Abstract
Expression of 15-lipoxygenase-1 (15-LOX-1) is decreased in many human cancers; however, the mechanistic significance of its decreased expression has been difficult to determine because its mouse homolog 12/15-LOX has opposing functions. We generated a mouse model in which expression of a human 15-LOX-1 transgene was targeted to the intestinal epithelium via the villin promoter. Targeted expression was confirmed by real-time reverse transcription–polymerase chain reaction and immunoblotting. When the 15-LOX-1 transgene was expressed in colonic epithelial cells of two independent mouse lines (B6 and FVB), azoxymethane-inducible colonic tumorigenesis was suppressed (mean number of tumors: wild type [WT] = 8.2, 15-LOX-1+/− = 4.91, 15-LOX-1+/+ = 3.57; WT vs 15-LOX-1+/− two-sided P = .003, WT vs 15-LOX-1+/+ two-sided P < .001; n = 10–14 mice per group). 15-LOX-1 transgene expression was always decreased in the tumors that did develop. In the presence of expression of the 15-LOX-1 transgene, expression of tumor necrosis factor alpha and its target inducible nitric oxide synthase were decreased and activation of nuclear factor-kappa B in colonic epithelial cells was inhibited.
CONTEXT AND CAVEATS
Prior knowledge
Expression of 15-lipoxygenase-1 (15-LOX-1) is reduced in many human cancers; however, it has been difficult to establish a mouse model to determine its function in tumorigenesis because its murine homolog has additional, and interfering, enzymatic activity. To clarify this issue, the authors studied azoxymethane (AOM)-induced colonic tumorigenesis in transgenic mice in which human 15-LOX-1 was expressed specifically in intestinal epithelial cells.
Study design
Transgenic mice that expressed human 15-LOX-1 from the villin promoter in colonic epithelial cells were established in two different genetic backgrounds. Tissue-specific expression of the transgene, susceptibility to AOM-induced colonic tumorigenesis, and activation of the tumor necrosis factor alpha (TNF-α), inducible nitric oxide synthase (iNOS), and nuclear factor-κB (NF-κB) pathways were examined in these mice and the parental wild-type strain.
Contribution
Mice in which human 15-LOX-1 was expressed in colonic epithelial cells were less susceptible than wild-type mice to AOM-induced colonic tumorigenesis. Expression of the 15-LOX-1 transgene was reduced in those tumors that did develop. Expression of the 15-LOX-1 was also associated with reduced expression of TNF-α and its target, iNOS, and with reduced activation of NF-κB.
Implication
15-LOX-1 appears to act as a tumor suppressor and an inhibitor of TNF-α–iNOS signaling and NF-κB activation.
Limitations
The conclusions are based on overexpression of a human gene in a mouse genetic background. The gene was overexpressed only in intestinal epithelial cells, so the effects of 15-LOX-1 expression in stromal cells and other tissues are unknown.
From the Editors
Emerging data support a mechanistic link between chronic inflammation and tumorigenesis, especially colonic tumorigenesis (1). Aberrant cell differentiation, especially suppression of terminal cell differentiation, is also thought to be an important mechanism for promoting tumorigenesis (2). 15-Lipoxygenase-1 (15-LOX-1) is an inducible and highly regulated enzyme in normal human cells (3), which has a key role in the production of lipid signaling mediators, for example, 13-hydroxyoctadecadienoic acid (13-HODE) from linoleic acid (4) and resolvins and protectins from docosahexaenoic acid (5). 15-LOX-1 is important to the resolution of inflammation (5) and to terminal differentiation of normal cells via degradation of organelles as the cells terminally mature (3).
Expression of 15-LOX-1 is decreased in various human cancers, including colon cancer (6–8), esophageal cancer (9), breast cancer (10), and pancreatic cancer (11). Re-expression of 15-LOX-1 in colon cancer cell lines by pharmaceutical or genetic means inhibits their proliferation in vitro and their growth as xenograft tumors in immunosuppressed mice (7,8,12,13). The role of 15-LOX-1 in tumorigenesis, however, continues to be debated (14). It has been difficult to study in murine models because its murine homolog, 12/15-LOX, produces both 12-hydroxyeicosatetraenoic acid (12-HETE) and 13-HODE, which have opposing biological effects on important physiological and pathological processes, including tumorigenesis (15,16). To determine the specific impact of 15-LOX-1 expression in colonic epithelial cells on colonic tumorigenesis, we developed a novel transgenic 15-LOX-1 mouse model, villin-15-LOX-1 mice, in which human 15-LOX-1 (ALOX15) expression is targeted to the intestinal epithelial cells via the villin promoter. Using this model, we examined the effects of targeted 15-LOX-1 expression on azoxymethane (AOM)-induced colonic tumorigenesis (17) and on tumor necrosis factor alpha (TNF-α) and inducible nitric oxide synthase (iNOS) signaling, which have been reported to promote colitis-associated colonic tumorigenesis (18).
