Abstract
The events of cell division are regulated by a complex interplay between kinases and phosphatases. Cyclin-dependent kinases (Cdks), polo-like kinases (Plks) and Aurora kinases play central roles in this process. Polo kinase (Plk1 in humans) regulates a wide range of events in mitosis and cytokinesis. To ensure the accuracy of these processes, polo activity itself is subject to complex regulation. Phosphorylation of polo in its T loop (or activation loop) increases its kinase activity several-fold. It has been shown that Aurora A kinase, with its co-factor Bora, activates Plk1 in G2, and that this is essential for recovery from cell cycle arrest induced by DNA damage. In a recent article published in PLoS Biology, we report that Drosophila polo is activated by Aurora B kinase at centromeres, and that this is crucial for polo function in regulating chromosome dynamics in prometaphase. Our results suggest that this regulatory pathway is conserved in humans. Here, we propose a model for the collaboration between Aurora B and polo in the regulation of kinetochore attachment to microtubules in early mitosis. Moreover, we suggest that Aurora B could also function to activate Polo/Plk1 in cytokinesis. Finally, we discuss recent findings and open questions regarding the activation of polo and polo-like kinases by different kinases in mitosis, cytokinesis and other processes.
Keywords: Aurora A, Aurora B, Bora, centromere, INCENP, kinase, Kinetochore, mitosis, phosphatase, Plk1, Polo
Polo Kinase in the Cell Cycle
The Polo kinase was discovered nearly 25 years ago in Drosophila.1,2 Polo and its closest orthologs in other species (Plk1 in humans) are now firmly established as central regulators of the cell cycle, playing crucial roles during mitosis and cytokinesis.3-5 Four other Polo-like kinases exist in humans (Plk2–5), which fulfill more divergent and specialized roles in cellular proliferation, development and tissue-specific functions.4,5 It has long been known that the activity of Plk1 increases several-fold in mitosis,6 but how this occurs mechanistically, temporally and spatially in the cell is not fully understood. As discussed in this paper, it is now clear that both Aurora A and Aurora B contribute to activate Polo/Plk1 for distinct roles in mitosis. Yet, other Plks could be activated by different kinases.
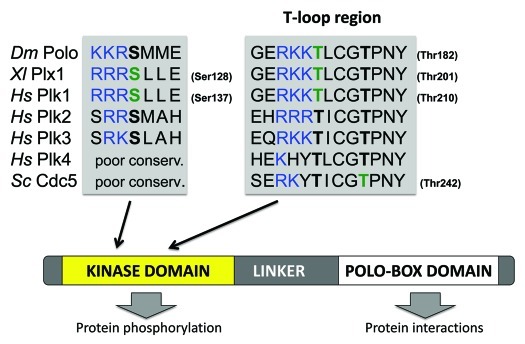
The multiplicity of tasks assigned to Plk1 is matched by the complex upstream regulation of this protein.4 First, all Plks comprise a C-terminal Polo-box domain (PBD) (Fig. 1) that allows them to interact with target proteins. In addition, Plk1 is transcriptionally regulated to allow maximal expression in G2- and M phases,7,8 while APCCdh1-mediated ubiquitination promotes Plk1 degradation in late M phase.9 Like many kinases, Plk1 is activated by phosphorylation in its T loop, which induces a conformational change to promote catalysis.10 Phosphorylation at that site is also thought to lock the protein in an open conformation, where the kinase domain and the PBD are dissociated.11 Phosphorylation occurs at Thr210 in human Plk1,12 and at the equivalent Thr201 in Xenopus Plx1.13 This site is highly conserved among Plks, from yeast to humans (Fig. 1).
Figure 1. Position of key activating phosphorylation sites in Polo-like kinases. Polo-like kinases are defined by the presence of a C-terminal Polo-Box Domain and an N-terminal kinase domain separated by a linker segment. Sequence alignments are shown for regions of interest. Phosphorylation sites with verified activating function are in green. Equivalent residues in other Plks are in bold black. Positively charged residues that precede those sites are in blue. The regions of Plk4 and Cdc5 equivalent to the Ser137 region of Plk1 are poorly conserved. Dm, Drosophila melanogaster; Xl, Xenopus leavis; Hs, Homo sapiens; Sc, Saccharomyces cerevisiae.
