Abstract
Spermatogenesis is regulated by a cascade of steroid regulated genes in the testis. Recent studies suggested that acupuncture may improve fertility in men with abnormal semen parameters. Yet, the underlying mechanisms in which acupuncture enhances spermatogenesis remain largely unknown. Here we used a scrotal heat-treated rat model to study the effect of electroacupuncture (EA) on recovery of spermatogenesis. In this model, spermatogenesis was disrupted by 30 min scrotal heat treatment at 43°C. Ten sessions of EA were given at Baihui (GV20), Guanyuan (CV4), Zusanli (ST36) and Sanyinjiao (SP6) from day 9 to day 36 post-treatment. Sperm motility and production, morphology of the germinal epithelium by Johnsen’s scoring, germ cell apoptosis by TUNEL staining, proliferation by proliferating cell nuclear antigen (PCNA) staining, as well as serum testosterone and inhibin B levels by immunoassays were evaluated on day 0, 1, 9, 25, 37, 46, 56 and 79. When compared with the heat-treated (H) group, the heat-treated plus EA (H+EA) group showed a significant increase (p < 0.05) in PCNA-positive cells and inhibin B levels on days 37 and 46, and a higher Johnsen’s score till day 56. On day 79, motile spermatozoa could be found in the vas deferens of H+EA group only. Consistently, there was a trend of improved motility and increased number of motile epididymal spermatozoa in the H+EA group than the H group; while apoptosis of germ cells and serum testosterone levels were similar between the two groups. Taken together, EA enhanced germ cell proliferation through improvement of Sertoli cell functions. This may facilitate the recovery of spermatogenesis and may restore normal semen parameters in subfertile patients.
Keywords: Apoptosis, electroacupuncture, proliferation, spermatogenesis, spermatozoa
Introduction
Subfertility affects 15% of couples worldwide, and half of the cases are attributed to male factors including impaired sperm production, activity and transport.1,2 The causes of male subfertility are multi-factorial. Genetic, epigenetic, environmental and lifestyle-related factors, as well as gene-environmental interactions are known to affect human fertility.3 Intervention includes surgical correction of varicoceles and obstructions of the male reproductive ductal system, hormonal therapy, antioxidant therapy, antibiotics, corticosteroids, methylxanthines, vitamins, minerals and amino acids, but only a few of them have been confirmed to be effective by randomized controlled studies.4,5
Acupuncture has a history of 3000 years in China and is increasingly used throughout the world especially in the UK,6 US,7 Australia8 and Japan,9 for pain relief and a variety of disorders, including subfertility.10-12 It is an effective alternative for pain relief during oocyte retrieval.10,13 Acupuncture on the day of embryo transfer may improve the live birth rate of IVF patients.10,14,15
Accumulating evidence suggests that acupuncture may improve sperm count,16-18 motility,19,20 morphology,21 ultra-structural integrity22 and fertilization rate after ICSI19 in subfertile men, partly by modulation of FSH, LH and testosterone levels.23 Acupuncture has been postulated to increase antioxidant supply by vasodilation21 and regulate immune defense and local inflammation.17 However, the underlying mechanism on how acupuncture improves male fertility remains largely unknown.
The heat-treated testis model in rats was successfully used to study spermatogenesis.24-26 Heating of the rat testes in a 43°C water bath for 15 min significantly reduced the testicular weight and increased serum FSH and LH levels on day 9.25 The decrease in testicular weight and sperm count was partially recovered by day 97.26,27 Heat-treatment caused apoptosis of germ cells in the testis. The number of apoptotic cells increased rapidly in a stage and cell specific manner one day after treatment but returned to normal level by day 9.25 The number of proliferating cells as detected by proliferating cell nuclear antigen (PCNA) staining decreased after heat-treatment28 or in experimentally induced cryptorchid testis in mice.29
In humans, a transient increase in scrotal temperatures by occupational exposure, lifestyle, clothing or cryptorchidism was correlated with low sperm concentrations,30,31 reduced sperm motility32 and reduced percentage of normal morphology,33 although some of the associations remained controversial.34,35 We hypothesize that electroacupuncture (EA) increases proliferation and decreases apoptosis of the germ cells in the heat-treated rat testis, thereby facilitates the recovery of spermatogenesis. Therefore, the objective of the present study is to investigate the changes in spermatogenesis and sperm count in heat-treated rats with or without EA treatment.
Results
Body weight, weight of testis, epididymis, seminal vesicle and coagulating gland
The weight of the rat, epididymis, testis and seminal vesicle plus coagulating gland were compared among the untreated control (C), heat-treated (H) and heat-treated plus electroacupuncture (H+EA) groups. No significant differences were found in the body weight and the weight of seminal vesicle plus coagulating gland among the three groups from day 37 onwards. The weights of the testis and the epididymis were significantly reduced (p < 0.05) on day 25 after heat treatment (H25). The weight of the testis, but not of the epididymis increased gradually from day 46 onward in both H and H+EA groups. However, there was no significant difference on the weight of the testis or the epididymis between the H and H+EA groups till day 79 (Table 1).
Table 1. Body weight, weight of testis, epididymis and seminal vesicle plus coagulating gland of control (C), heat-treated (H) and heat-treated plus electroacupuncture (H+EA) rats on day 37, 46, 56 and 79.
| Time (Day) | Symbol | Body Weight (BW, g) | Testis (g/kg BW) | Epididymis (g/kg BW) | Seminal vesicle + Coagulating gland (g/kg BW) |
|---|---|---|---|---|---|
| Day 0 |
C0 |
539 ± 42 |
3.41 ± 0.18 |
1.12 ± 0.08 |
1.50 ± 0.18 |
| Day 1 |
H1 |
494 ± 36 |
3.99 ± 0.56* |
1.28 ± 0.11 |
1.71 ± 0.15 |
| Day 9 |
H9 |
541 ± 26 |
1.71 ± 0.24* |
1.02 ± 0.11 |
1.36 ± 0.44 |
| Day 25 |
H25 |
525 ± 15 |
1.71 ± 0.40* |
0.79 ± 0.18* |
1.49 ± 0.17 |
| Day 37 |
C37 |
510 ± 59 |
3.66 ± 0.21a |
1.20 ± 0.11a |
1.60 ± 0.17 |
| |
H37 |
517 ± 46 |
1.67 ± 0.32b |
0.81 ± 0.05b |
1.59 ± 0.26 |
| |
H+EA37 |
503 ± 31 |
1.77 ± 0.04b |
0.84 ± 0.11b |
1.69 ± 0.45 |
| Day 46 |
C46 |
572 ± 25 |
3.31 ± 0.33a |
1.20 ± 0.07a |
1.46 ± 0.26 |
| |
H46 |
538 ± 54 |
1.76 ± 0.17b |
0.81 ± 0.10b |
1.72 ± 0.38 |
| |
H+EA46 |
557 ± 41 |
1.96 ± 0.31b |
0.81 ± 0.11b |
1.53 ± 0.32 |
| Day 56 |
C56 |
572 ± 34 |
3.30 ± 0.23a |
1.22 ± 0.07a |
1.79 ± 0.21 |
| |
H56 |
588 ± 60 |
1.88 ± 0.38b |
0.71 ± 0.07b |
1.66 ± 0.19 |
| |
H+EA56 |
597 ± 36 |
1.92 ± 0.29b |
0.76 ± 0.04b |
1.59 ± 0.18 |
| Day 79 |
C79 |
573 ± 77 |
3.39 ± 0.44a |
1.22 ± 0.08a |
1.60 ± 0.20 |
| |
H79 |
597 ± 33 |
2.11 ± 0.28b |
0.80 ± 0.05b |
1.63 ± 0.25 |
| H+EA79 | 635 ± 69 | 2.28 ± 0.55b | 0.80 ± 0.08b | 1.62 ± 0.23 |
Results were expressed as mean ± SD. a-b is significantly different (p < 0.05) between treatment groups of the same day. * is significantly different (p < 0.05) from C0.
