Abstract
Background
Even protective ventilation may aggravate or induce lung failure, particularly in preinjured lungs. Thus, new adjuvant pharmacologic strategies are needed to minimize ventilator-induced lung injury (VILI). Intermedin/Adrenomedullin-2 (IMD) stabilized pulmonary endothelial barrier function in vitro. We hypothesized that IMD may attenuate VILI-associated lung permeability in vivo.
Methodology/Principal Findings
Human pulmonary microvascular endothelial cell (HPMVEC) monolayers were incubated with IMD, and transcellular electrical resistance was measured to quantify endothelial barrier function. Expression and localization of endogenous pulmonary IMD, and its receptor complexes composed of calcitonin receptor-like receptor (CRLR) and receptor activity-modifying proteins (RAMPs) 1–3 were analyzed by qRT-PCR and immunofluorescence in non ventilated mouse lungs and in lungs ventilated for 6 h. In untreated and IMD treated mice, lung permeability, pulmonary leukocyte recruitment and cytokine levels were assessed after mechanical ventilation. Further, the impact of IMD on pulmonary vasoconstriction was investigated in precision cut lung slices (PCLS) and in isolated perfused and ventilated mouse lungs. IMD stabilized endothelial barrier function in HPMVECs. Mechanical ventilation reduced the expression of RAMP3, but not of IMD, CRLR, and RAMP1 and 2. Mechanical ventilation induced lung hyperpermeability, which was ameliorated by IMD treatment. Oxygenation was not improved by IMD, which may be attributed to impaired hypoxic vasoconstriction due to IMD treatment. IMD had minor impact on pulmonary leukocyte recruitment and did not reduce cytokine levels in VILI.
Conclusions/Significance
IMD may possibly provide a new approach to attenuate VILI.
Introduction
Mechanical ventilation (MV) is a life saving treatment without alternatives in acute respiratory failure, and MV is also employed following surgery or trauma. One third of all patients in intensive care units are receiving MV [1]. Notably, even a minimum of physical forces on lung tissue induced by MV may evoke ventilator-induced lung injury (VILI), an important undesirable effect of respirator therapy [2]. Minimization of MV-induced physical stress by reduction of tidal volumes to 6 ml/kg significantly improved clinical outcome of mechanically ventilated patients [3]. However, particularly preinjured lungs are sensitive for the development of VILI even in the setting of lung-protective ventilation [4], [5]. VILI is characterized by pulmonary inflammation with liberation of cytokines, recruitment of leukocytes to the lung and particularly increased lung permeability, resulting in lung edema, surfactant dysfunction, impaired lung compliance and deterioration of pulmonary gas exchange [6]. As the necessity to guarantee sufficient gas exchange frequently limits a further substantial reduction of tidal volumes and oxygen supply, new adjuvant pharmacological therapies enhancing pulmonary vascular barrier function in VILI/acute respiratory distress syndrome (ARDS) in addition to lung-protective ventilation are needed to prevent or ameliorate VILI.
We have previously shown that the potent barrier protective properties of the calcitonin receptor-like receptor (CRLR) agonist adrenomedullin reduced VILI in different murine VILI models [7]. Intermedin (IMD), alternatively named adrenomedullin-2, is an endogenous peptide also signaling via CRLR coupled to the receptor activity-modifying proteins (RAMP) 2 or 3 [8], [9]. Intermedin stabilized endothelial barrier function in vitro and in an isolated mouse lung model of hydrostatic lung edema [10], [11], and reduced pulmonary leukocyte infiltration in pulmonary ischemia/reperfusion injury [12]. Further, IMD had protective effects in cardiac ischemia/reperfusion injury and lowered systemic blood pressure [8], [13], [14].
Pulmonary hyperpermeability and hyperinflammation are hallmarks of VILI and ARDS, and pharmacologic attenuation of vascular barrier breakdown in these conditions may be a promising therapeutic approach to limit VILI in addition to lung protective ventilation strategies. We hypothesized that the CRLR ligand IMD has barrier protective effects in a murine model of VILI in vivo.