Plk1 is activated in G2 and promotes mitotic entry by indirectly stimulating cyclin B-Cdk1 activation.16 Plk1 phosphorylates and inactivates Wee1 and Myt1, two kinases that target Cdk1 to keep it inactive,17,18 while it also activates Cdc25C,19 the phosphatase that removes inhibitory phosphates on Cdk1. But what activates Plk1 in G2? It had been suggested that Plx1 was activated by xPlkk1/Slk,14 but it was later found that xPlkk1 acts downstream of Plk1, although it could contribute to further activate Plx1 in a positive feedback loop.15 Thus, convincing evidence for a kinase activating Plx1 or Plk1 was still lacking.
In 2008, two papers reported that Plk1 is activated in G2 by Aurora A kinase.20,21 This requires the protein Bora that appears to facilitate the recognition of Plk1 by Aurora A, which then phosphorylates Plk1 at Thr210 in its activation loop. This pathway was shown to dictate the normal timing of mitotic entry and to be essential for re-entry into mitosis in recovery from a DNA damage response arrest.21 Where exactly the activation of Plk1 by Aurora A occurs is unclear, but one could envision that it could take place at least in part at centrosomes, where both proteins co-localize and regulate spindle poles.22 However, Plk1, Aurora A and Bora are dispensable for mitotic entry in an unperturbed cell cycle.4,20,21
Bringing Active Polo to the Kinetochore
Polo/Plk1 activity is not necessary for mitotic entry but is essential for chromosome congression in prometaphase.4 While Polo and Plk1 localize at centromeres and kinetochores in early mitosis, Aurora A and Bora do not. We hypothesized that another kinase was required to activate Plk1 at kinetochores. Aurora B was an obvious candidate for the job. First, the T-loop activation site of Polo/ Plk1 kinase lies in a consensus motif for both Aurora A and B kinases, as it is preceded by positively charged residues (Fig. 1).23-25 Second, Aurora B is concentrated at centromeres in early mitosis and regulates several proteins of the kinetochore, where Plk1 is localized. The different pattern of localization between Aurora A and Aurora B is due to their interactions with completely distinct regulatory proteins that ensure their functional specificity.26 Aurora B is the enzymatically active subunit of the chromosomal passenger complex (CPC), which also includes INCENP, Survivin and Borealin.27 The CPC localizes to chromosomes in early mitosis, later becoming enriched at centromeres, where it is required for proper kinetochore function. After anaphase onset, the CPC relocalizes to the central spindle, where it colocalizes with Polo and is required for cytokinesis.28 Thus, if Aurora B activates Polo on chromosomes in early mitosis, it could potentially keep Polo active later on the central spindle in cytokinesis.
In our paper recently published in PLoS Biology,29 we used Drosophila as a model to test if Aurora B could activate Polo. We found that INCENP and Aurora B kinase are required for Polo activation by T-loop phosphorylation at centromeres and kinetochores in vivo and in cultured cells. Recruitment of total Polo to the centromere/kinetochore region did not depend on the CPC. We found that INCENP co-localizes with active Polo and physically interacts with Polo. Our results confirm that Aurora B indeed plays an important role in Polo activation in mitosis (Fig. 2) and places the protein INCENP in a unique position as a platform for the coordination of the activities of both kinases.30
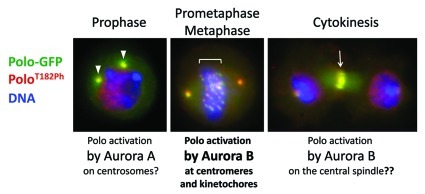
Figure 2. Localization of T-loop phosphorylated PoloT182Ph. Images of Drosophila cells in culture expressing Polo-GFP (green) and stained with a phospho-specific antibody against PoloT182Ph (red). Blue: DNA. In prophase, PoloT182Ph is on seen on centrosomes (arrowheads), where it could be phosphorylated by Aurora A. In prometaphase, PoloT182Ph appears on centromeres and kinetochores (under bracket), where it depends on phosphorylation by Aurora B.29 In cytokinesis, PoloT182Ph concentrates on the central spindle at the level of the cleavage furrow (arrow), where we hypothesize that it could again be phosphorylated by Aurora B. The red signal in interphase nuclei was verified to be non-specific to Polo.29
Inhibition, inactivation or mutation of Polo/Plk1 leads to a mitotic arrest with monopolar spindles and failures in correct kinetochore-microtubule (KT-MT) attachments.1,3,31-33 We found that chromosome congression was strongly dependent on T-loop activation of Polo. Interestingly, bipolar spindles could still be assembled without Polo activation at the same site. Thus, unphosphorylated Polo may provide sufficient activity for proper spindle regulation, but a higher level of Polo activity may be required at kinetochores, and brought about by the CPC.
