Abstract
p70 S6 kinase (p70S6K), a member of the AGC serine/threonine kinase family, was initially identified as a key player, together with its downstream effector S6, in the regulation of cellular growth and survival. The p70S6K protein has emerged in recent years as a multifunctional protein which also regulates the actin cytoskeleton and thus plays a role in cell migration. This new function is through two important activities of p70S6K, namely actin cross-linking and Rac1 and Cdc42 activation. The testis is critically dependent on an intricate balance of fundamental cellular processes such as adhesion, migration, and differentiation. It is increasingly evident that Rho GTPases and actin binding proteins play fundamental roles in regulating spermatogenesis within the testis. In this review, we will discuss current findings of p70S6K in the control of actin cytoskeleton dynamics. In addition, the potential role of p70S6K in spermatogenesis and testicular function will be highlighted.
Keywords: actin, cancer, Cdc42, germ cell, p70 S6 kinase, Rac1, spermatogenesis, testis
Introduction
Cell migration is an essential component of a variety of processes including wound repair,1 angiogenesis,2 immunity,3 and metastasis.4 Coordinated changes in actin cytoskeleton reorganization in response to microenvironmental signals result in migration. Thus, much effort has been made to understand the molecular machinery that drives the movement of the cell and has focused on the nature of cytoskeletal structures. Indeed, the actin cytoskeleton is essential and central to every step of the migration process.5 The 70 kDa ribosomal S6 kinase (p70S6K) is a serine/threonine kinase with a well-established role in protein synthesis.6 Although it was originally described as being exclusively involved in cell growth, our laboratory has recently published data describing p70S6K in other key aspects of cell functions, such as cellular migration. In this new role for p70S6K, the protein interacts with the actin cytoskeleton and activates the Rho GTPases to catalyze the formation of lamellipodia and filopodia.7 In this article, we will review the current data that may provide new insights to the potentially important role for p70S6K as a possible regulator of actin dynamics in the testis.
Overview of p70S6K
p70S6K belongs to the AGC subfamily of serine/threonine kinases, which also includes other important signaling molecules like Akt, protein kinase A and protein kinase C. p70S6K is encoded by the ribosomal protein S6 kinase, 70 kDa, polypeptide 1 gene (RPS6KB1), which is located on chromosome 17q23.1 in humans. p70S6K, with an apparent electrophoretic mobility of 70 kDa,8 consists of 502 amino acids and a molecular weight of 56,153 Da. The amino acid sequence of p70S6K has 100% similarity in all mammalians so far examined.9 S6K gene has also been identified in several invertebrate species, including Drosophila melanogaster10,11 and Caenorhabditis elegans.12 A novel S6K was recently found in the yeast Schizosaccharomyces pomba.13 All these indicate that S6K is evolutionary conserved among eukaryotes and therefore may represent a significant functional component.
Structure of p70S6K
p70S6K can be divided into five functional domains/regions: (1) the amino (N)-terminal domain, (2) the AGC-kinase conserved catalytic domain, (3) the linker region, (4) the putative autoinhibitory domain, and (5) the carboxyl (C)-terminal domain.14 At least eight phosphorylation sites have been mapped in endogenous kinase, including Ser411, Ser418, Thr421 and Ser424 in the autoinhibitory domain,15,16 Thr229 in the catalytic domain17 and Ser371, Thr389 and Ser404 in the linker region (Fig. 1).15 The kinase exists in two conformations, inactive and active state. In the inactive state of p70S6K, the carboxyl-terminal autoinhibitory domain, which has sequence similarity to the substrate region of the S6 protein, may act as a pseudosubstrate and interacts with the N-terminus (Fig. 1).14 According to the current model, p70S6K activation is initiated by the release of the autoinhibition exerted by the autoinhibitory domain.18 This is then followed by a series of phosphorylation of eight or more serine or threonine residues at the autoinhibitory domain, the linker region, and then the catalytic domain, to obtain full kinase activation.6,19-23
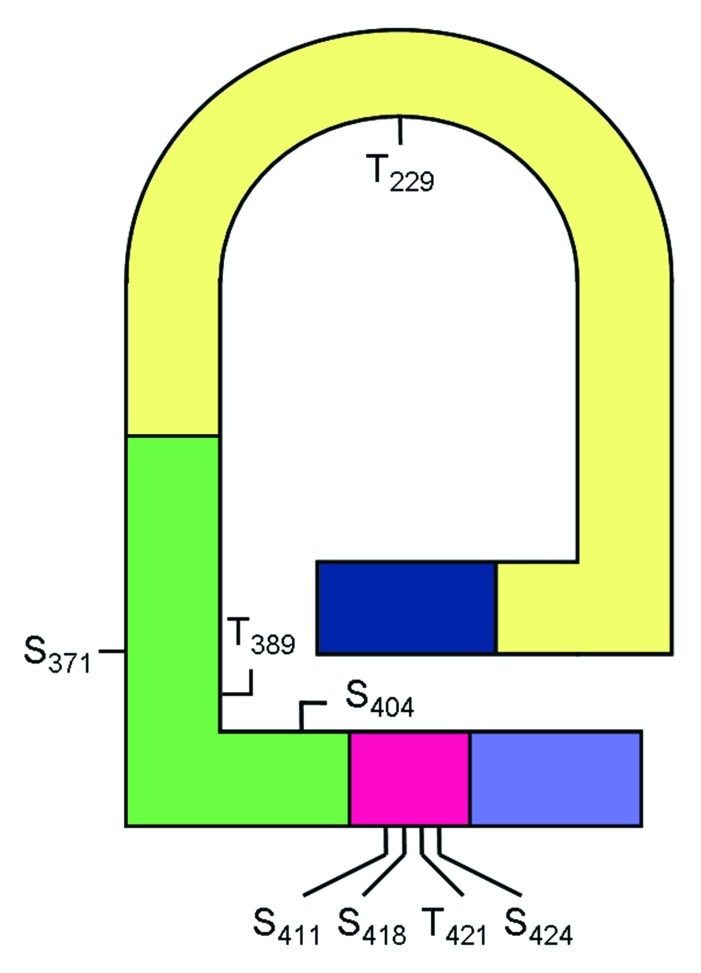
Figure 1. A model to illustrate domains and phosphorylation sites of p70S6K. p70S6K can be divided into five functional domains/regions: (1) the amino (N)-terminal domain (blue), (2) the AGC-kinase conserved catalytic domain (yellow), (3) the linker region (green), (4) the putative autoinhibitory domain (red), and (5) the carboxyl (C)-terminal domain (purple). Eight phosphorylation sites have been mapped.