In the current study we observed IMD-evoked barrier stabilization on endothelial cell layers in vitro. In a clinically relevant mouse model of VILI, continuously infused IMD reduced VILI-induced pulmonary hyperpermeability without affecting pulmonary and systemic inflammation. IMD revealed vasodilatatory effects on pulmonary vasculature in mouse lungs. Further, the influence of MV on pulmonary IMD expression and distribution as well as on expression of the IMD receptor components was investigated.
Methods
Ethics Statement
Animal experiments were approved by the animal ethics committee of the Charité-Universtätsmedizin Berlin and local governmental authorities (LAGeSo (Landesamt für Gesundheit und Soziales Berlin), approval ID: G 0100/10).
Transcellular Electrical Resistance (TER) of Endothelial Cells
Human pulmonary microvascular endothelial cells (HPMVEC) were purchased from PromoCell (Heidelberg, Germany) and grown on evaporated gold electrodes, connected to an electrical cell-substrate impedance system (Applied Biophysics, Troy, NY, USA). Cells were exposed to 0.01 or 0.1 µM human IMD (1–47, Phoenix, Burlingame, CA, USA) or solvent and TER values from each microelectrode were continuously monitored as described previously [15].
Mice
Female C57Bl/6 mice (8 to 10 weeks; 18 to 20 g; Charles River, Sulzfeld, Germany) were used.
Mechanical Ventilation and IMD Treatment
As described previously [16] mice were anesthetized by intraperitoneal injections of Fentanyl (75 µg/kg), Midazolam (1.5 mg/kg) and Medetomedin (0.75 mg/kg). Repetitively, Fentanyl (16 µg/kg), Midazolam (0.33 mg/kg) and Medetomedin (0.16 mg/kg) was supplied via an intraperitoneal catheter when required to guarantee adequate anaesthesia during the observation period. Body temperature was maintained at 37°C by a temperature-controlled heating pad. After tracheotomy and intubation, mice were ventilated (MiniVent, Hugo-Sachs-Electronics, March-Hugstetten, Germany) with 70% oxygen; tidal volume (VT) 7 ml/kg; respiratory rate (RR) 240 minute–1; positive end-expiratory pressure (PEEP) 6cmH2O. A carotid artery catheter was placed for blood pressure monitoring and infusion of NaCl 0.9% containing 100 mmol/l HCO3– (350 µl/h). There was no additional fluid support in any conducted experiment. A urinary catheter was inserted. VT, RR, airway pressure, peripheral oxygen saturation and urine output were monitored (Pulmodyn, Hugo-Sachs-Electronics, March-Hugstetten, Germany; MouseOx, STARRLife-Sciences, Oakmont, PA, USA). After preparation, a recruitment manoeuvre was performed (airway pressure 35cmH2O for 5 sec) before respirator settings were adjusted for 6 h to VT 12 ml/kg, RR 120 min–1, PEEP 2cmH2O. A second recruitment manoeuvre was performed 5 min before termination of the experiment. All mice survived the protocol. At termination of the experiments mice were sacrificed by exsanguination via the carotid artery catheter. Blood samples were analyzed for paO2 and lactate by blood gas analyzer (ABL-800, Radiometer, Copenhagen, Denmark). P/F ratio was calculated as paO2/FiO2. Non-ventilated mice served as controls. Murine IMD (1–47, Phoenix, Burlingame, CA, USA) (0.025 mg/kg/h) was continuously infused via the carotid artery catheter starting with onset of ventilation.
qRT-PCR
Lungs were flushed and snap frozen in liquid nitrogen. Total RNA was isolated from the lungs using RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. To remove genomic DNA contamination, isolated RNA samples were treated with 1 U DNase/µg RNA (Invitrogen, Karlsruhe, Germany) for 15 min at 37°C. One microgram of total RNA was used in a 20 µl reaction to synthesize cDNA using Superscript H- reverse transcriptase (200 U/µg RNA, Invitrogen) and oligo dTs as primers. Reverse transcription reaction was for 50 min at 42°C. Real-time quantitative PCR was performed using the I-Cycler IQ detection system (Bio-Rad, Munich, Germany) in combination with the IQ SYBR Green Real-Time PCR Supermix (Bio-Rad). The PCR conditions included initial denaturation in one cycle of 10 min at 95°C followed by 40 cycles of 20 s at 95°C, 20 s at 60°C, and 20 s at 72°C. The relative expressions were calculated as: 2-(ΔCT) × 1/mean control 2-(ΔCT), where ΔCT is calculated as: ΔCT = CTGOI-CTHKG (GOI: gene of interest, HKG: house keeping gene). Primer sequences are provided in Table S1.
