Abstract
Tubulobulbar complexes (TBCs) are actin-related double-membrane invaginations formed at intercellular junctions in the seminiferous epithelium of mammalian testis. They occur at basal junction complexes between neighboring Sertoli cells and at apical junctions between Sertoli cells and spermatids. They are proposed to internalize intercellular junctions during the translocation of spermatocytes from basal to adluminal compartments of the seminiferous epithelium, and during sperm release from Sertoli cells. Although TBCs are specific to the seminiferous epithelium, they morphologically resemble podosomes in osteoclasts. Previously, we have reported that a key group of proteins consisting of N-WASp, Arp2/3, cortactin and dynamin that occur at podosomes also is present at TBCs. Here we explore the prediction that zyxin, a focal adhesion protein known to be present at podosomes, also is present at apical TBCs. A rabbit polyclonal anti-zyxin antibody (B71) was used to label fixed fragments and frozen sections of testis. In both fragments and sections, B71 labeled tubular regions of TBCs at apical sites of attachment between Sertoli cells and spermatids, in addition to being localized at actin related intercellular adhesion junctions termed ectoplasmic specializations. Although the function of zyxin at TBCs has yet to be determined, the protein is known to interact with the cytoplasmic domain of integrins at focal adhesions, and integrins are known to be present in TBCs.
Keywords: endocytosis, intercellular junctions, podosomes, tubulobulbar complexes, vinculin, zyxin
Introduction
Tubulobulbar complexes are actin-related tubular structures that form at intercellular junctions in testis.1 Formation of tubulobulbar complexes is synchronized with the disappearance of unique actin-related intercellular adhesion junctions termed ectoplasmic specializations (ES).2 Based partly on this observation, and on the observation that basal TBCs in electron micrographs contain tight and gap junctions,2 it has been proposed that the function of tubulobulbar complexes is to internalize intercellular junctions.3 The recent finding that junction proteins (nectin and integrin) are concentrated in apical TBCs and are associated with EEA1 is consistent with this proposal.4
A tubulobulbar complex consists of a long tubular protrusion of the plasma membranes of either a spermatid or a Sertoli cell into a corresponding invagination of an adjacent Sertoli cell.2,3 In the latter Sertoli cell, a network of actin filaments cuffs the double-membrane tube. The distal part of the tube is bulbar in shape, lacks an association with actin and is closely related to a cistern of endoplasmic reticulum. At the terminus of each TBC is a clathrin coated pit.2,3
Tubulobulbar complexes are testis-specific and their double-membrane tubular structure is unique. Morphological studies have established the structure of tubulobulbar complexes but the mechanism by which they are formed remains unclear.
Despite their distinctive characteristics, tubulobulbar complexes resemble podosomes in some other systems.4 Podosomes formed by monocyte-derived cells, like osteoclasts, consist of a tubular plasma membrane core surrounded by a cuff of actin filaments.5 Rather than a projection of one cell into another, podosomes form at areas of cell/substrate attachment and consist of a single membrane invagination.5 In addition, osteoclast podosomes do not have bulbar regions nor are they associated with clathrin-coated pits or other vesicular elements of the endocytosis machinery. Previously, we have shown that tubulobulbar complexes contain similar actin related components including N-WASp, Arp2/3, cortactin, and dynamin 3 to those reported to be present at podosomes and we speculate that the two structures also may have other molecular components in common.4,6 One of these components is zyxin.
Zyxin is an 82 kDa protein that is known to concentrate at focal adhesions.7 It has been described as a mechanosensitive protein due to its ability to mobilize and relocate from focal adhesions to actin stress fibers in response to mechanical cues.7 Zyxin is reported to be present at smooth muscle podosomes.8
In smooth muscle cells, phorbol ester triggers the conversion of focal adhesions into podosomes.8 At discrete microdomains on the ventral surface of the plasma membrane, podosomes form near the sites where stress fibers insert into adhesion plaques.9 These structures are reported to contain α-actinin, F-actin, and vinculin and exhibit a tubular, column-like structure arising perpendicularly to the “bottom” of phorbol dibutyrate treated cells.10 The composition of smooth muscle podosomes has not been confirmed at the ultrastructural level. Despite this lack of morphological evidence, their structure has been included in the description of podosomes that have a central membrane invagination surrounded by an actin filament cuff that in turn is encircled by a larger, ring-shaped structure containing focal adhesion proteins such as α-actinin and vinculin.9 Also, podosome formation is dependent on Arp 2/3-dependent actin polymerization.8
Smooth muscle cell focal adhesions contain structural proteins zyxin and vinculin.8 During the simultaneous events of focal adhesion disassembly and podosome formation, cytoskeletal rearrangement takes place. At later stages of podosome formation, zyxin and vinculin redistribute to podosomes and co-localize at these sites.8 Both zyxin and vinculin have previously been reported to be present at ESs in the seminiferous epithelium.11,12 Furthermore, vinculin has been reported to be present at tubulobulbar complexes.13
In this study, we explore the prediction that these two major structural components, vinculin and zyxin, are co-distributed at tubulobulbar complexes. We immunologically probe cryosections and epithelial fragments of perfusion fixed testis for vinculin and zyxin. We use rat testis as the model system because tubulobulbar complexes are best characterized in this model.
