Abstract
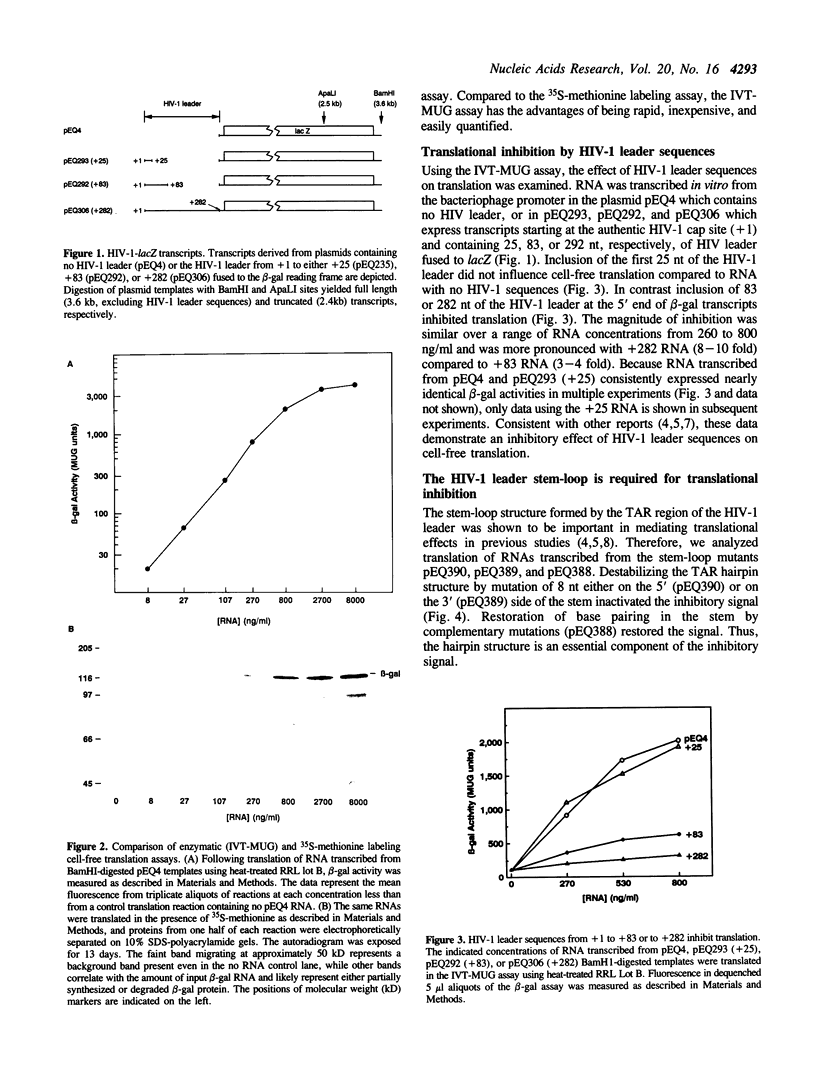
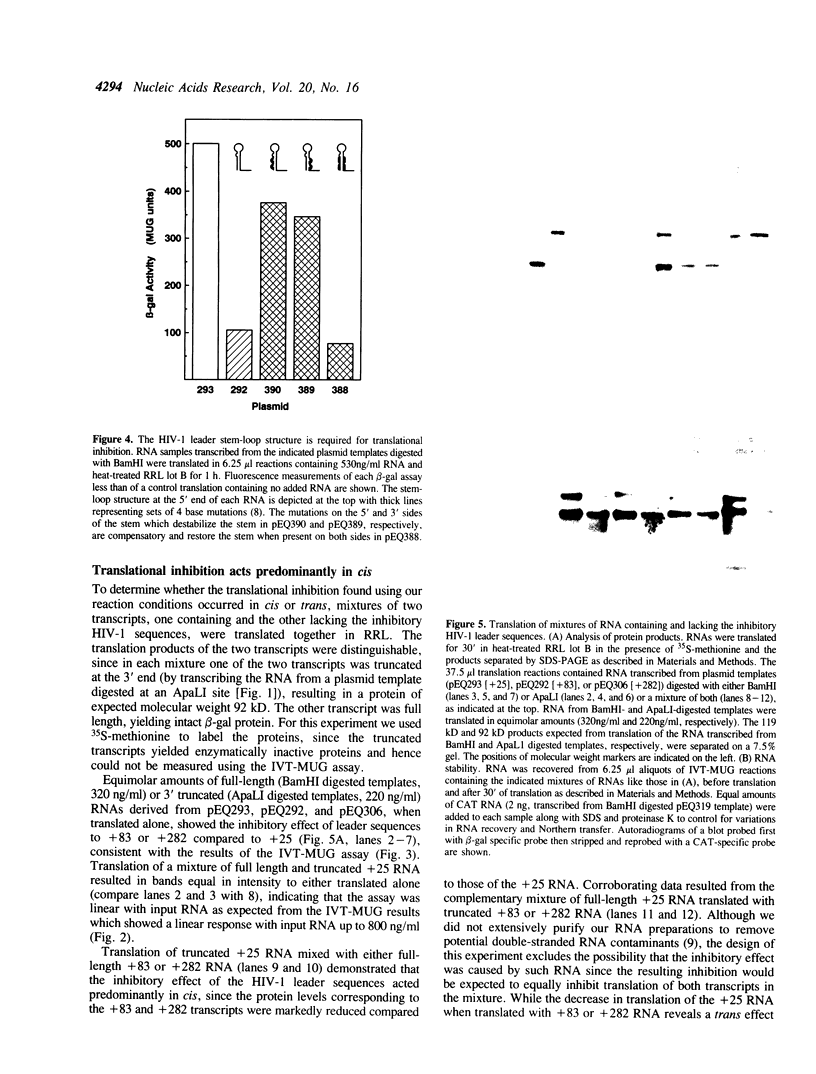
The 5' ends of all human immunodeficiency virus type I (HIV-1) transcripts have the potential to coordinately regulate translation of HIV-1 mRNAs. Conflicting observations of the translational impact of these sequences in various systems stimulated these analyses of translation in reticulocyte lysates. We report a sensitive, rapid, quantitative, and inexpensive cell-free translation assay in which translational efficiency is monitored by enzymatic assay of the translation products. Using this assay and conventional radiolabeling assays, we demonstrate that the HIV-1 transcript leader inhibits downstream translation and that the stem-loop structure is required. Under our assay conditions, this inhibition occurs predominantly in cis and is not mediated by the 68 kD, interferon-induced, double-stranded RNA-activated kinase (p68). However, under other assay conditions the HIV-1 leader may activate p68 and inhibit translation in trans. We show that variation between individual preparations of cell-free extracts can dramatically alter the magnitude of the translational inhibition by the HIV-1 leader. Further, we provide evidence that a heat-labile factor is required for efficient translation of transcripts containing the HIV-1 leader. These observations provide a foundation for identifying factors required for translation of HIV-1 transcripts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biegalke B. J., Geballe A. P. Sequence requirements for activation of the HIV-1 LTR by human cytomegalovirus. Virology. 1991 Jul;183(1):381–385. doi: 10.1016/0042-6822(91)90151-z. [DOI] [PubMed] [Google Scholar]
- Braddock M., Chambers A., Wilson W., Esnouf M. P., Adams S. E., Kingsman A. J., Kingsman S. M. HIV-1 TAT "activates" presynthesized RNA in the nucleus. Cell. 1989 Jul 28;58(2):269–279. doi: 10.1016/0092-8674(89)90841-6. [DOI] [PubMed] [Google Scholar]
- Braddock M., Thorburn A. M., Chambers A., Elliott G. D., Anderson G. J., Kingsman A. J., Kingsman S. M. A nuclear translational block imposed by the HIV-1 U3 region is relieved by the Tat-TAR interaction. Cell. 1990 Sep 21;62(6):1123–1133. doi: 10.1016/0092-8674(90)90389-v. [DOI] [PubMed] [Google Scholar]