Immunofluorescent Staining
Immunolabelling for IMD and CRLR immunolabelling was performed by overnight incubation at room temperature with previously characterized antibodies [11], [17], including double-labelling with biotinylated rat monoclonal anti-CD31 (1 µg/ml; clone MEC 13.3, BD Biosciences, Heidelberg, Germany), an endothelial marker [18], and biotinylated lycopersicon esculentum (LEA) lectin (1∶3200; Vector Laboratories, Burlingame, CA, USA), a type I alveolar epithelial cell marker [19]. Secondary reagents, each applied for 1 h, were Cy3-conjugated goat anti-human IgG F(ab)2 (1∶500; Dianova, Hamburg, Germany), Cy3-conjugated donkey anti-rabbit IgG (1∶2000; Dianova), and FITC-conjugated streptavidin (1∶500; Sigma, Deisenhofen, Germany). Optionally, nuclei were counterstained by adding 0.4 µg/ml 4′,6-diamidino-2′-phenylindole-dihydrochloride (DAPI; Sigma) to the secondary reagent solution.
Lung Permeability
At the end of the experiment BAL and plasma concentration of previously injected human serum albumin (cHSA) was determined and permeability was assessed by calculating the cHSA BAL/Plasma ratio as described previously [16].
Hypoxic Vasoconstriction in Precision Cut Lung Slices (PCLS)
PCLS were prepared as described previously [20], [21]. Briefly, mice were killed by cervical dislocation and the airways were filled with 1.5% low melting point agarose. After solidification of the agarose, the lungs were cut into 200 µm thick slices. The agarose was removed by incubation of the PCLS in phenolred-free minimal essential medium (MEM) continuously gassed with 21% O2, 5% CO2, 74% N2 for at least 2 h at 37°C. To analyze vasoreactivity of individual cross-sectioned intra-acinar arteries (minimal inner diameter up to 40 µm), PCLS were transferred into a flow-through superfusion chamber (Hugo Sachs Elektronik, March, Germany). At the beginning of each experiment the capability of the vessel to contract in response to the thromboxane analogue U46619 and to dilate after application of the NO donor sodium nitroprusside was checked. After washing out these drugs with normoxic gassed in phenolred-free MEM (21%O2, 5% CO2, 74% N2) PCLS were incubated with hypoxic gassed medium (1%O2, 5% CO2, 94% N2; 0.7 ml/min). After 15 min 500 nM of murine IMD was added to the hypoxic medium. Pictures of the artery were taken every 2 min using an inverted microscope mounted on the superfusion chamber. Changes of the luminal area of the vessels were evaluated by lining the inner boundaries by hand. The luminal area at the beginning of the experiment was defined as 100% and vasoreactivity was expressed as relative decrease or increase of this area. In the graph shown only the values obtained for the hypoxic incubation followed by the incubation in hypoxic medium +/- IMD are given. For a clear graphic presentation the values obtained at the beginning of hypoxia were defined as 100%.
Isolated Perfused and Ventilated Mouse Lungs
Lungs were prepared as described [22], [23]. Briefly, anesthetized mice were tracheotomised and ventilated. After sternotomy and cannulation of left atrium and pulmonary artery, lungs were perfused with 37°C sterile Krebs-Henseleit hydroxyethylamylopectine buffer (1mL/min) and ventilated by negative pressure (Pexp –4.5, Pins –9.0 cm H2O) in a humidified chamber. After a steady state period of 30 min, the thromboxane receptor agonist U46619 (3 × 10–8 M) was administered to the perfusate, evoking an increase in pulmonary arterial pressure. Murine IMD was administered to the perfusate for 30 seconds in 12-min intervals with IMD concentrations being increased. Maximum pressure response after IMD administration was determined.
