Abstract
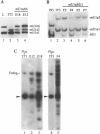
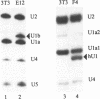
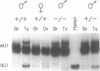
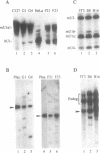
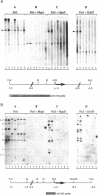
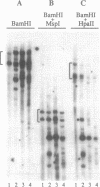
The expression of mouse embryonic U1 snRNA (mU1b) genes is subject to stage- and tissue-specific control, being restricted to early embryos and adult tissues that contain a high proportion of stem cells capable of further differentiation. To determine the mechanism of this control we have sought to distinguish between differential RNA stability and regulation of U1 gene promoter activity in several cell types. We demonstrate here that mU1b RNA can accumulate to high levels in permanently transfected mouse 3T3 and C127 fibroblast cells which normally do not express the endogenous U1b genes, and apparently can do so without significantly interfering with cell growth. Expression of transfected chimeric U1 genes in such cells is much more efficient when their promoters are derived from a constitutively expressed mU1a gene rather than from an mU1b gene. In transgenic mice, introduced U1 transgenes with an mU1b 5' flanking region are subject to normal tissue-specific control, indicating that U1b promoter activity is restricted to tissues that normally express U1b genes. Inactivation of the embryonic genes during normal differentiation is not associated with methylation of upstream CpG-rich sequences; however, in NIH 3T3 fibroblasts, the 5' flanking regions of endogenous mU1b genes are completely methylated, indicating that DNA methylation serves to imprint the inactive state of the mU1b genes in cultured cells. Based on these results, we propose that the developmental control of U1b gene expression is due to differential activity of mU1a and mU1b promoters rather than to differential stability of U1a and U1b RNAs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antequera F., Boyes J., Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell. 1990 Aug 10;62(3):503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- Bach M., Krol A., Lührmann R. Structure-probing of U1 snRNPs gradually depleted of the U1-specific proteins A, C and 70k. Evidence that A interacts differentially with developmentally regulated mouse U1 snRNA variants. Nucleic Acids Res. 1990 Feb 11;18(3):449–457. doi: 10.1093/nar/18.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Bird A., Taggart M., Frommer M., Miller O. J., Macleod D. A fraction of the mouse genome that is derived from islands of nonmethylated, CpG-rich DNA. Cell. 1985 Jan;40(1):91–99. doi: 10.1016/0092-8674(85)90312-5. [DOI] [PubMed] [Google Scholar]
- Blatt C., Saxe D., Marzluff W. F., Lobo S., Nesbitt M. N., Simon M. I. Mapping and gene order of U1 small nuclear RNA, endogenous viral env sequence, amylase, and alcohol dehydrogenase-3 on mouse chromosome 3. Somat Cell Mol Genet. 1988 Mar;14(2):133–142. doi: 10.1007/BF01534398. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Schenborn E. T. The human U1 snRNA promoter and enhancer do not direct synthesis of messenger RNA. Nucleic Acids Res. 1988 Jul 11;16(13):5827–5840. doi: 10.1093/nar/16.13.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell. 1983 Mar;32(3):669–680. doi: 10.1016/0092-8674(83)90053-3. [DOI] [PubMed] [Google Scholar]
- Forbes D. J., Kirschner M. W., Caput D., Dahlberg J. E., Lund E. Differential expression of multiple U1 small nuclear RNAs in oocytes and embryos of Xenopus laevis. Cell. 1984 Oct;38(3):681–689. doi: 10.1016/0092-8674(84)90263-0. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hanley B. A., Schuler M. A. Developmental expression of plant snRNAs. Nucleic Acids Res. 1991 Nov 25;19(22):6319–6325. doi: 10.1093/nar/19.22.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard E. F., Michael S. K., Dahlberg J. E., Lund E. Functional, developmentally expressed genes for mouse U1a and U1b snRNAs contain both conserved and non-conserved transcription signals. Nucleic Acids Res. 1986 Dec 22;14(24):9811–9825. doi: 10.1093/nar/14.24.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Wolkowicz M. J., Rideout W. M., 3rd, Gonzales F. A., Marziasz C. M., Coetzee G. A., Tapscott S. J. De novo methylation of the MyoD1 CpG island during the establishment of immortal cell lines. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6117–6121. doi: 10.1073/pnas.87.16.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Harada F. New U1 RNA species found in Friend SFFV (spleen focus forming virus)-transformed mouse cells. J Biol Chem. 1985 Jun 25;260(12):7775–7782. [PubMed] [Google Scholar]
- Kuo H. C., Nasim F. H., Grabowski P. J. Control of alternative splicing by the differential binding of U1 small nuclear ribonucleoprotein particle. Science. 1991 Mar 1;251(4997):1045–1050. doi: 10.1126/science.1825520. [DOI] [PubMed] [Google Scholar]
- Lea I., Moore H. D., Latchman D. S. Differential expression of the mouse U1a and U1b SnRNA genes is not dependent on sequence differences in the octamer motif. Biochem J. 1991 Aug 1;277(Pt 3):719–722. doi: 10.1042/bj2770719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo P. C., Mount S. M. Drosophila melanogaster genes for U1 snRNA variants and their expression during development. Nucleic Acids Res. 1990 Dec 11;18(23):6971–6979. doi: 10.1093/nar/18.23.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Bostock C. J., Dahlberg J. E. The transcription of Xenopus laevis embryonic U1 snRNA genes changes when oocytes mature into eggs. Genes Dev. 1987 Mar;1(1):47–56. doi: 10.1101/gad.1.1.47. [DOI] [PubMed] [Google Scholar]