- Braddock M., Thorburn A. M., Kingsman A. J., Kingsman S. M. Blocking of Tat-dependent HIV-1 RNA modification by an inhibitor of RNA polymerase II processivity. Nature. 1991 Apr 4;350(6317):439–441. doi: 10.1038/350439a0. [DOI] [PubMed] [Google Scholar]
- Chin D. J., Selby M. J., Peterlin B. M. Human immunodeficiency virus type 1 Tat does not transactivate mature trans-acting responsive region RNA species in the nucleus or cytoplasm of primate cells. J Virol. 1991 Apr;65(4):1758–1764. doi: 10.1128/jvi.65.4.1758-1764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Regulation of gene expression in the human immunodeficiency virus type 1. Adv Virus Res. 1991;40:1–17. doi: 10.1016/s0065-3527(08)60275-4. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. The HIV-1 Tat protein: an RNA sequence-specific processivity factor? Cell. 1990 Nov 16;63(4):655–657. doi: 10.1016/0092-8674(90)90129-3. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Inhibition of mRNA binding to ribosomes by localized activation of dsRNA-dependent protein kinase. Nature. 1984 Sep 6;311(5981):79–81. doi: 10.1038/311079a0. [DOI] [PubMed] [Google Scholar]
- Edery I., Petryshyn R., Sonenberg N. Activation of double-stranded RNA-dependent kinase (dsl) by the TAR region of HIV-1 mRNA: a novel translational control mechanism. Cell. 1989 Jan 27;56(2):303–312. doi: 10.1016/0092-8674(89)90904-5. [DOI] [PubMed] [Google Scholar]
- Gunnery S., Rice A. P., Robertson H. D., Mathews M. B. Tat-responsive region RNA of human immunodeficiency virus 1 can prevent activation of the double-stranded-RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8687–8691. doi: 10.1073/pnas.87.22.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- O'Malley R. P., Duncan R. F., Hershey J. W., Mathews M. B. Modification of protein synthesis initiation factors and the shut-off of host protein synthesis in adenovirus-infected cells. Virology. 1989 Jan;168(1):112–118. doi: 10.1016/0042-6822(89)90409-1. [DOI] [PubMed] [Google Scholar]
