Abstract
Understanding the mechanisms that lead to the differentiation of male germ cells from their spermatogonial stem cells through meiosis to give rise to mature haploid spermatozoa has been a major quest for many decades. Unlike most other cell types this differentiation process is more or less completely dependent upon the cells being located within the strongly structured niche provided by mature Sertoli cells within an intact seminiferous epithelium. While much new information is currently being obtained through the application and description of relevant gene mutations, there is still a considerable need for in vitro models with which to explore the mechanisms involved. Not only are systems of in vitro spermatogenesis important for understanding the basic science, they have marked pragmatic value in offering ex vivo systems for the artificial maturation of immature germ cells from male infertility patients, as well as providing opportunities for the transgenic manipulation of male germ cells. In this review, we have summarized literature relating to simplistic culturing of germ cells, co-cultures of germ cells with other cell types, especially with Sertoli cells, cultures of seminiferous tubule fragments, and briefly mention the opportunities of xenografting larger testicular pieces. The majority of methods are successful in allowing the differentiation of small steps in the progress of spermatogonia to spermatozoa; few tolerate the chromosomal reduction division through meiosis, and even fewer seem able to complete the complex morphogenesis which results in freely swimming spermatozoa. However, recent progress with complex culture environments, such as 3-d matrices, suggest that possibly success is now not too far away.
Introduction
The production of gametes has inspired scientists for many generations to develop methods by which to investigate and intervene in the complex differentiation process which leads to mature sperm and oocytes. Whereas for the latter, some progress has been made, for example in regard to in vitro oocyte maturation (IVM),1 the investigation of spermatogenesis has been hampered by a lack of appropriate in vitro techniques. As early as 1937, Martinovitch2 cultured testicular explants and observed the differentiation of spermatogonia into pachytene spermatocytes. Although explant cultures remain useful, with more understanding of the molecular mechanisms involved, there has also been development of germ cell monocultures and co-cultures. These comparatively minimalistic cultures, while less true to the in vivo situation, reduce culture complexity, which in turn aids the examination and understanding of testicular paracrine interactions. However, none of the current minimal systems have yet been able to induce meiotic division and subsequent differentiation of spermatogonia into fully functional mature spermatozoa, and thereby mimicking the in vivo situation. However, using a more complex organ culture system comprising neonatal testis fragments, Sato and colleagues have finally been able to achieve production of functional spermatozoa from spermatogonia.3 Mostly, minimal cultures possess the capacity to induce either production or maturation of haploid spermatids, but not both. This article looks at the various approaches in use by researchers attempting to address this problem.
In vivo Spermatogenesis
Mammalian spermatogenesis is governed by a complex system of paracrine and endocrine activity within a structurally well organized tissue (Figs. 1 and 2). During the process of spermatogenesis, diploid spermatogonial stem cells, as well as maintaining the stem cell pool, differentiate into spermatocytes, which then undergo meiosis and produce haploid daughter spermatids. These in turn undergo huge morphological and biochemical change in the process of spermiogenesis to become mature spermatozoa, which ultimately separate from the adherent Sertoli cells and, once released, passively migrate to the epididymis for further maturation. Central to this system are the Sertoli cells, which in response to endocrine and paracrine stimulation by factors such as FSH and testosterone5,6 provide both paracrine regulation and structural support to the differentiating germ cells. Sertoli cells adhere to germ cells to form a highly complex epithelium, in which various tight and adherent junctions form the blood-testis-barrier and regulate germ cell location and movement toward the lumen during differentiation.7 As secretory cells, Sertoli cells produce growth and anti-apoptotic factors such as Steel (kit-ligand), as well as seminiferous tubule fluid8 with its proteins and other constituents. Sertoli cells are essential to control the diverse environmental niche(s) in which male germ cells develop.
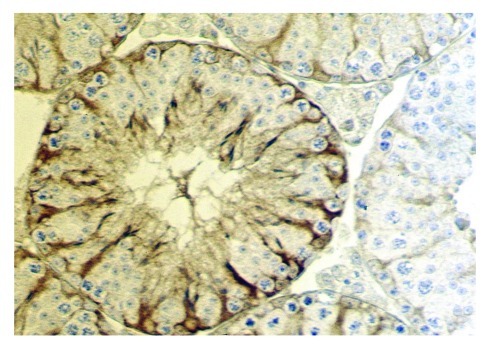
Figure 1. Cross-section of a seminiferous tubule from a mouse testis. Sertoli cells are specifically immunostained for transgenically overexpressed neurophysin.4 This image emphasizes clearly the different compartments (niches) in which Sertoli cells nurture specific groups of germ cells, and how the Sertoli cells effectively determine the architecture of the seminiferous epithelium.
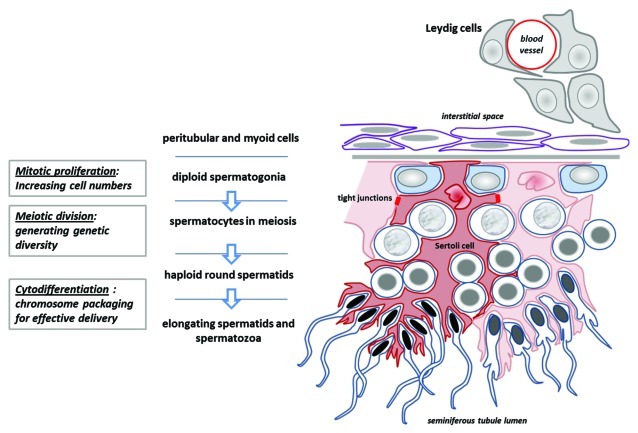
Figure 2. Schematic diagram to illustrate the essential structure of the spermatogenic epithelium, its relation to the Leydig cells and interstitial space, and the manner in which the Sertoli cells determine the architecture of germ cell differentiation, as they progress from the tubule-enclosing basement membrane to the place of mature spermatozoa release in the tubule lumen (below).
In vitro cultures seek to emulate and simplify this resulting environment and in turn reproduce the sequential progression of spermatogonia through spermatogenesis (including meiosis) and spermiogenesis. Fortunately, all of the functions of the testis do not need to be replicated in culture. So for example, the role of the tight junction barrier (blood-testis-barrier) to exclude immune cells from access to the genetically novel haploid germ cells is obviated. Nevertheless, the role of such junctions (also adherens and gap junctions) as mediators of important cell signaling events among Sertoli cells and between Sertoli and germ cells should not be ignored. Also, the lack of interstitial cells (largely Leydig cells) is an advantage since it allows factors derived from such cells to be added or manipulated exogenously.
Amphibian Spermatogenesis
Compared with mammals, amphibian testicular tissue appears to be easier to grow in vitro, growth proceeding from spermatogonia to the development of mid-stage spermatids in a simple medium without addition of hormones or growth factors.9,10 Within hours of culture, spermatocytes complete meiosis I, followed by meiosis II less than a day later.9 Addition of amphibian or mammalian FSH induces longer term survival,11 and increases spermatogonial proliferation12 and differentiation.13 While the process of spermatogenesis appears to be superficially similar in all vertebrates, there are some key anatomical and endocrinological differences which appear to be responsible for the relative ease, compared with mammals, with which amphibian germ cells can be cultured in vitro. Amphibian Sertoli cells encircle a single spermatogonium and resulting daughter cells, forming a cyst in which these cells differentiate. Depending on the species, the cyst ruptures either upon flagellum development or spermatozoan maturation,14 and therefore premeiotic germ cells are not exposed directly to any exogenous growth factors. As amphibian spermatocytes can differentiate in vitro without Sertoli cells present,9,10,13 spermatogenic progression appears to be determined solely by intrinsic factors, with some extrinsic factors (e.g., FSH, prolactin)15 merely modulating this process. Essentially similar results have also been obtained using cells from teleost fish,16-18 though these can still be improved by using a Sertoli cell feeder layer.18 By contrast, extrinsic factors are essential in mammalian systems, and some of the greatest culture difficulties lie in trying to duplicate in vitro the complex paracrine and endocrine environment of the normal testis.
The Significance of Developing Models of in vitro Spermatogenesis
The objectives of developing culture systems which reproduce male germ cell development in culture are various. On the one hand it is to follow the reductionist prerogative to reduce a complex process into its minimal parts in order to examine, manipulate and understand these at a cellular and molecular level. The minimalism of in vitro cultures allows for the manipulation of the paracrine environment and hence the exploration of the roles of individual growth factors in spermatogenesis. Such systems are of great value to study the regulation of sperm phenotype and morphology, as well as specific germ cell gene expression at different stages of differentiation.