Importantly, our experiments confirmed that the CPC-Plk1 activation pathway is active in human cells. The activation of Polo kinase by Aurora B at mitotic centromeres may be widely conserved in animals. Although the role of Aurora A and Bora in Plk1 activation is solidly established in human cells, this mechanism may not be universally conserved and could also serve tissue-specific functions. In Drosophila cells, Aurora A knockdown led to a modest reduction in active Polo levels at centrosomes.29 Although bora is an essential gene in flies, no mitotic defects have been reported in bora mutants. Instead, bora mutant clones were shown to produce defects in asymmetric cell division, reminiscent of some aurora a mutants.35 As both Polo and Aurora A are required for asymmetric cell division,36,37 Aurora A and Bora could function to activate Polo specifically in that context. However, while human Bora binds both Aurora A and Plk1, Drosophila Bora has been found to bind Aurora A35 but not Polo. Thus it remains unclear how Bora and Polo are functionally connected in flies. No Bora orthologs have been identified in the yeasts. It seems that Aurora B in the CPC plays a more essential and perhaps better conserved role in activating Polo in mitosis.
Controlling Kinetochore-Microtubule Attachment: A Close Collaboration Between Polo and Aurora B Kinase
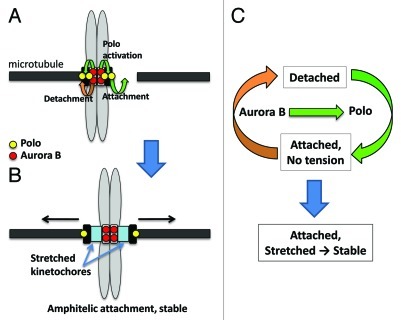
More Polo is on kinetochores than centromeres in prometaphase and metaphase, while the CPC is mainly at inner centromeres.29 So how and where does the CPC activate Polo? In cultured cells, we observed that Polo is initially recruited to centromeres before INCENP is seen there.29 The direct interaction between Polo and INCENP could help the recruitment of the CPC or stabilize its localization at centromeres. Centromeric CPC is then required for its activation of Polo and its functions in regulating kinetochore-microtubule attachment. Consistent with this model, active Polo is seen at centromeres only after INCENP has been recruited.29 Once activated at centromeres, active Polo could relocalize to kinetochores. But diffusible Aurora B emanating from centromeres could also keep Polo phosphorylated and active at that site (Fig. 3A).
Figure 3. Model for the collaboration between Aurora B and Polo kinases in the regulation of kinetochore-microtubule attachments. (A) Aurora B (red) phosphorylation of kinetochore subunits promotes the detachment of kinetochores that are not under tension (orange arrow). Polo (yellow) at the kinetochore promotes kinetochore attachment to microtubules (large green arrow). Aurora B also phosphorylates and activates Polo at centromeres, and possibly on kinetochores (or activated Polo relocalizes from centromeres to kinetochores). (B) Once amphitelic attachment is reached, tension stretches kinetochores, separating Polo on the outer kinetochore from Aurora B on the inner centromere. (C) The activation of Polo by Aurora B could facilitate the attachment/detachment cycle that takes place between kinetchores and microtubules in prometaphase.