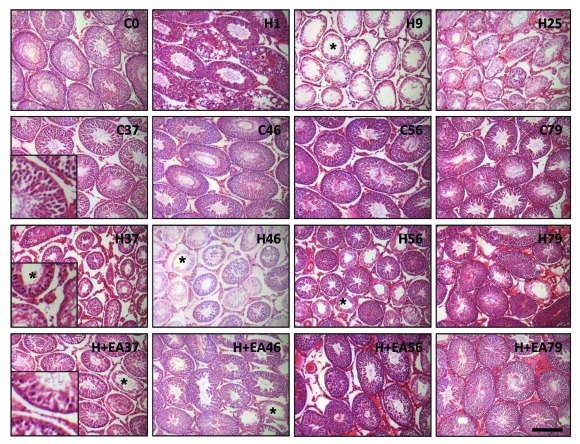
Histology of the seminiferous tubules
The representative histology of the seminiferous tubules of the three groups was shown in Figure 2. There was a drastic change in histology one day after heat exposure (H1). These changes included a decrease in the epithelial thickness, loosening and vacuolization of the germinal epithelium, presence of cellular debris in the lumen, appearing of multinucleated giant cells, and pyknotic cells with fragmented nucleus. On day 9 (H9) and 25 (H25), majority of the seminiferous tubules contained spermatogonia with or without an incomplete layer of primary spermatocytes and no spermatids. Spermatogenesis recovered steadily from day 37 onwards. There were an increasing proportion of normal tubules containing all types of germ cells. Compared with the H group, the H+EA group exhibited a faster recovery in spermatogenesis, though both groups showed variation in the extent of recovery among individual animals within the same treatment group and among seminiferous tubules within the same testis manifested as the presence of some poorly-recovered tubules scattering among normal tubules. The Johnsen’s score (Table 2) was used to quantify the difference in spermatogenesis in the three groups. There was a significant decrease in Johnsen’s score on day 9 (H9) and 25 (H25) after heat treatment when compared with the control (C0). On day 37, the Johnsen’s score of the H+EA group (H+EA37) was comparable to that of the control group (C37) indicating a recovery of spermatogenesis, while the Johnsen’s score of the H group did not reach a normal value until day 79. From day 37 to day 79, the Johnsen’s score was higher in the H+EA group than the H group, albeit the differences were not statistically significant.
Figure 2. Morphology of the seminiferous tubules from the control (C), heat-treated (H) and heat-treated plus electroacupuncture (H+EA) rats. (A) Morphological changes in the seminiferous tubules of the rat testis collected at different time points (day 1, 9, 25, 37, 46, 56 and 79). On day 9 (H9), the germinal epithelium was reduced to spermatogonia or primary spermatocytes. Round spermatids were all missing (seminiferous tubule marked with asterisk). Spermatogenesis gradually recovered from day 37 (H37 and H+EA37). Scale bar = 100 µm.
Table 2. Johnsen’s score, number of proliferating and apoptotic germ cells per tubule, inhibin B and testosterone levels of control (C), heat-treated (H) and heat-treated plus electroacupuncture (H+EA) rats on day 37, 46, 56 and 79.
| Time (Day) | Symbol | Johnsen's score | Number of PCNA positive cells per tubule | Number of TUNEL positive cells per tubule | Inhibin B (pg/ml) | Testosterone (ng/ml) |
|---|---|---|---|---|---|---|
| Day 0 |
C0 |
10 (10–10) |
22.8 ± 3.1 |
0.2 (0.2–0.2) |
29.75 ± 2.31 |
2.12 (1.60–4.00) |
| Day 1 |
H1 |
9.3 (9.1–9.4) |
25.8 ± 3.4 |
63.7 (59.1–66.7)* |
18.88 ± 1.13* |
1.89 (1.31–4.38) |
| Day 9 |
H9 |
4.1 (4–4.2)* |
18.3 ± 0.8 |
0.7 (0.4–1) |
3.93 ± 2.52* |
2.88 (2.26–6.85) |
| Day 25 |
H25 |
3.7 (3.1–5.2)* |
6.8 ± 2.1* |
0.2 (0–0.6) |
2.71 ± 1.66* |
2.71 (2.59–4.38) |
| Day 37 |
C37 |
10.0 (9.8–10) a |
22.9 ± 2.4 a |
0.1 (0.1–0.2) |
27.94 ± 4.16a |
2.19 (1.07–6.86) |
| |
H37 |
5.3 (2.9–7.8) b |
11.2 ± 2.8 b |
0.5 (0–0.7) |
8.52 ± 4.69b |
3.50 (1.53–8.75) |
| |
H+EA37 |
6.7 (4.6–8.1) a,b |
22.7 ± 0.5 a |
0.4 (0.2–0.8) |
20.29 ± 2.88a,b |
2.72 (1.02–4.64) |
| Day 46 |
C46 |
10.0 (9.9–10) a |
20.3 ± 1.4 a |
0.2 (0.2–0.2) |
30.38 ± 3.21a |
4.31 (3.43–5.98) |
| |
H46 |
4.4 (3–8.6) b |
8.6 ± 2.6 b |
0.6 (0.3–1.2) |
9.53 ± 3.59 b |
4.98 (1.55–5.48) |
| |
H+EA46 |
7.7 (6.2–8.9) a,b |
21.2 ± 2.1 a |
0.5 (0.3–0.8) |
19.67 ± 2.26a |
3.48 (1.65–15.00) |
| Day 56 |
C56 |
10.0 (9.9–10) a |
25.3 ± 1.1 |
0.3 (0.3–0.3) |
27.98 ± 5.37 |
4.10 (2.62–20.38) |
| |
H56 |
7.0 (2.9–9.2) b |
14.6 ± 4.3 |
0.4 (0.3–3.4) |
13.79 ± 3.46 |
6.28 (2.51–13.42) |
| |
H+EA56 |
8.2 (3.5–9.3) a,b |
17.5 ± 4.1 |
0.4 (0.2–0.8) |
17.44 ± 0.76 |
6.85 (5.11–18.75) |
| Day 79 |
C79 |
10.0 (10–10) |
25.4 ± 1.1 |
0.3 (0.1–0.3) |
31.77 ± 4.64 |
3.20 (2.11–8.93) |
| |
H79 |
7.6 (3.2–9.4) |
17.4 ± 3.7 |
0.5 (0.4–1.1) |
24.07 ± 4.18 |
2.41 (1.54–13.69) |
| H+EA79 | 8.9 (2.7–9.8) | 16.0 ± 4.0 | 0.2 (0.2–0.3) | 20.24 ± 8.97 | 5.81 (1.40–23.06) |
Results were expressed as mean ± SEM for normally distributed data and median (range) for skewed data. a-b is significantly different (p < 0.05) between treatment groups of the same day. * is significantly different (p < 0.05) from C0.