But there is also a highly pragmatic reason: namely to develop an in vitro system by which relatively undifferentiated (diploid) germ cells, which might originate from an infertile patient, or which have been experimentally (transgenically) manipulated, can be induced to develop further (particularly through meiosis) to give rise to haploid spermatids or spermatozoa with the capacity to fertilize an oocyte and create a new and healthy organism. While the latter aim can be achieved in part through in vivo experimentation, the in vitro approach allows manipulation at the single cell level and hence, for example, the selection of a single resulting spermatozoon for an ICSI (intracytoplasmic sperm injection) procedure.
A system capable of reliably producing haploid sperm would be of benefit to both researchers and animal ethics. Production of transgenic animals is difficult, requiring both successful parental transgenesis and then breeding offspring expressing the gene. It is typically costly, time consuming, restricted to certain species only, and requires sacrificing many animals in order to guarantee success. Genetic manipulation of male germ cells directly for subsequent use in ICSI should result in guaranteed heterozygous offspring with the transfected gene present in every cell of their body, which can in turn be bred for homozygous offspring.
Finally, a perfected in vitro spermatogenesis culture system would benefit IVF treatment for patients with non-obstructive azoospermia (NOA), for example with Sertoli cell disorders and consequent spermatogenic arrest. In general, IVF treats obstructive fertility disorders by removing physical obstacles to pregnancy, but male germ cells from many NOA patients are either absent, under-developed or malformed, and hence unsuitable for conventional IVF. Transferring germ cells from damaging in vivo microenvironments to controlled in vitro cultures could enable appropriate germ cell maturation and the production of viable spermatozoa, suitable for ICSI.
There is thus a substantial need for in vitro spermatogenesis systems. Attempts to address this need have made use of a range of different approaches. These vary from germ cell monocultures to tissue and tubule cultures; with variations in medium, culture surface and use of feeder cells.
Monocultures
Germ cell monocultures represent an ideal approach for understanding gene expression and the roles of testicular factors in differentiation. Biological systems contain a great deal of redundancy and it is only by removing confounding influences that the roles of individual factors can be fully understood.
The simplest cultures lack hormones and growth factor supplementation, and are short-term by necessity with, after 24 h, more than 50% of cells losing viability.19,20 Such cultures are often used to examine immediate signs of differentiation21,22 or cell suitability for IVF.23 Longer term cultures (weeks to months) have become possible following experimentation with media,24,25 such as varying concentration of fetal calf serum (FCS) and addition of growth factors.24,26,27 For example, the Sertoli cell secreted factor, glial cell derived neurotrophic factor (GDNF), controls spermatogonial self-renewal and differentiation,28,29 and manipulation of its concentration places this control in the researcher’s hands. Spermatogonia in established monocultures and in feeder-germ cell cocultures lacking GDNF cease proliferating but continue differentiating,30 and proliferation cannot be restored through supplementation of other growth factors (e.g., EGF, FGF, LIF).31 High GDNF levels do the reverse, allowing for maintenance of long-term and extremely pure spermatogonial stem cell (SSC) cultures.32
Spermatocytes and spermatids are not sensitive to GDNF levels, as these express different membrane receptors to spermatogonia and are therefore responsive to different factors. Spermatogonia express stem cell specific factors which are not expressed in the more differentiated spermatocytes or spermatids, such as EpCAM,33 Thy-134 and GDNF family receptor α 1 (GFRA1).28,29 Receptors for factors promoting meiotic division are typically only expressed following differentiation, e.g., c-Kit (the receptor for Steel factor), which is expressed in late spermatocytes and round spermatids in humans35; and differentiating spermatogonia and spermatocytes in rodents.36-39 Survival factors likewise appear important at this stage, with suggested factors including ERα, expressed largely in pachytene spermatocytes and elongating spermatids40 and INSL3,41 whose receptor, Rxfp2, is increased 5-fold in haploid compared with tetraploid spermatocytes or diploid spermatids.42 These stage-specific differences are useful differentiation markers and are revealing about differences in cell needs, suggesting that media optimised to promote survival and differentiation of cells at one stage of development may not support later stages. Although growth factors can be supplemented into media, under normal circumstances spermatids are exposed to completely different environments compared with spermatocytes and spermatogonia, largely due to the blood-testis-barrier. Most emphasis recently has been placed on the monoculture of spermatogonia, although there is at least one excellent study on the short-term culture of purified spermatocytes,43 which can be shown to pass through meiosis in the absence of other cell types.
Direct cell-cell contact between germ and feeder cells also appears to be important for normal spermatogenesis and is lacking in germ cell monocultures. Germ cell monocultures have decreased cell viability44 and proliferation31,45 compared with feeder-germ cell co-cultures, one study finding culture doubling-time increasing to 112 h with monocultured germline stem cells compared with 65 h in co-cultures with mouse embryonic fibroblast feeders.46
Feeder Cells
Whereas monocultures facilitate the study of individual growth factors, Sertoli-germ cell co-cultures are useful to examine paracrine influences as a whole, such as the role of testosterone or dihydrotestosterone (DHT), which act via Sertoli cells as germ cell survival factors.47,48 Germ cells themselves lack the androgen receptor (AR).49 The use of Sertoli cell monocultures suggests that testosterone deprivation prevents the generation of the tight-junction constituent Claudin 11,50 transferrin,51 and various other proteins associated with Sertoli cell function. Sertoli-germ cell co-cultures in turn illustrate the resulting effects on germ cells, with Sertoli cell-specific AR-knockout (SCARKO) mice having reduced capacity for germ cell support,52 resulting in spermatogenic arrest.53 Sertoli-germ cell co-cultures also have the advantage of being easier to maintain than monocultures, since as long as the Sertoli cells survive, many growth factors are continually produced within the culture, instead of requiring exogenous supplementation. Accordingly some of the more successful in vitro cultures of the last decades utilized this approach,54-56 which is well suited for tracking gene expression over time57 and producing post-meiotic germ cells for IVF, with haploid germ cells produced from mice,58 neonatal bulls,59 and non-obstructive azoospermia patients.60,61
However, primary adult-type Sertoli cells are sensitive to handling and prone to lysis during removal from seminiferous tubules; separation of cells for culture requires extensive enzymatic digestion, which alters the cell morphology and removes cytoplasmic extensions.45 As a result, in vitro cultured adult-type Sertoli cells may not form correct cell junctions with germ cells at all stages of differentiation.62 An alternative is to use immature Sertoli cells from prepubertal testes, which do not adhere as strongly to the few germ cells present and require less digestion to gain a relatively pure fraction.62–<,64 Prepubertal Sertoli cells are also mitotically active, whereas adult Sertoli cells do not divide in vitro56 and therefore may become depleted, although their longevity can be increased by testosterone and FSH supplementation.63 However, immature Sertoli cells do not express a full adult cell phenotype and therefore, though still able to respond to FSH and androgens, may not express certain growth factors or proteins required for germ cell adherence and survival.
Another alternative is to make use of immortalised cell-lines derived from Sertoli cells such as the mouse TM4, 15P-1 or SK11 cells, all of which express a range of Sertoli cell-specific genes,38,65-68 and therefore are suitable for conditioning media for culture use. 15P-1 cells aggregate with and bind to germ cells in a similar way to normal Sertoli cells,38,69 whereas SK11 cells seem to lack this capacity,68 and so appear to be less suited for studies of germ cell binding, although detailed studies using this cell type have not been performed. While SK11 cells do express the androgen receptor, the follicle stimulating hormone receptor (FSHR), which regulates expression of many Sertoli cell products, appears to be relatively downregulated compared with in vivo Sertoli cells.66 A line of SK11 cells have been transfected to overexpress FSHR.70 This does not appear to resolve the problem, though such cells may be useful for studies of chronic FSH over-exposure such as can occur during spermatogenic failure.