Regulation of p70S6K
The activity of p70S6K is regulated through phosphorylation/dephosphorylation events. The phosphorylation events are stimulated by a variety of mitogenic factors.24,25 Several upstream in vivo signaling pathways have been identified to regulate the phosphorylation and activation of p70S6K. One pathway that has been widely accepted is the phosphatidylinositol 3-kinase (PI3K)/Akt pathway.26-28 Following stimulation, PI3K is recruited to plasma membrane and activated by G-protein coupled receptors or receptor tyrosine kinase. Active PI3K then phosphorylates the membrane lipid phosphatidylinositol 4,5-biphosphate PIP2 to produce phosphatidylinositol 3,4,5-biphosphate PIP3 which recruits and activates 3-phosphoinositide-dependent kinase 1 (PDK1). PDK1 has been shown to markedly phosphorylate p70S6K to acquire full kinase activation.18,26,29,30 Alternatively, PDK1 phosphorylates and activates Akt, which is recruited by PIP2/PIP3 to plasma membrane, and active Akt subsequently activates p70S6K.31 Mammalian target of rapamycin (mTOR) is another signaling protein downstream of PI3K pathway that phosphorylates p70S6K in vivo.20,32 Dunfner et al. has proposed that an additional signaling pathway is required for full p70S6K activation as PI3K, PDK1, and mTOR only partial activate p70S6K in vivo.26 Extracellular signal-regulated kinases 1/2 (ERK1/2) under the mitogen-activated protein kinase (MAPK) signaling has been shown to phosphorylate p70S6K in vivo.33 However, the involvement of ERK1/2 in p70S6K activation is controversial since there are studies providing evidence that ERK1/2 is neither necessary nor sufficient for p70S6K activation.34,35 As the serine/threonine sites in the autoinhibitory domain of p70S6K are of consensus sequences similar to those recognized by MAPKs and that phosphorylation at the autoinhibitory domain is an early step required for p70S6K activation, MAPK has been suggested to be essential for p70S6K activation similar to PI3K.27,36 Moreover, there are reports revealing that p70S6K can be activated by the Raf/MEK/ERK signaling under specific physiological conditions.27,37 In addition to ERK1/2, p38 MAPK and Jun N-terminal kinase (JNK) are also putative kinases regulating p70S6K activation in the cell.18,38
Functions of p70S6K
1. Protein synthesis
Ribosomal S6 protein (S6), a component of the 40S ribosomal subunit, is the first identified downstream target of p70S6K.6 Through phosphorylation of S6, p70S6K regulates the translation of a subset of mRNA containing an oligopyrimidines tract at the 5′ untranslated region, 5′ TOP mRNA.39 These 5′TOP mRNAs account for 20% of total cellular mRNA and most of them encode ribosomal proteins, elongation factors, and poly(A)-binding protein, which are essential components for the translation machinery.40,41 In addition to S6, p70S6K also regulates both the initiation and elongation phases of translation by phosphorylating eukaryotic translation initiation factor 4B (eIF4B), a cofactor of an RNA helicase, eukaryotic translation initiation factor 4A (eIF4A).42 Phosphorylation of eIF4B leads to the assembly of eIF4A to form the translation initiation complex, which subsequently increases the rate of translation. Furthermore, p70S6K can regulate translation elongation by inhibiting the kinase activity of eukaryotic elongation factor 2 kinase (eEF2K), a negative regulator of eukaryotic elongation factor 2 (eEF2).43
2. Cell growth and cell size
The regulation of p70S6K on cell growth has been studied in Drosophila and mice. Montagne et al. has demonstrated that loss of S6K gene is semi-lethal to Drosophila, in which the few surviving adults had a severely reduced body size due to a decrease in cell size rather than a decrease in cell number.44 However, in mice the deficiency of the S6K gene is not lethal, although their body size at birth was reduced due to a decrease in organ weight, including the testis.45 Cell growth modulated by p70S6K may be independent of S6-mediated protein translation because the level of S6 phosphorylation remains intact in the S6K knockout mice.45 S6K1 Aly/REF-like target (SKAR), a mRNA processing protein, is later revealed as a downstream regulator of p70S6K on cell growth.46
3. Cell cycle progression
Evidence for a role of p70S6K in cell cycle progression comes from studies using the p70S6K neutralizing antibodies47 or the immunosuppressant rapamycin.48-50 These inhibitory effects cause G1 arrest and significantly delay S phase entry. p70S6K may also promote G1 to S phase transition by increasing the translation of cyclin D1 and p21 which are critical proteins forming the cyclin-CDK complex in G1 phase required for Rb phosphorylation and the subsequent entry of S phase.51
4. Cell survival
Bcl-2-associated death promoter (BAD), a proapoptotic protein, is a downstream effector of p70S6K that regulates cell survival. The apoptotic effect of BAD can be abrogated upon phosphorylation.52 BAD would be hypophosphorylated in the absence of p70S6K.53 Moreover, overexpression of p70S6K can rescue cell from apoptosis by expressing either BAD wildtype or S112A.53 p70S6K may also promote cell survival through phosphorylation of Mdm2, a p53 ubiquitin ligase.54
In addition to the major functions mentioned above, p70S6K can regulate other cellular functions through downstream effectors, such as cAMP-responsive element modulator τ (CREMτ) for gene transcription55 and insulin receptor substrate 1 (IRS1) for homeostasis.56
Actin Filament Dynamics