As predicted, zyxin and vinculin co-distribute at apical tubulobulbar complexes. Rather than the staining of individual complexes that was observed with actin assembly proteins, vinculin and zyxin display a more diffuse, although specific association with tubulobulbar complexes.
Results
The zyxin antibody labels ectoplasmic specializations and tubulobulbar complexes
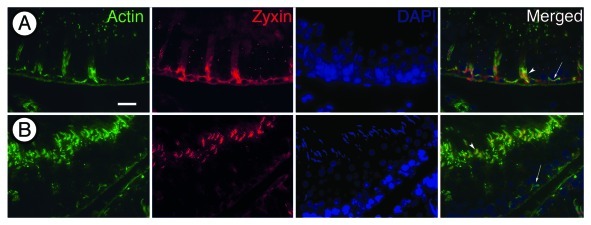
In sectioned tissue, the zyxin antibody reacted at tubulobulbar complexes, in addition to reacting with ectoplasmic specializations as previously been reported.11 Interestingly, the staining varies according to the stage of the tubule that is observed. At stage V of spermatogenesis when spermatids are situated deep in apical Sertoli cell crypts, the zyxin antibody clearly labels ectoplasmic specializations associated with the crypts as indicated by intense staining with phalloidin (Fig. 1A). In stage VII tubules, staining is present adjacent to the convex or dorsal face of late spermatids where ectoplasmic specializations are known to occur, and around tubulobulbar complexes clustered adjacent to the convex face of the hook-shaped spermatid heads (Fig. 1B).
Figure 1. Fluorescence micrographs of rat seminiferous epithelium triple labeled for actin (phalloidin), zyxin and DNA (DAPI). (A) Stage V of spermatogenesis. The phalloidin (green) stains actin filament bundles in ectoplasmic specializations near the base of the epithelium and at sites of attachment between Sertoli cells and spermatids. Zyxin (red) displays a similar staining pattern localizing to ectoplamic specializations and at sites of attachment between Sertoli cells and spermatids. (B) Stage VII of spermatogenesis. Phalloidin stains tubulobulbar complexes in a similar pattern as zyxin. Zyxin is redistributed from ectoplamic specializations to tubulobulbar complexes in stage VII of spermatogenesis. Arrows indicate location of basal ES. Arrowheads indicate location of apical ES. Bar = 20 μm.
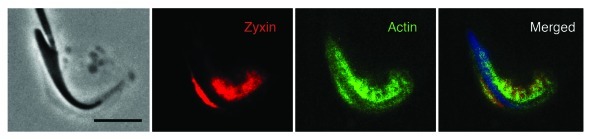
Fragments of seminiferous epithelium viewed at 100X reveal intense staining associated with the dorsal curvature of spermatid heads and diffuse staining around tubulobulbar complexes (Fig. 2) although staining of individual complexes also was observed. Phalloidin can be seen labeling the actin cuff around individual complexes (Fig. 2).
Figure 2. Phase and immunofluorescence micrographs of a rat spermatid and attached Sertoli cell regions labeled for zyxin (red), actin (green), and DNA (blue). Actin can be seen outlining individual tubulobulbar complexes that are adjacent to the concave surface of the spermatid head. Zyxin displays a more diffuse, although specific staining around tubulobulbar complexes. Bar = 5 μm.