We generated villin-15-LOX-1 mice by subcloning human 15-LOX-1 cDNA into a villin promoter–driven expression construct (p12.4Kvill-15-LOX-1), which was then used for pronuclear injection into C57BL/6 (B6) and FVB/N (FVB) fertilized oocytes (Supplementary Methods, available online). Four villin-15-LOX-1 founder mice, one with a B6 and three with an FVB background, were identified with genomic quantitative real-time polymerase chain reaction (qPCR) testing using specific primers and probes for the human 15-LOX-1 sequence that had no cross-reactivity with mouse DNA sequences including 12/15-LOX (Supplementary Figure 1, A and B, available online). Because B6 mice are resistant to AOM-induced tumorigenesis, the villin-15-LOX-1 B6 mouse line was backcrossed, using marker-assisted accelerated backcrossing, to mice with 95% or higher FVB background and then bred for three more generations with FVB wild-type (WT) mice to generate villin-15-LOX-1-A mice to test AOM-induced colonic tumorigenesis. We also selected an FVB mouse line with high 15-LOX-1 expression level in the colon, referred to as villin-15-LOX-1-B (Supplementary Figure 1, B, available online), for AOM testing. Heterozygous and homozygous villin-15-LOX-1-A and villin-15-LOX-1-B mice were identified by measuring levels of genomic DNA for the 15-LOX-1 transgene (Supplementary Figure 1, C and D, available online). (Experiments were conducted according to protocols approved by the M. D. Anderson Institutional Animal Care and Use Committee. The mice were treated in accordance with the US Public Health Service “Guide for the Care and Use of Laboratory Animals.”)
15-LOX-1 expression was proportional to the levels of genomic 15-LOX-1 transgenic DNA in both villin-15-LOX-1-A mice (Supplementary Figure 1, E, available online) and villin-15-LOX-1-B mice (Supplementary Figure 1, F, available online). We detected 15-LOX-1 expression at the mRNA level (by quantitative reverse transcription–real-time PCR [qRT-PCR]) and at the protein level (by immunoblotting) in isolated intestinal crypts but not in other organs (for villin-15-LOX-1-A mice, see Supplementary Figure 1, G and I [available online] and for villin-15-LOX-1-B mice, see Supplementary Figure 1, H and J [available online]). Thus, we confirmed that 15-LOX-1 expression was targeted to the intestinal epithelial cells in our transgenic model, as expected (19).
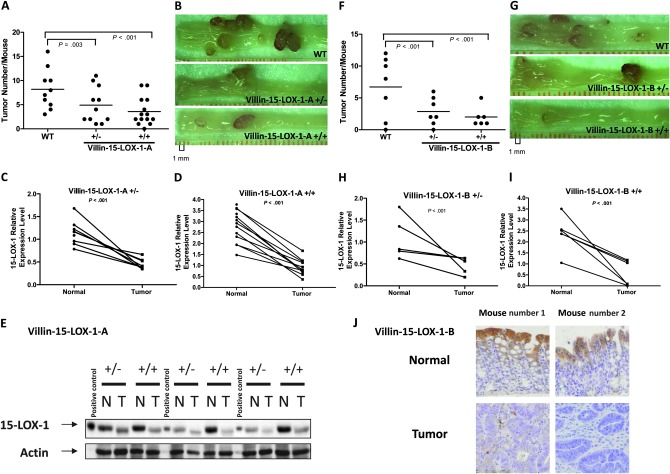
In the presence of targeted 15-LOX-1 expression, AOM-induced colorectal tumorigenesis was statistically significantly inhibited in both independent transgenic mouse lines. Villin-15-LOX-1-A transgenic mice had a statistically significantly reduced number of colon tumors per mouse compared with WT mice (mean for WT = 8.2, heterozygotes = 4.91, homozygotes = 3.57: tumor numbers were, on average, 40.13% [95% confidence interval, CI, = 15.6% to 57.54%; P = .003] lower in heterozygotes and 56.54% [95% CI = 38.09% to 69.36%; P < .001] lower in homozygotes compared with WT; Figure 1, A and B). Similarly, villin-15-LOX-1-B transgenic mice had a statistically significantly reduced number of colon tumors per mouse compared with WT mice (mean for WT = 6.71, heterozygotes = 2.86, homozygotes = 2; tumor numbers were, on average, 57.45% [95% CI = 28.19% to 74.78%; P = .001] lower in heterozygotes and 70.21% [95% CI = 43.85% to 84.2%; P < .001] lower in homozygotes compared with WT; Figure 1, F and G). One WT mouse and one villin-15-LOX-1-A heterozygous mouse were killed 17 days before the prespecified day for killing the mice because large rectal tumors caused bleeding and distress. These findings—that two unrelated mouse lines established via two separate pronuclear injections into two different genetic backgrounds showed similar results with respect to colonic tumorigenesis—indicated that the effects of 15-LOX-1 transgenic expression on tumor formation were not mouse line specific.
Figure 1.