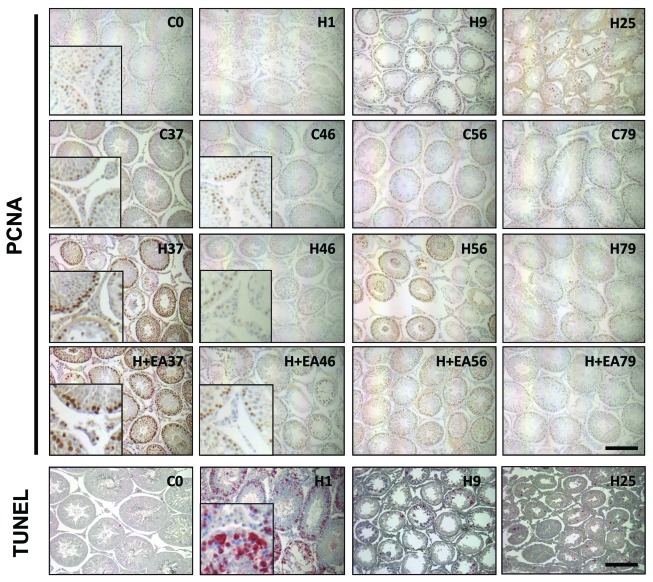
Germ cell proliferation
The proliferation of germ cells was detected by PCNA staining (Fig. 3). The number of PCNA-positive cells per tubule was significantly reduced on day 25, 37 and 46 after heat treatment (H25, H37 and H46) (p < 0.05, Table 2) when compared with the controls (C0, C37 and C46). On day 37 and 46, the number of PCNA-positive cells of the H+EA group was comparable to that of the control group (C37 and C46), but was significantly higher than that of the H group (H37 and H46, p < 0.05). Interestingly, the germ cell proliferation was comparable among the three groups from day 56 onwards.
Figure 3. Immunohistochemical staining for the proliferation and apoptotic marker in the testis. The spermatogonia and preleptotene primary spermatocytes at the cycle of active DNA synthesis were labeled with the anti-proliferating cell nuclear antigen antibody (brown). The number of PCNA-positive cells (per tubule) reduced remarkably on day 25 (H25) after heat treatment, and was higher in the H+EA group than the H group on days 37 and 46. The number of apoptotic (TUNEL-positive) cells increased greatly on day 1 (H1) but was not prominent on day 9 (H9), in which heat treatment caused a dramatic loss of the germ cells. Scale bar = 100 µm.
Germ cell apoptosis (TUNEL assay)
The number of apoptotic cells per tubule increased from 0.2 in the control group on day 0 (C0) to 63.7 one day after heat exposure (H1) (p < 0.05, Table 2 and Figure 3). The value returned to normal on day 9 due to the depletion of germ cells, and remained low during the recovery of spermatogenesis. No statistical difference (p > 0.05) in the number of apoptotic cells was observed among the control, H and H+EA groups from day 37 to day 79.
Serum inhibin B and testosterone concentration
As shown in Table 2, serum inhibin B levels decreased significantly one day after heat treatment (H1). The inhibin B levels were higher in the H+EA group than the H group on day 37 (p = 0.053) and 46 (p < 0.05). No difference was detected in serum testosterone levels after heat treatment or among the three groups throughout the study (Table 2).
Number of motile spermatozoa in the vas deferens
Scrotal heat treatment significantly decreased the number of motile spermatozoa in the vas deferens as no motile spermatozoa were detected in both H and H+EA groups from day 25 to day 56 (Table 3). Interestingly, some motile spermatozoa appeared in the vas deferens of the H+EA group from day 79, and the number was not significantly different from that of the control group (p > 0.05). No motile spermatozoa were found in the H group till the end of the study on day 79 (Table 3).
Table 3. Vas deferens sperm count and motility of epididymal spermatozoa of control (C), heat-treated (H) and heat-treated plus electroacupuncture (H+EA) rats on day 37, 46, 56 and 79.
| Table (Day) | Symbol | Number of motile spermatozoa (million per cm vas deferens) | Epididymal spermatozoa progressive motile (a+b)(%) | Epididymal spermatozoa non-progressive motile (c) (%) |
|---|---|---|---|---|
| Day 0 |
C0 |
1.54 (1.19–2.69) |
27 (11–42) |
49 (35–63) |
| Day 1 |
H1 |
1.88 (1.02–3.22) |
27 (18–44) |
41 (37–50) |
| Day 9 |
H9 |
1.51 (0.43–2.51) |
27 (2–40) |
23 (20–69) |
| Day 25 |
H25 |
0 (0–0)* |
0 (0–0)* |
0 (0–0)* |
| Day 37 |
C37 |
0.89 (0.44–2.09)a |
21 (6–29)a |
49 (23–56)a |
| |
H37 |
0 (0–0)b |
0 (0–0)b |
0 (0–0)b |
| |
H+EA37 |
0 (0–0)b |
0 (0–0)b |
0 (0–0)b |
| Day 46 |
C46 |
1.65 (0.5–5.85)a |
24 (17–36)a |
40 (11–45)a |
| |
H46 |
0 (0–0)b |
0 (0–0)b |
0 (0–0)b |
| |
H+EA46 |
0 (0–0)b |
0 (0–2)b |
0 (0–13)b |
| Day 56 |
C56 |
1.27 (0.32–2.77)a |
24 (19–33)a |
40 (36–45)a |
| |
H56 |
0 (0–0)b |
1 (0–5)b |
1 (0–27)b |
| |
H+EA56 |
0 (0–0)b |
0 (0–1)b |
0 (0–16)b |
| Day 79 |
C79 |
1.46 (0.68–3.21)a |
30 (19–51)a |
48 (30–49)a |
| |
H79 |
0 (0–0)b |
4 (0–12)b |
11 (0–24)b |
| H+EA79 | 0.02 (0–0.4)a,b | 14 (0–23)b | 22 (0–41)a,b |
Results were expressed as median (range). a-b is significantly different (p < 0.05) between treatment groups of the same day. * is significantly different (p < 0.05) from C0.