Of the many other cell lines which have been developed as feeder cells, one of the more successful lines used for cultivation of testicular cells are Vero cells; these are reported to produce a conditioned medium which performs better than ordinary medium in spermatozoa maturation cultures,44 an effect which is further improved if FSH and testosterone are supplemented.20,61 Although derived from human kidney, Vero cells have been successfully used to nurture tissue derived from NOA patients, resulting in spermatogenesis71 and round spermatid maturation20,72,73 in culture. It is possible that Vero cells, being of mesonephric origin, are behaving here somewhat like epididymal cells. The degree to which Vero cells mimic Sertoli cell contact is unclear. Although Vero cells appear to maintain spermatozoa motility via cell-cell contact,44 the capacity of Vero cells to bind to male germ cells at other stages of differentiation has not been studied in detail. For spermatogonial stem cell cultures, STO mouse fibroblasts also appear to be sufficient to promote differentiation, probably through their secretion of LIF and Wnt family members.74,75 Bovine embryonic fibroblasts may function in a similar way.76
The choice for optimal feeder cells remains dependent on desired outcomes, and all have specific advantages and disadvantages. Nagano et al. compared colonisation capacity of germ cells cultured with different feeder cells and determined that Sertoli cell derived lines performed poorly in vitro compared with other feeders, encouraging germ cell differentiation over proliferation, and therefore slowing colony growth.77 Vero cells have many advantages but grow optimally at 37°C, whereas spermatogenesis occurs optimally at the scrotal temperature of 32–34°C, and therefore culture temperature is always to the disadvantage of either spermatogenesis or feeder cell maintenance. Sertoli cells are superior for studies requiring a system most representative of the in vivo situation, but in terms of clinical outcomes, e.g., production of haploid germ cells to be used for ICSI, other feeder cell-lines appear superior. If post-meiotic cells are to be used subsequently for fertilization, then the question also arises as to whether the choice of feeder cells could epigenetically influence the health of any offspring. While still premature, a recent study has suggested that porcine fetal Sertoli cells, which are potentially available in large quantities, might indeed be suitable to promote the development of human spermatids.78
Seminiferous Tubule Culture
Testicular explant cultures can vary in size from small tubular fragments,79-83 to sections of the entire testis, including interstitial tissue.84-86 What these have in common is the avoidance of many of the problems associated with mono- or co-culture. First, the testicular paracrine environment is partly or mostly preserved, as every cell type within the seminiferous tubules is present, as are also often cells from the interstitial tissue. It appears to be comparatively straightforward to maintain these systems using relatively simple media.79,87 The culture is inherently capable of supporting all stages of spermatogenesis, and permits simultaneous observation of the entire tissue and spermatogenic cycle.
Second, unless tissue is enzymatically digested, the anatomical support between Sertoli and germ cells remains intact. This in turn nurtures germ cells, prevents breakage of differentiating spermatogonial intercellular bridges, and keeps the culture more closely representative of the in vivo situation. Sertoli cells have been reported to compartmentalise culture chambers and condition each with different levels of transferrin, suggesting functional tight junctions as per the in vivo blood-testis barrier.88 These advantages have been exploited in studies concerned with interactions between endocrine, paracrine and autocrine systems on testicular development.89 Moreover, maintaining different stages of germ cell differentiation together might encourage additional autocrine/paracrine interactions which are mutually beneficial. Very little is known about such interactions, except that microarray studies of purified spermatogonia and spermatocytes (GEO database) emphasize the quite different transcriptional and presumably secretory profile of each cell type.
Maintenance of tubule cultures for more than a few days can be difficult, with inevitable collapse of the tubule lumen and hence altered diffusion kinetics for growth factors and metabolites (see Figs. 3 and 4).83,90 This can lead to a rapid in vitro degeneration of larger tubules, with progressive loss of spermatocytes and spermatids from the first day of culture.90 In combination with steps taken to maintain intratubular structure such as tubular sealing,90 medium flow to cells is disrupted. Cultures of smaller tubule fragments do not experience this problem, as in addition to the smaller area for medium to perfuse, the gross tubular structure often dissolves, leaving only the epithelium with Sertoli-germ cell connections as appropriate to donor age.88 Therefore, although there is still rapid cell death following entry to culture, cell viability quickly plateaus at 70%82 or 90%,88 and spermatogenesis resumes. Cell survival and viability in cultures of larger tubules has increased in recent studies,83 but generally these cultures are relatively short-term (2–3 d).
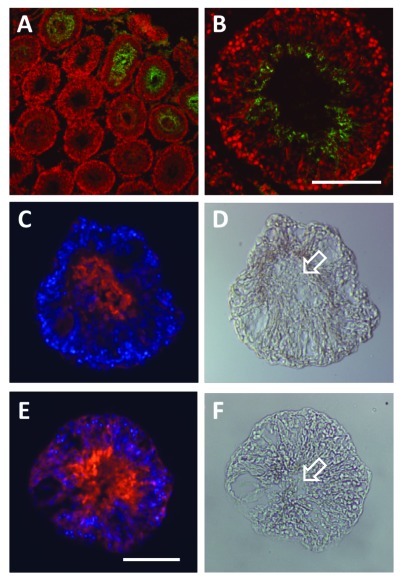
Figure 3. Immunofluorescence microscopy to illustrate the gradual structural deterioration of the seminiferous epithelium in rat seminiferous tubules cultured in vitro for 24 h and 48 h. (A and B) are immunofluorescence images of intact cryosectioned adult rat testis, using the post-meiotic marker Dbil5 (previously endozepine-like peptide, ELP; green fluorophore).91 Sections were counterstained with the nuclei marker TO-PRO-3 Iodide (here red fluorophore). (Scale bar, 50µm). (C and D) represent cryosections of adult rat seminiferous tubules cultured for 24 h, as described,83 using the post-meiotic marker Dbil5 (red fluorophore) counterstained with DAPI (blue fluorophore), to show collapse of the tubule lumen and initial gradual mixing of different cell types within the seminiferous epithelium. (D) represents the phase-contrast image of the section in (C) in direct illumination. (E and F), as in (C and D), here cultured for 48 h. Note that now there is more substantial disruption of the epithelial structure, and the appearance of vacuoles. Nevertheless, the fact that the late spermatid marker Dbil5 is still quantitatively expressed in these tubules, shows that there has been no substantial cell death (also shown using apoptotic markers, not shown) and loss of sensitive late germ cell stages, even though there has been loss of lumen (arrows).
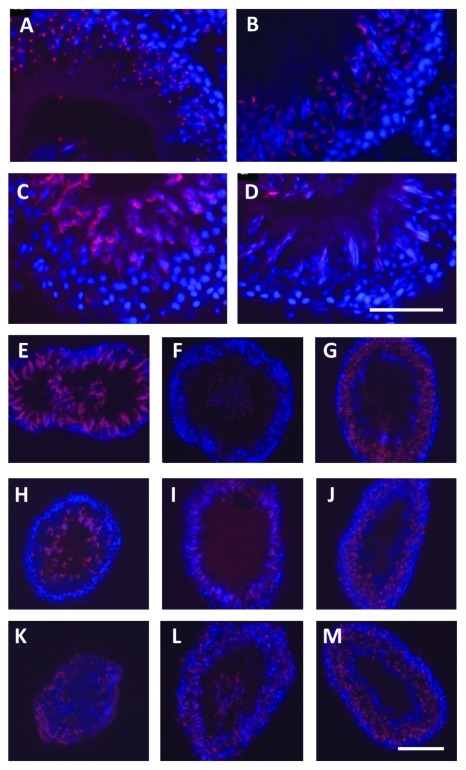
Figure 4. Intact adult rat testis (A-D) and in vitro cultured seminiferous tubules from adult rats (E-M), immunofluorescently labeled using the acrosome-specific lectin peanut agglutinin (PNA; red fluorophore) counterstained using the nuclear fluorophore DAPI (blue). (A) spermatogenic stage II-III; (B) spematogenic stage V-VI; (C) spermatogenic stage X-XI; (D) spermatogenic stage XIII-XIV. Note that the PNA highlights clearly the morphologically diverse phenotypes of the differentiating spermatids and the normal organization of the seminiferous epithelium. E-M: Cryosections from tubules cultured in vitro for 2 h (E-G), 24 h (H-J), and 48 h (K-M), as described.83 (E, H and K) represent so-called ‘pale’ sections of tubules, equivalent to spermatogenic stages IX to XIII; (F, I and L) correspond to ‘spot’, stages XIV to IV; (G, J and M) correspond to ‘dark’, stages VI to VIII. Note here that while there has been a degree of disruption to the organization of the seminiferous epithelium, this is not severe, although there is still loss of cells to the lumen and some disorganization of the epithelial structure.
Experimental manipulation of tubules is also more difficult than for conventional cell cultures. Danner and colleagues83 developed a procedure for introducing DNA constructs into cells within intact seminiferous tubules in vitro. This procedure involved first microinjection of DNA into the tubule lumen, followed by square-wave electroporation. Tubules were then cultivated for up to 48 h to assess expression from the new DNA. The aim was to analyze promoter regions for genes expressed only in post-meiotic haploid germ cells, since no appropriate cell lines exist for this purpose. While total transfection as assessed by expression of green fluorescent protein could be quite high (up to 80%), transfection efficiency varied considerably depending upon the tubular stage of spermatogenesis. Then transfection only occurred on one side of the tubule, with all cell types, including Sertoli cells being transfected, making cell sorting necessary, if a cell-specific response was required. The most important finding from this study, however, was that supposedly cell-type specific gene promoters, based on work in transgenic animals, appeared to lose this specificity when expressed as described in these in vitro tubule cultures, suggesting that in vivo expression of a transgene somehow introduced a more specific epigenetic environment than could be attained within the short term context of these in vitro transfections.