Zyxin is present with vinculin at tubulobulbar complexes
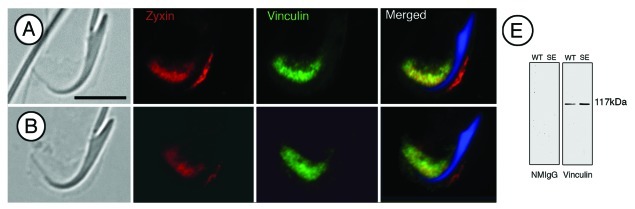
Zyxin and vinculin are present at tubulobulbar complexes. The staining pattern for both proteins appears diffuse, and in some cases outlines individual complexes that are adjacent to the concave surface of mature spermatid heads. A western blot of whole testis and seminiferous epithelium confirms the presence of vinculin (Fig. 3). Interestingly, vinculin stain is not concentrated with zyxin stain in relationship to the dorsal aspect of spermatid heads.
Figure 3. (A and B) Paired phase and immunofluorescence micrographs of rat spermatids and attached Sertoli cell regions triple labeled for zyxin, vinculin and DAPI (blue). Zyxin and vinculin display nearly identical staining patterns—confirmation that the two proteins co-localize. (E) Western blot of whole rat testis (WT) and seminiferous epithelium (SE) labeled for vinculin. A single band in each lysate can be observed at 117 kDa, the appropriate molecular weight of vinculin. Bar = 5 μm.
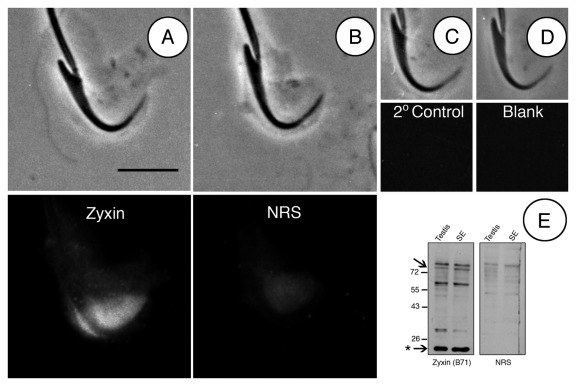
Tubulobulbar complexes are best resolved in fragmented tissue.15 Zyxin can be seen diffusely labeling the Sertoli cell cuff in regions where tubulobulbar complexes are present. These staining patterns are absent in controls (Fig. 4).
Figure 4. Controls for zyxin labeling of rat seminiferous epithelial fragments. (A) late spermatid and attached Sertoli cell cuff labeled for zyxin. Diffuse staining is present around tubulobulbar complexes. (B) late spermatid and attached Sertoli cell treated with Normal Rabbit Serum (NRS) instead of primary antibody. (C) secondary antibody control. (D) blank control for autofluorescence. (E) western blot of seminiferous epithelium and whole testis lysates probed with Zyxin (B71) antibody. A number of immunoreactive bands are visible. The intense band at 20 kDa (asterisk) indicates the HED-2 zyxin splice variant. The 82 kDa band is the expected molecular weight of full length zyxin (arrowhead). Bar = 5 μm.
In testis, the zyxin antibody is specific for zyxin and splice variant HED-2
The zyxin antibody B71 (Beckerle Lab) that was used in these studies reacted on western blots of whole testis and seminiferous epithelium at the appropriate molecular weight of 82 kDa (Fig. 4E). Interestingly, a second, more intense band at 20 kDa was present (Fig. 4E). A band of this weight has been previously reported to be present in human seminiferous epithelium and represents zyxin splice variant HED-2.14 Similar gene splicing may be occurring in rat although this remains to be determined. Like full-length zyxin, HED-2 contains a proline rich domain and three LIM domains. HED-2 is present in Sertoli cells and other tissues.14 A band also is present at 66kDa. Hoffman and coworkers have reported this band previously in platelets as a protein that may have similarity to zyxin.20 Although we believe zyxin is being immunolocalized in Figures 1–4, it is unclear which isoform is being expressed at each specific location.
Discussion
In this study we demonstrate that zyxin is present at tubulobulbar complexes in the seminiferous epithelium of the testis. Furthermore, zyxin co-localizes with vinculin.
