Powell MB, Blohm-Mangone KA, Holubec H, Guillén-Rodriguez JM, et al. APC-dependent changes in expression of genes influencing polyamine metabolism, and consequences for gastrointestinal carcinogenesis, in the Min mouse. Carcinogenesis. 1999;20:1709–13. doi: 10.1093/carcin/20.9.1709. [DOI] [PubMed] [Google Scholar]
- 36.Ignatenko NA, Besselsen DG, Roy UK, Stringer DE, Blohm-Mangone KA, Padilla-Torres JL, et al. Dietary putrescine reduces the intestinal anticarcinogenic activity of sulindac in a murine model of familial adenomatous polyposis. Nutr Cancer. 2006;56:172–81. doi: 10.1207/s15327914nc5602_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ignatenko NA, Besselsen DG, Stringer DE, Blohm-Mangone KA, Cui H, Gerner EW. Combination chemoprevention of intestinal carcinogenesis in a murine model of familial adenomatous polyposis. Nutr Cancer. 2008;60(Suppl 1):30–5. doi: 10.1080/01635580802401317. [DOI] [PubMed] [Google Scholar]
- 38.Jacoby RF, Cole CE, Tutsch K, Newton MA, Kelloff G, Hawk ET, et al. Chemopreventive efficacy of combined piroxicam and difluoromethylornithine treatment of Apc mutant Min mouse adenomas, and selective toxicity against Apc mutant embryos. Cancer Res. 2000;60:1864–70. [PubMed] [Google Scholar]
- 39.Tucker JM, Murphy JT, Kisiel N, Diegelman P, Barbour KW, Davis C, et al. Potent modulation of intestinal tumorigenesis in Apcmin/+ mice by the polyamine catabolic enzyme spermidine/spermine N1-acetyltransferase. Cancer Res. 2005;65:5390–8. doi: 10.1158/0008-5472.CAN-05-0229. [DOI] [PubMed] [Google Scholar]
- 40.Bailey HH, Kim K, Verma AK, Sielaff K, Larson PO, Snow S, et al. A randomized, double-blind, placebo-controlled phase 3 skin cancer prevention study of alpha-difluoromethylornithine in subjects with previous history of skin cancer. Cancer Prev Res (Phila) 2010;3:35–47. doi: 10.1158/1940-6207.CAPR-09-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyskens FL, Jr., McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [Phila Pa] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikeguchi Y, Wang X, McCloskey DE, Coleman CS, Nelson P, Hu G, et al. Characterization of transgenic mice with widespread overexpression of spermine synthase. Biochem J. 2004;381:701–7. doi: 10.1042/BJ20040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feith DJ, Shantz LM, Shoop PL, Keefer KA, Prakashagowda C, Pegg AE. Mouse skin chemical carcinogenesis is inhibited by antizyme in promotion-sensitive and promotion-resistant genetic backgrounds. Mol Carcinog. 2007;46:453–65. doi: 10.1002/mc.20294. [DOI] [PubMed] [Google Scholar]
- 44.Pegg AE, Wang X, Schwartz CE, McCloskey DE. Spermine synthase activity affects the content of decarboxylated S-adenosylmethionine. Biochem J. 2011;433:139–44. doi: 10.1042/BJ20101228. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Feith DJ, Welsh P, Coleman CS, Lopez C, Woster PM, et al. Studies of the mechanism by which increased spermidine/spermine N1-acetyltransferase activity increases susceptibility to skin carcinogenesis. Carcinogenesis. 2007;28:2404–11. doi: 10.1093/carcin/bgm162. [DOI] [PubMed] [Google Scholar]
- 46.Coleman CS, Pegg AE, Megosh LC, Guo Y, Sawicki JA, O’Brien TG. Targeted expression of spermidine/spermine N1-acetyltransferase increases susceptibility to chemically induced skin carcinogenesis. Carcinogenesis. 2002;23:359–64. doi: 10.1093/carcin/23.2.359. [DOI] [PubMed] [Google Scholar]
- 47.Guo Y, Cleveland JL, O’Brien TG. Haploinsufficiency for odc modifies mouse skin tumor susceptibility. Cancer Res. 2005;65:1146–9. doi: 10.1158/0008-5472.CAN-04-3244. [DOI] [PubMed] [Google Scholar]
- 48.Nilsson JA, Keller UB, Baudino TA, Yang C, Norton S, Old JA, et al. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell. 2005;7:433–44. doi: 10.1016/j.ccr.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 49.Kee K, Foster BA, Merali S, Kramer DL, Hensen ML, Diegelman P, et al. Activated polyamine catabolism depletes acetyl-CoA pools and suppresses prostate tumor growth in TRAMP mice. J Biol Chem. 2004;279:40076–83. doi: 10.1074/jbc.M406002200. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Ikeguchi Y, McCloskey DE, Nelson P, Pegg AE. Spermine synthesis is required for normal viability, growth, and fertility in the mouse. J Biol Chem. 2004;279:51370–5. doi: 10.1074/jbc.M410471200. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Levic S, Gratton MA, Doyle KJ, Yamoah EN, Pegg AE. Spermine synthase deficiency leads to deafness and a profound sensitivity to alpha-difluoromethylornithine. J Biol Chem. 2009;284:930–7. doi: 10.1074/jbc.M807758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seiler N. Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 1. Selective enzyme inhibitors. Curr Drug Targets. 2003;4:537–64. doi: 10.2174/1389450033490885. [DOI] [PubMed] [Google Scholar]