Effects of 15-lipoxygenase-1 (15-LOX-1) transgenic expression on axoxymethane (AOM)-induced colonic tumorigenesis. See Supplementary Methods (available online). A–E) Wild-type (WT) FVB mice (n = 10), villin-15-LOX-1-A heterozygotes (n = 11), and villin-15-LOX-1-A homozygotes (n = 14) were treated with AOM for 6 weeks. Mice were killed 20 weeks after the end of AOM treatment and examined for tumor formation. A) Scatter plot of tumor incidence. The mean number of tumors per mouse decreased as villin-15-LOX-1 expression increased (WT vs heterozygotes, P = .003; WT vs homozygotes, P < .001, two-sided Poisson regression). B) Dissected colons of mice after treatment with AOM. Photographs were taken with a SMZ800 stereoscopic zoom microscope (Nikon Instruments Inc, Melville, NY) at ×10 magnification. C and D) 15-LOX-1 mRNA expression in normal colon mucosa vs colon tumors. 15-LOX-1 mRNA in colonic epithelial cells was measured by quantitative reverse transcription–polymerase chain reaction. Values are means of triplicate measurements from normal mucosa and tumor tissue from each mouse (normal mucosa vs tumor, P < .001, two-sided paired t test for both heterozygotes and homozygotes). E) 15-LOX-1 protein expression in normal colon mucosa vs colon tumors. 15-LOX-1 protein expression was measured by immunoblotting of intestinal crypt lysates. 15-LOX-1 protein expression in HCT-116 cells transfected with a 15-LOX-1 expression vector served as a positive control. N = normal mucosal crypts; T = tumor mucosal crypts. F–I) Same as panels (A–D), except in villin-15-LOX-1-B mice. J) 15-LOX-1 protein expression in villin-15-LOX-1-B treated with AOM. Representative photographs of normal and tumor colonic mucosa stained for 15-LOX-1 by immunohistochemistry.
15-LOX-1 mRNA expression and protein expression were uniformly decreased in colon tumors formed in both lines of transgenic villin-15-LOX-1 mice. In villin-15-LOX-1-A mice, 15-LOX-1 mRNA expression was higher in normal mucosa than in tumors in each mouse that developed colon tumors (in heterozygous villin-15-LOX-1-A mice, mean levels of 15-LOX-1 mRNA in nonmalignant colonic epithelial cells = 1.14 vs in tumor cells = 0.45, difference = 0.63 [calculated on log transformed data], 95% CI = 0.42 to 0.94, P < .001; in homozygous villin-15-LOX-1-A mice, mean expression in nonmalignant cells = 2.78 vs in tumor cells = 0.92, difference = 1.63, 95% CI = 1.24 to 2.14, P < .001; Figure 1, C and D). 15-LOX-1 protein expression was also higher in nonmalignant than in malignant colonic epithelial cells from the same mouse (Figure 1, E). Similarly, in both heterozygous and homozygous villin-15-LOX-1-B mice, there was more 15-LOX-1 mRNA expressed in nonmalignant than in malignant colonic epithelial cells (in heterozygotes, mean relative expression level in normal cells = 1.081 vs in tumor cells = 0.467, difference = 0.44, 95% CI = 0.14 to 1.37, P = 0.036; in homozygotes, mean expression in normal cells = 2.39 vs in tumor cells = 0.59, difference = 1.64, 95% CI = 1.01 to 2.67, P = 0.02; Figure 1, H and I). In 15-LOX-1-B mice, 15-LOX-1 protein expression was detected by immunohistochemistry in normal colonic crypts—especially in the upper part of the crypts, where cells undergo differentiation and apoptosis—but it was markedly decreased in paired tumor colonic epithelial cells (Figure 1, J). These novel findings suggest that decreased 15-LOX-1 expression was required for tumor formation even when 15-LOX-1 was expressed under the control of a constitutively active promoter in epithelial cells.
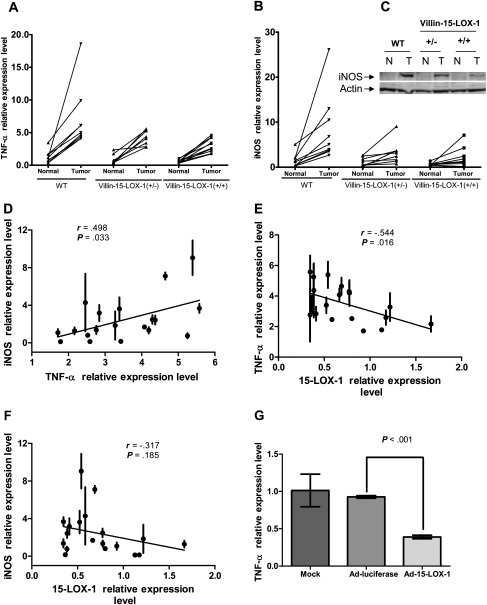
We next set out to examine whether the mechanism of 15-LOX-1-inhibited tumorigenesis includes suppression of TNF-α–iNOS signaling. TNF-α is a major proinflammatory cytokine that contributes to the pathogenesis of human colitis; TNF-α-blocking agents are approved for the treatment of ulcerative colitis (20). Furthermore, TNF-α activates iNOS transcription (21), and this signaling is linked to colitis-associated colonic tumorigenesis in mice (18). To investigate the effect of the 15-LOX-1 transgene on TNF-α-iNOS signaling, we measured TNF-α and iNOS mRNA expression levels in tumor and normal colonic epithelial cells of WT and villin-15-LOX-1 mice. Both TNF-α and iNOS expression were markedly increased in colon tumors induced by AOM and their levels in these tumors were statistically significantly inversely associated with 15-LOX-1 expression levels (Supplementary Table 1, available online; Figure 2, A, B, and C). In WT mice, both TNF-α and iNOS mRNA expression were higher in tumors than in paired normal mucosa (Supplementary Table 1, available online; Figure 2, A and B). These results suggest that TNF-α–iNOS signaling is increased during colonic tumorigenesis not only in colitis-associated colon cancer mouse models but also in the AOM-induced colonic tumorigenesis model, which is more representative of the human colonic tumorigenesis in general.