Spermatogenesis and spermiogenesis have continued in tubule cultures grown in media designed to be specifically favorable to Sertoli cells, although success varied dependent on the spermatogenic stage of the tubule.80,81 Most experiments concerned with medium optimisation for tubule cultures have been concerned with production of haploid germ cells suitable for IVF. These studies rarely lasted beyond 2492 or 4893 hours and therefore were less concerned with survival and morphological status of somatic cells, instead concentrating on acute effects of hormones on spermatogenesis and spermiogenesis rates.48
Gohbara and colleagues85 have recently repeated some of the longer-term 1960s testis culture experiments using more modern technology and examined effects of varying medium, oxygen concentrations and temperature. Other studies have examined the roles of adding growth factors on tubule health and morphology in vitro, for example determining that addition of nerve growth factor in combination with FCS helps restore in vivo morphology to cultured Sertoli cells.90
However, tubule cultures have proven extremely useful for IVF with NOA patients. In addition to spermatogenesis occurring, spermatids already within tubules from biopsies matured to produce elongating spermatids.48 However, this system appears less efficient than healthy in vivo spermatogenesis, with the ratio of differentiation to apoptosis apparently decreasing in vitro, as suggested by high rates of diploid and tetraploid cell death in tubules cultures from healthy rats.82-85,88
Explant Culture of Testicular Fragments
The full potential of explant cultures is only now being realized. Few such studies have addressed the optimization of media as thoroughly as for mono- and Sertoli-germ cell co-cultures, in which medium, hormone and growth factor supplementation is vital for cell survival. The distinction here is provided by the exciting new report by Sato and colleagues who used neonatal mouse testis fragments and have achieved fully functional spermatozoa starting from spermatogonia.3 However, all the constituents of the testicular paracrine system, including androgen-producing Leydig cells, are present within explant cultures and can sustain germ cell proliferation in quite basic media, such as Dulbecco’s Modified Eagle Medium (DMEM) supplemented only with FCS and antibiotics.87
Until recently,3,85 organ culture experiments have had limited success inducing spermatogenesis beyond pachytene spermatocytes.84 Besides the recent work of Sato and colleagues, using pieces of neonatal mouse testis,3 the greatest spermatogenic success has occurred in smaller, mechanically processed fragments, such as the NOA testicular biopsy samples used by Tesarik and colleagues.92 These were mechanically disintegrated, separating samples into more permeable germ and Sertoli cell aggregates,92,93 and showed more advanced spermatogenesis following culture than samples enzymatically digested.92 Resulting spermatids have given rise to apparently healthy offspring produced via ICSI.94
Limitations of the Culture Environment
Despite the multiple approaches available, it has not yet been possible to use pre-meiotic germ cells to produce viable, free-swimming, morphologically normal spermatozoa. Various systems appear to support spermatogenesis to the round spermatid stage, or spermiogenesis of immature spermatids already present in excised tissue, but never the full process. Underpinning this is that two different and apparently incompatible endpoints are being sought: the capacity to produce functional spermatozoa, occurring most successfully in tubule cultures; and the capacity to understand the process by which spermatozoa are generated, where most success occurs in mono- and co-culture systems, in which the spermatogenic process is impaired but more readily observable.
Cells at different stages of spermatogenesis appear to have different culture requirements, with no one culture condition permitting the entire process, while still allowing for study of paracrine and/or autocrine activity. Moreover, the roles of the extracellular matrix and other paracrine factors, such as those secreted by peritubular and Leydig cells, remain neglected. For example, it is known that there are peritubular myoid cell secreted factors (PMoDs) that modulate the effects of testosterone on Sertoli cells, in turn altering the secretion of transferrin and inhibin.95,96 The identity of these factors is still unknown, though they are likely to be important regulators of spermatogenesis in vivo.
One variable which has been studied in detail is the role of FCS in medium, although its presence clouds the interpretation of effects from experimentally manipulated factors, and makes comparisons with serum-free cultures and in vivo observations very difficult. Its use continues because male germ cells often appear to grow better in monocultures,97 feeder-germ cell co-cultures,98 and tubule cultures85,90 with FCS than without, in terms of colonisation capability and proliferation. Note that this does not necessarily translate to greater cell viability.26 The specific benefits bestowed by FCS are unknown, as even in serum-free cultures, cell viability varies dependent on age and origin of cells.26 FCS contains a complex mixture of factors and hormones which vary between batches due to donor sex and age. Batch variations alone make FCS-containing cultures hard to consider as experimentally controlled and are quite difficult to replicate. Comparisons between serum-free and FCS-containing cultures indicate that the entire composition and morphology of the cultures differ,26 meaning that FCS definitely does more than simply induce cell proliferation. Kanatsu-Shinohara et al. have observed that effects of FCS on germline stem cells vary depending on whether it is a freshly establishing culture, in which FCS encouraged colony establishment, or an established co-culture of germline stem cells and feeder cells, in which FSC slowed the proliferation rate.31 The need for a standardised substitute has been in demand for some time, and assuming one is found, it remains clear that further optimisation of media is needed to cater for differences in age and differentiation status in donor samples.
Endpoints and Epigenetics
If IVF and the production of healthy offspring is the desired outcome, then the present situation is still far from ideal. While round spermatids can be generated from pre-meiotic germ cells, such haploid cells may still not be suitable for forming a zygote, even using ICSI. The haploid male gamete brings more to the forming zygote than simply a copy of the male genome. It also brings epigenetic information in the form of male imprinted genes, as well as RNA, and more recently potentially active heterochromatin.99,100 While the male imprint in terms of DNA methylation is probably already established before meiosis, both sperm RNA and the small percentage of non-protamine containing active heterochromatin are probably determined during spermatogenesis and may only be fully matured after the sperm have left the testes. It is thus probably too simplistic to assume that because the protamine-containing sperm DNA is globally demethylated (except for imprinted genes) soon after fertilization, any epigenetic modifications are of no consequence. In fact, new studies show that the small amount of histone-based heterochromatin transported with the mature spermatozoa to the oocyte may be important for establishing the first spectrum of early development genes within the embryo before the embryonic genome is activated,99 thus paving the way for correct early embryonic differentiation. Even imprinting disorders such as Angelman and Beckwith-Wiedermann syndromes have been linked to assisted reproductive technology (ART), with higher frequencies in offspring born following ART compared with the rest of the population.101 This remains controversial102 and difficult to verify due to the rareness of these conditions in the general population (roughly 1 in 15000).103 Moreover, association between ART and imprinting disorders does not imply causality104; these disorders could be the result of or even the cause of the parental infertility. Indeed, several recent studies have linked male infertility with altered methylation of key male fertility genes.105 The use of immature spermatids for ICSI94 is thus doubly problematic. On the one hand, natural epigenetic maturation may not yet have occurred and, on the other, such spermatids derive anyway from pathological tissues. Also, such spermatid maturation cultures contain disproportionately large numbers of spermatids with abnormal morphology, such as extruding nuclei, a lack of nuclear condensation, and abnormal flagellar development.93 Furthermore, even assuming good quality spermatids are selected, these immature sperm lack the condensed DNA of mature spermatozoa. This in turn might influence the rate of global demethylation within the zygote, which appears to be driven at least in part by the process of chromatin reorganization itself.106 There is thus still a substantial need for more research before we can assume that in vitro spermatogenesis to produce haploid gametes for infertile couples can be considered both safe and effective.
Novel Developments and Strategies
There are a growing number of alternatives currently being explored to help improve in vitro spermatogenesis culture systems. One of the most important of these has been to try and substitute FCS in the medium. Kanatsu-Shinohara and colleagues107 successfully cultured SSCs without FCS by supplementing to the medium 1mg/ml fetuin, the major protein constituent of FCS; 3mg/ml lipid-rich bovine serum albumin (BSA); and a mixture of lipids, lipoproteins and cholesterol. Fetuin supplemented media induced cell binding to laminin coated plates and resulting colonies yielded germline stem cells of the same morphology and gene expression as control colonies cultured in 1% FCS medium. Following grafting and differentiation in a donor mouse, resulting spermatozoa were healthy, functional, and capable of fertilisation. The system is not truly simplified yet - as Nagano108 points out, the StemPro medium used is a complex medium already containing a mixture of growth factors and steroid hormones, and as a proprietary medium may also contain undisclosed components. It is also not perfect - cell proliferation still remained faster in 1% FCS medium (4-fold amplification compared with 3-fold amplification with lipid, BSA and fetuin substituted medium); and concentration of SSCs within the culture remained lower than FCS-cultured controls. Nevertheless, this offers the possibility of a monoculture system in which most variables can be regulated and controlled, circumstances ideal for examining individual paracrine and autocrine factors, particularly if in future some of the newer animal free media substitutes can be used.