In this regard, a gradient in Aurora B activity concentrated at centromeres has been proposed to serve as a mechanism whereby kinetochores can sense bipolar attachment.38-41 In this model, once under tension, a kinetochore is stretched away from its centromere, and the Aurora B substrates at the kinetochore become less efficiently phosphorylated. Since Aurora B phosphorylation of kinetochore components results in destabilization of kinetochore-microtubule attachments, the outcome of this mechanism is preferential stabilization of bipolar attachments (Fig. 3B). Until then, incorrectly attached kinetochores detach under the effect of Aurora B, and their re-attachment requires Polo activity. Thus, a close collaboration between Aurora B and Polo keeps the attachment/detachment cycle active in prometaphase, and the coupling between Aurora B and Polo activities appears logical (Fig. 3C).
Such a cycle would not be possible without phosphatase activity. Recently, protein phosphatase 2A in complex with its B56 regulatory subunit (PP2A-B56) has been shown to antagonize both Aurora B and Plk1 activities during prometaphase. A balance between Aurora B and Plk1 kinases vs. PP2A-B56 is required for proper chromosome attachment and congression in prometaphase.42 PP2A-B56 is enriched at centromeres/kinetochores, and substrates of both Aurora B and Plk1 become hyperphosphorylated when B56 isoforms are depleted, while kinetochores fail to reach correct attachments. Thus PP2A-B56 appears as an essential phosphatase in the attachment/detachment cycle. When kinetochores are bipolarly attached and under tension, less Aurora B activity can reach the kinetochores, and PP1 is then recruited to the kinetochores, further dephosphorylating Aurora B substrates and stabilizing attachments.43 Less Plk1 is also seen on KTs that have attached correctly.42,44 The complex interplay between kinases and phosphatases in kinetochore regulation is slowly coming to light.
What Turns Polo On during Cytokinesis?
Polo is essential for cytokinesis.3,45 In human cells, Plk1 promotes RhoA activity at the contractile ring, which drives cleavage furrow ingression.46,47 Polo activity also regulates several other effectors of cytokinesis.48 We found that T loop-activated Polo is enriched at the cleavage furrow and the midbody ring (Fig. 2).29 This activation of Polo is likely to be important for cytokinesis, but this has not been fully investigated yet. Although activated Polo could, in principle, transit from the kinetochore to the central spindle, it seems unlikely to survive phosphatase activities after anaphase onset. There again, Aurora B in the CPC would be in a good position to phosphorylate Polo, as they are in close proximity on the central spindle during cytokinesis. Conversely, Aurora A and Bora are unlikely to mediate Polo/Plk1 activation in cytokinesis. In human cells, Bora has been shown to be degraded during mitosis, and this is triggered by Plk1,49,50 the same kinase that it contributes to activate in G2. More experiments are required to determine if Polo/Plk1 T-loop phosphorylation is essential for cytokinesis, as well as the role of Aurora B and the CPC in this activation.
Other Sites of Activation and Other Plks
Polo-like kinase activation is surely more complex than one phosphorylation site and two kinases. Ser137 is an alternative site of activation in the kinase domain of Plk1.12 It has been shown to be phosphorylated briefly in mitosis and has been proposed to contribute to regulate the activity of the spindle assembly checkpoint.51 How phosphorylation at Ser137 increases Plk1 activity and how it differs from Thr210 phosphorylation is unclear. Ser137 is conserved in Plk2, Plk3 as well as in Drosophila Polo (Fig. 1), but the identity of the kinase(s) targeting it is unknown. Interestingly, Ser137 is preceded by positively charged residues, making it a possible target of Aurora kinases (Fig. 1). Aurora kinase activation of a Plk outside its kinase domain has also been reported recently in the fission yeast S. pombe. In this case, Ark1 (Aurora A) phosphorylates Plo1 (Polo) in the linker region (Fig. 1) to promote its recruitment to the spindle pole bodies, and this contributes to trigger mitosis in response to nutrient availability sensed by the TOR pathway.52
If Aurora kinases are ideally poised to activate Polo/Plk1 in G2, mitosis and cytokinesis, can they also contribute to regulating other Plk family members? Little or nothing has been published on T-loop activation of Plk2, Plk3 and Plk4. Based on the presence of positively charged residues preceding the equivalent site to Thr210 (Plk1) and Thr182 (Polo) in Plk2 and Plk3, these too could be targets of Aurora kinases (Fig. 1). However, Plk2 and Plk3 are known to function mostly in interphase and in differentiated cells,4,5 where Aurora kinases are largely inactive. It will be interesting to investigate whether Plk2, Plk3 and Plk4 require T-loop phosphorylation at all for their functions in vivo, and, if so, the identity of the kinase(s) responsible for their activation.