A dynamic actin cytoskeleton is essential for many cellular functions, such as maintenance of cell shape,57 cell junction,57 and cell motility.5 Actin in cells exists in three different forms: monomeric (globular-actin; G-actin), oligomeric, and polymeric (filamentous actin; F-actin).58 F-actin is polarized with a fast-growing plus end, also known as barbed end, and a slow-grown minus end, also known as pointed end.59 Reorganization of the actin cytoskeleton, including polymerization, depolymerization, nucleation, bundling/ cross-linking, capping, severing, and branching, is facilitated by the actin binding proteins.58 The Rho family GTPases, including Rac1–3, Cdc42, and RhoA-C, play a central role in coordinating the actin binding proteins for cytoskeleton reorganization.60 Rac1 and Cdc42 transduce signals to the actin binding proteins through two major types of interacting proteins: (1) Wiskott-Aldrich syndrome protein (WASP)/suppressor of cAMP receptor (Scar)/WASP family verprolin homology protein (WAVE) and (2) p21-activated kinase 1 (PAK1). WASP/Scar/WAVE promotes F-actin nucleation upon Rac1 and Cdc42 activation. While WASP family protein is activated by Cdc42,61,62 Scar/WAVE family protein is indirectly activated by Rac1 through the Nck-adaptor complex.63,64 Active WASP/WAVE then undergoes conformational change and binds with the ATP-G-actin binding protein, profilin, and the actin-related protein2/3 (Arp2/3) complex. These work synergistically to speed up actin branching and polymerization through actin nucleation of ATP-G-actin to the pre-existing actin filaments,65,66 thereby facilitating the building of the dendritic actin network.67 On the other hand, PAK1, a downstream effector of Rac1 and Cdc42, phosphorylates and activates LIM kinase (LIMK). LIMK phosphorylates cofilin, an actin filament serving protein,68,69 leading to its inactivation, which in turn inhibits depolymerization and severing of actin filaments. LIMK can also be phosphorylated by p160 Rho-associated coiled-coil-containing protein kinase (ROCK), a downstream effector of Rho.70 Apart from the LIMK, Rho can also regulate actin polymerization through another downstream effector diaphanous-related formin, which promotes the polymerization of unbranched filaments.71,72
p70S6K in the control of actin cytoskeleton
The Rho GTPases and p70S6K were first shown to coexist in the same pathway in a study by Chou et al.73 Rac1 and Cdc42, but not RhoA, complex with and activate p70S6K, which can be blocked by the mTOR inhibitor, rapamycin and the PI3K inhibitor, wortmannin.73 A role of p70S6K in actin cytoskeleton reorganization was further hinted by Berven et al. and through to colocalize with the stress fibers and actin arc at the leading edge of Swiss3T3 fibroblasts under growth factor stimulation.74 This colocalization of p70S6K and stress fibers was suggested to regulate actin polymerization as rapamycin treatment could inhibit the elongation and organization of actin stress fibers via inhibition of p70S6K.75 However, the biological function of such an interaction is not known. Recently, our lab not only identified p70S6K as a critical regulator of the actin cytoskeleton but also showed that it is pivotal for the directional migration of cancer cells, which is a prerequisite of metastasis (Fig. 2).7 Our findings provide several insights into the regulation of p70S6K on the actin cytoskeleton. First, we have demonstrated for the first time that p70S6K can directly bind with and cross-link F-actin in vitro. Moreover, active p70S6K colocalizes with the actin filaments at the leading edge of motile cells in vivo and p70S6K-F-actin colocalization is cytochalasin D-sensitive. However, unlike some actin bundling/cross-linking proteins, p70S6K does not change the rate of actin polymerization, but stabilizes actin filaments by decreasing the rate and extent of ADP/cofilin-dependent actin depolymerization.
Figure 2. Schematic illustration on the mechanism by which p70S6K regulates actin cytoskeleton in ovarian cancer cells. p70S6K directly binds/cross links with F-actin and activates PAK1 through Rac1 and Cdc42 to modulate actin cytoskeleton dynamics for cell migration.
Second, our results suggest that the correlation of p70S6K with Rho GTPases and cell migration depends on the cell type or on the signaling context, leading to differential functions. In fibroblasts, p70S6K acts as a downstream effector of Rac1 and Cdc42. It is interesting to note that this activation of p70S6K by Rac1 and Cdc42 appears to be independent of the ability of these Rho GTPases to regulate the cytoskeleton during cell cycle progression.73 By contrast, in carcinomas and epithelial cells, we have shown that p70S6K functions upstream of both Rac1 and Cdc42 to regulate actin cytoskeleton reorganization and thus cell migration, in which Rac1 and Cdc42 are known to function. We also show an essential role for PAK1 in this process.
Third, the regulation of the actin cytoskeleton by p70S6K reveals that many oncogenic signals could mediate cancer cell invasion and metastasis by modulating p70S6K activity. For example, p70S6K is a downstream effector of the PI3K/Akt pathway, which is frequently activated in human cancers. Moreover, p70S6K is also well known to be activated by hormones, cytokines, and growth factors. Our finding that p70S6K binding to the actin cytoskeleton is more effective in the presence than in the absence of p70S6K phosphorylation indicates that it is a dynamic regulation of the actin cytoskeleton, reinforcing the notion that cell migration is a finely tuned event. This also implies that the actin-binding domain in p70S6K in an inactive conformation may be unseen and suggests an additional regulation inside the cell.
Actin Cytoskeleton Reorganization and Spermatogenesis
Spermatogenesis is a process in which diploid spermatogonia (germ cells) go through a series of stages and differentiate into mature spermatozoa between Sertoli cells within the seminiferous tubule. Many cellular events are involved in this process, including cell division, differentiation, cell movement, reconstructing of cell junctions,76 changes in cell shape and size such as differentiation of elongated spermatids from round spermatocytes, all of which require dramatic reorganization of the actin cytoskeleton. Since actin cytoskeleton dynamics and some of the regulatory proteins in spermatogenesis have been comprehensively reviewed,76,77 we will focus on the activities of actin bundling/cross-linking and Rho GTPases.
Actin cytoskeletal network in Sertoli cell
Sertoli cells in the seminiferous tubule extend from the basal lamina to the lumen of the tubule and are able to alter their cell shape to accommodate morphological changes of germ cells, thereby providing both structural and nutritional support to the germ cells throughout their development.78 In Sertoli cells, F-actin are abundantly detected and are concentrated at the adherens junctions (AJ), the ectoplasmic specialization (ES) and the tubulobulbar complex (TBC).79 The arrangement of F-actin in the AJs, ES and TBC, is drastically different from that in other polarized epithelia which will be discussed in the following sections.