Zyxin is a significant component of focal adhesions.16 The protein contains a number of dynamic structural features including three C-terminal LIM domains involved in protein-protein interactions,17 a proline rich domain that can interact with Ena/VASP family members18 and a nuclear export signal that allows translocation between the nucleus and cytoplasm.19
Zyxin-null 3T3 cells display increased motility in comparison to wildtype cells that express zyxin. This phenotype is due to weaker stress fibers that result from a lack of zyxin expression.20 Re-expression of zyxin suppresses the migratory phenotype.21
A number of experiments have shown that zyxin distribution in a cell is mechanosensitive. In mouse fibroblasts, changes in the actin cytoskeleton were observed upon the application of uniaxial cyclic stretch. Filamentous actin realigned itself to be perpendicular to the stretch vector.7 Zyxin mobilized from focal adhesions to stress fibers when cells were subject to this stretch assay. At stress fibers, zyxin acts to reinforce the actin cytoskeleton in response to mechanical stimulation.
At focal adhesions in mouse fibroblasts zyxin and vinculin are colocalized. During cell stretching assays, zyxin translocates from the focal adhesion to stress fibers; however, vinculin stays at the focal adhesion.7 This is in contrast to what we have observed at tubulobulbar complexes and what Gimona and coworkers (Gimona et al. 2003) observed at smooth muscle podosomes where zyxin and vinculin both relocate from sites of adhesion to the actin cytoskeleton.8
Tubulobulbar complexes are testis-specific structures that we propose engage in a sort of clathrin-mediated bulk endocytosis of intercellular junctions during sperm release and translocation of spermatocytes. “Clathrin-mediated bulk endocytosis” is a description that was recently coined in reference to the behavior of reconstituted membranes in a cell free system.22 Upon fission disruption with a G-protein analog, clathrin-mediated tubulation was observed resulting in structures that morphologically resemble tubulobulbar complexes.22
Tubulobulbar complexes that develop at junctions between Sertoli cells and spermatids consist of a long tubular protrusion of spermatid plasma membrane into a corresponding invagination of the adjacent Sertoli cell.2,3 A network of actin filaments cuffs the double-membrane tube and the entire structure ends in a clathrin-coated pit.4
TBCs have features in common with podosomes. Podosomes are subcellular, actin-containing structures formed at cell/matrix contacts.23 Although the function of podosomes is not entirely clear, adhesion and matrix degradation are likely.24 The formation of podosomes is dependent on an actin assembly complex involving N-WASp, Arp2/3, cortactin and dynamin.5 During this formation, zyxin and vinculin redistribute from focal adhesions to podosomes.8 A similar sort of redistribution appears to occur during the formation of tubulobulbar complexes in regions of intercellular attachment in the seminiferous epithelium.
In apical regions of the seminiferous epithelium, tubulobulbar complexes form in regions where unique actin-related intercellular adherens-like junctions (ectoplasmic specializations) occur at sites of Sertoli cell attachment to spermatids. Vinculin and zyxin have both been reported to be present at these actin-related junctions.11,12 The actin assembly proteins N-WASp, Arp2/3, cortactin and dynamin 3 are present at tubulobulbar complexes and presumably are involved with their formation. Vinculin and zyxin appear to redistribute from ectoplasmic specializations to tubulobulbar complexes as the former structures disassemble. The exact role of zyxin and vinculin at TBCs remains to be identified.
Materials and Methods
Animals used in this study were reproductively active male Sprague Dawley rats. They were obtained from Charles River animal colonies and were maintained according to the guidelines established by the Canadian Council on Animal Care. All experiments were done at least in duplicate using tissue from different animals.
Reagents
Unless indicated otherwise, all reagents used in the studies were obtained from Sigma-Aldrich Canada. The paraformaldehyde was obtained from Fisher Scientific (04042–500).
Secondary antibodies and phallotoxins conjugated to Alexa fluorochromes were obtained from Invitrogen (A11008, A11036) and those conjugated to HRP were purchased from Jackson ImmunoResearch Laboratories Inc. (170–5046). Normal serums or immunoglobulins used in specificity controls were purchased from Jackson ImmunoResearch Laboratories, Inc. (011–000–120, 011–000–003).
Primary antibodies
Primary antibodies were obtained from the following sources and used at the following concentrations for immuno-staining and western blots: mouse-anti-vinculin (Sigma Aldrich, V 9131))[1:2500 ]WB [1:250] IF; B71 rabbit-anti-zyxin (gift from Beckerle Lab, Huntsman Cancer Institute) [1:1250] WB, [1:400] IF.