Motility of epididymal spermatozoa
No motile spermatozoa were found in the epididymis by day 25 post-heat treatment. Newly produced motile spermatozoa were found in the H+EA group from day 46 and in the H group from day 56, but the percentage of progressive motile (a+b) and non-progressive motile (c) spermatozoa in these groups were significantly lower than that of the control group (p < 0.05). On day 79, the H+EA group had a percentage of non-progressive motile spermatozoa comparable to while that of the H group remained significantly lower (p < 0.05) than that of the control group. Yet, the difference in the percentages was not statistically significant between the H and H+EA group (p > 0.05).
Discussion
In the present study, the effects of acupuncture in spermatogenesis and semen parameters were studied using a rat model with heat-treated testes. When compared with the H group, the H+EA group exhibited a faster recovery of spermatogenesis as shown by a higher Johnsen’s score and an improved sperm production and motility. This improvement by EA is associated with an enhanced germ cell proliferation (PCNA staining) and Sertoli cell function (inhibin B level), but not with germ cell apoptosis and Leydig cell function.
The heat-treated testis model is commonly used for spermatogenesis study. A single heat exposure of the rat scrotal testis at 43°C for 30 min significantly reduced the number of motile spermatozoa on day 25 post-treatment. This is consistent with others that the number of spermatozoa in the heat-treated testis and epididymis decreased significantly by day 35 and gradually recovered thereafter.26,27 In a surgical cryptorchid rat model, the number of intact spermatozoa in the testis and epididymis decreased remarkably and no intact spermatozoa was found on day 15.36 In addition, the percentage of motile spermatozoa in the epididymis decreased from day 3 after the surgery, and the sperm motility was significantly impaired from day 5.37 In mice, scrotal heat-treatment at 40°C for 60 min increased the percentage of abnormal spermatozoa in cauda epididymis from day 7 to day 35.38
Heat treatment decreased the number of PCNA positive cells in the testis. It was likely that the total number of spermatogonia and spermatocytes was reduced. In sheep, heat treatment reduced the production of A1 spermatogonia after 20 d,39 and the spermatogonia were arrested.40 Moreover, heat treatment compromised DNA synthesis and reduced DNA polymerases activities in the germ cells of a cryptochid rat model.28
In the present study, almost all secondary spermatocytes and round spermatids were depleted on day 9. The depletion of germ cells was mediated by apoptosis and formation of multinucleated giant cells.25,36 We also found that the proliferation of germ cells was reduced at day 25, but returned to normal level on day 56 onward after heat treatment.
The Johnsen’s score in the H+EA group was comparable to that of the control group on day 37 after treatment, indicating a full recovery of spermatogenesis after 10 sessions of EA treatment, while the scores in the H group on days 37, 46 and 56 were significantly lower than that of the control. Although the differences in the Johnsen’s score and the percentage of advanced tubules between the H+EA and H groups were not statistically significant, probably due to the small sample size and the large sample variation within the same group, a trend of faster recovery in the H+EA group was consistently seen throughout the study.
We observed an increase in germ cell proliferation in the H+EA group when compared with the H group, and a positive correlation between the proliferation index and the Johnsen’s score (Spearman’s rank correlation coefficient: 0.716, p < 0.001). Although there is no evidence on a stimulatory effect of EA on germ cell proliferation, EA facilitates neurogenesis and maturation of newborn neurons in the striatum after transient global ischemia.41 Moreover, EA induces differential expression of genes related to cell differentiation, cell proliferation, muscle repair and hyperplasia in mouse skeletal muscles.42 Furthermore, pre-treatment with moxibustion at one of the acupoints, Zusanli (ST36), of this study, was reported to promote proliferation of gastric mucosal cells in stress-induced gastric ulcer.43
EA facilitates the recovery of spermatogenesis by increasing the motility and production of spermatozoa in the epididymis. Clinical evidence suggests that EA increases sperm count in oligozoospermic patients.16-18,22 In the present study, sperm production was measured by the number of motile spermatozoa in the vas deferens as the immotile spermatozoa lost their fertilization ability in vivo. On day 79, a small amount of motile spermatozoa were only present in the H+EA group but not in the H group. Yet, the increase in sperm motility may result from an enhanced epididymal function by EA, since epididymis is the major place for sperm maturation and storage.44 It may be worthwhile to investigate whether the improved sperm motility after EA was related to changes in the water, pH value or protein profiles in the epididymal fluid that affect sperm maturation and storage.45
How EA stimulates spermatogenesis and increases spermatogonial proliferation remains obscure. It is possible that EA modulates the hypothalamus-pituitary-gonadal axis by altering FSH and LH secretion. Increased FSH secretion induced the production of follistatin and inhibin, which facilitated the transition of gonocytes to spermatogonia in culture of 3-d-old rat testicular fragments.46 Furthermore, FSH was considered as a prerequisite for the completion of spermatogonial mitosis and the differentiation of spermatocytes into spermatids.47 In the heat-treated testis model, the elevation of FSH level from day 9 to day 2825 is possibly a response to facilitate spermatogonial recruitment and proliferation when most of the apoptotic cells have been removed. Thus, the improved basal cell proliferation in the present study may be partly mediated via EA-induced upregulation of FSH. Accumulating evidence suggest that acupuncture restored the lowered FSH to normal level in subfertile men.23 However, whether EA played a similar role in the present study on regulation of FSH needs further investigations.
The serum testosterone levels as well as the weight of the androgen-responsive glands (seminal vesicles and coagulating glands) did not change with or without EA. It was reported that heat treatment did not affect plasma and testicular testosterone concentrations,25 although an increased in serum LH may drive the Leydig cells to produce enough testosterone to maintain normal spermatogenesis.48,49 In line with this, both acupuncture and Chinese herbs were found to restore the reduced LH and testosterone concentrations in subfertile men and rats.23,50
It is possible that GnRH may be involved in the regulation of FSH and LH by EA. In fact, EA was reported to increase the number of GnRH neurons, the GnRH mRNA level in the hypothalamus, and the GnRH-receptor mRNA level in the pituitary in the ovariectomized rats.51 In normal female rats, the expression of hypothalamic GnRH was higher after EA treatment.52 It may be worthwhile to investigate the GnRH level if the FSH and LH levels are confirmed to be changed after EA.