Ectoplasmic specialization (ES)
ESs are localized at two sites in seminiferous epithelium: (1) junctions between adjacent Sertoli cells near the basal lamina of the seminiferous epithelium, namely basal ES; (2) and adhesion between Sertoli cells and elongating/elongated spermatids at the apical region of the seminiferous epithelium, namely apical ES. F-actin at the ES forms a hexagonal array between the plasma and endoplasmic reticulum membranes of Sertoli cells.80 Although myosin VIIa, an actin motor protein, has been found to be enriched at the apical ES, the actin bundles at ES are thought to be non-contractile. Instead, these actin bundles may structurally contribute to the stability of the intercellular adhesion at ES. The mechanisms underlying the above processes are largely unknown. However, based on the actin bundle structure at the ES, formin that has been shown to be abundant in Sertoli cells has been proposed to be important in the regulation of actin polymerization at the ES.79,81 Ena/VASP family proteins that promote actin elongation at pointed end by tethering the filaments to sites of active actin assembly may also be involved. While the localization of formin and Ena/VASP at the ES is still unknown, several actin bundling/crosslinking proteins have been found at the ES site. These include espin,82 fimbrin,83 vinculin,83 Eps8,84 and α-actinin.85 The temporal and spatial expression of Eps8 and Arp2 at the Sertoli cell-spermatid interface (apical ES) coincides with the onset of spermatid elongation.82 The expression of Eps8 at the blood-testis barrier (BTB) is high at all stages of the epithelial cycle, except at stage VIII when the BTB undergoes extensive restructuring to facilitate the transit of preleptotene spermatocytes.84 Interestingly, the expression of Arp3 is also significantly induced at the BTB at stage VIII of the spermatogenesis cycle.86 Although the activity of actin bundling proteins at the ES has not been directly demonstrated, treatment of developing rats with adjudin, a drug that disrupts AJ at the Sertoli-germ cell interface, showed that espin appears to function with establishment of BTB.87 Small Rho GTPases known to regulate actin are also implicated in regulating spermatogenesis within the testis. For example, RhoB was found at the apical ES in region in association with elongating spermatids. RhoB expression was decreased markedly surrounding the elongated spermatids at late stages VII to VIII of the cycle.88
Tubulobulbar complex (TBC)
Similar to ES, TBC is localized to both the basal and apical region of the Sertoli cell: (1) basal TBC is between Sertoli cells near the basal lamina of the seminiferous epithelium; (2) apical TBC is at adhesions between Sertoli cells and the concave side of the head of elongated spermatids at the apical region of the seminiferous epithelium and appears just a few days before spermiation at late stage VIII.89 Various functions of TBC has been proposed, such as to anchor spermatids to the Sertoli cells, to remove cytoplasm from spermatids,90 to facilitate shaping of spermatid heads,91 and to internalize junction proteins during movement of spermatocytes through basal TBC and the release of sperms.92 F-actin at the TBC exists as three-dimensional highly branched networks.79,93 Although the detailed mechanism mediating the actin network at TBC is still unknown, Arp2/3-based branching of actin filaments has been proposed to play a role in the formation of the actin network. Arp3 and WASP that are essential for actin branching have been shown to localize to the apical TBC,94 supporting the proposed mechanism of the actin network formation. Rac1 and Cdc42 that activate the Arp2/3 mechanism have been detected in the cytoplasm of Sertoli cells around the spermatid head95and inactivation of Rho GTPases by toxin A causes disaggregation of actin cytoskeleton in Sertoli cells.96 In addition to the actin binding proteins mentioned above, cofilin,97 and Eps884 have also been shown to localize to the TBC, suggesting that actin bundling and actin severing and/or depolymerization are required in the formation of actin network at the TBC.
Actin regulatory proteins in germ cells
During spermatogenesis, round germ cells undergo dramatic morphological changes and remodel into mature spermatozoa with head and tail. F-actin in spermatids is concentrated in the intercellular junctions, the subacrosomal space, the acroplaxome, and the manchette. Actin and its regulatory proteins therefore have important functions in regulating the development and morphogenesis of germ cells. The actin bundling protein testis fascin has been shown to express in the head of elongating spermatids. Another actin bundling protein Eps8 has also been detected in germ cells although its expression is less than that in Sertoli cells.84 In addition to the actin bundling proteins, the actin polymerization and branching promoters mDia1/281 and WAVE198 have also been detected in spermatocytes or spermatids, suggesting actin bundling, polymerization, and branching may be involved in spermatid development.
p70S6K in Spermatogenesis
Several studies have suggested that p70S6K may have a role in spermatogenesis by regulating the development of primary Sertoli cells under follicle stimulating hormone (FSH) and luteinizing hormone (LH) stimulation.99,100 Although there are no studies demonstrating that p70S6K may play a role in actin cytoskeleton reorganization in Sertoli cells, a report by Riera et al., which focused on the interleukin (IL)-1β-stimulated lactate production in Sertoli cells,101 may provide hints on this role. The results showed that IL-1β increases phosphorylation of p70S6K, but the activation is not related to lactate production. Recent studies have also shown that IL-1 can regulate the dynamics of actin cytoskeleton and cell junctions in addition to its well-known role in innate immunity102 and tissue homeostasis,103 suggesting that p70S6K may be a regulator of IL-1 on actin cytoskeleton reorganization. Some other cytokines and growth factors which are potent activator of p70S6K in other cell types, such as transforming growth factor (TGF)-β, and hepatocyte growth factor (HGF), also have important functions in testicular development and spermatogenesis (Table 1). Cytokines involved in spermatogenesis have been reviewed by Xia et al.89 These cytokines mostly activate p70S6K through PI3K/Akt and MAPK pathways, which have been shown to regulate AJ dynamics and spermatogenesis. The expression or activities of the key components in PI3K/Akt and MAPK pathways, including the 85α and p110α subunits of PI3K, Akt, and ERK1/2, have been shown to increase during the AJ assembly of Sertoli cells and germ cells (i.e., apical ES).104 Both PI3K and active Akt are abundant at the site of apical ES from stages IV to VIII and are detected at the basal ES.104 Inhibition of PI3K using inhibitors is able to disrupt the AJ.105 All these suggest that the PI3K/Akt and MAPK pathways may be required for AJ assembly (for ERK pathway review, ref. 106). Siu et al. has also shown the expression of PAK2 during AJ assembly and suggested a role of PAK in AJ formation. Although the expression of PAK2 but not PAK1 increases during AJ assembly, the activity of PAK1 was not detected in the study.104 Recently, Wong et al. has revealed that Cdc42 mediates TGF-β3-induced BTB disruption by enhancing endocytosis of integral membrane proteins at BTB.107 This suggests a possible mechanism by which p70S6K may regulate the BTB restructuring at stage VIII through mediating the activation of Cdc42. Thus, extracellular stimuli may activate p70S6K via PI3K/Akt and MAPK pathways, which in turn may lead to actin cytoskeleton reorganization at AJs and BTB restructuring through Rac1/Cdc42 activation. A possible perspective of p70S6K in regulating the actin cytoskeleton dynamics of spermatogenesis in Sertoli cells is shown in Figure 3.
