Abstract
Fifteen curcumin analogs were synthesized and tested for in-vitro cytotoxicity towards B16 and L1210 murine cancer cell lines using an MTT assay. Significant activity was discovered for two analogs: 8 (B16 IC50 = 1.6 μM; L1210 IC50 = 0.35 μM) and 9 (B16 IC50 = 0.51 μM; L1210 IC50 = 1.2 μM). Several other analogs exhibited notable cytotoxicity. The data from quantitative structure-activity relationships suggest that large electron-withdrawing substituents placed in the meta-position of the arylidene aryl rings enhance potencies. Compounds 8 and 9 were found using a cell-based assay to have virtually no effects on microtubules at concentrations up to 40 μM. These results suggest that tubulin inhibition is not the principal mechanism by which the curcumin analogs act.
Keywords: B16, Curcumin analogs, L1210, Microtubules, Structure-activity relationships
Introduction
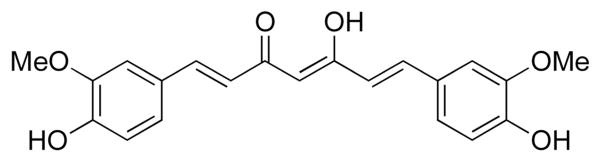
Curcumin (Fig. 1), a bis-α,β-unsaturated β-diketone found in the rhizomes of the herbaceous plant turmeric, has been extensively studied due to its biological effects, especially its anticancer properties. Curcumin has significant anti-proliferative effects towards various human cancer cell lines derived from prostate, large intestine, bone, and white blood cells [1–4]. In addition, curcumin has been shown to have a degree of tumor specificity, targeting malignant cells in preference to non-malignant cells [5] and it is effective at low micromolar concentrations [6].
Figure 1.
Structure of curcumin.
The mechanisms by which curcumin acts to control malignant cell growth are varied, and have been proposed to be tissue-specific [6]. Cellular apoptosis is one of the main mechanisms that has been studied [7, 8]. Curcumin is also known to slow angiogenesis by blocking AP-1 [9] and is known to down-regulate the oncogene MDM2 in prostate cancer [10]. It has also been reported that cur-cumin can inhibit the polymerization of tubulin for spindle- fiber formation during mitotic cell division [11]. This is of particular interest to one of the author’s laboratory, which has extensively studied the effects of combretasta-tin-A4 analogs, such as chalcones and diarylheterocycles, as mitotic inhibitors. Presumably, cytotoxicity is mediated, at least in part, by microtubule depolymerization and inhibition of tubulin polymerization [12–17].
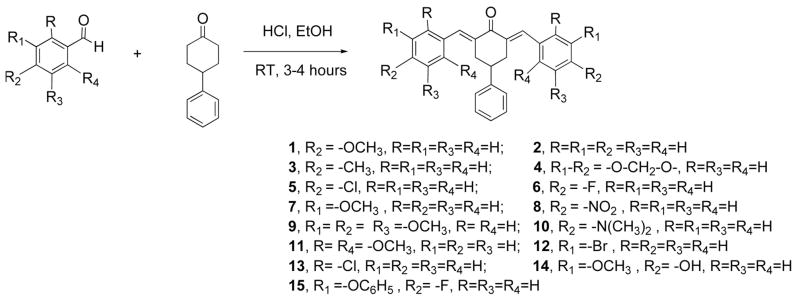
The current study reports the synthesis of fifteen cur-cumin analogs 1–15 for evaluation as cytotoxins. These analogs replaced the diketone moiety of curcumin with a 4-phenylcyclohexanone moiety. Cyclohexanone analogs that lack the 4-phenyl group have recently been reported and they were discovered to have significant anti-angiogenic activity, measured by the inhibition of the growth of SVR endothelial cells in culture [18]. Substituted heterocyclic analogs of curcumin namely various 3,5-bis(benzylidene)-4-piperidones have also been reported and they were found to be active in inhibiting the growth of cancer cells in culture [19, 20]. From molecular modeling and X-ray crystallography studies, a relationship between the conformation of the diaryl moieties and cytotoxicity, as well as the Hammett constant σ, was reported [20].