EA affects ovarian blood flow (OBF) and it was found that low-frequency (2 or 10 Hz) EA increased the OBF by the ovarian sympathetic nerves via supra-spinal pathways in normal rats.53 Similarly, heat treatment of rat testes to 43°C for 30 min resulted in a significant reduction in blood flow per testis.49 Since the testes and ovaries share similar innervations, EA with a frequency of 3 Hz/9 Hz in the present study may also improve the testicular blood flow, facilitating the delivery of nutrients for germ cell repopulation and tissue repair. Future studies should be performed to examine the effect of EA on testicular blood flow with a laser-Doppler flow probe.54
Although manual acupuncture is more common in clinical practice, EA was mostly used in research for the sake of standardization of stimulation. The present EA protocol was chosen based on its positive effect on spermatogenesis, semen parameters and hormone levels.16,17,19 The selected acupoints were commonly used in both research and clinical practice related to the reproductive system. According to the Chinese medicine theory, Baihui belongs to the Du meridian and Guanyuan to the Ren meridian. Acupuncture on the two points helps to restore the balance between Yin and Yang. Acupuncture also nourishes the Kidney Yin at Sanyinjiao and improves production of Qi and blood at Zusanli, which are regarded as necessaries for fertility. Although a Deqi sensation (i.e., a feeling of soreness, numbness, distension or pain by patients) represents adequacy of stimulation in humans, the sensation cannot be assessed in rats. A widely accepted criterion for successful stimulation in animal acupuncture is the local muscle twist, which reflects the activation of muscle-nerve afferents.51-53 The adequacy of EA stimulation in animals may also be confirmed by the blood β-endorphine level, which was elevated by 30 min and remained high even at 24-h after acupuncture in humans.55 The dosage of EA (10 sessions) may be inadequate since in standard clinical practice, it usually takes several months to treat subfertility.56 The effect of EA on spermatogenesis may be more prominent if additional sessions are given.
In conclusion, EA improves sperm production after scrotal heat treatment. EA facilitates the recovery of spermatogenesis by enhancing germ cell proliferation and restoring normal inhibin B levels. The underlying mechanism may be related to the changes in hormone levels, testicular blood flow as well as Sertoli cell and Leydig cell functions after EA and warrants further investigations.
Materials and Methods
Animals
Adult SD rats at 9–11 weeks old were used. The rats were housed at the Laboratory Animal Unit of the University of Hong Kong and were acclimatized for at least 7 d before experimentation. They were kept under controlled temperature (22°C) and 12 h light-dark cycles, with free access to water and food. The experiment protocol was approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong (No. 1554–07), and was conducted according to its guidelines for the use of experimental animals.
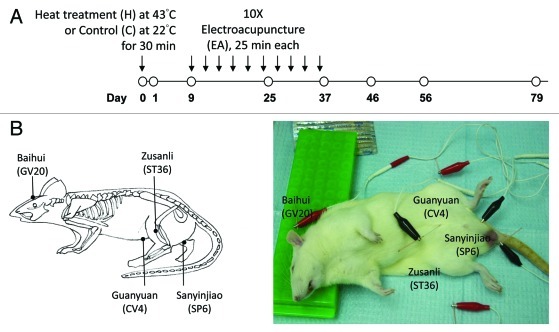
Scrotal heat treatment and electroacupuncture
Rats were divided into 3 groups (C: control; H: heat-treated; H+A: heat-treated plus electroacupuncture, n = 3–6 at each time point). Before heat treatment, the rats were anesthetized. The lower body and scrota containing testes were immersed in a thermostatically controlled water bath (Grant Instruments, model SS40–2) at 43°C (H and H+EA) or 22°C (C) for 30 min. Animals in the C and H group were sacrificed on day 0, 1, 9, 25, 37, 46, 56 and 79 after heat exposure (Fig. 1A). Animals in the H+EA group received additional EA under general anesthesia every 3 d from day 9 to day 36 (10 sessions in total) after heat treatment, and were sacrificed on day 37, 46, 56 and 79 (Fig. 1A). Each EA session lasted for 25 min. Disposable stainless steel needles (0.25 × 40 mm) (Suzhou Medical Appliance, catalog number: AY1981) were inserted into 6 acupoints in the scalp, abdomen and hind limbs [Baihui (GV20), Guanyuan (CV4), Sanyinjiao (SP6; bilateral), Zusanli (ST36; bilateral)], which were then connected to an electrical stimulator (ITO, Model ES-160) (Fig. 1B). The acupoints were electrically stimulated with alternating frequencies of 3 Hz and 9 Hz to prevent desensitization of tissue to stimulation. Individual pulses were symmetric, bi-phasic square wave pulses with alternating polarities and a pulse duration of 0.25 ms. The intensity was the minimal level to induce local muscle contractions (normally 1.5–2 mA), which indicated the activation of muscle-nerve afferents.53 Animals in the control and H group received anesthesia without EA.
Figure 1. Schematic diagram showing electroacupuncture (EA) treatment of the Sprague-Dawley (SD) rat. (A) SD rats were either treated with scrotal heat (43 °C) or control (22 °C) for 30 min. On day 9, EA were performed every three days for 10 sessions (25 min each). Tissue samples were collected on day 0, 1, 9, 25, 37, 46, 56 and 79. (B) EA was performed at six acupoints in the scalp, abdomen and hind limbs [Baihui (GV20), Guanyuan (CV4), Sanyinjiao (SP6; bilateral), Zusanli (ST36; bilateral)]. A schematic diagram showing the acupoints in rat was shown on the left. The acupoints were electrically stimulated with alternating frequencies of 3 Hz and 9 Hz to prevent desensitization of tissue to stimulation.
Blood collection, tissue preparation and release of spermatozoa from the vas deferens and epididymis
The serum from scarified rats was obtained from clotted blood by centrifugation at 4000 rpm for 30 min and stored at -80°C until used. The serum concentration of testosterone and inhibin B was measured by the enzyme linked immunosorbent assay (Diagnostic Systems Laboratories, catalog number: DSL-10–4000 for testosterone and DSL-10–84100i for inhibin B). Each vas deferens was removed with a length of 3 cm from the end joining the prostate gland, and was placed in a Petri dish containing 0.2 ml pre-warmed (37°C) modified Biggers, Whitten, and Whittingham (BWW) medium.57 The spermatozoa were flushed out with 0.8 ml pre-warmed modified BWW from a syringe fitted with a blunt ended needle. The sperm suspension from vas deferens of both sides was then pooled in a 2 ml eppendorf tube. Meanwhile, the left testis was weighed, cut transversely from the center and fixed in 4% paraformaldehyde. The right testis and epididymis, bilateral seminal vesicles plus coagulating glands were also weighed before frozen at -80°C. The distal half of the left cauda epididymis was cut into 3 portions and incubated in 2 ml modified BWW at 37°C for 10 min to facilitate release of spermatozoa and recovery of sperm motility.58
Histological evaluation of spermatogenesis by the Johnsen’s score
Paraffin-embedded testis were cut in 5 µm and stained with hematoxylin (Sigma-aldrich) and eosin (Sigma-aldrich, E6003). More than 100 cross-sections of the seminiferous tubules from each animal were scored from 1 (no cells in the tubule) to 10 (complete spermatogenesis), according to the most advanced cell type in the tubule. The Johnsen’s score was calculated as the mean of these scores.59 The assessor was blinded to the assignment of treatment.