Table 1. Potent activators of p70S6K in testis.
| Function of cytokines in testis | Activation of p70S6K in testis | References | |
|---|---|---|---|
|
Hormones |
|
|
|
| FSH |
Regulate the development of Sertoli cells |
Yes |
100 |
|
Cytokines |
|
|
|
| IL-1α |
Regulate Sertoli-germ cell adhesion |
n.d. |
99,100 |
| IL-1β |
Regulate lactate production in Sertoli cells |
Yes |
101 |
| BMP-4 |
Maintain spermatogenesis; promote differentiation of spermatogonia |
n.d. |
110–112 |
|
Growth factors |
|
|
|
| EGF |
Enhance spermatogonia proliferation and differentiation |
n.d. |
113,114 |
| FGF2 |
Induce testosterone production in Leydig cells |
Yes |
115 |
| HGF |
Modulate Sertoli-Sertoli tight junction dynamics; increase steroidogenetic activity of Leydig cell |
n.d. |
116–118 |
| PDGF |
Regulate the development of the Leydig cell lineage and spermatogenesis |
n.d. |
110,111 |
| SCF |
Promote spermatogonia proliferation |
Yes |
109 |
| TGF-β1 | Inhibit steroidogenesis in Leydig cells | n.d. | 119,120 |
Abbreviations: BMP-4, bone morphogenetic protein-4; EGF, epidermal growth factor; FGF2, fibroblast growth factor 2; FSH, follicle stimulating hormone; HGF, hepatocyte growth factor; IL-1β, interleukin-1β; n.d., not determined; PDGF, platelet-derived growth factor; SCF, stem cell factor; TGF β1, transforming growth factor-β1.
Figure 3. Schematic perspective of p70S6K on spermatogenesis regulation in Sertoli cells. p70S6K activation through the PI3K/Akt or MAPK pathways may have a possible role in regulating the actin cytoskeleton at AJ and BTB restructuring through Rac1/Cdc42-activating activities.
In germ cells, the expression of p70S6K is relatively constant during its maturation; however, the kinase activity of p70S6K is increased. Immunohistochemistry detection of p70S6K also showed that there is a nucleus-to-cytoplasm translocation of p70S6K during spermatogenesis.108 Moreover, p70S6K has been shown to mediate cytokine-induced signaling to stimulate proliferation of type A spermatogonia, which may play a role in the biosynthesis and preparation of germ cells for fertilization.109
Concluding Remarks and Perspectives
The regulation of ES recently has received a great deal of attention because they may shed light on male contraceptive development. However, the signaling pathways that regulate actin polymerization and depolymerization at the ES have not been studied in detail. It is suspected that different cytokines and hormones may be involved in changes in the dynamics of actin filaments in the testis. Our new findings have linked p70S6K to the actin cytoskeleton and have led to the suggestion that this widely studied kinase may play a key role in epithelial cell motility. Altered expression of p70S6K and several actin bundling/cross-linking proteins are being reported in the testis. Rho GTPases activities are important for the maintenance and formation of the actin cytoskeleton in Sertoli cells. Further demonstration of this intriguing phenomenon of a new role for p70S6K in regulating the actin dynamics in the testis would have interesting and important consequences. In addition, a better understanding of how the different networks of p70S6K functional interactions are orchestrated in a stimulus or context-specific way, as well as the functional roles on actin reorganization in specific cellular and animal experimental models are essential. This knowledge likely will contribute to a new and important piece in the complex jigsaw of spermatogenesis and male infertility.
Acknowledgments
A.S.T.W. is supported by the Research Grant Council grant HKU7599/05M and the HKU Outstanding Young Researcher Award.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/19413
References
- 1.Winter GD. Movement of epidermal cells over the wound surface. In: Montagna W, Billingham RE, eds. Advances in Biology of Skin - Vol V, Wound Healing. Oxford: Pergamon Press, 1964:133-27. [Google Scholar]
- 2.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–94. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 3.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–90. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–52. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Sci. 2005;118:4917–9. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- 6.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5’TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ip CK, Cheung AN, Ngan HY, Wong AS. p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene. 2011;30:2420–32. doi: 10.1038/onc.2010.615. [DOI] [PubMed] [Google Scholar]
- 8.Jenö P, Ballou LM, Novak-Hofer I, Thomas G. Identification and characterization of a mitogen-activated S6 kinase. Proc Natl Acad Sci U S A. 1988;85:406–10. doi: 10.1073/pnas.85.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montagne J, Thomas G, eds. S6K integrates nutrient and mitogen signals to control cell growth. New York: Cold Spring Harbor Laboratory Press, 2004. [Google Scholar]
- 10.Stewart MJ, Berry CO, Zilberman F, Thomas G, Kozma SC. The Drosophila p70s6k homolog exhibits conserved regulatory elements and rapamycin sensitivity. Proc Natl Acad Sci U S A. 1996;93:10791–6. doi: 10.1073/pnas.93.20.10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson KL, Chou MM, Blenis J, Gelbart WM, Erikson RL. A Drosophila gene structurally and functionally homologous to the mammalian 70-kDa s6 kinase gene. Proc Natl Acad Sci U S A. 1996;93:13694–8. doi: 10.1073/pnas.93.24.13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long X, Spycher C, Han ZS, Rose AM, Müller F, Avruch J. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Curr Biol. 2002;12:1448–61. doi: 10.1016/S0960-9822(02)01091-6. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo T, Kubo Y, Watanabe Y, Yamamoto M. Schizosaccharomyces pombe AGC family kinase Gad8p forms a conserved signaling module with TOR and PDK1-like kinases. EMBO J. 2003;22:3073–83. doi: 10.1093/emboj/cdg298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/S0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari S, Bannwarth W, Morley SJ, Totty NF, Thomas G. Activation of p70s6k is associated with phosphorylation of four clustered sites displaying Ser/Thr-Pro motifs. Proc Natl Acad Sci U S A. 1992;89:7282–6. doi: 10.1073/pnas.89.15.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price DJ, Mukhopadhyay NK, Avruch J. Insulin-activated protein kinases phosphorylate a pseudosubstrate synthetic peptide inhibitor of the p70 S6 kinase. J Biol Chem. 1991;266:16281–4. [PubMed] [Google Scholar]