Figure 2.
Effects of 15-lipoxygenase-1 (15-LOX-1) transgene expression on tumor necrosis factor alpha (TNF-α) and inducible nitric oxide synthetase (iNOS) expression during colonic tumorigenesis. See Supplementary Methods (available online). A and B) Effects of 15-LOX-1 transgene expression on TNF-α and iNOS expression during axoxymethane (AOM)-induced colonic tumorigenesis. TNF-α and iNOS mRNA expression levels were measured by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) in isolated epithelial cells of normal colon mucosa and colon tumors of individual WT mice (n = 9), and heterozygous (n = 9) and homozygous (n = 13) villin-15-LOX-1-A transgenic mice that were treated with AOM for 6 weeks and killed 20 weeks after the end of AOM treatment. Values are means from triplicate measurements for each mouse. P values were determined by two-sided two-way analysis of variance tests. C) Effect of 15-LOX-1 transgene expression on iNOS protein expression in intestinal crypt lysates, as measured by immunoblotting. N = normal mucosal crypts; T = tumor mucosal crypts. D) Correlation between TNF-α and iNOS expression levels in colon tumors. Scatter plot of TNF-α levels and iNOS mRNA relative expression levels in tumors from each of the individual villin-15-LOX-1 heterozygotes and homozygotes treated with AOM as described for panels (A) and (B); mRNA levels were measured by qRT-PCR. Values are the means and 95% confidence intervals (CIs) of triplicate measurements from each mouse. r = Spearman correlation coefficient. E and F) Correlation between 15-LOX-1 transgenic expression and TNF-α and iNOS expression levels in colon tumors. Scatter plots show 15-LOX-1 mRNA expression in relation to TNF-α level (E) or iNOS level (F) in tumors from each of the individual villin-15-LOX-1 heterozygotes and homozygotes treated with AOM as described for panels (A) and (B). mRNA levels were measured by qRT-PCR. Values are the means and 95% CIs of triplicate measurements from each mouse. r = Spearman correlation coefficient. G) Effects of 15-LOX-1 expression on TNF-α expression in a colon cancer cell line. LoVo colon cancer cells were transfected with either an adenoviral vector that expresses 15-LOX-1 (Ad-15-LOX-1) or with the same vector expressing luciferase (Ad-Luciferase) as a control. Cells were harvested 48 hours after transfection, and TNF-α mRNA was measured by qRT-PCR. Values are the means and 95% CIs of triplicate experiments. P values were determined by two-sided, one-way analysis of variance tests. Three independent experiments showed similar results.
More importantly, the differences in TNF-α mRNA levels between malignant and normal colonic epithelial cells were statistically significantly smaller in the homozygous and heterozygous villin-15-LOX-1 mice compared with WT mice (Supplementary Table 1, available online; Figure 2, A). Similarly, there were statistically significantly lesser increases in iNOS mRNA in tumor vs normal tissue of heterozygous and homozygous villin-15-LOX-1 mice compared with WT mice (Supplementary Table 1, available online; Figure 2, B). We used additional statistical modeling to test for 15-LOX-1 trend effects on the tumor–normal differences for TNF-α and iNOS by computing an interaction between the linear orthogonal polynomial in genotype vs tumor–normal interaction. We found the interaction to be statistically significant for both TNF-α (P = .04) and for iNOS (P < .001); the tumor–normal differences were greater in WT than in heterozygous or homozygous villin-15-LOX-1 mice, with the decrease being proportional to the heterozygous or homozygous status of 15-LOX-1 transgene expression.
In the presence of 15-LOX-1 transgene expression, iNOS protein expression was also reduced in tumors (Figure 2, C). The expression of iNOS mRNA was proportional to TNF-α mRNA expression (Figure 2, D). Furthermore, we observed an inverse correlation between 15-LOX-1 mRNA levels in colon tumors of villin-15-LOX-1 transgenic heterozygote and homozygote mice and TNF-α (r = −0.544, P = .016, Figure 2, E) and a trend for iNOS (r = −0.317, P = .185, Figure 2, F), which supports the notion that 15-LOX-1 transgene expression inhibited both TNF-α and iNOS expression during colonic tumorigenesis.