In the budding yeast, Cdc5 is the sole Plk, and it plays very similar roles to Drosophila Polo and human Plk1. However, its activation has been shown to depend on phosphorylation at a cyclin-dependent kinase (Cdk) site in the T loop (Thr242, corresponding to Thr214 in Plk1) (Fig. 1).53 The same study found that Cdc5 Thr238, corresponding to Plk1 Thr210 (Fig. 1) is phosphorylated in vivo, but its mutation did not affect Cdc5’s essential functions. Thr242 of Cdc5 is conserved in all Plks of humans and Drosophila, where it is followed by a proline residue. It is tempting to speculate that proline-directed kinases, including Cdks and MAP kinases, could contribute to activate other Plks at this alternative T-loop site.
The field has come a long way in deciphering the astonishing complexity of functions of the Polo-like kinases in the cell cycle, mitosis, cytokinesis and even outside cell division.3,4,54 While T-loop phosphorylation of Plk1 by Aurora A/Bora can help the cell enter mitosis, Polo activation at the same site by the CPC is required in mitosis. It should not be surprising, but rather expected, that Plks have found multiple activating partner kinases to help regulate them in their various functions, by phosphorylating them at both the same and different residues. Much remains to be done before a full understanding of Plk activation can be reached, but let’s be prepared to see the picture expand and complexify.
Acknowledgements
We thank Benjamin Kwok and Paul Maddox for comments on the manuscript. Research in V.A.’s lab on Polo regulation is supported by the Canadian Institutes of Health Research, of which V.A. is also a New Investigator Award recipient. M.C.’s work is supported by Wellcome Trust Program Grant to W.C.E.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/19724
References
- 1.Sunkel CE, Glover DM. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988;89:25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Llamazares S, Moreira A, Tavares A, Girdham C, Spruce BA, Gonzalez C, et al. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991;5(12A):2153–65. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- 3.Petronczki M, Lénárt P, Peters JM. Polo on the Rise-from Mitotic Entry to Cytokinesis with Plk1. Dev Cell. 2008;14:646–59. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–75. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- 5.de Cárcer G, Manning G, Malumbres M. From Plk1 to Plk5: functional evolution of polo-like kinases. Cell Cycle. 2011;10:2255–62. doi: 10.4161/cc.10.14.16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golsteyn RM, Mundt KE, Fry AM, Nigg EA. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J Cell Biol. 1995;129:1617–28. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchiumi T, Longo DL, Ferris DK. Cell cycle regulation of the human polo-like kinase (PLK) promoter. J Biol Chem. 1997;272:9166–74. doi: 10.1074/jbc.272.14.9166. [DOI] [PubMed] [Google Scholar]
- 8.Martin BT, Strebhardt K. Polo-like kinase 1: target and regulator of transcriptional control. Cell Cycle. 2006;5:2881–5. doi: 10.4161/cc.5.24.3538. [DOI] [PubMed] [Google Scholar]
- 9.Lindon C, Pines J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J Cell Biol. 2004;164:233–41. doi: 10.1083/jcb.200309035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kothe M, Kohls D, Low S, Coli R, Cheng AC, Jacques SL, et al. Structure of the catalytic domain of human polo-like kinase 1. Biochemistry. 2007;46:5960–71. doi: 10.1021/bi602474j. [DOI] [PubMed] [Google Scholar]
- 11.Jang YJ, Lin CY, Ma S, Erikson RL. Functional studies on the role of the C-terminal domain of mammalian polo-like kinase. Proc Natl Acad Sci U S A. 2002;99:1984–9. doi: 10.1073/pnas.042689299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang YJ, Ma S, Terada Y, Erikson RL. Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J Biol Chem. 2002;277:44115–20. doi: 10.1074/jbc.M202172200. [DOI] [PubMed] [Google Scholar]