In the present study, the hydroxyl and methoxy substituents present on the aryl rings in curcumin were retained and, in addition, other groups were introduced in order to examine the possible relationships between stereo-electronic and hydrophobic factors and cytotoxicity. The cytotoxicity studies on the compounds were performed with a 72 h continuous exposure MTT assay, using murine B16 (melanoma) and L1210 (leukemia) cell lines. Two of the most potent compounds, 8 and 9, underwent further evaluation for effects on cellular microtubules.
Results and discussion
Chemistry
The curcumin analogs depicted in Table 1 were synthesized using an acid catalyzed aldol condensation of the appropriate aryl aldehyde with 4-phenylcyclohexanone (Scheme 1). The products were purified by recrystallization from ethanol and were obtained in yields of 45–80%. The structures were ascertained by IR, NMR, and mass spectrometry. Compounds 1 and 10 have been described previously but only their photophysical properties were studied [21].
Table 1.
Cytotoxicity results of synthesized curcumin analogs in B16 and L1210 cell lines as determined by MTT assay.
| Compound | IC50 (μM) | |
|---|---|---|
| B16 | L1210 | |
 1
1
|
15.0 ± 2.8 | >100 |
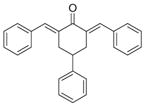 2
2
|
3.4 ± 0.2 | 3.6 ± 0.4 |
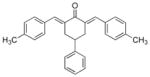 3
3
|
>100 | >100 |
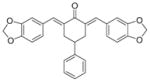 4
4
|
12.3 ± 7.7 | 8.6 ± 3.6 |
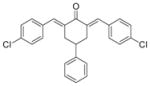 5
5
|
3.4 ± 0.5 | 4.5 ± 1.8 |
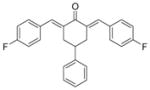 6
6
|
3.3 ± 0.3 | 5.6 ± 0.4 |
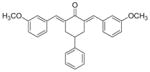 7
7
|
3.5 ± 0.3 | 3.5 ± 0.2 |
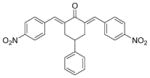 8
8
|
1.6 ± 0.6 | 0.35 ± 0.0 |
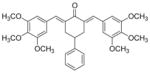 9
9
|
0.51 ± 0.1 | 1.2 ± 0.9 |
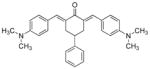 10
10
|
Insoluble in DMSO | |
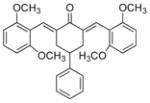 11
11
|
>100 | >100 |
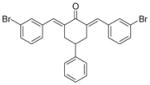 12
12
|
2.1 ± 0.4 | 2.9 ± 0.6 |
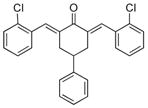 13
13
|
5.3 ± 0.4 | 4.5 ± 0.5 |
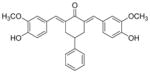 14
14
|
3.2 ± 0.3 | 3.2 ± 0.3 |
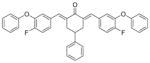 15
15
|
>100 | >100 |
Scheme 1.
Synthesis of the 2,6-bis(benzylidene)-4-phenylcyclohexanones or curcumin analogs 1–15.
Cytotoxic activity in murine cell lines
The cytotoxicity of curcumin analogs 1–9, 11–15 was assessed in B16 (murine melanoma) and L1210 (murine leukemia) cell lines using a 72 h continuous exposure MTT assay as previously described [14]. The concentration at which 50% cell growth was inhibited (IC50, μM) was determined for each compound in triplicate experiments. Data could not be obtained for compound 10 because it was insoluble in different solvents including dimethylsulfoxide. The results from the cytotoxicity studies are presented in Table 1.
The following general observations were made. First, the IC50 values of a number of the compounds are in the low micromolar range (1–10 μM) in 61% of the bioassays. In addition, the IC50 figures of both 8 and 9 in one of the bioassays are sub-micromolar and they are clearly lead molecules. Second, with the exception of 1, the IC50 values of the compounds are similar in both screens.