This mechanistic link was further confirmed by an experiment in human LoVo colon cancer cells. Expression of 15-LOX-1 via an adenoviral vector in LoVo cells statistically significantly inhibited TNF-α expression in comparison with expression of a control adenoviral vector (Ad-Luciferase) or mock transfection (Figure 2, G). These findings suggest that 15-LOX-1 inhibits TNF-α–iNOS signaling as a mechanism to suppress colonic tumorigenesis.
Nuclear factor-kappa B (NF-κB) transcription factors are heterodimeric proteins composed of different combinations of members of the Rel/NF-κB family of transcription factors, and they play important roles in promoting chronic inflammation and tumorigenesis, especially in the case of colon cancer (22). TNF-α activates NF-κB (22); iNOS is a downstream target for NF-κB to promote tumorigenesis (23). On the basis of our observation that 15-LOX-1 inhibited both TNF-α and iNOS, we sought to determine whether 15-LOX-1 transgene–mediated modulation of NF-κB signaling might contribute to 15-LOX-1-mediated inhibition of colonic tumorigenesis.
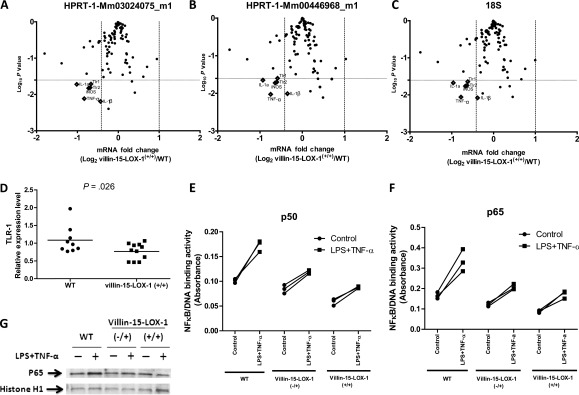
We examined the effects of 15-LOX-1 on NF-κB signaling using TaqMan low-density arrays (Applied Biosystems, Foster City, CA) to measure mRNA expression of 88 genes involved in the NF-κB signaling pathway to determine which of these genes were differentially expressed in the normal colonic epithelial cells of AOM-treated homozygous 15-LOX-1 mice, in which tumorigenesis was inhibited, compared with that of AOM-treated WT mice (Supplementary Methods, available online). An exploratory analyses of these measurements using a cutoff P value of .05 showed that 15-LOX-1 altered the expression of approximately 22% of the tested genes in the normal colonic epithelial cells of villin-15-LOX-1-A mice compared with that of WT controls (Supplementary Table 2, available online). In a further exploratory analysis using volcano plots of the distributions of fold change (log2 [fold change]) for biological significance and Student's t test P values (−log10 [P value]) for statistical significance, we identified six genes with at least 0.25-fold less expression in homozygous villin-15-LOX-1 mice compared with WT mice (two-sided Student's t test P values < .025): IL-1β, TNF-α, Toll-like receptor 1 (TLR-1), Toll-like receptor 2 (TLR-2), iNOS, and IL-1α (Figure 3, A–C).
Figure 3.
Effects of 15-lipoxygenase-1 (15-LOX-1) transgenic expression on nuclear factor-kappa B (NF-κB) signaling in colonic epithelial cells. See Supplementary Methods (available online). A–C) Effects of 15-LOX-1 transgenic expression on NF-κB signaling pathways. Homozygous villin-15-LOX-1-A transgenic mice (villin-15-LOX-1+/+) and control (wild-type [WT]) mice were treated with azoxymethane (AOM) and killed 20 weeks later as described in Supplementary Methods (available online). Gene expression was measured in isolated normal colonic crypt epithelial cells using TaqMan low-density array assays. Data were normalized to levels obtained with either HPRT-1 mRNA probe A (Mm00446968_m1), HPRT-1 mRNA probe B (Mm03024075_m1), or 18S ribosomal RNA. C). Volcano plots are shown that relate the fold changes in NF-κB signaling pathway–related gene expression in nonmalignant colonic mucosa between these two groups of mice. Diamonds indicate genes with more than 25% reduced expression levels (P values were determined by two-sided Student's t test P values < .025). D) Effects of 15-LOX-1 transgenic expression on Toll-like receptor-1 (TLR-1) expression in colonic epithelial cells. TLR-1 mRNA expression was measured using quantitative reverse transcription real-time polymerase chain reaction in normal colonic crypt epithelial cells of individual villin-15-LOX-1-A transgenic and control WT mice treated with AOM as described for panels (A–C). Values are the means of triplicate measurements for each mouse. P values were determined by two-sided Student's t tests. E and F) Effects of 15-LOX-1 transgene expression on NF-κB activation. Primary colonic epithelial cells were isolated from the indicated mice and were treated with either 10 μg/mL lipopolysaccharides (LPS) and 10 ng/mL tumor necrosis factor alpha (TNF-α) or with vehicle solution as a control. NF-κB binding to its DNA consensus site was measured by TransAM ELISA assays with specific p50 (E) or p65 (F) antibodies. Values shown are the means of triplicate measurements for each mouse. G) Effects of 15-LOX-1 transgenic expression on p65 protein levels in the nucleus. Nuclear protein fractions were extracted from isolated colonic epithelial cells treated with either LPS and TNF-α or control vehicle solution as described for panels (E) and (F). Proteins were immunoblotted and probed with p65 and histone H1 antibodies. Representative blots for the three indicated types of mice are shown.