Other improvements have aimed to create cultures more like the in vivo situation, increasing rates of cell survival, proliferation and differentiation. Alternative two-dimensional systems have been tested, such as paraffin submerged microdrop cultures, which ensure greater culture stability in terms of pH, temperature and evaporation.109 These also place cells under pressure, for in vivo the tunica albuginea keeps the testes and its tissue under constant pressure. Other systems have concentrated on exploring the role of cell oxygenation and improved liquid gas interfaces by culturing cells on top of partially submerged agar.85 Additional studies have focused on the role of testicular anatomy, and have resulted in Sertoli-germ cell co-cultures and tubule fragments grown on or inside substrates resembling the extracellular matrix and basement membrane surrounding the seminiferous tubules. Such studies have included laminin,110-112 matrigel,39,55 agar,39,85 bioactive polymers,113 and collagen114,115 gels. Sertoli cells, in particular, cultured on a mixture of laminin and type I collagen display polarity and gene expression more strongly resembling the in vivo situation than those cultured on plastic.110 Sertoli cell monocultures even have been observed to form cords and tubes when cultured on or in laminin111 and matrigel,112 as have SF7 cells,39 a Sertoli cell derived cell-line.
Culture in a three-dimensional collagen gel improves viability and differentiation rates of rat testicular tissue compared with those grown in traditional flat cultures, and is further improved by addition of matrigel as a gel component.114 This system has successfully induced meiotic divisions in tissue originating from NOA patients, with rates varying in proportion to patient FSH serum levels.115 Cultures within poly(D,L-lactic-co-glycolic acid)-based porous scaffolds likewise show improved rates of germ cell viability and proliferation, and maintained these over time, compared with those in two-dimensional cultures.113 The main disadvantage to this system is the extra mechanical and enzymatic processing that needs to be undertaken to extract cultured tissue or cells from the substrate before use.
A further interesting alternative to conventional in vitro culture is that of xenografting into immuno-compromised mice, in which the host essentially acts as both incubator and medium. Methods include retrograde injection of germ cells into germ cell stripped tubules, and grafting of entire pieces of testicular tissue into the host body. Successful spermatogenesis has been recorded from testis grafts from rats,116 mice, cattle,87 cats117 and humans. This system may be appropriate for producing germ cells for IVF, or for extracting germ cells from an environment which may be toxic or lacking in required hormones. Experimentally, such systems do not permit tight control over the factors to which the germ cells are exposed, but do provide an excellent means of testing germ cell viability and proliferative status after culture,25,109 success being measured by tubule recolonization. Another promising application is in the evaluation of specific gene ablation: for example, testes from wild type and connexin 43 knockout mice have been transplanted into nude mice to assess the role of this protein in normal spermatogenesis.118
Frontiers and Conclusions
There are many options being explored at present, and innovative new techniques are being created and tested. Further research into medium, substrates and extra-tubular paracrine factors may hold the key toward improvement of all of the various culture systems currently being utilized.
The differing goals in research—namely inducing spermatogenesis and understanding it—may only strengthen the reproductive field. It has led to a diversity of techniques being developed and a wealth of information that can be utilized by those interested in both aims. As both the clinical and research communities have vested interests in developing systems of in vitro spermatogenesis, and with this flow of information between the two, it remains only a matter of time before it becomes a reality.
Acknowledgments
We should like to thank the many colleagues in Australia and Germany who have contributed to our thoughts here with advice and suggestions. We are also grateful to the German Research Council (DFG), to the Australian National Health and Medical Research Council (NHMRC; grant APP1009243), and to the University of Adelaide scholarship scheme for financially supporting much of the work that has provided the science to support this review.
Disclosure of Potential Conflicts of Interest
The authors do not have any conflicts of interest to disclose.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/19383
References
- 1.Gilchrist RB, Thompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology. 2007;67:6–15. doi: 10.1016/j.theriogenology.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Martinovitch PN. Development in vitro of the mammalian gonad. Nature. 1937;139:413. doi: 10.1038/139413a0. [DOI] [Google Scholar]
- 3.Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–7. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- 4.Ang HL, Ivell R, Walther N, Nicholson H, Ungefroren H, Millar M, et al. Over-expression of oxytocin in the testes of a transgenic mouse model. J Endocrinol. 1994;140:53–62. doi: 10.1677/joe.0.1400053. [DOI] [PubMed] [Google Scholar]
- 5.Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- 6.Petersen C, Söder O. The sertoli cell—a hormonal target and ‘super’ nurse for germ cells that determines testicular size. Horm Res. 2006;66:153–61. doi: 10.1159/000094142. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–74. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 8.Rato L, Socorro S, Cavaco JE, Oliveira PF. Tubular fluid secretion in the seminiferous epithelium: ion transporters and aquaporins in Sertoli cells. J Membr Biol. 2010;236:215–24. doi: 10.1007/s00232-010-9294-x. [DOI] [PubMed] [Google Scholar]
- 9.Abé S, Asakura S, Ukeshima A. Formation of flagella during interphase in secondary spermatocytes from Xenopus laevis in vitro. J Exp Zool. 1988;246:65–70. doi: 10.1002/jez.1402460109. [DOI] [PubMed] [Google Scholar]
- 10.Abé S, Hiyoshi H. Synthesis of sperm-specific basic nuclear proteins (SPs) in cultured spermatids from Xenopus laevis. Exp Cell Res. 1991;194:90–4. doi: 10.1016/0014-4827(91)90134-G. [DOI] [PubMed] [Google Scholar]
- 11.Yazawa T, Yamamoto T, Jin Y, Abé S. Follicle-stimulating hormone is indispensable for the last spermatogonial mitosis preceding meiosis initiation in newts (Cynops pyrrhogaster) Biol Reprod. 2002;66:14–20. doi: 10.1095/biolreprod66.1.14. [DOI] [PubMed] [Google Scholar]
- 12.Maekawa K, Ji ZS, Abé S. Proliferation of newt spermatogonia by mammalian FSH via Sertoli cells in vitro. J Exp Zool. 1995;272:363–73. doi: 10.1002/jez.1402720506. [DOI] [PubMed] [Google Scholar]
- 13.Ji ZS, Kubokawa K, Abé S. Promotion of differentiation of newt primary spermatocytes into spermatids by mammalian FSH via Sertoli cells. J Exp Zool. 1995;272:374–83. doi: 10.1002/jez.1402720507. [DOI] [PubMed] [Google Scholar]
- 14.Abé S. Hormonal control of meiosis initiation in the testis from Japanese newt, Cynops pyrrhogaster. Zoolog Sci. 2004;21:691–704. doi: 10.2108/zsj.21.691. [DOI] [PubMed] [Google Scholar]
- 15.Yazawa T, Yamamoto K, Kikuyama S, Abé SI. Elevation of plasma prolactin concentrations by low temperature is the cause of spermatogonial cell death in the newt, Cynops pyrrhogaster. Gen Comp Endocrinol. 1999;113:302–11. doi: 10.1006/gcen.1998.7207. [DOI] [PubMed] [Google Scholar]