Leukocytes in Lung Tissue, BAL Fluid and Blood
Lungs were flushed. The left lung was digested in RPMI containing collagenase and DNAse for 1 h. Leukocytes were extracted by meshing the lung tissue through a cell strainer (100 µm). BAL cells were isolated by centrifugation of BAL fluid gained as described above. Lung and BAL leukocytes were counted by haemocytometer and blood leukocytes were quantified using TruCount-Tubes. Leukocytes were differentiated by flow cytometry according to their sidescatter/forward-scatter properties and CD45, Gr-1 and F4-80 expression (FACSCalibur, BD, Heidelberg, Germany).
Quantification of Cytokines
Cytokines were quantified from total protein of flushed homogenized left lungs and from plasma samples (BioRad, Hercules, CA, USA).
Data Analyses
Data are expressed as mean ± SEM. For comparison between groups, Mann-Whitey U-test was used. For the comparison of continuously measured data between groups, 2-way ANOVA was applied. P values <0.05 were considered statistically significant.
Results
IMD Stabilized Endothelial Barrier Function in HPMVECs
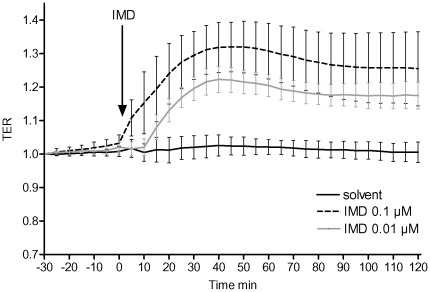
Confluent monolayers of HPMVECs stimulated with 0.01 or 0.1 µM IMD showed a dose dependent increase in TER displaying improved endothelial barrier integrity. This effect lasted for the whole observation period of 120 min (Fig. 1). Comparable improvement of barrier function was observed in primary human umbilical vein endothelial cell monolayers (data not shown in detail).
Figure 1. IMD improved endothelial barrier function in endothelial cells.
HPMVECs grown to confluence on gold microelectrodes to measure transcellular electrical resistance (TER) were stimulated with 0.01 or 0.1 µM IMD or with solvent. IMD dose dependently improved endothelial barrier function as displayed by TER increase. (n = 6).
Pulmonary RAMP3 mRNA Levels were Down Regulated in VILI
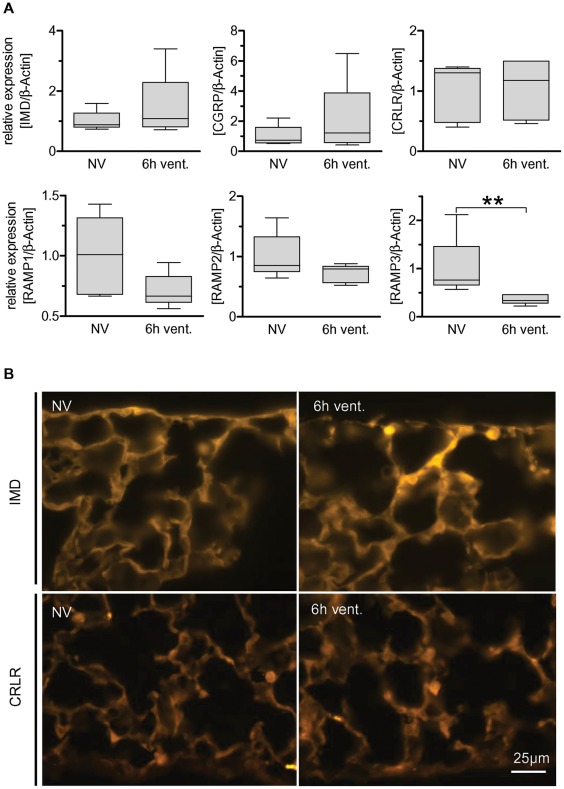
The impact of mechanical ventilation on endogenous IMD and its receptors was determined prior to exogenous IMD application. The influence of mechanical ventilation on the mRNA expression of IMD and its receptor compounds CRLR, RAMP1-3 as well as on the CRLR/RAMP1 agonist calcitonin gene-related peptide (CGRP) was assessed by qRT-PCR. VILI caused down regulation of pulmonary RAMP3 mRNA levels while the mRNA levels of IMD, CRLR, CGRP and RAMP1-2 were not affected by MV (Fig. 2A). IMD and CRLR protein expression and distribution in lung tissue was not altered by MV.