Immunohistochemical staining for PCNA
The assay was performed as published with minor modification.60,61 Briefly, after deparaffinization and antigen retrieval, the sections were quenched with 3% H2O2 for 30 min, followed by blocking with 10% rabbit serum (Sigma-aldrich, R9133) in phosphate-buffered saline for 30 min. The sections were then incubated sequentially with PCNA primary antibody (PC-10, DAKO, catalog number: M0879) at 1:5000 in the blocking solution for 1 h, biotinylated rabbit anti-mouse IgG (DAKO, catalog number: E0354) at 1:3000 in the blocking solution for 30 min and ABC (Vector Laboratories, Inc., catalog number: PK-6100) for 30 min. The peroxidase activity was visualized with 3,3′-diaminobenzidine-4 HCl (DAB) (DAKO). The slides were counterstained with hematoxylin, dehydrated, and mounted with the Permount™ (Electron Microscopy Sciences, catalog number: 17986–01). In the negative control, blocking solution was used in place of the primary antibody.
The proliferation index was calculated as the total number of PCNA-positive cells (spermatogonia and preleptotene primary spermatocytes) per tubule. Only basal germ cells were counted because they are the cells at the cycle of active DNA synthesis.60 Twenty randomly chosen tubules were evaluated for each slide, because our unpublished data showed that the proliferation index from 20 tubules was representative of that from more tubules. The assessor was blinded to the assignment of the treatment.
In situ terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay
The TUNEL assay was performed on the deparaffinized sections with the In Situ Cell Death Detection Kit, AP (Roche Diagnostics), according to the manufacturer’s instructions. The sections were then counterstained with hematoxylin (Sigma-aldrich) and mounted with DAKO mounting solution (DAKO). Positive control sections were pretreated with deoxyribonuclease I (40 IU/mL) (USB, 78411) for 10 min at 37°C before labeling. Sections for the negative control were incubated in the staining solution free of the terminal deoxynucleotidyl transferase.
The level of germ cell apoptosis was expressed as the total number of TUNEL-positive germ cells (red signal) per tubule.62 More than 100 randomly chosen, round tubules from each specimen were evaluated in a blinded manner. Since the aim was to assess germ cell apoptosis during recovery, tubules from day 37 to day 79 were evaluated only if more than 5 spermatocytes or more advanced cells were present; this would exclude the condition in which the low incidence of apoptosis was due to loss of germ cells. The staging of seminiferous tubules was performed according to the criteria previously published.63
Sperm motility and number
Eight microliter of sperm suspension was placed in a Cell VU chamber with a chamber depth of 20 µm (Millennium Sciences Inc.) on a heated (37°C) microscope stage to facilitate free movement of the spermatozoa. The assessment was performed in line with the WHO guidelines for semen analysis64 and ESHRE guidelines for sperm motility.65 The concentration of spermatozoa from the vas deferens was determined with the improved Neubauer hemocytometer according to the WHO criteria.64 Sperm number was calculated as sperm concentration × 2 ml / 6 cm (million per cm vas deferens). The number of motile spermatozoa was obtained by multiplying the sperm count with the percentage of motile spermatozoa in the vas deferens.
Statistical analysis
The results were expressed as mean ± SEM for normally distributed data and median (range) for skewed data. Statistical analysis was done by One Way ANOVA for normally distributed data and Kruskal-Wallis One Way ANOVA on Ranks for skewed data with SigmaStat (Version 3.10, Systat Software, Inc.). Differences with a p value less than 0.05 were statistically significant.
Acknowledgments
The authors would like to thank Prof. B. Chen and Dr. X. An for their advice on the electroacupuncture protocol. This work was supported in part by grants from the Committee on Research and Conference Grant, The University of Hong Kong to K.F. Lee.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/19282
References
- 1.Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab. 2011;25:271–85. doi: 10.1016/j.beem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Wong EW, Cheng CY. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci. 2011;32:290–9. doi: 10.1016/j.tips.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giwercman A, Giwercman YL. Environmental factors and testicular function. Best Pract Res Clin Endocrinol Metab. 2011;25:391–402. doi: 10.1016/j.beem.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 4.de Kretser DM. Male infertility. Lancet. 1997;349:787–90. doi: 10.1016/S0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- 5.Crimmel AS, Conner CS, Monga M. Withered Yang: a review of traditional Chinese medical treatment of male infertility and erectile dysfunction. J Androl. 2001;22:173–82. [PubMed] [Google Scholar]
- 6.Thomas KJ, Nicholl JP, Coleman P. Use and expenditure on complementary medicine in England: a population based survey. Complement Ther Med. 2001;9:2–11. doi: 10.1054/ctim.2000.0407. [DOI] [PubMed] [Google Scholar]
- 7.Burke A, Upchurch DM, Dye C, Chyu L. Acupuncture use in the United States: findings from the National Health Interview Survey. J Altern Complement Med. 2006;12:639–48. doi: 10.1089/acm.2006.12.639. [DOI] [PubMed] [Google Scholar]
- 8.Xue CC, Zhang AL, Lin V, Myers R, Polus B, Story DF. Acupuncture, chiropractic and osteopathy use in Australia: a national population survey. BMC Public Health. 2008;8:105. doi: 10.1186/1471-2458-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita H, Tsukayama H, Sugishita C. Popularity of complementary and alternative medicine in Japan: a telephone survey. Complement Ther Med. 2002;10:84–93. doi: 10.1054/ctim.2002.0519. [DOI] [PubMed] [Google Scholar]
- 10.Ng EH, So WS, Gao J, Wong YY, Ho PC. The role of acupuncture in the management of subfertility. Fertil Steril. 2008;90:1–13. doi: 10.1016/j.fertnstert.2008.02.094. [DOI] [PubMed] [Google Scholar]
- 11.Madaschi C, Braga DP, Figueira RdeC, Iaconelli A, Jr., Borges E., Jr Effect of acupuncture on assisted reproduction treatment outcomes. Acupunct Med. 2010;28:180–4. doi: 10.1136/aim.2009.002022. [DOI] [PubMed] [Google Scholar]