- 16.Saiki A, Tamura M, Matsumoto M, Katowgi J, Watanabe A, Onitake K. Establishment of in vitro spermatogenesis from spermatocytes in the medaka, Oryzias latipes. Dev Growth Differ. 1997;39:337–44. doi: 10.1046/j.1440-169X.1997.t01-2-00009.x. [DOI] [PubMed] [Google Scholar]
- 17.Miura T, Ando N, Miura C, Yamauchi K. Comparative studies between in vivo and in vitro spermatogenesis of Japanese eel (Anguilla japonica) Zoolog Sci. 2002;19:321–9. doi: 10.2108/zsj.19.321. [DOI] [PubMed] [Google Scholar]
- 18.Sakai N. In vitro male germ cell cultures of zebrafish. Methods. 2006;39:239–45. doi: 10.1016/j.ymeth.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Aslam I, Fishel S. Short-term in-vitro culture and cryopreservation of spermatogenic cells used for human in-vitro conception. Hum Reprod. 1998;13:634–8. doi: 10.1093/humrep/13.3.634. [DOI] [PubMed] [Google Scholar]
- 20.Movahedin M, Ajeen A, Ghorbanzadeh N, Tiraihi T, Valojerdi MR, Kazemnejad A. In vitro maturation of fresh and frozen-thawed mouse round spermatids. Andrologia. 2004;36:269–76. doi: 10.1111/j.1439-0272.2004.00617.x. [DOI] [PubMed] [Google Scholar]
- 21.Gerton GL, Millette CF. Generation of flagella by cultured mouse spermatids. J Cell Biol. 1984;98:619–28. doi: 10.1083/jcb.98.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi MS, Anakwe OO, Gerton GL. Preparation and short-term culture of enriched populations of guinea pig spermatocytes and spermatids. J Androl. 1990;11:120–30. [PubMed] [Google Scholar]
- 23.Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R, et al. In-vitro culture of spermatozoa induces motility and increases implantation and pregnancy rates after testicular sperm extraction and intracytoplasmic sperm injection. Hum Reprod. 1999;14:2808–11. doi: 10.1093/humrep/14.11.2808. [DOI] [PubMed] [Google Scholar]
- 24.Dirami G, Ravindranath N, Pursel V, Dym M. Effects of stem cell factor and granulocyte macrophage-colony stimulating factor on survival of porcine type A spermatogonia cultured in KSOM. Biol Reprod. 1999;61:225–30. doi: 10.1095/biolreprod61.1.225. [DOI] [PubMed] [Google Scholar]
- 25.Jeong D, McLean DJ, Griswold MD. Long-term culture and transplantation of murine testicular germ cells. J Androl. 2003;24:661–9. doi: 10.1002/j.1939-4640.2003.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 26.Creemers LB, den Ouden K, van Pelt AM, de Rooij DG. Maintenance of adult mouse type A spermatogonia in vitro: influence of serum and growth factors and comparison with prepubertal spermatogonial cell culture. Reproduction. 2002;124:791–9. doi: 10.1530/rep.0.1240791. [DOI] [PubMed] [Google Scholar]
- 27.Yan W, Suominen J, Toppari J. Stem cell factor protects germ cells from apoptosis in vitro. J Cell Sci. 2000;113:161–8. doi: 10.1242/jcs.113.1.161. [DOI] [PubMed] [Google Scholar]
- 28.Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–93. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–7. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–94. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, et al. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985–91. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 32.Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, Testart J, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–6. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 33.Anderson R, Schaible K, Heasman J, Wylie C. Expression of the homophilic adhesion molecule, Ep-CAM, in the mammalian germ line. J Reprod Fertil. 1999;116:379–84. doi: 10.1530/jrf.0.1160379. [DOI] [PubMed] [Google Scholar]
- 34.Ryu BY, Orwig KE, Kubota H, Avarbock MR, Brinster RL. Phenotypic and functional characteristics of spermatogonial stem cells in rats. Dev Biol. 2004;274:158–70. doi: 10.1016/j.ydbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 35.He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363–72. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140:5894–900. doi: 10.1210/en.140.12.5894. [DOI] [PubMed] [Google Scholar]
- 37.Prabhu SM, Meistrich ML, McLaughlin EA, Roman SD, Warne S, Mendis S, et al. Expression of c-Kit receptor mRNA and protein in the developing, adult and irradiated rodent testis. Reproduction. 2006;131:489–99. doi: 10.1530/rep.1.00968. [DOI] [PubMed] [Google Scholar]
- 38.Vincent S, Segretain D, Nishikawa S, Nishikawa SI, Sage J, Cuzin F, et al. Stage-specific expression of the Kit receptor and its ligand (KL) during male gametogenesis in the mouse: a Kit-KL interaction critical for meiosis. Development. 1998;125:4585–93. doi: 10.1242/dev.125.22.4585. [DOI] [PubMed] [Google Scholar]
- 39.van der Wee K, Hofmann MC. An in vitro tubule assay identifies HGF as a morphogen for the formation of seminiferous tubules in the postnatal mouse testis. Exp Cell Res. 1999;252:175–85. doi: 10.1006/excr.1999.4630. [DOI] [PubMed] [Google Scholar]
- 40.Pentikäinen V, Erkkilä K, Suomalainen L, Parvinen M, Dunkel L. Estradiol acts as a germ cell survival factor in the human testis in vitro. J Clin Endocrinol Metab. 2000;85:2057–67. doi: 10.1210/jc.85.5.2057. [DOI] [PubMed] [Google Scholar]
- 41.Kawamura K, Kumagai J, Sudo S, Chun SY, Pisarska M, Morita H, et al. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci U S A. 2004;101:7323–8. doi: 10.1073/pnas.0307061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anand-Ivell RJK, Relan V, Balvers M, Coiffec-Dorval I, Fritsch M, Bathgate RA, et al. Expression of the insulin-like peptide 3 (INSL3) hormone-receptor (LGR8) system in the testis. Biol Reprod. 2006;74:945–53. doi: 10.1095/biolreprod.105.048165. [DOI] [PubMed] [Google Scholar]
- 43.La Salle S, Sun F, Handel MA. Isolation and short-term culture of mouse spermatocytes for analysis of meiosis. Methods Mol Biol. 2009;558:279–97. doi: 10.1007/978-1-60761-103-5_17. [DOI] [PubMed] [Google Scholar]
- 44.Chen HF, Ho HN, Chen SU, Lien YR, Chao KH, Lin HR, et al. Co-culture with Vero cell monolayer maintains the motility of asthenozoospermic semen samples. Hum Reprod. 1994;9:1276–80. doi: 10.1093/oxfordjournals.humrep.a138694. [DOI] [PubMed] [Google Scholar]
- 45.Koruji M, Movahedin M, Mowla SJ, Gourabi H, Arfaee AJ. Efficiency of adult mouse spermatogonial stem cell colony formation under several culture conditions. In Vitro Cellular and Development Biology - Animal 45: 281–289. [DOI] [PubMed] [Google Scholar]
- 46.Kanatsu-Shinohara M, Inoue K, Lee J, Miki H, Ogonuki N, Toyokuni S, et al. Anchorage-independent growth of mouse male germline stem cells in vitro. Biol Reprod. 2006;74:522–9. doi: 10.1095/biolreprod.105.046441. [DOI] [PubMed] [Google Scholar]
- 47.Erkkilä K, Henriksén K, Hirvonen V, Rannikko S, Salo J, Parvinen M, et al. Testosterone regulates apoptosis in adult human seminiferous tubules in vitro. J Clin Endocrinol Metab. 1997;82:2314–21. doi: 10.1210/jc.82.7.2314. [DOI] [PubMed] [Google Scholar]
- 48.Tesarik J, Guido M, Mendoza C, Greco E. Human spermatogenesis in vitro: respective effects of follicle-stimulating hormone and testosterone on meiosis, spermiogenesis, and Sertoli cell apoptosis. J Clin Endocrinol Metab. 1998;83:4467–73. doi: 10.1210/jc.83.12.4467. [DOI] [PubMed] [Google Scholar]
- 49.Shan LX, Bardin CW, Hardy MP. Immunohistochemical analysis of androgen effects on androgen receptor expression in developing Leydig and Sertoli cells. Endocrinology. 1997;138:1259–66. doi: 10.1210/en.138.3.1259. [DOI] [PubMed] [Google Scholar]
- 50.Kaitu’u-Lino TJ, Sluka P, Foo CF, Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction. 2007;133:1169–79. doi: 10.1530/REP-06-0385. [DOI] [PubMed] [Google Scholar]
- 51.Sylvester SR, Griswold MD. The testicular iron shuttle: a “nurse” function of the Sertoli cells. J Androl. 1994;15:381–5. [PubMed] [Google Scholar]
- 52.Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, et al. The role of androgens in sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology. 2005;146:2674–83. doi: 10.1210/en.2004-1630. [DOI] [PubMed] [Google Scholar]