Our findings that 15-LOX-1-inhibited expression of TNF-α and iNOS in the low-density array assays were in agreement with our earlier findings with single-gene analyses. Among the other genes that were differentially expressed, IL-1β is a major proinflammatory cytokine that has been linked to colonic tumorigenesis and is repressed by 15-LOX-1 re-expression in colon cancer cells (24). The TLRs (eg, TLR-1 and TLR-2) activate NF-κB signaling via the MyD88 signaling pathway (23). To confirm the low-density array results, we performed single-gene quantitative RT-PCR measurements and found that IL-1β relative expression levels, calibrated to the expression level in WT normal colonic epithelial cells, were lower in AOM-treated homozygous villin-15-LOX-1-A mice (mean = 0.54, 95% CI = 0.36 to 0.72) than in AOM-treated WT mice (mean = 1.77, 95% CI = 0.17 to 3.36) (mean difference = 0.71, 95% CI = 0.51 to 0.99, P = .046). In a further confirmatory follow-up analysis using single-gene quantitative RT-PCR, TLR-1 expression was also reduced in the normal mucosa of AOM-treated homozygous villin-15-LOX-1-A mice compared with WT mice (Figure 3, D), suggesting that 15-LOX-1 suppresses TLR and NF-κB gene expression, which influence both proinflammatory and protumorigenic signaling pathways.
We next tested whether 15-LOX-1 directly effects NF-κB activation in colonic epithelial cells by measuring its effects on the binding activity of p65 and p50, the proteins that usually form the NF-κB heterodimer (25). Specifically, we measured active NF-κB binding to its consensus site using specific antibodies to p65 and p50 (26). In the presence of 15-LOX-1 transgene expression, lipopolysaccharide- and TNF-α-mediated activation of NF-κB-DNA-binding activity (p50 enzyme-linked immunosorbent assay [ELISA]) was reduced in ex vivo experiments of isolated colonic crypt epithelial cells (Supplementary Methods, available online) from villin-15-LOX-1 mice compared with those in WT mice (in homozygotes, mean reduction in NF-κB activity = 58%, 95% CI = 51% to 66%, P < .001; in heterozygotes, mean reduction in NF-κB activity = 50%; 95% CI = 44% to 57%, P < .001; Figure 3, E). 15-LOX-1 transgenic expression also reduced NF-κB/DNA binding activation (p65 ELISA assay) by lipopolysaccharides and TNF-α in colonic epithelial cells in homozygous villin-15-LOX-1 mice by a mean of 50% compared with WT (95% CI = 42% to 58%, P < .001, two-way analysis of variance) and heterozygous villin-15-LOX-1 mice by a mean of 49% (95% CI = 43% to 56%, P < .001, two-way analysis of variance) (Figure 3, F). 15-LOX-1 transgenic expression also reduced p65 protein expression levels in heterozygous and homozygous villin-15-LOX-1 mice (Figure 3, G).
Our mouse model, in which human 15-LOX-1 transgene expression is targeted specifically to the intestinal epithelial cells, shows that tumorigenesis is inhibited in the presence of transgenic 15-LOX-1. Because 15-LOX-1 expression is reduced whenever AOM-induced tumors do form in these transgenic mice, in a way, they experimentally mimic the finding that 15-LOX-1 expression is decreased in human colon cancer and polyp epithelial cells (6–8,27) and thus provide an experimental model that demonstrates the mechanistic contribution of 15-LOX-1 to colonic tumorigenesis. Our observation that transgenic villin–driven 15-LOX-1 expression levels were lower in tumors than in paired normal colonic mucosa from the same mice is likely related to tumorigenesis-induced genetic and/or epigenetic changes that suppressed the nonnative villin promoter. The nonnative villin promoter had no effects on villin expression in colonic epithelial cells as the transgenic construct contained no villin coding sequence. This was confirmed by measuring villin expression in the normal mucosa of villin-15-LOX-1 transgenic and WT mice (data not shown).
In previous studies using 12/15-LOX murine models, the mixed function of 12/15-LOX precluded the dissection of 15-LOX-1 function. For example, 12/15-LOX overexpression in mouse skin promoted and inhibited tumorigenesis in the same mouse depending on the 12/15-LOX expression level, which increased either 12-HETE or 13-HODE levels (16). Similarly, although mice with deletions of the 12/15-LOX locus are predisposed to development of myelofibrosis, a preleukemic disease (28), they have reduced levels of both 12-HETE and 13-HODE, which preclude determination of whether myelofibrosis develops in response to the absence of 12-LOX or 15-LOX-1 function. In another model, targeted transgenic 15-LOX-1 expression in blood vessels of mice (using the preproendothelin-1 promoter) suppressed the growth of transplanted cancer cell lines (29). However, this model could only be used to study the effects of 15-LOX-1 on tumor angiogenesis. Whereas angiogenesis is one important mechanism for promotion of tumorigenesis, especially in its late stages, tumorigenesis develops via various other crucial mechanisms, especially in its early stages. In our current mouse model, we specifically show the effects of 15-LOX-1 expression in colonic epithelial cells on tumorigenesis.