- Lund E., Bostock C., Robertson M., Christie S., Mitchen J. L., Dahlberg J. E. U1 small nuclear RNA genes are located on human chromosome 1 and are expressed in mouse-human hybrid cells. Mol Cell Biol. 1983 Dec;3(12):2211–2220. doi: 10.1128/mcb.3.12.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Differential accumulation of U1 and U4 small nuclear RNAs during Xenopus development. Genes Dev. 1987 Mar;1(1):39–46. doi: 10.1101/gad.1.1.39. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. True genes for human U1 small nuclear RNA. Copy number, polymorphism, and methylation. J Biol Chem. 1984 Feb 10;259(3):2013–2021. [PubMed] [Google Scholar]
- Lund E., Kahan B., Dahlberg J. E. Differential control of U1 small nuclear RNA expression during mouse development. Science. 1985 Sep 20;229(4719):1271–1274. doi: 10.1126/science.2412294. [DOI] [PubMed] [Google Scholar]
- Lund E., Nesbitt M. N. The embryonic and adult mouse U1 snRNA genes map to different chromosomal loci. Somat Cell Mol Genet. 1988 Mar;14(2):143–148. doi: 10.1007/BF01534399. [DOI] [PubMed] [Google Scholar]
- Mangin M., Ares M., Jr, Weiner A. M. U1 small nuclear RNA genes are subject to dosage compensation in mouse cells. Science. 1985 Jul 19;229(4710):272–275. doi: 10.1126/science.2409601. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Brown D. T., Lobo S., Wang S. S. Isolation and characterization of two linked mouse U1b small nuclear RNA genes. Nucleic Acids Res. 1983 Sep 24;11(18):6255–6270. doi: 10.1093/nar/11.18.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias P. D., Bernard H. U., Scott A., Brady G., Hashimoto-Gotoh T., Schütz G. A bovine papilloma virus vector with a dominant resistance marker replicates extrachromosomally in mouse and E. coli cells. EMBO J. 1983;2(9):1487–1492. doi: 10.1002/j.1460-2075.1983.tb01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael S. K., Hilgers J., Kozak C., Whitney J. B., 3rd, Howard E. F. Characterization and mapping of DNA sequence homologous to mouse U1a1 snRNA: localization on chromosome 11 near the Dlb-1 and Re loci. Somat Cell Mol Genet. 1986 May;12(3):215–223. doi: 10.1007/BF01570780. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Pettersson I., Hinterberger M., Karmas A., Steitz J. A. The U1 small nuclear RNA-protein complex selectively binds a 5' splice site in vitro. Cell. 1983 Jun;33(2):509–518. doi: 10.1016/0092-8674(83)90432-4. [DOI] [PubMed] [Google Scholar]
- Moussa N. M., Lobo S. M., Marzluff W. F. Expression of a mouse U1b gene in mouse L cells. Gene. 1985;36(3):311–319. doi: 10.1016/0378-1119(85)90186-6. [DOI] [PubMed] [Google Scholar]
- Munns T. W., Liszewski M. K., Tellam J. T., Sims H. F., Rhoads R. E. Antibody-nucleic acid complexes. Immunospecific retention of globin messenger ribonucleic acid with antibodies specific for 7-methylguanosine. Biochemistry. 1982 Jun 8;21(12):2922–2928. doi: 10.1021/bi00541a018. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. A precursor of globin messenger RNA. J Mol Biol. 1976 Sep 15;106(2):403–420. doi: 10.1016/0022-2836(76)90093-0. [DOI] [PubMed] [Google Scholar]
- Saffer J. D., Jackson S. P., Annarella M. B. Developmental expression of Sp1 in the mouse. Mol Cell Biol. 1991 Apr;11(4):2189–2199. doi: 10.1128/mcb.11.4.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C., Marzluff W. F. Expression of the U1 RNA gene repeat during early sea urchin development: evidence for a switch in U1 RNA genes during development. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2572–2576. doi: 10.1073/pnas.86.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Nienhuis A. W. Only the promoter region of the constitutively expressed normal and amplified human dihydrofolate reductase gene is DNase I hypersensitive and undermethylated. J Biol Chem. 1985 Feb 25;260(4):2468–2474. [PubMed] [Google Scholar]
- Siliciano P. G., Guthrie C. 5' splice site selection in yeast: genetic alterations in base-pairing with U1 reveal additional requirements. Genes Dev. 1988 Oct;2(10):1258–1267. doi: 10.1101/gad.2.10.1258. [DOI] [PubMed] [Google Scholar]
- Séraphin B., Kretzner L., Rosbash M. A U1 snRNA:pre-mRNA base pairing interaction is required early in yeast spliceosome assembly but does not uniquely define the 5' cleavage site. EMBO J. 1988 Aug;7(8):2533–2538. doi: 10.1002/j.1460-2075.1988.tb03101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Lai J. S., Herr W. Promoter-selective activation domains in Oct-1 and Oct-2 direct differential activation of an snRNA and mRNA promoter. Cell. 1992 Feb 21;68(4):755–767. doi: 10.1016/0092-8674(92)90150-b. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Brown D. D. Developmental regulation of two 5S ribosomal RNA genes. Science. 1988 Sep 23;241(4873):1626–1632. doi: 10.1126/science.241.4873.1626. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Weiner A. M. A compensatory base change in U1 snRNA suppresses a 5' splice site mutation. Cell. 1986 Sep 12;46(6):827–835. doi: 10.1016/0092-8674(86)90064-4. [DOI] [PubMed] [Google Scholar]