- 17.Weng QP, Andrabi K, Klippel A, Kozlowski MT, Williams LT, Avruch J. Phosphatidylinositol 3-kinase signals activation of p70 S6 kinase in situ through site-specific p70 phosphorylation. Proc Natl Acad Sci U S A. 1995;92:5744–8. doi: 10.1073/pnas.92.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng QP, Kozlowski M, Belham C, Zhang A, Comb MJ, Avruch J. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. J Biol Chem. 1998;273:16621–9. doi: 10.1074/jbc.273.26.16621. [DOI] [PubMed] [Google Scholar]
- 19.Alessi DR, Kozlowski MT, Weng QP, Morrice N, Avruch J. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol. 1998;8:69–81. doi: 10.1016/S0960-9822(98)70037-5. [DOI] [PubMed] [Google Scholar]
- 20.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A. 1998;95:1432–7. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennis PB, Pullen N, Kozma SC, Thomas G. The principal rapamycin-sensitive p70(s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol Cell Biol. 1996;16:6242–51. doi: 10.1128/mcb.16.11.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han JW, Pearson RB, Dennis PB, Thomas G. Rapamycin, wortmannin, and the methylxanthine SQ20006 inactivate p70s6k by inducing dephosphorylation of the same subset of sites. J Biol Chem. 1995;270:21396–403. doi: 10.1074/jbc.270.36.21396. [DOI] [PubMed] [Google Scholar]
- 23.Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, et al. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 1995;14:5279–87. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novak-Hofer I, Thomas G. An activated S6 kinase in extracts from serum- and epidermal growth factor-stimulated Swiss 3T3 cells. J Biol Chem. 1984;259:5995–6000. [PubMed] [Google Scholar]
- 25.Thomas G, Hall MN. TOR signalling and control of cell growth. Curr Opin Cell Biol. 1997;9:782–7. doi: 10.1016/S0955-0674(97)80078-6. [DOI] [PubMed] [Google Scholar]
- 26.Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res. 1999;253:100–9. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- 27.Eguchi S, Iwasaki H, Ueno H, Frank GD, Motley ED, Eguchi K, et al. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. J Biol Chem. 1999;274:36843–51. doi: 10.1074/jbc.274.52.36843. [DOI] [PubMed] [Google Scholar]
- 28.Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344:427–31. doi: 10.1042/0264-6021:3440427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balendran A, Currie R, Armstrong CG, Avruch J, Alessi DR. Evidence that 3-phosphoinositide-dependent protein kinase-1 mediates phosphorylation of p70 S6 kinase in vivo at Thr-412 as well as Thr-252. J Biol Chem. 1999;274:37400–6. doi: 10.1074/jbc.274.52.37400. [DOI] [PubMed] [Google Scholar]
- 30.Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, et al. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–10. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- 31.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 32.Isotani S, Hara K, Tokunaga C, Inoue H, Avruch J, Yonezawa K. Immunopurified mammalian target of rapamycin phosphorylates and activates p70 S6 kinase alpha in vitro. J Biol Chem. 1999;274:34493–8. doi: 10.1074/jbc.274.48.34493. [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay NK, Price DJ, Kyriakis JM, Pelech S, Sanghera J, Avruch J. An array of insulin-activated, proline-directed serine/threonine protein kinases phosphorylate the p70 S6 kinase. J Biol Chem. 1992;267:3325–35. [PubMed] [Google Scholar]
- 34.Chung J, Grammer TC, Lemon KP, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370:71–5. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 35.Ming XF, Burgering BM, Wennström S, Claesson-Welsh L, Heldin CH, Bos JL, et al. Activation of p70/p85 S6 kinase by a pathway independent of p21ras. Nature. 1994;371:426–9. doi: 10.1038/371426a0. [DOI] [PubMed] [Google Scholar]
- 36.Papst PJ, Sugiyama H, Nagasawa M, Lucas JJ, Maller JL, Terada N. Cdc2-cyclin B phosphorylates p70 S6 kinase on Ser411 at mitosis. J Biol Chem. 1998;273:15077–84. doi: 10.1074/jbc.273.24.15077. [DOI] [PubMed] [Google Scholar]
- 37.Iijima Y, Laser M, Shiraishi H, Willey CD, Sundaravadivel B, Xu L, et al. c-Raf/MEK/ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells. J Biol Chem. 2002;277:23065–75. doi: 10.1074/jbc.M200328200. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Dong Z, Nomura M, Zhong S, Chen N, Bode AM, et al. Signal transduction pathways involved in phosphorylation and activation of p70S6K following exposure to UVA irradiation. J Biol Chem. 2001;276:20913–23. doi: 10.1074/jbc.M009047200. [DOI] [PubMed] [Google Scholar]
- 39.Peterson RT, Schreiber SL. Translation control: connecting mitogens and the ribosome. Curr Biol. 1998;8:R248–50. doi: 10.1016/S0960-9822(98)70152-6. [DOI] [PubMed] [Google Scholar]
- 40.Bischoff SR, Tsai S, Hardison NE, York AM, Freking BA, Nonneman D, et al. Identification of SNPs and INDELS in swine transcribed sequences using short oligonucleotide microarrays. BMC Genomics. 2008;9:252. doi: 10.1186/1471-2164-9-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hornstein E, Git A, Braunstein I, Avni D, Meyuhas O. The expression of poly(A)-binding protein gene is translationally regulated in a growth-dependent fashion through a 5′-terminal oligopyrimidine tract motif. J Biol Chem. 1999;274:1708–14. doi: 10.1074/jbc.274.3.1708. [DOI] [PubMed] [Google Scholar]
- 42.Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, et al. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004;23:1761–9. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 2001;20:4370–9. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G. Drosophila S6 kinase: a regulator of cell size. Science. 1999;285:2126–9. doi: 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- 45.Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 1998;17:6649–59. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson CJ, Bröenstrup M, Fingar DC, Jülich K, Ballif BA, Gygi S, et al. SKAR is a specific target of S6 kinase 1 in cell growth control. Curr Biol. 2004;14:1540–9. doi: 10.1016/j.cub.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 47.Lane HA, Fernandez A, Lamb NJ, Thomas G. p70s6k function is essential for G1 progression. Nature. 1993;363:170–2. doi: 10.1038/363170a0. [DOI] [PubMed] [Google Scholar]
- 48.Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–36. doi: 10.1016/0092-8674(92)90643-Q. [DOI] [PubMed] [Google Scholar]
- 49.Kuo CJ, Chung J, Fiorentino DF, Flanagan WM, Blenis J, Crabtree GR. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992;358:70–3. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- 50.Price DJ, Grove JR, Calvo V, Avruch J, Bierer BE. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992;257:973–7. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- 51.Viñals F, Chambard JC, Pouysségur J. p70 S6 kinase-mediated protein synthesis is a critical step for vascular endothelial cell proliferation. J Biol Chem. 1999;274:26776–82. doi: 10.1074/jbc.274.38.26776. [DOI] [PubMed] [Google Scholar]