Immunofluorescence
Tissue preparation
All testicular material was perfusion fixed by organ perfusion. Animals were anesthetized with isoflurane, and their testes removed. The spermatic arteries were canulated with 26-gauge needles and perfused with warm PBS (1.5 M NaCl, 0.2M NaKPO4, and 1.5 M KCl, pH 7.3) for 2 min followed by warm fixative (3% paraformaldehyde, 1.5 M NaCl, 0.2 M NaKPO4, and 1.5 M KCl, pH 7.3) for 30 min. The perfusion was changed back to PBS for the last 30 min to complete the fixation.
Fixed frozen zections
Fixed testes were placed on aluminum stubs in a pool of OCT (Optimal Cutting Temperature) Compound (Sakura Finetek, 4583) and then frozen with liquid nitrogen. Frozen sections (5μM) were cut, collected on poly-l-lysine coated slides and immediately plunged into cold (-20°C) acetone. After 5 min, the slides were removed from the acetone, allowed to air dry, and then immunostained.
Fragmented material
Fixed testes were decapsulated in PBS, and the seminiferous tubules were minced into small pieces using scalpels. The material was gently aspirated, first through an 18-gauge needle and then a 21-gauge needle to fragment the seminiferous epithelium. Larger material was allowed to sediment for 10 min and then the supernant, containing epithelial fragments, was collected and concentrated using low-speed centrifugation. The fragments were resuspended in a small amount of PBS, and drops of the material were placed on poly-l-lysine (Sigma, P8920) coated slides. Excess fluid was removed, and the slides were plunged into cold (-20°C) acetone. After 5 min, the slides were removed from the acetone, air-dried, and immunostained.
Staining protocol
Slides with attached sections or fragments were rehydrated with 5% normal goat serum (NGS)(Sigma, G9023) in TPBS-BSA (PBS containing 0.5% Tween-20 and 0.1% BSA) for 20 min. Primary antibodies were diluted with TPBS-BSA containing 1% NGS, added to the tissue, and then the slides were incubated overnight at 4°C. The slides were washed with TPBS-BSA, and the slides were incubated for 1 h at 37°C with secondary antibodies conjugated to Alexa 488 or 568 and diluted 1:100 in TPBS-BSA. Slides were washed and coverslips mounted using Vectashield (Vector Labs (H-1000)) or Vectashield containing 4′, 6′-diamidino-2-phenylindole (DAPI)(H-1200). Staining was evaluated and photographed using a Zeiss Axiophot microscope fitted with appropriate filter sets for detecting fluorescence.
To double immunostain samples, the two primary antibodies were added together as a cocktail to the slides, as were the two secondary antibodies. To label filamentous actin, the slides were stained for 20 min at room temperature with Alexa 568 or 488 phalloidin made up in TPBS-BSA, and then extensively washed with TPBS-BSA.
Western blotting
Western Blots were done using both whole testis and isolated seminiferous epithelium lysates. Preparation of the lysates and staining protocols are described in detail elsewhere (Vaid, Guttman 2007). Lysates included protease inhibitor Complete, mini (Roche, 1–836–170)). Controls for staining consisted of replacing the primary antibody with normal immunoglobulin or serum at the same concentration and from the same animal source as the primary antibody.
Acknowledgments
We would like to thank Mary Beckerle for the generous gift of Zyxin antibody (B71). This article was supported by the Natural Sciences and Engineering Research Council of Canada grant RGPIN 155397–08 to A.W.V.