Specifically, the following comparisons were made in attempting to discern correlations between the nature of the aryl substituents and cytotoxic potencies. First, the mode of action of conjugated unsaturated ketones includes alkylation of cellular thiols [22]. Hence, the electronic nature of the aryl substituents will affect the charge densities on the olefinic carbon atoms, which, in turn, will control the rate and extent of thiol alkylation. Linear and semilogarithmic plots were made between the Hammett s and Taft σ* values (Table 2) of the aryl substituents and the IC50 values which were less than 100 μM in the B16 assay. A trend towards a negative correlation was noted in the linear plot (p = 0.083). The analyses were repeated using the biological data in the L1210 screen, which indicated a negative correlation in the semilogarithmic plot (p = 0.060) and a linear plot (p = 0.117). Thus, cytotoxic potencies rise as the electron-withdrawing capacity of the aryl substituents increases. This observation is in accord with the view that a mode of action of these compounds is likely by reaction with cellular thiols.
Table 2.
Physical constants of the substituents in analogs 1–15: Hammett σ and Taft σ*, Hansch π, and molar refractivity MR.
| Analogs | Substituents | σ/σ* | π | MR |
|---|---|---|---|---|
| 1 | 4-OCH3 | −0.28 | −0.02 | 9.93 |
| 2 | H | 0.00 | 0.00 | 3.09 |
| 4 | 3,4-O-CH2-O | −0.27 | −0.05 | 9.99 |
| 5 | 4-Cl | 0.24 | 0.71 | 8.09 |
| 6 | 4-F | 0.06 | 0.14 | 2.98 |
| 7 | 3-OCH3 | 0.11 | −0.02 | 9.93 |
| 8 | 4-NO2 | 0.78 | −0.28 | 9.42 |
| 9 | 3,4,5-(OCH3) | −0.06 | −0.06 | 23.61 |
| 12 | 3-Br | 0.39 | 0.86 | 10.94 |
| 13 | 2-Cl | 0.37 | 0.71 | 8.09 |
| 14 | 3-OCH-3,4-OH | −0.27 | −0.69 | 11.75 |
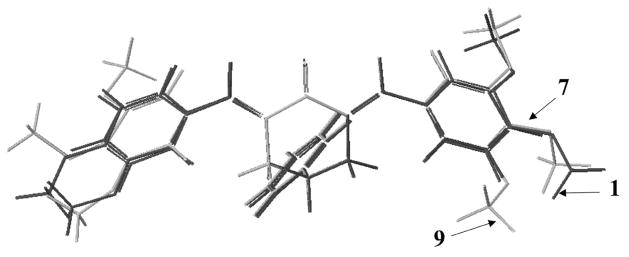
Second, the hydrophobic and steric properties of aryl substituents can influence the magnitude of biological responses [23]. Linear and semilogarithmic plots were constructed between the IC50 values of 1, 2, 4 – 9, and 12 – 14 in the B16 screen and both the Hansch π and molar refractivity (MR) values (Table 2). The process was repeated using the biological data for 2, 4–9, and 12–14 in the L1210 assay. No correlations were noted (p > 0.05), although a trend towards a negative correlation was observed in the semilogarithmic plot between the IC50 values in the B16 screen and the MR figures (p = 0.089). This observation suggests that potency is enhanced by increasing the size of the aryl substituents. A further steric feature of these molecules, which could influence cytotoxic potencies are the torsion angles θ1 and θ2 between the aryl rings and the adjacent olefinic linkages. Consequently, models of 1, 2, 4–9, and 12–14 were built and the θ1 and θ2 angles recorded. Linear and semilogarithmic plots between the θ1 and θ2 values of these compounds and the IC50 figures in the B16 screen were made. The process was repeated using the data from the L1210 test except 1 was omitted (IC50 value of > 100 μM). No correlations were observed (p > 0.05). Thus, these torsion angles are unlikely to exert a major influence on cytotoxic potencies. Models revealed that the locations of the methoxy group of 7 and the 3-methoxy substituent of 9 virtually overlapped (Fig. 2) which may explain the significant potencies of both compounds. In addition, since the IC50 values of 1 are greater than 2, the 4-methoxy group lowers cytotoxic potencies. Figure 2 reveals that the 4-methoxy substituents of 1 and 9 are in similar locations, suggesting that this group in 9 has an adverse effect on potency and that the 3,5-dimethoxy analog may well exceed the potencies of 9.