- 53.Ikeguchi Y, Mackintosh CA, McCloskey DE, Pegg AE. Effect of spermine synthase on the sensitivity of cells to anti-tumour agents. Biochem J. 2003;373:885–92. doi: 10.1042/BJ20030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Astrup EG, Paulsen JE. Changes in epidermal polyamine biosynthesis and specific activity of DNA following a single application of 12-O-tetradecanoyl-phorbol-13-acetate to hairless mouse skin. Carcinogenesis. 1981;2:545–51. doi: 10.1093/carcin/2.6.545. [DOI] [PubMed] [Google Scholar]
- 55.Feith DJ, Origanti S, Shoop PL, Sass-Kuhn S, Shantz LM. Tumor suppressor activity of ODC antizyme in MEK-driven skin tumorigenesis. Carcinogenesis. 2006;27:1090–8. doi: 10.1093/carcin/bgi343. [DOI] [PubMed] [Google Scholar]
- 56.Feith DJ, Shantz LM, Pegg AE. Targeted antizyme expression in the skin of transgenic mice reduces tumor promoter induction of ornithine decarboxylase and decreases sensitivity to chemical carcinogenesis. Cancer Res. 2001;61:6073–81. [PubMed] [Google Scholar]
- 57.Meyskens FL, Jr., Emerson SS, Pelot D, Meshkinpour H, Shassetz LR, Einspahr J, et al. Dose de-escalation chemoprevention trial of alpha-difluoromethylornithine in patients with colon polyps. J Natl Cancer Inst. 1994;86:1122–30. doi: 10.1093/jnci/86.15.1122. [DOI] [PubMed] [Google Scholar]
- 58.Shi C, Welsh PA, Sass-Kuhn S, Wang X, McCloskey DE, Pegg AE, et al. Characterization of transgenic mice with overexpression of spermidine synthase. Amino Acids. 2011 doi: 10.1007/s00726-011-1028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riggs PK, Angel JM, Abel EL, DiGiovanni J. Differential gene expression in epidermis of mice sensitive and resistant to phorbol ester skin tumor promotion. Mol Carcinog. 2005;44:122–36. doi: 10.1002/mc.20127. [DOI] [PubMed] [Google Scholar]
- 60.Xiao L, Cui YH, Rao JN, Zou T, Liu L, Smith A, et al. Regulation of cyclin-dependent kinase 4 translation through CUG-binding protein 1 and microRNA-222 by polyamines. Mol Biol Cell. 2011;22:3055–69. doi: 10.1091/mbc.E11-01-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayes CS, Defeo K, Dang H, Trempus CS, Morris RJ, Gilmour SK. A prolonged and exaggerated wound response with elevated ODC activity mimics early tumor development. Carcinogenesis. 2011;32:1340–8. doi: 10.1093/carcin/bgr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keough MP, Hayes CS, DeFeo K, Gilmour SK. Elevated epidermal ornithine decarboxylase activity suppresses contact hypersensitivity. J Invest Dermatol. 2011;131:158–66. doi: 10.1038/jid.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackintosh CA, Pegg AE. Effect of spermine synthase deficiency on polyamine biosynthesis and content in mice and embryonic fibroblasts, and the sensitivity of fibroblasts to 1,3-bis-(2-chloroethyl)-N-nitrosourea. Biochem J. 2000;351:439–47. doi: 10.1042/0264-6021:3510439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nisenberg O, Pegg AE, Welsh PA, Keefer K, Shantz LM. Overproduction of cardiac S-adenosylmethionine decarboxylase in transgenic mice. Biochem J. 2006;393:295–302. doi: 10.1042/BJ20051196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becerra-Solano LE, Butler J, Castañeda-Cisneros G, McCloskey DE, Wang X, Pegg AE, et al. A missense mutation, p.V132G, in the X-linked spermine synthase gene (SMS) causes Snyder-Robinson syndrome. Am J Med Genet A. 2009;149A:328–35. doi: 10.1002/ajmg.a.32641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dietrich WF, Lander ES, Smith JS, Moser AR, Gould KA, Luongo C, et al. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell. 1993;75:631–9. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]