- 13.Qian YW, Erikson E, Maller JL. Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol Cell Biol. 1999;19:8625–32. doi: 10.1128/mcb.19.12.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian YW, Erikson E, Maller JL. Purification and cloning of a protein kinase that phosphorylates and activates the polo-like kinase Plx1. Science. 1998;282:1701–4. doi: 10.1126/science.282.5394.1701. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Maller JL. Xenopus Polo-like kinase Plx1: a multifunctional mitotic kinase. Oncogene. 2005;24:238–47. doi: 10.1038/sj.onc.1208220. [DOI] [PubMed] [Google Scholar]
- 16.van Vugt MA, Medema RH. Getting in and out of mitosis with Polo-like kinase-1. Oncogene. 2005;24:2844–59. doi: 10.1038/sj.onc.1208617. [DOI] [PubMed] [Google Scholar]
- 17.Inoue D, Sagata N. The Polo-like kinase Plx1 interacts with and inhibits Myt1 after fertilization of Xenopus eggs. EMBO J. 2005;24:1057–67. doi: 10.1038/sj.emboj.7600567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe N, Arai H, Nishihara Y, Taniguchi M, Watanabe N, Hunter T, et al. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc Natl Acad Sci U S A. 2004;101:4419–24. doi: 10.1073/pnas.0307700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–80. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- 20.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–8. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macůrek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–23. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 22.Eckerdt F, Maller JL. Kicking off the polo game. Trends Biochem Sci. 2008;33:511–3. doi: 10.1016/j.tibs.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Honda R, Körner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–41. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, Yates JR, 3rd, et al. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–72. doi: 10.1016/S0092-8674(02)00973-X. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari S, Marin O, Pagano MA, Meggio F, Hess D, El-Shemerly M, et al. Aurora-A site specificity: a study with synthetic peptide substrates. Biochem J. 2005;390:293–302. doi: 10.1042/BJ20050343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmena M, Ruchaud S, Earnshaw WC. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr Opin Cell Biol. 2009;21:796–805. doi: 10.1016/j.ceb.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 28.Hümmer S, Mayer TU. Cdk1 negatively regulates midzone localization of the mitotic kinesin Mklp2 and the chromosomal passenger complex. Curr Biol. 2009;19:607–12. doi: 10.1016/j.cub.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 29.Carmena M, Pinson X, Platani M, Salloum Z, Xu Z, Clark A, et al. The chromosomal passenger complex activates polo kinase at centromeres. PLoS Biol. 2012;10:e1001250. doi: 10.1371/journal.pbio.1001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmena M, Earnshaw WC. INCENP at the kinase crossroads. Nat Cell Biol. 2006;8:110–1. doi: 10.1038/ncb0206-110. [DOI] [PubMed] [Google Scholar]
- 31.Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–13. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steegmaier M, Hoffmann M, Baum A, Lénárt P, Petronczki M, Krssák M, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17:316–22. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 33.Lénárt P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17:304–15. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 34.Goto H, Kiyono T, Tomono Y, Kawajiri A, Urano T, Furukawa K, et al. Complex formation of Plk1 and INCENP required for metaphase-anaphase transition. Nat Cell Biol. 2006;8:180–7. doi: 10.1038/ncb1350. [DOI] [PubMed] [Google Scholar]