- Parkin N. T., Cohen E. A., Darveau A., Rosen C., Haseltine W., Sonenberg N. Mutational analysis of the 5' non-coding region of human immunodeficiency virus type 1: effects of secondary structure on translation. EMBO J. 1988 Sep;7(9):2831–2837. doi: 10.1002/j.1460-2075.1988.tb03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Agy M., Hovanessian A. G., Sonenberg N., Katze M. G. The integrity of the stem structure of human immunodeficiency virus type 1 Tat-responsive sequence of RNA is required for interaction with the interferon-induced 68,000-Mr protein kinase. J Virol. 1991 Feb;65(2):632–640. doi: 10.1128/jvi.65.2.632-640.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Katze M. G., Parkin N. T., Edery I., Hovanessian A. G., Sonenberg N. Control of the interferon-induced 68-kilodalton protein kinase by the HIV-1 tat gene product. Science. 1990 Mar 9;247(4947):1216–1219. doi: 10.1126/science.2180064. [DOI] [PubMed] [Google Scholar]
- Schleiss M. R., Degnin C. R., Geballe A. P. Translational control of human cytomegalovirus gp48 expression. J Virol. 1991 Dec;65(12):6782–6789. doi: 10.1128/jvi.65.12.6782-6789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SenGupta D. N., Berkhout B., Gatignol A., Zhou A. M., Silverman R. H. Direct evidence for translational regulation by leader RNA and Tat protein of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7492–7496. doi: 10.1073/pnas.87.19.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SenGupta D. N., Silverman R. H. Activation of interferon-regulated, dsRNA-dependent enzymes by human immunodeficiency virus-1 leader RNA. Nucleic Acids Res. 1989 Feb 11;17(3):969–978. doi: 10.1093/nar/17.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmeen L., Bass B., Sonenberg N., Weintraub H., Groudine M. Tat-dependent adenosine-to-inosine modification of wild-type transactivation response RNA. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8096–8100. doi: 10.1073/pnas.88.18.8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Marciniak R. A. HIV TAR: an RNA enhancer? Cell. 1989 Oct 20;59(2):229–230. doi: 10.1016/0092-8674(89)90279-1. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Gilbert C. S., Kerr I. M. The respective roles of the protein kinase and pppA2' p5' A2' p5 A-activated endonuclease in the inhibition of protein synthesis by double stranded RNA in rabbit reticulocyte lysates. Nucleic Acids Res. 1979 Apr;6(4):1335–1350. doi: 10.1093/nar/6.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]