- 53.De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–32. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tres LL, Kierszenbaum AL. Viability of rat spermatogenic cells in vitro is facilitated by their coculture with Sertoli cells in serum-free hormone-supplemented medium. Proc Natl Acad Sci U S A. 1983;80:3377–81. doi: 10.1073/pnas.80.11.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hadley MA, Byers SW, Súrez-Quian CA, Kleinman HK, Dym M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J Cell Biol. 1985;101:1511–22. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Magueresse-Battistoni B, Gérard N, Jégou B. Pachytene spermatocytes can achieve meiotic process in vitro. Biochem Biophys Res Commun. 1991;179:1115–21. doi: 10.1016/0006-291X(91)91935-6. [DOI] [PubMed] [Google Scholar]
- 57.Smith FF, Tres LL, Kierszenbaum AL. Expression of testis-specific histone genes during the development of rat spermatogenic cells in vitro. Dev Dyn. 1992;193:49–57. doi: 10.1002/aja.1001930108. [DOI] [PubMed] [Google Scholar]
- 58.Marh J, Tres LL, Yamazaki Y, Yanagimachi R, Kierszenbaum AL. Mouse round spermatids developed in vitro from preexisting spermatocytes can produce normal offspring by nuclear injection into in vivo-developed mature oocytes. Biol Reprod. 2003;69:169–76. doi: 10.1095/biolreprod.102.015099. [DOI] [PubMed] [Google Scholar]
- 59.Lee DR, Kaproth MT, Parks JE. In vitro production of haploid germ cells from fresh or frozen-thawed testicular cells of neonatal bulls. Biol Reprod. 2001;65:873–8. doi: 10.1095/biolreprod65.3.873. [DOI] [PubMed] [Google Scholar]
- 60.Lee DR, Kim KS, Yang YH, Oh HS, Lee SH, Chung TG, et al. Isolation of male germ stem cell-like cells from testicular tissue of non-obstructive azoospermic patients and differentiation into haploid male germ cells in vitro. Hum Reprod. 2006;21:471–6. doi: 10.1093/humrep/dei319. [DOI] [PubMed] [Google Scholar]
- 61.Sousa M, Cremades N, Alves C, Silva J, Barros A. Developmental potential of human spermatogenic cells co-cultured with Sertoli cells. Hum Reprod. 2002;17:161–72. doi: 10.1093/humrep/17.1.161. [DOI] [PubMed] [Google Scholar]
- 62.Ś R, Neves R, Fernandes S, Alves C, Carvalho F, Silva J, et al. Cytological and expression studies and quantitative analysis of the temporal and stage-specific effects of follicle-stimulating hormone and testosterone during cocultures of the normal human seminiferous epithelium. Biol Reprod. 2008;79:962–75. doi: 10.1095/biolreprod.107.067546. [DOI] [PubMed] [Google Scholar]
- 63.Dorrington JH, Roller NF, Fritz IB. Effects of follicle-stimulating hormone on cultures of Sertoli cell preparations. Mol Cell Endocrinol. 1975;3:57–70. doi: 10.1016/0303-7207(75)90031-3. [DOI] [PubMed] [Google Scholar]
- 64.Bucci LR, Brock WA, Johnson TS, Meistrich ML. Isolation and biochemical studies of enriched populations of spermatogonia and early primary spermatocytes from rat testes. Biol Reprod. 1986;34:195–206. doi: 10.1095/biolreprod34.1.195. [DOI] [PubMed] [Google Scholar]
- 65.Mather JP. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol Reprod. 1980;23:243–52. doi: 10.1095/biolreprod23.1.243. [DOI] [PubMed] [Google Scholar]
- 66.Walther N, Jansen M, Ergün S, Kascheike B, Ivell R. Sertoli cell lines established from H-2Kb-tsA58 transgenic mice differentially regulate the expression of cell-specific genes. Exp Cell Res. 1996;225:411–21. doi: 10.1006/excr.1996.0192. [DOI] [PubMed] [Google Scholar]
- 67.Sneddon SF, Walther N, Saunders PT. Expression of androgen and estrogen receptors in sertoli cells: studies using the mouse SK11 cell line. Endocrinology. 2005;146:5304–12. doi: 10.1210/en.2005-0914. [DOI] [PubMed] [Google Scholar]
- 68.Wolski KM, Feig C, Kirchhoff C, Cameron DF. Immortalized Sertoli cell lines sk11 and sk9 and binding of spermatids in vitro. Asian J Androl. 2007;9:312–20. doi: 10.1111/j.1745-7262.2007.00256.x. [DOI] [PubMed] [Google Scholar]
- 69.Rassoulzadegan M, Paquis-Flucklinger V, Bertino B, Sage J, Jasin M, Miyagawa K, et al. Transmeiotic differentiation of male germ cells in culture. Cell. 1993;75:997–1006. doi: 10.1016/0092-8674(93)90543-Y. [DOI] [PubMed] [Google Scholar]
- 70.Strothmann K, Simoni M, Mathur P, Siakhamary S, Nieschlag E, Gromoll J. Gene expression profiling of mouse Sertoli cell lines. Cell Tissue Res. 2004;315:249–57. doi: 10.1007/s00441-003-0834-x. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka A, Nagayoshi M, Awata S, Mawatari Y, Tanaka I, Kusunoki H. Completion of meiosis in human primary spermatocytes through in vitro coculture with Vero cells. Fertil Steril. 2003;79(Suppl 1):795–801. doi: 10.1016/S0015-0282(02)04833-1. [DOI] [PubMed] [Google Scholar]
- 72.Cremades N, Bernabeu R, Barros A, Sousa M. In-vitro maturation of round spermatids using co-culture on Vero cells. Hum Reprod. 1999;14:1287–93. doi: 10.1093/humrep/14.5.1287. [DOI] [PubMed] [Google Scholar]
- 73.Cremades N, Sousa M, Bernabeu R, Barros A. Developmental potential of elongating and elongated spermatids obtained after in-vitro maturation of isolated round spermatids. Hum Reprod. 2001;16:1938–44. doi: 10.1093/humrep/16.9.1938. [DOI] [PubMed] [Google Scholar]
- 74.Matsui Y, Toksoz D, Nishikawa S, Nishikawa S, Williams D, Zsebo K, et al. Effect of Steel factor and leukaemia inhibitory factor on murine primordial germ cells in culture. Nature. 1991;353:750–2. doi: 10.1038/353750a0. [DOI] [PubMed] [Google Scholar]
- 75.Yeh JR, Zhang X, Nagano MC. Wnt5a is a cell-extrinsic factor that supports self-renewal of mouse spermatogonial stem cells. J Cell Sci. 2011;124:2357–66. doi: 10.1242/jcs.080903. [DOI] [PubMed] [Google Scholar]
- 76.Oatley JM, Reeves JJ, McLean DJ. Biological activity of cryopreserved bovine spermatogonial stem cells during in vitro culture. Biol Reprod. 2004;71:942–7. doi: 10.1095/biolreprod.104.028894. [DOI] [PubMed] [Google Scholar]
- 77.Nagano M, Ryu BY, Brinster CJ, Avarbock MR, Brinster RL. Maintenance of mouse male germ line stem cells in vitro. Biol Reprod. 2003;68:2207–14. doi: 10.1095/biolreprod.102.014050. [DOI] [PubMed] [Google Scholar]
- 78.Menegazzo M, Zuccarello D, Luca G, Ferlin A, Calvitti M, Mancuso F, et al. Improvements in human sperm quality by long-term in vitro co-culture with isolated porcine Sertoli cells. Hum Reprod. 2011;26:2598–605. doi: 10.1093/humrep/der248. [DOI] [PubMed] [Google Scholar]
- 79.Toppari J, Brown WR, Parvinen M. Rat spermatogenesis in vitro traced by live cell squashes and monoclonal antibodies. Ann N Y Acad Sci. 1984;438:515–8. doi: 10.1111/j.1749-6632.1984.tb38321.x. [DOI] [PubMed] [Google Scholar]
- 80.Toppari J, Parvinen M. In vitro differentiation of rat seminiferous tubular segments from defined stages of the epithelial cycle morphologic and immunolocalization analysis. J Androl. 1985;6:334–43. doi: 10.1002/j.1939-4640.1985.tb03289.x. [DOI] [PubMed] [Google Scholar]
- 81.Toppari J, Mali P, Eerola E. Rat spermatogenesis in vitro traced by quantitative flow cytometry. J Histochem Cytochem. 1986;34:1029–35. doi: 10.1177/34.8.3734416. [DOI] [PubMed] [Google Scholar]
- 82.Hue D, Staub C, Perrard-Sapori MH, Weiss M, Nicolle JC, Vigier M, et al. Meiotic differentiation of germinal cells in three-week cultures of whole cell population from rat seminiferous tubules. Biol Reprod. 1998;59:379–87. doi: 10.1095/biolreprod59.2.379. [DOI] [PubMed] [Google Scholar]
- 83.Danner S, Kirchhoff C, Ivell R. Seminiferous tubule transfection in vitro to define post-meiotic gene regulation. Reprod Biol Endocrinol. 2009;7:67. doi: 10.1186/1477-7827-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aizawa S, Nishimune Y. In-vitro differentiation of type A spermatogonia in mouse cryptorchid testis. J Reprod Fertil. 1979;56:99–104. doi: 10.1530/jrf.0.0560099. [DOI] [PubMed] [Google Scholar]