Figure 2.
Superimposed structures of 1, 7, and 9.
Third, the positions of the substituents on the benzylidene aryl rings in relation to cytotoxic potencies were addressed. A review of the IC50 values for compounds which possess a single para substituent namely 1, 3, 5, 6, 8 indicates that those molecules with electron-withdrawing groups (5, 6, 8) are more potent than the analogs with electron-repelling substituents (1, 3). This observation confirms that in developing the cluster of unsaturated ketones, strongly electron-attracting groups should be attached to the benzylidene aryl rings. The meta position appears to be optimal for the methoxy group. Thus, the potency of 7 which possesses a single meta-methoxy substituent is greater than 1 having a single para-methoxy group. The addition of a 4-hydroxy moiety to 7 led to 14, which has similar IC50 values as 7. The number of meta-methoxy groups was increased in 9 which possesses high potency. On the other hand, placing methoxy groups in the ortho position as in 11 eliminates significant cytotoxic potency. The possibility that the meta position may be the optimal location was enhanced by the fact that the 3-bromo analog 12 had the lowest IC50 values apart from the lead compounds 8 and 9. One may note, however, that the size of the group in the meta position is a consideration since the placing of the bulky 3-phenoxy substituent into 6 leading to 15 led to a marked reduction in potency. In summary, the available evidence in regard to structure-activity relationships in this series of compounds reveals that in general (i) the compounds are potent cytotoxins, (ii) large electron-withdrawing groups should be placed in the benzylidene aryl rings, and (iii) the meta position is likely the preferred location in producing analogs with low IC50 values.
Effects on cellular microtubules
Since curcumins have been reported to cause microtubule depolymerization and inhibit tubulin polymerization and angiogenesis, the effects of the two active compounds 8 and 9 on cellular microtubules were investigated [15, 24]. Results from these studies showed that compounds 8 and 9 were virtually inactive at concentrations up to 40 μM. These results suggest that the mechanism of cytotoxicity is unrelated to the disruption of microtubules.
Conclusion
The results from this study confirm that readily accessible curcumin analogs are potential cytotoxins for anti-cancer-drug discovery. It is concluded that large electron-withdrawing groups at the meta positions enhance cytotoxic potencies.
Experimental
Chemistry
All fifteen analogs were synthesized according to the following general procedure. Aqueous hydrochloric acid (35%, 0.5 mL) was added dropwise to a solution of 4-phenylcyclohexanone (500 mg) and the appropriate aryl aldehyde (2 equivalents) in ethanol (20 mL) at room temperature. The reaction mixture was stirred under reflux for 3–4 h monitoring by silica gel TLC plates (hexane/ethyl acetate, 9 : 1). The precipitate formed upon cooling to room temperature was filtered, washed with chilled ethanol, and recrystallized from ethanol (with the exception of 15 which was purified over silica eluting with ethyl acetate/hexane, 5 : 95). The isolated chemical yields were between 70–80%, except compound 8 was isolated in 45% yield.
2,6-Bis[(4-methoxyphenyl)methinyl]-4-phenylcyclohexanone 1
Yellow solid. M.p. 1150C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 2.98 (m, 3H), 3.28 (m, 2H), 3.81 (s, 6H), 6.88 (d, 4H, J = 8.8 Hz), 7.25–7.37 (m, 5H), 7.42 (d, 4H, J = 8.8 Hz), 7.83 (s, 2H). IR (KBr pellets cm− 1) ν = 3029, 2954, 2904, 2836, 2042, 1663, 1599, 1509, 1460, 1254, 1175, 1030, 988, 833, 703. ESI(APCI)-MS: m/z = 411 [M + 1].