Glossary
Abbreviations:
- TBC
tubulobulbar complex
- ES
ectoplasmic specialization
- SE
seminiferous epithelium
- WT
whole testis
Disclosure of Potential Conflicts of Interest
The authors have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/19391
References
- 1.Guttman JA, Takai Y, Vogl AW. Evidence that tubulobulbar complexes in the seminiferous epithelium are involved with internalization of adhesion junctions. Biol Reprod. 2004;71:548–59. doi: 10.1095/biolreprod.104.028803. [DOI] [PubMed] [Google Scholar]
- 2.Russell LD. Observations on the inter-relationships of Sertoli cells at the level of the blood- testis barrier: evidence for formation and resorption of Sertoli-Sertoli tubulobulbar complexes during the spermatogenic cycle of the rat. Am J Anat. 1979;155:259–79. doi: 10.1002/aja.1001550208. [DOI] [PubMed] [Google Scholar]
- 3.Russell LD, Clermont Y. Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec. 1976;185:259–78. doi: 10.1002/ar.1091850302. [DOI] [PubMed] [Google Scholar]
- 4.Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod. 2009;80:162–74. doi: 10.1095/biolreprod.108.070623. [DOI] [PubMed] [Google Scholar]
- 5.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–57. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 6.Vaid KS, Guttman JA, Babyak N, Deng W, McNiven MA, Mochizuki N, et al. The role of dynamin 3 in the testis. J Cell Physiol. 2007;210:644–54. doi: 10.1002/jcp.20855. [DOI] [PubMed] [Google Scholar]
- 7.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol. 2005;171:209–15. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaverina I, Stradal TEB, Gimona M. Podosome formation in cultured A7r5 vascular smooth muscle cells requires Arp2/3-dependent de-novo actin polymerization at discrete microdomains. J Cell Sci. 2003;116:4915–24. doi: 10.1242/jcs.00818. [DOI] [PubMed] [Google Scholar]
- 9.Webb BA, Eves R, Crawley SW, Zhou S, Côté GP, Mak AS. PAK1 induces podosome formation in A7r5 vascular smooth muscle cells in a PAK-interacting exchange factor-dependent manner. Am J Physiol Cell Physiol. 2005;289:C898–907. doi: 10.1152/ajpcell.00095.2005. [DOI] [PubMed] [Google Scholar]
- 10.Hai C-M, Hahne P, Harrington EO, Gimona M. Conventional protein kinase C mediates phorbol-dibutyrate-induced cytoskeletal remodeling in a7r5 smooth muscle cells. Exp Cell Res. 2002;280:64–74. doi: 10.1006/excr.2002.5592. [DOI] [PubMed] [Google Scholar]
- 11.Lee NP, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25:200–15. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 12.Grove BD, Vogl AW. Sertoli cell ectoplasmic specializations: A type of actin-associated adhesion junction? J Cell Sci. 1989;93:309–23. doi: 10.1242/jcs.93.2.309. [DOI] [PubMed] [Google Scholar]
- 13.Kusumi N, Watanabe M, Yamada H, Li SA, Kashiwakura Y, Matsukawa T, et al. Implication of amphiphysin 1 and dynamin 2 in tubulobulbar complex formation and spermatid release. Cell Struct Funct. 2007;32:101–13. doi: 10.1247/csf.07024. [DOI] [PubMed] [Google Scholar]
- 14.Yang JX, Miao SY, Wu YW, Zhang Z, Zong SD, Wang LF, et al. Gene encoding a human testis Sertoli cell component related to LIM domain protein. Biochem Mol Biol Int. 1998;46:11–9. doi: 10.1080/15216549800203502. [DOI] [PubMed] [Google Scholar]
- 15.Young JS, Guttman JA, Vaid KS, Shahinian H, Vogl AW. Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod. 2009;80:153–61. doi: 10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- 16.Crawford AW, Beckerle MC. Purification and characterization of zyxin, an 82,000-dalton component of adherens junctions. J Biol Chem. 1991;266:5847–53. [PubMed] [Google Scholar]
- 17.Schmeichel KL, Beckerle MC. The LIM domain is a modular protein-binding interface. Cell. 1994;79:211–9. doi: 10.1016/0092-8674(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 18.Drees B, Friederich E, Fradelizi J, Louvard D, Beckerle MC, Golsteyn RM. Characterization of the interaction between zyxin and members of the Ena/vasodilator-stimulated phosphoprotein family of proteins. J Biol Chem. 2000;275:22503–11. doi: 10.1074/jbc.M001698200. [DOI] [PubMed] [Google Scholar]
- 19.Nix DA, Beckerle MC. Nuclear-cytoplasmic shuttling of the focal contact protein, zyxin: a potential mechanism for communication between sites of cell adhesion and the nucleus. J Cell Biol. 1997;138:1139–47. doi: 10.1083/jcb.138.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman LM, Nix DA, Benson B, Boot-Hanford R, Gustafsson E, Jamora C, et al. Targeted disruption of the murine zyxin gene. Mol Cell Biol. 2003;23:70–9. doi: 10.1128/MCB.23.1.70-79.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amsellem V, Kryszke M-H, Hervy M, Subra F, Athman R, Leh H, et al. The actin cytoskeleton-associated protein zyxin acts as a tumor suppressor in Ewing tumor cells. Exp Cell Res. 2005;304:443–56. doi: 10.1016/j.yexcr.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Wu M, Huang B, Graham M, Raimondi A, Heuser JE, Zhuang X, et al. Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system. Nat Cell Biol. 2010;12:902–8. doi: 10.1038/ncb2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–85. doi: 10.1016/S0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 24.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–57. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]