- 35.Hutterer A, Berdnik D, Wirtz-Peitz F, Zigman M, Schleiffer A, Knoblich JA. Mitotic activation of the kinase Aurora-A requires its binding partner Bora. Dev Cell. 2006;11:147–57. doi: 10.1016/j.devcel.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Berdnik D, Knoblich JA. Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr Biol. 2002;12:640–7. doi: 10.1016/S0960-9822(02)00766-2. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Ouyang Y, Somers WG, Chia W, Lu B. Polo inhibits progenitor self-renewal and regulates Numb asymmetry by phosphorylating Pon. Nature. 2007;449:96–100. doi: 10.1038/nature06056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, et al. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–29. doi: 10.1016/S0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 39.Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, et al. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–68. doi: 10.1016/S1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- 40.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323:1350–3. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lampson MA, Cheeseman IM. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011;21:133–40. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foley EA, Maldonado M, Kapoor TM. Formation of stable attachments between kinetochores and microtubules depends on the B56-PP2A phosphatase. Nat Cell Biol. 2011;13:1265–71. doi: 10.1038/ncb2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Wulf P, Montani F, Visintin R. Protein phosphatases take the mitotic stage. Curr Opin Cell Biol. 2009;21:806–15. doi: 10.1016/j.ceb.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Ahonen LJ, Kallio MJ, Daum JR, Bolton M, Manke IA, Yaffe MB, et al. Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr Biol. 2005;15:1078–89. doi: 10.1016/j.cub.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 45.Carmena M, Riparbelli MG, Minestrini G, Tavares AM, Adams R, Callaini G, et al. Drosophila polo kinase is required for cytokinesis. J Cell Biol. 1998;143:659–71. doi: 10.1083/jcb.143.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burkard ME, Maciejowski J, Rodriguez-Bravo V, Repka M, Lowery DM, Clauser KR, et al. Plk1 self-organization and priming phosphorylation of HsCYK-4 at the spindle midzone regulate the onset of division in human cells. PLoS Biol. 2009;7:e1000111. doi: 10.1371/journal.pbio.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfe BA, Takaki T, Petronczki M, Glotzer M. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol. 2009;7:e1000110. doi: 10.1371/journal.pbio.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Wang J, Jiao H, Liao J, Xu X. Cytokinesis and cancer: Polo loves ROCK’n’ Rho(A) J Genet Genomics. 2010;37:159–72. doi: 10.1016/S1673-8527(09)60034-5. [DOI] [PubMed] [Google Scholar]
- 49.Chan EH, Santamaria A, Silljé HH, Nigg EA. Plk1 regulates mitotic Aurora A function through betaTrCP-dependent degradation of hBora. Chromosoma. 2008;117:457–69. doi: 10.1007/s00412-008-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seki A, Coppinger JA, Du H, Jang CY, Yates JR, 3rd, Fang G. Plk1- and beta-TrCP-dependent degradation of Bora controls mitotic progression. J Cell Biol. 2008;181:65–78. doi: 10.1083/jcb.200712027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van de Weerdt BC, van Vugt MA, Lindon C, Kauw JJ, Rozendaal MJ, Klompmaker R, et al. Uncoupling anaphase-promoting complex/cyclosome activity from spindle assembly checkpoint control by deregulating polo-like kinase 1. Mol Cell Biol. 2005;25:2031–44. doi: 10.1128/MCB.25.5.2031-2044.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hálová L, Petersen J. Aurora promotes cell division during recovery from TOR-mediated cell cycle arrest by driving spindle pole body recruitment of Polo. J Cell Sci. 2011;124:3441–9. doi: 10.1242/jcs.083683. [DOI] [PubMed] [Google Scholar]
- 53.Mortensen EM, Haas W, Gygi M, Gygi SP, Kellogg DR. Cdc28-dependent regulation of the Cdc5/Polo kinase. Curr Biol. 2005;15:2033–7. doi: 10.1016/j.cub.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 54.Takaki T, Trenz K, Costanzo V, Petronczki M. Polo-like kinase 1 reaches beyond mitosis--cytokinesis, DNA damage response, and development. Curr Opin Cell Biol. 2008;20:650–60. doi: 10.1016/j.ceb.2008.10.005. [DOI] [PubMed] [Google Scholar]