- 12.Moy I, Milad MP, Barnes R, Confino E, Kazer RR, Zhang X. Randomized controlled trial: effects of acupuncture on pregnancy rates in women undergoing in vitro fertilization. Fertil Steril. 2011;95:583–7. doi: 10.1016/j.fertnstert.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Stener-Victorin E. The pain-relieving effect of electro-acupuncture and conventional medical analgesic methods during oocyte retrieval: a systematic review of randomized controlled trials. Hum Reprod. 2005;20:339–49. doi: 10.1093/humrep/deh595. [DOI] [PubMed] [Google Scholar]
- 14.Manheimer E, Zhang G, Udoff L, Haramati A, Langenberg P, Berman BM, et al. Effects of acupuncture on rates of pregnancy and live birth among women undergoing in vitro fertilisation: systematic review and meta-analysis. BMJ. 2008;336:545–9. doi: 10.1136/bmj.39471.430451.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheong YC, Hung Yu Ng E, Ledger WL. Acupuncture and assisted conception. Cochrane Database Syst Rev. 2008:CD006920. doi: 10.1002/14651858.CD006920.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Siterman S, Eltes F, Wolfson V, Zabludovsky N, Bartoov B. Effect of acupuncture on sperm parameters of males suffering from subfertility related to low sperm quality. Arch Androl. 1997;39:155–61. doi: 10.3109/01485019708987914. [DOI] [PubMed] [Google Scholar]
- 17.Siterman S, Eltes F, Wolfson V, Lederman H, Bartoov B. Does acupuncture treatment affect sperm density in males with very low sperm count? A pilot study. Andrologia. 2000;32:31–9. [PubMed] [Google Scholar]
- 18.Siterman S, Eltes F, Schechter L, Maimon Y, Lederman H, Bartoov B. Success of acupuncture treatment in patients with initially low sperm output is associated with a decrease in scrotal skin temperature. Asian J Androl. 2009;11:200–8. doi: 10.1038/aja.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, Huang G, Lu F, Paulus WE, Sterzik K. Influence of acupuncture on idiopathic male infertility in assisted reproductive technology. J Huazhong Univ Sci Technolog Med Sci. 2002;22:228–30. doi: 10.1007/BF02828187. [DOI] [PubMed] [Google Scholar]
- 20.Dieterle S, Li C, Greb R, Bartzsch F, Hatzmann W, Huang D. A prospective randomized placebo-controlled study of the effect of acupuncture in infertile patients with severe oligoasthenozoospermia. Fertil Steril. 2009;92:1340–3. doi: 10.1016/j.fertnstert.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 21.Gurfinkel E, Cedenho AP, Yamamura Y, Srougi M. Effects of acupuncture and moxa treatment in patients with semen abnormalities. Asian J Androl. 2003;5:345–8. [PubMed] [Google Scholar]
- 22.Pei J, Strehler E, Noss U, Abt M, Piomboni P, Baccetti B, et al. Quantitative evaluation of spermatozoa ultrastructure after acupuncture treatment for idiopathic male infertility. Fertil Steril. 2005;84:141–7. doi: 10.1016/j.fertnstert.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 23.Fischl F, Riegler R, Bieglmayer C, Nasr F, Neumark J. [Modification of semen quality by acupuncture in subfertile males] Geburtshilfe Frauenheilkd. 1984;44:510–2. doi: 10.1055/s-2008-1036707. [DOI] [PubMed] [Google Scholar]
- 24.Setchell BP. The Parkes Lecture. Heat and the testis. J Reprod Fertil. 1998;114:179–94. doi: 10.1530/jrf.0.1140179. [DOI] [PubMed] [Google Scholar]
- 25.Lue YH, Hikim AP, Swerdloff RS, Im P, Taing KS, Bui T, et al. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology. 1999;140:1709–17. doi: 10.1210/en.140.4.1709. [DOI] [PubMed] [Google Scholar]
- 26.Setchell BP, Plöen L, Ritzen EM. Effect of local heating of rat testes after suppression of spermatogenesis by pretreatment with a GnRH agonist and an anti-androgen. Reproduction. 2002;124:133–40. doi: 10.1530/rep.0.1240133. [DOI] [PubMed] [Google Scholar]
- 27.Setchell BP, Plöen L, Ritzen EM. Reduction of long-term effects of local heating of the testis by treatment of rats with a GnRH agonist and an anti-androgen. Reproduction. 2001;122:255–63. doi: 10.1530/rep.0.1220255. [DOI] [PubMed] [Google Scholar]
- 28.Fujisawa M, Hayashi A, Okada H, Arakawa S, Kamidono S. Enzymes involved in DNA synthesis in the testes are regulated by temperature in vitro. Eur Urol. 1997;31:237–42. doi: 10.1159/000474457. [DOI] [PubMed] [Google Scholar]
- 29.Kazusa K, Namiki Y, Asano A, Kon Y, Endoh D, Agui T. Differences in spermatogenesis in cryptorchid testes among various strains of mice. Comp Med. 2004;54:179–84. [PubMed] [Google Scholar]
- 30.Banks S, King SA, Irvine DS, Saunders PT. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction. 2005;129:505–14. doi: 10.1530/rep.1.00531. [DOI] [PubMed] [Google Scholar]
- 31.Hjollund NH, Storgaard L, Ernst E, Bonde JP, Olsen J. Impact of diurnal scrotal temperature on semen quality. Reprod Toxicol. 2002;16:215–21. doi: 10.1016/S0890-6238(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 32.Brindley GS. Deep scrotal temperature and the effect on it of clothing, air temperature, activity, posture and paraplegia. Br J Urol. 1982;54:49–55. doi: 10.1111/j.1464-410X.1982.tb13510.x. [DOI] [PubMed] [Google Scholar]
- 33.Figà-Talamanca I, Cini C, Varricchio GC, Dondero F, Gandini L, Lenzi A, et al. Effects of prolonged autovehicle driving on male reproduction function: a study among taxi drivers. Am J Ind Med. 1996;30:750–8. doi: 10.1002/(SICI)1097-0274(199612)30:6<750::AID-AJIM12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Bonde JP. Semen quality in welders exposed to radiant heat. Br J Ind Med. 1992;49:5–10. doi: 10.1136/oem.49.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Figà-Talamanca I, Dell’Orco V, Pupi A, Dondero F, Gandini L, Lenzi A, et al. Fertility and semen quality of workers exposed to high temperatures in the ceramics industry. Reprod Toxicol. 1992;6:517–23. doi: 10.1016/0890-6238(92)90036-S. [DOI] [PubMed] [Google Scholar]
- 36.Chaki SP, Misro MM, Ghosh D, Gautam DK, Srinivas M. Apoptosis and cell removal in the cryptorchid rat testis. Apoptosis. 2005;10:395–405. doi: 10.1007/s10495-005-0813-7. [DOI] [PubMed] [Google Scholar]
- 37.Ren L, Medan MS, Ozu M, Li C, Watanabe G, Taya K. Effects of experimental cryptorchidism on sperm motility and testicular endocrinology in adult male rats. J Reprod Dev. 2006;52:219–28. doi: 10.1262/jrd.17073. [DOI] [PubMed] [Google Scholar]
- 38.Sailer BL, Sarkar LJ, Bjordahl JA, Jost LK, Evenson DP. Effects of heat stress on mouse testicular cells and sperm chromatin structure. J Androl. 1997;18:294–301. [PubMed] [Google Scholar]