2,6-bis(phenylmethinyl)-4-phenylcyclohexanone 2
Yellow solid. M.p. 132°C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 2.99 (m, 3H), 3.29 (m, 2H), 7.26–7.45 (m, 15H), 7.87 (s, 2H). IR (KBr pellets cm− 1) ν = 3054, 3026, 2881, 1961, 1816, 1659, 1603, 1566, 1493, 1446, 1294, 1235, 1189, 1159, 986, 935, 762, 697. ESI(APCI)-MS: m/z = 351 [M + 1].
2,6-Bis[(4-methylphenyl)methinyl]-4-phenylcyclohexanone 3
Yellow solid. M.p. 1890C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) =2.35 (s, 6H), 2.98 (m, 3H), 3.27 (m, 2H), 7.16 (d, 4H, J = 8.0 Hz), 7.23–7.36 (m, 9H), 7.84 (s, 2H). IR (KBr pellets cm− 1) ν = 3026, 2919, 2732, 1917, 1661, 1599, 1563, 1510, 1316, 1291, 1239, 1179, 1149, 989, 818, 750, 700. ESI(APCI)-MS: m/z = 379 [M + 1].
2,6-Bis[(3,4-methylenedioxyphenyl)methinyl]-4-phenylcyclohexanone 4
Yellow solid. M.p. 196°C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 2.96 (m, 3H), 3.25 (m, 2H), 5.97 (s, 4H), 6.81 (d, 2H, J = 8.0 Hz), 6.94–6.99 (m, 4H), 7.25–7.37 (m, 5H), 7.77 (s, 2H). IR (KBr pellets cm− 1) ν = 3076, 3003, 2896, 2778, 1855, 1657, 1589, 1556, 1500, 1435, 1359, 1336, 1293, 1224, 1146, 1096, 1039, 989, 934, 867, 814, 759. ESI(APCI)-MS: m/z = 439 [M + 1].
2,6-Bis[(4-chlorophenyl)methinyl]-4-phenylcyclohexanone 5
Yellow solid. M.p. 171°C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 2.97 (m, 3H), 3.22 (m, 2H), 7.25–7.28 (m, 4H), 7.33–7.38 (m, 9H), 7.80(s, 2H). IR (KBr pellets cm− 1) ν = 3064, 3028, 2899, 2564, 1911, 1666, 1604, 1490, 1407, 1309, 1281, 1237, 1185, 1146, 1094, 1011, 989, 833, 762, 698. ESI(APCI)-MS: m/z = 419 [M + 1].
2,6-Bis[(4-fluorophenyl)methinyl]-4-phenylcyclohexanone 6
Yellow solid. M.p. 159°C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 2.96 (m, 3H), 3.22 (m, 2H), 7.02–7.06 (m, 4H), 7.24–7.35 (m, 5H), 7.39 (m, 4H), 7.81 (s, 2H). IR (KBr pellets cm− 1) ν = 3043, 2899, 2838, 1946, 1666, 1600, 1566, 1508, 1414, 1292, 1232, 1187, 1148, 1101, 989, 832, 754, 702. ESI(APCI)-MS: m/z = 387 [M + 1].
2,6-Bis[(3-methoxyphenyl)methinyl]-4-phenylcyclohexanone 7
Yellow solid. M.p. 97°C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 2.96 (m, 3H), 3.27 (m, 2H), 3.78 (s, 6H), 6.83 (m, 2H), 6.94 (s, 2H), 7.00(m, 2H), 7.21–7.33 (m, 7H), 7.81 (s, 2H). IR (KBr pellets cm− 1) ν = 3068, 3017, 2952, 2891, 2837, 2577, 1938, 1660, 1601, 1575, 1481, 1433, 1246, 1196, 1215, 1157, 1051, 947, 899, 785, 743, 695. ESI(APCI)-MS: m/z = 411 [M + 1].