Our results suggest that 15-LOX-1 suppresses TNF-α–iNOS signaling as a mechanism for inhibiting tumorigenesis. Previous data have been inconsistent with regard to the effects of 12/15-LOX on this pathway, showing that 12/15-LOX both inhibits (30) and activates TNF-α–iNOS signaling (31) in nonmalignant mouse models. Again, the discordance among previous results might be attributable to the two different products of 12/15-LOX, because TNF-α expression is increased by 12-HETE (31) but inhibited by 13-HODE (32). Our current results clearly show that 15-LOX-1 suppresses TNF-α and iNOS as a mechanism to inhibit colonic tumorigenesis
The currently available literature is very limited with regard to information on the effects of 15-LOX-1 on NF-κB in relation to tumorigenesis. The only reported data that we could identify are from in vitro studies of colon cancer cell lines showing 15-LOX-1-inhibited NF-κB, which is in agreement with our new in vivo data (these findings were published while this article was under review) (33). To our knowledge, there is no other information regarding the effects of 15-LOX-1 on NF-κB in relation to tumorigenesis. More importantly, our findings are the first, to our knowledge, to show that 15-LOX-1 inhibits NF-κB during tumorigenesis in vivo. Our data should provide important new insights because they suggest that 15-LOX-1 expression in colonic epithelial cells inhibits NF-κB activation to inhibit tumorigenesis.
Some of the potential limitations for the currently reported study include the possibility that transgenic expression of human 15-LOX-1 in mouse epithelial cells is limited in simulating human colorectal tumorigenesis because of species differences. Also, targeting transgenic 15-LOX-1 expression to epithelial intestinal cells provides no information on the possible contribution of 15-LOX-1 expression in stromal cells to colorectal tumorigenesis; future studies that target 15-LOX-1 expression in stromal cells will be required.
In conclusion, our current data demonstrate that 15-LOX-1 expression in colonic epithelial cells plays an important role in modulating colonic tumorigenesis via the suppression of critical regulators including TNF-α–iNOS signaling and NF-κB activation. Further studies to better define the mechanisms underlying the 15-LOX-1 suppression in cancer cells could lead to the development of molecularly based interventions to restore 15-LOX-1 expression in epithelial cells and thus inhibit tumorigenesis.
Funding
National Cancer Institute (R01-CA137213 to IS); the American Cancer Society Research Scholar Award (RSG-04-020-01-CNE); University of Texas M. D. Anderson Cancer Center Institutional Bridge Funding and the Caroline Wiess Law Endowment for Cancer Prevention. This study made use of the M. D. Anderson Cancer Center Genetically Engineered Mouse Facility, DNA Analysis Facility, and Research Animal Support Facility—Smithville, Genetic Services, supported by Cancer Center Support Grant (CA016672).
Supplementary Material
Footnotes
Z. Peng and Y. Wu have contributed equally to the work. The funding agencies had no involvement in the design of the study; the collection, analysis, or interpretation of the data; the writing of the article, and the decision to submit the article for publication. The authors were scientifically fully independent in the conduct of the study and these activities.
We are in debt to Dr Bernard Levin for his support and thoughtful feedback. We thank Dr Deborah L. Gumucio for providing us with the villin promoter–driven expression construct (p12.4Kvill).
References
- 1.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118(6):671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Mueller E, Sarraf P, Tontonoz P, et al. Terminal differentiation of human breast cancer through PPAR gamma. Mol Cell. 1998;1(3):465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68–69:263–290. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 4.Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci U S A. 1997;94(12):6148–6152. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Ann Rev Immunol. 2007;25(1):101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 6.Shureiqi I, Wojno KJ, Poore JA, et al. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis. 1999;20(10):1985–1995. doi: 10.1093/carcin/20.10.1985. [DOI] [PubMed] [Google Scholar]
- 7.Nixon JB, Kim KS, Lamb PW, Bottone FG, Eling TE. 15-Lipoxygenase-1 has anti-tumorigenic effects in colorectal cancer. Prostaglandins Leukot Essent Fatty Acids. 2004;70(1):7–15. doi: 10.1016/j.plefa.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Heslin MJ, Hawkins A, Boedefeld W, et al. Tumor-associated down-regulation of 15-lipoxygenase-1 is reversed by celecoxib in colorectal cancer. Ann Surg. 2005;241(6):941–946. doi: 10.1097/01.sla.0000164177.95620.c1. discussion 946–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shureiqi I, Xu X, Chen D, et al. Nonsteroidal anti-inflammatory drugs induce apoptosis in esophageal cancer cells by restoring 15-lipoxygenase-1 expression. Cancer Res. 2001;61(12):4879–4884. [PubMed] [Google Scholar]