- 52.del Peso L, González-García M, Page C, Herrera R, Nuñez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–9. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 53.Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci U S A. 2001;98:9666–70. doi: 10.1073/pnas.171301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang J, Meng Q, Vogt PK, Zhang R, Jiang B-H. A downstream kinase of the mammalian target of rapamycin, p70S6K1, regulates human double minute 2 protein phosphorylation and stability. J Cell Physiol. 2006;209:261–5. doi: 10.1002/jcp.20749. [DOI] [PubMed] [Google Scholar]
- 55.de Groot RP, Ballou LM, Sassone-Corsi P. Positive regulation of the cAMP-responsive activator CREM by the p70 S6 kinase: an alternative route to mitogen-induced gene expression. Cell. 1994;79:81–91. doi: 10.1016/0092-8674(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 56.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–23. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luna EJ, Hitt AL. Cytoskeleton--plasma membrane interactions. Science. 1992;258:955–64. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- 58.dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, et al. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–73. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- 59.Egelman EH, Orlova A. New insights into actin filament dynamics. Curr Opin Struct Biol. 1995;5:172–80. doi: 10.1016/0959-440X(95)80072-7. [DOI] [PubMed] [Google Scholar]
- 60.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 61.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–31. doi: 10.1016/S0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 62.Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol. 2000;150:1299–310. doi: 10.1083/jcb.150.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–3. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- 64.Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem. 2001;276:26448–52. doi: 10.1074/jbc.M103856200. [DOI] [PubMed] [Google Scholar]
- 65.Blanchoin L, Pollard TD, Mullins RD. Interactions of ADF/cofilin, Arp2/3 complex, capping protein and profilin in remodeling of branched actin filament networks. Curr Biol. 2000;10:1273–82. doi: 10.1016/S0960-9822(00)00749-1. [DOI] [PubMed] [Google Scholar]
- 66.Yang C, Huang M, DeBiasio J, Pring M, Joyce M, Miki H, et al. Profilin enhances Cdc42-induced nucleation of actin polymerization. J Cell Biol. 2000;150:1001–12. doi: 10.1083/jcb.150.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weaver AM, Young ME, Lee WL, Cooper JA. Integration of signals to the Arp2/3 complex. Curr Opin Cell Biol. 2003;15:23–30. doi: 10.1016/S0955-0674(02)00015-7. [DOI] [PubMed] [Google Scholar]
- 68.Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–9. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 69.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–9. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 70.Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–82. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- 71.Tominaga T, Sahai E, Chardin P, McCormick F, Courtneidge SA, Alberts AS. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol Cell. 2000;5:13–25. doi: 10.1016/S1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- 72.Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–43. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 73.Chou MM, Blenis J. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85:573–83. doi: 10.1016/S0092-8674(00)81257-X. [DOI] [PubMed] [Google Scholar]
- 74.Berven LA, Willard FS, Crouch MF. Role of the p70(S6K) pathway in regulating the actin cytoskeleton and cell migration. Exp Cell Res. 2004;296:183–95. doi: 10.1016/j.yexcr.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 75.Crouch MF. Regulation of thrombin-induced stress fibre formation in Swiss 3T3 cells by the 70-kDa S6 kinase. Biochem Biophys Res Commun. 1997;233:193–9. doi: 10.1006/bbrc.1997.6419. [DOI] [PubMed] [Google Scholar]
- 76.Lie PP, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–92. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao X, Yang WX. Actin-based dynamics during spermatogenesis and its significance. J Zhejiang Univ Sci B. 2007;8:498–506. doi: 10.1631/jzus.2007.B0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jégou B. The Sertoli cell in vivo and in vitro. Cell Biol Toxicol. 1992;8:49–54. doi: 10.1007/BF00130510. [DOI] [PubMed] [Google Scholar]
- 79.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 80.Vogl AW, Pfeiffer DC, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- 81.Mironova E, Millette CF. Expression of the diaphanous-related formin proteins mDia1 and mDia2 in the rat testis. Dev Dyn. 2008;237:2170–6. doi: 10.1002/dvdy.21622. [DOI] [PubMed] [Google Scholar]
- 82.Chen B, Li A, Wang D, Wang M, Zheng L, Bartles JR. Espin contains an additional actin-binding site in its N terminus and is a major actin-bundling protein of the Sertoli cell-spermatid ectoplasmic specialization junctional plaque. Mol Biol Cell. 1999;10:4327–39. doi: 10.1091/mbc.10.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grove BD, Vogl AW. Sertoli cell ectoplasmic specializations: a type of actin-associated adhesion junction? J Cell Sci. 1989;93:309–23. doi: 10.1242/jcs.93.2.309. [DOI] [PubMed] [Google Scholar]
- 84.Lie PP, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–67. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yazama F, Sawada H, Hirosawa K, Hayashi Y, Nishida T. Deep-etch visualization of the Sertoli cell (blood-testis) barrier in the boar. Tissue Cell. 1991;23:235–46. doi: 10.1016/0040-8166(91)90078-8. [DOI] [PubMed] [Google Scholar]
- 86.Lie PP, Chan AY, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci U S A. 2010;107:11411–6. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kopera IA, Su L, Bilinska B, Cheng CY, Mruk DD. An in vivo study on adjudin and blood-testis barrier dynamics. Endocrinology. 2009;150:4724–33. doi: 10.1210/en.2008-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lui WY, Lee WM, Cheng CY. Rho GTPases and spermatogenesis. Biochim Biophys Acta. 2003;1593:121–9. doi: 10.1016/S0167-4889(02)00348-8. [DOI] [PubMed] [Google Scholar]
- 89.Xia W, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring during spermatogenesis—a lesson to learn from the testis. Cytokine Growth Factor Rev. 2005;16:469–93. doi: 10.1016/j.cytogfr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 90.Russell LD. Spermatid-Sertoli tubulobulbar complexes as devices for elimination of cytoplasm from the head region late spermatids of the rat. Anat Rec. 1979;194:233–46. doi: 10.1002/ar.1091940205. [DOI] [PubMed] [Google Scholar]