2,6-Bis[(4-nitrophenyl)methinyl]-4-phenylcyclohexanone 8
Yellow solid. M.p. 81°C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 3.04 (m, 3H), 3.22 (m, 2H), 7.24–7.37 (m, 5H), 7.56 (d, 4H, J = 8.4 Hz), 7.87 (s, 2H), 8.22 (d, 4H, J = 8.8 Hz). IR (KBr pellets cm− 1) ν = 3070, 2924, 2847, 2447, 1935, 1665, 1594, 1518, 1345, 1300, 1240, 1192, 1152, 1110, 995, 908, 855, 761. ESI(APCI)-MS: m/z = 439 [M − 1].
2,6-Bis[(3,4,5-trimethoxyphenyl)methinyl]-4-phenylcyclohexanone 9
Yellow solid. M.p. 183°C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 3.03 (m, 3H), 3.33 (m, 2H), 3.85 (s, 12H), 3.88 (s, 6H), 6.69 (s, 4H), 7.26–7.35 (m, 5H), 7.81 (s, 2H). IR (KBr pellets cm− 1) ν = 2995, 2941, 2838, 2000, 1657, 1578, 1503, 1454, 1417, 1346, 1286, 1243, 1127, 1020, 936, 836, 733. ESI(APCI)-MS: m/z = 531 [M + 1].
2,6-Bis[(4-dimethylaminophenyl)methinyl]-4-phenylcyclohexanone 10
Orange solid. M.p. 70°C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 2.99 (m, 15H), 3.31 (m, 2H), 6.66 (d, 4H, J = 8.8Hz), 7.24–7.35 (m, 5H), 7.41 (d, 4H, J = 8.8 Hz), 7.83 (s, 2H); IR (KBr pellets cm− 1) ν = 3027, 2892, 2812, 2530, 1883, 1651, 1586, 1522, 1444, 1367, 1300, 1230, 1168, 1066, 988, 945, 818. ESI(APCI)-MS: m/z = 437 [M + 1].
2,6-Bis[(2,6-dimethoxyphenyl)methinyl]-4-phenylcyclohexanone 11
Yellow solid. M.p. 210°C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 2.62 (m, 4H), 2.97 (m, 1H), 3.79 (s, 12H), 6.51 (d, 4H, J = 8.4 Hz), 7.13–7.25 (m, 7H), 7.73 (s, 2H). IR (KBr pellets cm− 1) ν = 2997, 2937, 2837, 2531, 1910, 1671, 1615, 1582, 1469, 1433, 1294, 1254, 1138, 1107, 1033, 988 761. ESI(APCI)-MS: m/z = 471 [M + 1].
2,6-Bis[(3-bromophenyl)methinyl]-4-phenylcyclohexanone 12
Yellow solid. M.p. 171°C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 2.98 (m, 3H), 3.21 (m, 2H), 7.22–7.45 (m, 11H), 7.54 (s, 2H), 7.76 (s, 2H). IR (KBr pellets cm− 1) ν = 3056, 3027, 2908, 1947, 1745, 1662, 1605, 1574, 1475, 1411, 1286, 1239, 1188, 1153, 1103, 994, 788, 763. ESI(APCI)-MS: m/z = 506 [M + 1].
2,6-Bis[(2-chlorophenyl)methinyl]-4-phenylcyclohexanone 13
Yellow solid. M.p. 164°C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 2.86–3.11 (m, 5H), 7.20–7.32 (m, 11H), 7.41–7.43 (m, 2H), 7.98 (s, 2H). IR (KBr pellets cm− 1) ν = 3060, 3026, 2870, 1960, 1812, 1671, 1609, 1587, 1467, 1436, 1295, 1230, 1189, 1150, 1047, 989, 919, 867, 760, 701. ESI(APCI)-MS: m/z = 419 [M + 1].
2,6-Bis[(3-hydroxy-4-methoxyphenyl)methinyl]-4-phenylcyclohexanone 14
Yellow solid. M.p. 161°C. 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 2.99 (m, 3H), 3.29 (m, 2H), 3.88 (s, 6H), 5.78 (s, 2H), 6.89 (d, 2H, J = 8.4 Hz), 6.96 (d, 2H, J = 1.6 Hz), 7.04 (m, 2H), 7.24–7.36 (m, 5H), 7.80 (s, 2H). IR (KBr pellets cm− 1) ν = 3525, 3209, 2928, 1641, 1579, 1514, 1422, 1250, 1166, 1126, 1035, 1007, 936, 911, 856, 819. ESI(APCI)-MS: m/z = 443 [M + 1].