- 39.Hochereau-de Reviers MT, Locatelli A, Perreau C, Pisselet C, Setchell BP. Effects of a single brief period of moderate heating of the testes on seminiferous tubules in hypophysectomized rams treated with pituitary extract. J Reprod Fertil. 1993;97:381–7. doi: 10.1530/jrf.0.0970381. [DOI] [PubMed] [Google Scholar]
- 40.de Rooij DG, Okabe M, Nishimune Y. Arrest of spermatogonial differentiation in jsd/jsd, Sl17H/Sl17H, and cryptorchid mice. Biol Reprod. 1999;61:842–7. doi: 10.1095/biolreprod61.3.842. [DOI] [PubMed] [Google Scholar]
- 41.Yang ZJ, Shen DH, Guo X, Sun FY. Electroacupuncture enhances striatal neurogenesis in adult rat brains after a transient cerebral middle artery occlusion. Acupunct Electrother Res. 2005;30:185–99. doi: 10.3727/036012905815901244. [DOI] [PubMed] [Google Scholar]
- 42.Takaoka Y, Ohta M, Ito A, Takamatsu K, Sugano A, Funakoshi K, et al. Electroacupuncture suppresses myostatin gene expression: cell proliferative reaction in mouse skeletal muscle. Physiol Genomics. 2007;30:102–10. doi: 10.1152/physiolgenomics.00057.2006. [DOI] [PubMed] [Google Scholar]
- 43.Yi SX, Peng Y, Chang XR, Peng N, Yan J, Lin YP. Effect of pre-moxibustion on apoptosis and proliferation of gastric mucosa cells. World J Gastroenterol. 2007;13:2174–8. doi: 10.3748/wjg.v13.i15.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borg CL, Wolski KM, Gibbs GM, O’Bryan MK. Phenotyping male infertility in the mouse: how to get the most out of a ‘non-performer’. Hum Reprod Update. 2010;16:205–24. doi: 10.1093/humupd/dmp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buffone MG, Ijiri TW, Cao W, Merdiushev T, Aghajanian HK, Gerton GL. Heads or tails? Structural events and molecular mechanisms that promote mammalian sperm acrosomal exocytosis and motility. Mol Reprod Dev. 2012;79:4–18. doi: 10.1002/mrd.21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sofikitis N, Giotitsas N, Tsounapi P, Baltogiannis D, Giannakis D, Pardalidis N. Hormonal regulation of spermatogenesis and spermiogenesis. J Steroid Biochem Mol Biol. 2008;109:323–30. doi: 10.1016/j.jsbmb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Yazawa T, Yamamoto T, Jin Y, Abé S. Follicle-stimulating hormone is indispensable for the last spermatogonial mitosis preceding meiosis initiation in newts (Cynops pyrrhogaster) Biol Reprod. 2002;66:14–20. doi: 10.1095/biolreprod66.1.14. [DOI] [PubMed] [Google Scholar]
- 48.Damber JE, Bergh A, Janson PO. Leydig cell function and morphology in the rat testis after exposure to heat. Andrologia. 1980;12:12–9. doi: 10.1111/j.1439-0272.1980.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 49.Galil KA, Setchell BP. Effects of local heating of the testis on testicular blood flow and testosterone secretion in the rat. Int J Androl. 1988;11:73–85. doi: 10.1111/j.1365-2605.1988.tb01218.x. [DOI] [PubMed] [Google Scholar]
- 50.Yue GP, Chen Q, Dai N. [Experimental study on effect of bushen shengjing decoction on kidney yang and testicular dysfunction in rats] Zhongguo Zhong Xi Yi Jie He Za Zhi. 1997;17:289–91. [PubMed] [Google Scholar]
- 51.Zhao H, Tian ZZ, Chen BY. Electroacupuncture stimulates hypothalamic aromatization. Brain Res. 2005;1037:164–70. doi: 10.1016/j.brainres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Wang SJ, Zhu B, Jin ZG. [Study on effects of electroacupuncture on the expression of GnRH in the rat at different estrous cycles] Zhongguo Zhen Jiu. 2007;27:273–8. [PubMed] [Google Scholar]
- 53.Stener-Victorin E, Waldenström U, Wikland M, Nilsson L, Hägglund L, Lundeberg T. Electro-acupuncture as a peroperative analgesic method and its effects on implantation rate and neuropeptide Y concentrations in follicular fluid. Hum Reprod. 2003;18:1454–60. doi: 10.1093/humrep/deg277. [DOI] [PubMed] [Google Scholar]
- 54.Setchell BP, Bergh A, Widmark A, Damber JE. Effect of testicular temperature on vasomotion and blood flow. Int J Androl. 1995;18:120–6. doi: 10.1111/j.1365-2605.1995.tb00397.x. [DOI] [PubMed] [Google Scholar]
- 55.Petti F, Bangrazi A, Liguori A, Reale G, Ippoliti F. Effects of acupuncture on immune response related to opioid-like peptides. J Tradit Chin Med. 1998;18:55–63. [PubMed] [Google Scholar]
- 56.Anderson BJ, Haimovici F, Ginsburg ES, Schust DJ, Wayne PM. In vitro fertilization and acupuncture: clinical efficacy and mechanistic basis. Altern Ther Health Med. 2007;13:38–48. [PubMed] [Google Scholar]
- 57.Biggers JD, Whitten WK, Whittingham DG. The culture of mouse embryos in vitro. In: Daniel JCJ (ed). Methods in Mammalian Embryology. San Francisco: Freeman Press, 1971, 86–116. [Google Scholar]
- 58.Pérez-Crespo M, Pintado B, Gutiérrez-Ad´n A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol Reprod Dev. 2008;75:40–7. doi: 10.1002/mrd.20759. [DOI] [PubMed] [Google Scholar]
- 59.Johnsen SG. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 60.Salama M, Tsuji M, Tamura M, Kagawa S. Impact of aging and diabetes mellitus on the expression of the proliferating cell nuclear antigen in rat testicular tissue. Arch Androl. 1998;40:95–107. doi: 10.3109/01485019808987932. [DOI] [PubMed] [Google Scholar]
- 61.Lee KF, Tam YT, Zuo Y, Cheong AW, Pang RT, Lee NP, et al. Characterization of an acrosome protein VAD1.2/AEP2 which is differentially expressed in spermatogenesis. Mol Hum Reprod. 2008;14:465–74. doi: 10.1093/molehr/gan041. [DOI] [PubMed] [Google Scholar]
- 62.Matsuki S, Iuchi Y, Ikeda Y, Sasagawa I, Tomita Y, Fujii J. Suppression of cytochrome c release and apoptosis in testes with heat stress by minocycline. Biochem Biophys Res Commun. 2003;312:843–9. doi: 10.1016/j.bbrc.2003.10.191. [DOI] [PubMed] [Google Scholar]
- 63.Hess RA. Quantitative and qualitative characteristics of the stages and transitions in the cycle of the rat seminiferous epithelium: light microscopic observations of perfusion-fixed and plastic-embedded testes. Biol Reprod. 1990;43:525–42. doi: 10.1095/biolreprod43.3.525. [DOI] [PubMed] [Google Scholar]
- 64.World Health Organization. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction, (4th ed.), Cambridge University Press, New York (1999), Appendix 1A:61. [Google Scholar]
- 65.ESHRE Andrology Special Interest Group. European Society for Human Reproduction and Embryology Guidelines on the application of CASA technology in the analysis of spermatozoa. Hum Reprod. 1998;13:142–5. doi: 10.1093/humrep/13.1.142. [DOI] [PubMed] [Google Scholar]