Abstract
Comment on: Coupé B, et al. Cell Metab 2012; 15:247–55, Kaushik S, et al. EMBO Rep 2012; 13:258-65 and Quan W, et al. Endocrinology 2012; 153: In press
Autophagy has received considerable attention over the past decade owing to the fact that alteration of this cellular process, which degrades cytoplasmic materials, including organelles and misfolded proteins, contributes to a variety of diseases, such as cancer, muscular disorders and neurodegeneration.1 Recent studies using conditional, cell-specific gene-targeting methods have also revealed the importance of autophagy in the regulation of energy balance. For example, the deletion of essential autophagy genes, such as the autophagy-related gene (Atg) 7, in the liver, pancreas or adipose tissue produces alterations in body weight, adiposity and glucose homeostasis.2-6 Appetite, energy expenditure and metabolism are also carefully regulated by the central nervous system (CNS), particularly the pro-opiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus. These neurons act as major negative regulators of energy balance by reducing food intake and increasing energy expenditure. However, the role of CNS autophagy in the regulation of energy balance is largely unknown.
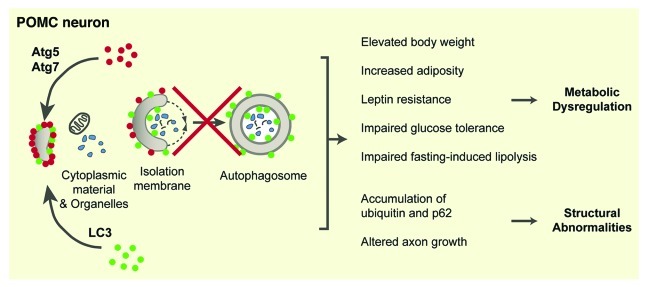
Three recent studies, including one from our laboratory, have implicated CNS autophagy in the pathogenesis of obesity (Fig. 1).7-9 These studies used a conditional cre-loxP approach to specifically delete Atg7 from POMC neurons. Pomc-Cre; Atg7loxploxp mice display higher body weights, hyperphagia and impaired glucose tolerance.7-9 These mice also exhibit an increased adiposity that is associated with leptin resistance.7-9 Mice lacking autophagy in POMC neurons develop an increased sensitivity to weight gain when they are fed a high-fat/high-energy diet.8,9
Figure 1. Schematic diagram illustrating the metabolic and structural effects of autophagy deletion in hypothalamic POMC neurons. POMC, pro-opiomelanocortin; Atg, autophagy-related gene.
Autophagy plays a particularly important role in biological processes that involve massive cellular elimination, such as neural development.10 Autophagy is constitutively present in the hypothalamus during important periods of development, and the loss of Atg7 in POMC neurons produces marked structural alterations during the first weeks of postnatal life and prior to the development of obesity(Fig. 1).7 Pomc-Cre; Atg7loxPloxP mice exhibit a reduced density of POMC-containing projections to each of their target nuclei, including the paraventricular nucleus of the hypothalamus, as early as postnatal day 14. These abnormalities in POMC neural projections persist throughout adult life and appear to be the result of diminished capacity of POMC neurons to extend axons.7 However, all developmental processes are not affected by autophagy deficiency. No changes in POMC cell numbers between Pomc-Cre; Atg7loxploxp and control mice are observed,7-9 which suggests that autophagy does not influence neurogenesis or programmed cell death, but that it specifically affects axonal growth. However, autophagy may be involved in hypothalamic neurodegeneration, because aging is associated with a decline in hypothalamic autophagy and the accumulation of p62 (a polyubiquitin-binding protein that is normally degraded by autophagy) specifically in POMC neurons.8 In addition, axonal swelling, which is a hallmark of neurodegeneration, is observed in the hypothalamus of mice that lack autophagy in POMC neurons (Coupé and Bouret, unpublished data).
Together, these recent studies suggest that autophagy is required for the proper development and function of POMC neurons, and that autophagy deficiency in POMC neurons causes marked metabolic and structural alterations. Autophagy is highly regulated by nutrient availability,11 including during perinatal life, and further studies may provide novel mechanistic insights on the influence of perinatal dietary changes on the susceptibility to metabolic diseases.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20046
References
- 1.Shintani T, et al. Science. 2004;306:990–5. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komatsu M, et al. Cell. 2007;131:1149–63. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 3.Jung HS, et al. Cell Metab. 2008;8:318–24. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Ebato C, et al. Cell Metab. 2008;8:325–32. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Singh R, et al. J Clin Invest. 2009;119:3329–39. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, et al. Proc Natl Acad Sci U S A. 2009;106:19860–5. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coupé B, et al. Cell Metab. 2012;15:247–55. doi: 10.1016/j.cmet.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaushik S, et al. EMBO Rep. 2012;13:258–65. doi: 10.1038/embor.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quan W, et al. Endocrinology. 2012 doi: 10.1210/en.2011-1882. [DOI] [PubMed] [Google Scholar]
- 10.Cecconi F, et al. Dev Cell. 2008;15:344–57. doi: 10.1016/j.devcel.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He C, et al. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]