2,6-Bis[(4-fluoro-3-phenoxyphenyl)methinyl]-4-phenylcyclohexanone 15
Yellow semisolid. 1H-NMR (CDCl3, 400 MHz) δ (ppm) = 2.77 (m, 3H), 3.10 (m, 2H), 6.95–7.35 (m, 21H), 7.69 (s, 2H). IR (KBr pellets) ν = 3030, 2928, 2854, 1943, 1741, 1670, 1586, 1508, 1418, 1271, 1211, 1149, 1117, 1003, 818, 752, 694. ESI(APCI)-MS: m/z = 571 [M + 1].
Statistical analyses
The Hammett s values were taken from the literature [25] and the Taft σ* figure has been reported previously [26]. The Hansch π and molar refractivity (MR) figures were obtained from published data [27]. The MR value of hydrogen is 1.03. Hence, in order that the relative bulk of the substituents was compared accurately, this figure was added to the MR value of the two groups in disubstituted compounds and 2.06 (2×1.03) for the monosubstituted analogs. The MR value for the unsubstituted compound 2 is 3.09. The linear and semilogarithmic plots were made using a commercial software package [28].
Molecular modeling
Models of 1,2,4–9, and 12–14 were built using BioMedCache 6.1 for Windows [29]. The lowest energy conformations were obtained from optimized geometry calculations in MOPAC using AM1 parameters. The torsion angles for these compounds are as follows 1: 46.7, −42.1; 2: 48.7, −44.6; 4: 49.0, −45.6; 5: 51.0, −44.5; 6: 48.7, −44.1; 7: 47.4, −48.0; 8: 49.5, −45.2; 9: 53.4, −45.7; 12: 47.8, −46.5; 13: 92.9, −50.4 and 14: 48.3, −45.6. Energy minimized structures of 1, 7 and 9 were superimposed (the five carbon atoms, C=C-C-C=C) and are depicted in Fig. 2.
Biology
Curcumin analogs 1–15 were subjected to a continuous exposure 72 h MTT assay as described previously [14]. L1210 and B16 cell lines were purchased from ATCC (Manassas, VA, USA) and were maintained as previously reported [14]. The microtubule disrupting effects were evaluated in A-10 cells by indirect immunofluorescent techniques as previously described [24].
Acknowledgments
The authors thank Taiho Pharmaceutical Co. and Hope College for support.
Footnotes
The authors have declared no conflict of interest.
References
- 1.Chen AP, Xu J. Am J Physiol. 2005;288:G447–G456. doi: 10.1152/ajpgi.00209.2004. [DOI] [PubMed] [Google Scholar]
- 2.Dorai T, Gehani N, Katz A. Prostate Cancer Prostatic Dis. 2000;3:84–93. doi: 10.1038/sj.pcan.4500399. [DOI] [PubMed] [Google Scholar]
- 3.Kuo ML, Huang TS, Lin JK. Biochim Biophys Acta. 1996;1317:95–100. doi: 10.1016/s0925-4439(96)00032-4. [DOI] [PubMed] [Google Scholar]
- 4.Ozaki K, Kawata Y, Amano S, Hanazawa S. Biochem Pharmacol. 2000;59:1577–1581. doi: 10.1016/s0006-2952(00)00277-x. [DOI] [PubMed] [Google Scholar]
- 5.Plummer S, Wakelin D, Bouer M. Br J Cancer. 2000;83(Suppl 1):20. [Google Scholar]