- 91.Kierszenbaum AL, Tres LL. The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch Histol Cytol. 2004;67:271–84. doi: 10.1679/aohc.67.271. [DOI] [PubMed] [Google Scholar]
- 92.Guttman JA, Takai Y, Vogl AW. Evidence that tubulobulbar complexes in the seminiferous epithelium are involved with internalization of adhesion junctions. Biol Reprod. 2004;71:548–59. doi: 10.1095/biolreprod.104.028803. [DOI] [PubMed] [Google Scholar]
- 93.Russell LD. Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cells in the rat testis. Anat Rec. 1979;194:213–32. doi: 10.1002/ar.1091940204. [DOI] [PubMed] [Google Scholar]
- 94.Young JS, Guttman JA, Vaid KS, Shahinian H, Vogl AW. Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod. 2009;80:153–61. doi: 10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- 95.Chapin RE, Wine RN, Harris MW, Borchers CH, Haseman JK. Structure and control of a cell-cell adhesion complex associated with spermiation in rat seminiferous epithelium. J Androl. 2001;22:1030–52. doi: 10.1002/j.1939-4640.2001.tb03444.x. [DOI] [PubMed] [Google Scholar]
- 96.Freeman EA, Jani P, Millette CE. Expression and potential function of Rho family small G proteins in cells of the mammalian seminiferous epithelium. Cell Commun Adhes. 2002;9:189–204. doi: 10.1080/15419060216016. [DOI] [PubMed] [Google Scholar]
- 97.Guttman JA, Obinata T, Shima J, Griswold M, Vogl AW. Non-muscle cofilin is a component of tubulobulbar complexes in the testis. Biol Reprod. 2004;70:805–12. doi: 10.1095/biolreprod.103.022723. [DOI] [PubMed] [Google Scholar]
- 98.Rawe VY, Ramalho-Santos J, Payne C, Chemes HE, Schatten G. WAVE1, an A-kinase anchoring protein, during mammalian spermatogenesis. Hum Reprod. 2004;19:2594–604. doi: 10.1093/humrep/deh513. [DOI] [PubMed] [Google Scholar]
- 99.Chen L, Jia JM, Zhong W, Zhang YL, Lü J, Wang HW, et al. [P70S6K is involved in the inhibition of testosterone production in TM3 mouse Leydig cells overexpressing Cox7a2] Zhonghua Nan Ke Xue. 2011;17:291–5. [PubMed] [Google Scholar]
- 100.Lécureuil C, Tesseraud S, Kara E, Martinat N, Sow A, Fontaine I, et al. Follicle-stimulating hormone activates p70 ribosomal protein S6 kinase by protein kinase A-mediated dephosphorylation of Thr 421/Ser 424 in primary Sertoli cells. Mol Endocrinol. 2005;19:1812–20. doi: 10.1210/me.2004-0289. [DOI] [PubMed] [Google Scholar]
- 101.Riera MF, Galardo MN, Pellizzari EH, Meroni SB, Cigorraga SB. Participation of phosphatidyl inositol 3-kinase/protein kinase B and ERK1/2 pathways in interleukin-1beta stimulation of lactate production in Sertoli cells. Reproduction. 2007;133:763–73. doi: 10.1530/rep.1.01091. [DOI] [PubMed] [Google Scholar]
- 102.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 103.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 104.Siu MK, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–47. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 105.Somasiri A, Wu C, Ellchuk T, Turley S, Roskelley CD. Phosphatidylinositol 3-kinase is required for adherens junction-dependent mammary epithelial cell spheroid formation. Differentiation. 2000;66:116–25. doi: 10.1046/j.1432-0436.2000.660206.x. [DOI] [PubMed] [Google Scholar]
- 106.Wong CH, Cheng CY. Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: a review of recent data. Dev Biol. 2005;286:1–15. doi: 10.1016/j.ydbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 107.Wong EW, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGF-beta3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci U S A. 2010;107:11399–404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu BZ, Song YT, Yu DH, Su WH, Gasana V, Li YX, et al. Expression and immunohistochemical localization of Cdc2 and P70S6K in different stages of mouse germ cells. Cell Biochem Funct. 2006;24:113–7. doi: 10.1002/cbf.1306. [DOI] [PubMed] [Google Scholar]
- 109.Feng LX, Ravindranath N, Dym M. Stem cell factor/c-kit up-regulates cyclin D3 and promotes cell cycle progression via the phosphoinositide 3-kinase/p70 S6 kinase pathway in spermatogonia. J Biol Chem. 2000;275:25572–6. doi: 10.1074/jbc.M002218200. [DOI] [PubMed] [Google Scholar]
- 110.Hu J, Chen YX, Wang D, Qi X, Li TG, Hao J, et al. Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Dev Biol. 2004;276:158–71. doi: 10.1016/j.ydbio.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 111.Pellegrini M, Grimaldi P, Rossi P, Geremia R, Dolci S. Developmental expression of BMP4/ALK3/SMAD5 signaling pathway in the mouse testis: a potential role of BMP4 in spermatogonia differentiation. J Cell Sci. 2003;116:3363–72. doi: 10.1242/jcs.00650. [DOI] [PubMed] [Google Scholar]
- 112.Kozawa O, Matsuno H, Uematsu T. Involvement of p70 S6 kinase in bone morphogenetic protein signaling: vascular endothelial growth factor synthesis by bone morphogenetic protein-4 in osteoblasts. J Cell Biochem. 2001;81:430–6. doi: 10.1002/1097-4644(20010601)81:3<430::AID-JCB1056>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 113.Abé K, Eto K, Abé S. Epidermal growth factor mediates spermatogonial proliferation in newt testis. Reprod Biol Endocrinol. 2008;6:7. doi: 10.1186/1477-7827-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nomura M, He Z, Koyama I, Ma WY, Miyamoto K, Dong Z. Involvement of the Akt/mTOR pathway on EGF-induced cell transformation. Mol Carcinog. 2003;38:25–32. doi: 10.1002/mc.10140. [DOI] [PubMed] [Google Scholar]
- 115.Bailly K, Soulet F, Leroy D, Amalric F, Bouche G. Uncoupling of cell proliferation and differentiation activities of basic fibroblast growth factor. FASEB J. 2000;14:333–44. [PubMed] [Google Scholar]
- 116.Catizone A, Ricci G, Galdieri M. Hepatocyte growth factor modulates Sertoli-Sertoli tight junction dynamics. J Cell Physiol. 2008;216:253–60. doi: 10.1002/jcp.21400. [DOI] [PubMed] [Google Scholar]
- 117.Del Bravo J, Catizone A, Ricci G, Galdieri M. Hepatocyte growth factor-modulated rat Leydig cell functions. J Androl. 2007;28:866–74. doi: 10.2164/jandrol.107.002865. [DOI] [PubMed] [Google Scholar]
- 118.Zhou HY, Wong AST. Activation of p70S6K induces expression of matrix metalloproteinase 9 associated with hepatocyte growth factor-mediated invasion in human ovarian cancer cells. Endocrinology. 2006;147:2557–66. doi: 10.1210/en.2005-1404. [DOI] [PubMed] [Google Scholar]
- 119.Chen YJ, Hsiao PW, Lee MT, Mason JI, Ke FC, Hwang JJ. Interplay of PI3K and cAMP/PKA signaling, and rapamycin-hypersensitivity in TGFbeta1 enhancement of FSH-stimulated steroidogenesis in rat ovarian granulosa cells. J Endocrinol. 2007;192:405–19. doi: 10.1677/JOE-06-0076. [DOI] [PubMed] [Google Scholar]
- 120.Avallet O, Vigier M, Perrard-Sapori MH, Saez JM. Transforming growth factor beta inhibits Leydig cell functions. Biochem Biophys Res Commun. 1987;146:575–81. doi: 10.1016/0006-291X(87)90567-5. [DOI] [PubMed] [Google Scholar]