- 10.Jiang WG, Watkins G, Douglas-Jones A, Mansel RE. Reduction of isoforms of 15-lipoxygenase (15-LOX)-1 and 15-LOX-2 in human breast cancer. Prostaglandins Leukot Essent Fatty Acids. 2006;74(4):235–245. doi: 10.1016/j.plefa.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Hennig R, Kehl T, Noor S, et al. 15-Lipoxygenase-1 production is lost in pancreatic cancer and overexpression of the gene inhibits tumor cell growth. Neoplasia. 2007;9(11):917–926. doi: 10.1593/neo.07565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shureiqi I, Jiang W, Zuo X, et al. The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100(17):9968–9973. doi: 10.1073/pnas.1631086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Fang B, Yang XQ, et al. Therapeutic molecular targeting of 15-lipoxygenase-1 in colon cancer. Mol Ther. 2008;16(5):886–892. doi: 10.1038/mt.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pidgeon GP, Lysaght J, Krishnamoorthy S, et al. Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metastasis Rev. 2007;26(3–4):503–524. doi: 10.1007/s10555-007-9098-3. [DOI] [PubMed] [Google Scholar]
- 15.Liu B, Khan WA, Hannun YA, et al. 12(S)-hydroxyeicosatetraenoic acid and 13(S)-hydroxyoctadecadienoic acid regulation of protein kinase C-alpha in melanoma cells: role of receptor-mediated hydrolysis of inositol phospholipids. Proc Natl Acad Sci U S A. 1995;92(20):9323–9327. doi: 10.1073/pnas.92.20.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller K, Siebert M, Heidt M, Marks F, Krieg P, Furstenberger G. Modulation of epidermal tumor development caused by targeted overexpression of epidermis-type 12S-lipoxygenase. Cancer Res. 2002;62(16):4610–4616. [PubMed] [Google Scholar]
- 17.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protocol. 2007;2(8):1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 18.Erdman SE, Rao VP, Poutahidis T, et al. Nitric oxide and TNF-α trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci U S A. 2009;106(4):1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132(2):279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 20.Lawson MM, Thomas AG, Akobeng AK. Tumour necrosis factor alpha blocking agents for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD005112.pub2. CD005112. [DOI] [PubMed] [Google Scholar]
- 21.Lowenstein CJ, Alley EW, Raval P, et al. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90(20):9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 24.Shureiqi I, Chen D, Day RS, et al. Profiling lipoxygenase metabolism in specific steps of colorectal tumorigenesis. Cancer Prev Res (Phila). 2010;3(7):829–838. doi: 10.1158/1940-6207.CAPR-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeuerle PA, Henkel T. Function and activation of NF-kappaB in the immune system. Ann Rev Immunol. 1994;12(1):141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz PA, Kim SC, Sartor RB, Haller D. 15-Deoxy-Δ12,14-prostaglandin J2-mediated ERK signaling inhibits gram-negative bacteria-induced RelA phosphorylation and interleukin-6 gene expression in intestinal epithelial cells through modulation of protein phosphatase 2A activity. J Biol Chem. 2004;279(34):36103–36111. doi: 10.1074/jbc.M405032200. [DOI] [PubMed] [Google Scholar]
- 27.Shureiqi I, Wu Y, Chen D, et al. The critical role of 15-lipoxygenase-1 in colorectal epithelial cell terminal differentiation and tumorigenesis. Cancer Res. 2005;65(24):11486–11492. doi: 10.1158/0008-5472.CAN-05-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Middleton MK, Zukas AM, Rubinstein T, et al. Identification of 12/15-lipoxygenase as a suppressor of myeloproliferative disease. J Exp Med. 2006;203(11):2529–2540. doi: 10.1084/jem.20061444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harats D, Ben-Shushan D, Cohen H, et al. Inhibition of carcinogenesis in transgenic mouse models over-expressing 15-lipoxygenase in the vascular wall under the control of murine preproendothelin-1 promoter. Cancer Lett. 2005;229(1):127–134. doi: 10.1016/j.canlet.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Paintlia AS, Paintlia MK, Singh I, Singh AK. IL-4-induced peroxisome proliferator-activated receptor {gamma} activation inhibits NF-{kappa}B trans activation in central nervous system (CNS) glial cells and protects oligodendrocyte progenitors under neuroinflammatory disease conditions: implication for CNS-demyelinating diseases. J Immunol. 2006;176(7):4385–4398. doi: 10.4049/jimmunol.176.7.4385. [DOI] [PubMed] [Google Scholar]
- 31.Wen Y, Gu J, Chakrabarti SK, et al. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-{alpha} in macrophages. Endocrinology. 2007;148(3):1313–1322. doi: 10.1210/en.2006-0665. [DOI] [PubMed] [Google Scholar]
- 32.Ricote M, Welch JS, Glass CK. Regulation of macrophage gene expression by the peroxisome proliferator-activated receptor-γ. Hormone Res. 2000;54(5/6):275–280. doi: 10.1159/000053271. [DOI] [PubMed] [Google Scholar]
- 33.Cimen I, Astarci E, Banerjee S. 15-lipoxygenase-1 exerts its tumor suppressive role by inhibiting nuclear factor-kappa B via activation of PPAR gamma. J Cell Biochem. 2011;112(9):2490–2501. doi: 10.1002/jcb.23174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.