- 6.Sharma RA, Gescher AJ, Steward WP. Eur J Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Kumar A, Bharti AC. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 8.Tsvetkov P, Asher G, Reiss V, Shaul Y, et al. Proc Natl Acad Sci USA. 2005;102:5535–5540. doi: 10.1073/pnas.0501828102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang TS, Lee SC, Lin JK. Proc Natl Acad Sci USA. 1991;88:5292–5296. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Zhang Z, Hill DL, Wang H, Zhang R. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 11.Gupta KK, Bharne SS, Rathinasamy K, Naik NR, Panda D. FEBS J. 2006;273:5320–5332. doi: 10.1111/j.1742-4658.2006.05525.x. [DOI] [PubMed] [Google Scholar]
- 12.Pati HN, Holt HL, LeBlanc R, Dickson J, et al. Med Chem Res. 2005;14:19–25. [Google Scholar]; Pati HN, Wicks M, Holt HL, LeBlanc R, et al. Heterocyclic Commun. 2005;11:117–120. [Google Scholar]
- 13.Holt HL, Jr, LeBlanc R, Dickson J, Brown T, et al. Heterocyclic Commun. 2005;11:465–470. [Google Scholar]
- 14.LeBlanc R, Dickson J, Brown T, Stewart M, et al. Bioorg Med Chem. 2005;13:6025–6034. doi: 10.1016/j.bmc.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Ruprich J, Prout A, Dickson J, Younglove B, et al. Lett Drug Des Discov. 2007;4:144–148. [Google Scholar]
- 16.Johnson M, Younglove B, Lee L, LeBlanc R, et al. Bioorg Med Chem Lett. 2007;17:5897–5901. doi: 10.1016/j.bmcl.2007.07.105. [DOI] [PubMed] [Google Scholar]
- 17.Lee L, Davis R, Vanderham J, Hills P, et al. Eur J Med Chem. 2008 doi: 10.1016/j.ejmech.2007.11.030. in press. [DOI] [PubMed] [Google Scholar]
- 18.Robinson TP, Ehlers T, Hubbard RB, Bai XH, et al. Bioorg Med Chem Lett. 2003;13:115–117. doi: 10.1016/s0960-894x(02)00832-6. [DOI] [PubMed] [Google Scholar]
- 19.Dimmock JR, Padmanilayam MP, Puthucode RN, Nazarali AJ, et al. J Med Chem. 2001;44:586–593. doi: 10.1021/jm0002580. [DOI] [PubMed] [Google Scholar]
- 20.Dimmock JR, Jha A, Zello GA, Quail JW, et al. Eur J Med Chem. 2002;37:961–972. doi: 10.1016/s0223-5234(02)01414-9. [DOI] [PubMed] [Google Scholar]
- 21.Badaeva A, Timoteera TV, Masunov A, Tretiak S. J Phys Chem A. 2005;109:7276–7284. doi: 10.1021/jp0521510. [DOI] [PubMed] [Google Scholar]
- 22.Pati HN, Das U, Sharma RK, Dimmock JR. Mini Rev Med Chem. 2007;7:131–139. doi: 10.2174/138955707779802642. [DOI] [PubMed] [Google Scholar]
- 23.Thomas G. Medicinal Chemistry An Introduction. 50–54. John Wiley and Sons, Ltd; Chichester: 2000. pp. 57–59. [Google Scholar]
- 24.Kong Y, Grembecka J, Edler MC, Hamel E, et al. ACS Chem Biol. 2005;12:1007–1014. doi: 10.1016/j.chembiol.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Perrin DD, Dempsey B, Serjeant EP. pKa Prediction for Organic Acids and Bases. Chapman and Hall; London: 1981. pp. 109–112.pp. 120 [Google Scholar]
- 26.Taft RW., Jr . In: Steric Effects in Organic Chemistry. Newman MS, editor. John Wiley and Sons, Inc; New York: 1956. p. 591. [Google Scholar]
- 27.Hansch C, Leo AJ. Substituent Constants for Correlation Analysis in Chemistry and Biology. John Wiley and Sons; New York: 1979. p. 49.p. 84. [Google Scholar]
- 28.Statistical Package for Social Sciences, SPSS for Windows, Standard Version, release 13.0. Chicago: SPSS Inc; 2004. [Google Scholar]
- 29.BioMedCache 6.1 Windows, BioMedCache. Fujitsu America, Inc; 2003. [Google Scholar]