- 85.Gohbara A, Katagiri K, Sato T, Kubota Y, Kagechika H, Araki Y, et al. In vitro murine spermatogenesis in an organ culture system. Biol Reprod. 2010;83:261–7. doi: 10.1095/biolreprod.110.083899. [DOI] [PubMed] [Google Scholar]
- 86.Mitchell RT, Saunders PT, Childs AJ, Cassidy-Kojima C, Anderson RA, Wallace WH, et al. Xenografting of human fetal testis tissue: a new approach to study fetal testis development and germ cell differentiation. Hum Reprod. 2010;25:2405–14. doi: 10.1093/humrep/deq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oatley JM, de Avila DM, Reeves JJ, McLean DJ. Testis tissue explant culture supports survival and proliferation of bovine spermatogonial stem cells. Biol Reprod. 2004;70:625–31. doi: 10.1095/biolreprod.103.022483. [DOI] [PubMed] [Google Scholar]
- 88.Staub C, Hue D, Nicolle JC, Perrard-Sapori MH, Segretain D, Durand P. The whole meiotic process can occur in vitro in untransformed rat spermatogenic cells. Exp Cell Res. 2000;260:85–95. doi: 10.1006/excr.2000.4998. [DOI] [PubMed] [Google Scholar]
- 89.Meehan T, Schlatt S, O’Bryan MK, de Kretser DM, Loveland KL. Regulation of germ cell and Sertoli cell development by activin, follistatin, and FSH. Dev Biol. 2000;220:225–37. doi: 10.1006/dbio.2000.9625. [DOI] [PubMed] [Google Scholar]
- 90.Seidl K, Holstein AF. Organ culture of human seminiferous tubules: a useful tool to study the role of nerve growth factor in the testis. Cell Tissue Res. 1990;261:539–47. doi: 10.1007/BF00313533. [DOI] [PubMed] [Google Scholar]
- 91.Pusch W, Balvers M, Weinbauer GF, Ivell R. The rat endozepine-like peptide gene is highly expressed in late haploid stages of male germ cell development. Biol Reprod. 2000;63:763–8. doi: 10.1095/biolreprod63.3.763. [DOI] [PubMed] [Google Scholar]
- 92.Tesarik J, Mendoza C, Anniballo R, Greco E. In-vitro differentiation of germ cells from frozen testicular biopsy specimens. Hum Reprod. 2000;15:1713–6. doi: 10.1093/humrep/15.8.1713. [DOI] [PubMed] [Google Scholar]
- 93.Tesarik J, Balaban B, Isiklar A, Alatas C, Urman B, Aksoy S, et al. In-vitro spermatogenesis resumption in men with maturation arrest: relationship with in-vivo blocking stage and serum FSH. Hum Reprod. 2000;15:1350–4. doi: 10.1093/humrep/15.6.1350. [DOI] [PubMed] [Google Scholar]
- 94.Tesarik J, Bahceci M, Ozcan C, Greco E, Mendoza C. Restoration of fertility by in-vitro spermatogenesis. Lancet. 1999;353:555–6. doi: 10.1016/S0140-6736(98)04784-9. [DOI] [PubMed] [Google Scholar]
- 95.Verhoeven G, Hoeben E, De Gendt K. Peritubular cell-Sertoli cell interactions: factors involved in PmodS activity. Andrologia. 2000;32:42–5. [PubMed] [Google Scholar]
- 96.Albrecht M. Insights into the nature of human testicular peritubular cells. Ann Anat. 2009;191:532–40. doi: 10.1016/j.aanat.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 97.Izadyar F, Den Ouden K, Creemers LB, Posthuma G, Parvinen M, De Rooij DG. Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod. 2003;68:272–81. doi: 10.1095/biolreprod.102.004986. [DOI] [PubMed] [Google Scholar]
- 98.Kubota H, Avarbock MR, Brinster RL. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod. 2004;71:722–31. doi: 10.1095/biolreprod.104.029207. [DOI] [PubMed] [Google Scholar]
- 99.Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction. 2010;139:287–301. doi: 10.1530/REP-09-0281. [DOI] [PubMed] [Google Scholar]
- 100.Trasler JM. Epigenetics in spermatogenesis. Mol Cell Endocrinol. 2009;306:33–6. doi: 10.1016/j.mce.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 101.Sutcliffe AG, Peters CJ, Bowdin S, Temple K, Reardon W, Wilson L, et al. Assisted reproductive therapies and imprinting disorders—a preliminary British survey. Hum Reprod. 2006;21:1009–11. doi: 10.1093/humrep/dei405. [DOI] [PubMed] [Google Scholar]
- 102.Bowdin S, Allen C, Kirby G, Brueton L, Afnan M, Barratt C, et al. A survey of assisted reproductive technology births and imprinting disorders. Hum Reprod. 2007;22:3237–40. doi: 10.1093/humrep/dem268. [DOI] [PubMed] [Google Scholar]
- 103.Biliya S, Bulla LA., Jr. Genomic imprinting: the influence of differential methylation in the two sexes. Exp Biol Med (Maywood) 2010;235:139–47. doi: 10.1258/ebm.2009.009251. [DOI] [PubMed] [Google Scholar]
- 104.Niemitz EL, Feinberg AP. Epigenetics and assisted reproductive technology: a call for investigation. Am J Hum Genet. 2004;74:599–609. doi: 10.1086/382897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Navarro-Costa P, Nogueira P, Carvalho M, Leal F, Cordeiro I, Calhaz-Jorge C, et al. Incorrect DNA methylation of the DAZL promoter CpG island associates with defective human sperm. Hum Reprod. 2010;25:2647–54. doi: 10.1093/humrep/deq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, et al. Active demethylation of the paternal genome in the mouse zygote. Curr Biol. 2000;10:475–8. doi: 10.1016/S0960-9822(00)00448-6. [DOI] [PubMed] [Google Scholar]
- 107.Kanatsu-Shinohara M, Inoue K, Ogonuki N, Morimoto H, Ogura A, Shinohara T. Serum- and feeder-free culture of mouse germline stem cells. Biol Reprod. 2011;84:97–105. doi: 10.1095/biolreprod.110.086462. [DOI] [PubMed] [Google Scholar]
- 108.Nagano MC. Techniques for culturing spermatogonial stem cells continue to improve. Biol Reprod. 2011;84:5–6. doi: 10.1095/biolreprod.110.088864. [DOI] [PubMed] [Google Scholar]
- 109.Araki Y, Sato T, Katagiri K, Kubota Y, Araki Y, Ogawa T. Proliferation of mouse spermatogonial stem cells in microdrop culture. Biol Reprod. 2010;83:951–7. doi: 10.1095/biolreprod.109.082800. [DOI] [PubMed] [Google Scholar]
- 110.Súrez-Quian CA, Hadley MA, Dym M. Effect of substrate on the shape of Sertoli cells in vitro. Ann N Y Acad Sci. 1984;438:417–34. doi: 10.1111/j.1749-6632.1984.tb38303.x. [DOI] [PubMed] [Google Scholar]
- 111.Gassei K, Schlatt S, Ehmcke J. De novo morphogenesis of seminiferous tubules from dissociated immature rat testicular cells in xenografts. J Androl. 2006;27:611–8. doi: 10.2164/jandrol.05207. [DOI] [PubMed] [Google Scholar]
- 112.Hadley MA, Weeks BS, Kleinman HK, Dym M. Laminin promotes formation of cord-like structures by Sertoli cells in vitro. Dev Biol. 1990;140:318–27. doi: 10.1016/0012-1606(90)90082-T. [DOI] [PubMed] [Google Scholar]
- 113.Lee JH, Oh JH, Lee JH, Kim MR, Min CK. Evaluation of in vitro spermatogenesis using poly(D,L-lactic-co-glycolic acid) (PLGA)-based macroporous biodegradable scaffolds. J Tissue Eng Regen Med. 2011;5:130–7. doi: 10.1002/term.297. [DOI] [PubMed] [Google Scholar]
- 114.Lee JH, Kim HJ, Kim H, Lee SJ, Gye MC. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials. 2006;27:2845–53. doi: 10.1016/j.biomaterials.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 115.Lee JH, Gye MC, Choi KW, Hong JY, Lee YB, Park DW, et al. In vitro differentiation of germ cells from nonobstructive azoospermic patients using three-dimensional culture in a collagen gel matrix. Fertil Steril. 2007;87:824–33. doi: 10.1016/j.fertnstert.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 116.Gassei K, Ehmcke J, Wood MA, Walker WH, Schlatt S. Immature rat seminiferous tubules reconstructed in vitro express markers of Sertoli cell maturation after xenografting into nude mouse hosts. Mol Hum Reprod. 2010;16:97–110. doi: 10.1093/molehr/gap081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Snedaker AK, Honaramooz A, Dobrinski I. A game of cat and mouse: xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J Androl. 2004;25:926–30. doi: 10.1002/j.1939-4640.2004.tb03163.x. [DOI] [PubMed] [Google Scholar]
- 118.Roscoe WA, Barr KJ, Mhawi AA, Pomerantz DK, Kidder GM. Failure of spermatogenesis in mice lacking connexin43. Biol Reprod. 2001;65:829–38. doi: 10.1095/biolreprod65.3.829. [DOI] [PubMed] [Google Scholar]