Figure 2. Regulation of IMD and its receptor complexes in VILI.
Mice were ventilated with a tidal volume of 12 ml/kg for 6 h (6h vent.). Non ventilated individuals (NV) served as controls. A) Regulation of IMD, CGRP and RAMP1-3 was quantified by qRT-PCR in the lungs of ventilated (6h vent.) and non ventilated mice (NV). (** p<0.01, n = 5). B) Immunofluorescence analysis of subpleural peripheral lung. IMD-immunolabelling was slightly enhanced in ventilated (6h vent.) compared to non-ventilated mice (NV) while CRLR-immunolabelling did not differ between groups. Tissue sections depicting NV and 6h vent groups were processed simultaneously and images were taken at the same exposure time (30 ms for IMD and 150 ms for CRLR).
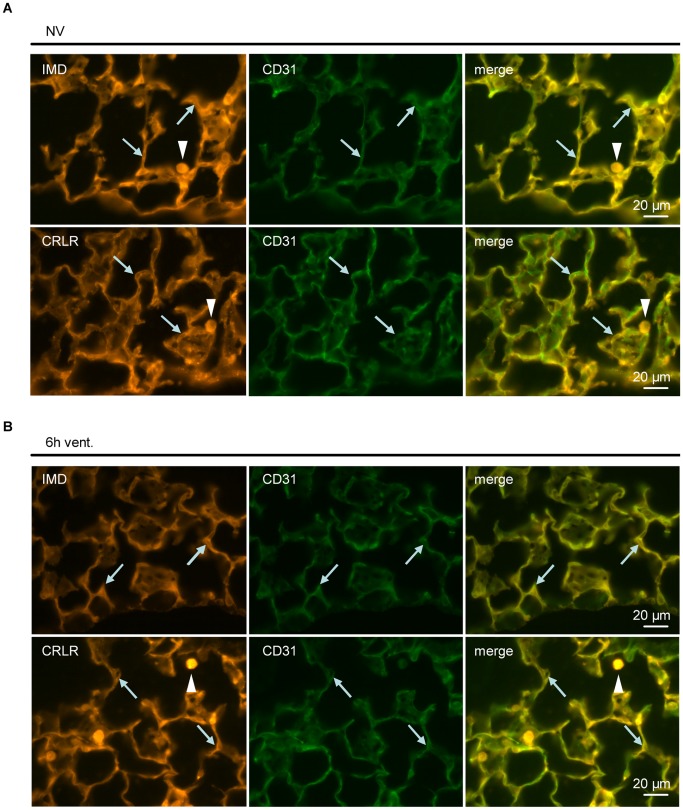
To investigate the effect of VILI on pulmonary IMD and CRLR protein expression and distribution in the lungs, immunofluorescence analysis was performed. The IMD specificity of the employed antibody was validated in preabsorption experiments (Fig. S1). A trend towards increased pulmonary IMD expression in VILI was observed (Fig. 2B). CRLR expression was not altered by MV (Fig. 2B). IMD and CRLR protein were located mainly, although not exclusively, in alveolar macrophages and in the pulmonary endothelium, which was confirmed by double staining of IMD or CRLR and the endothelial marker CD31 (Fig. 3A). These expression patterns were not altered by MV (Fig. 3B). Co-labelling with LEA, a marker for type I alveolar epithelial cells, demonstrated limited colocalization. Instead, large epithelial stretches without IMD- or CRLR-immunolabelling were evident (Fig S2).
Figure 3. Pulmonary distribution of IMD and CRLR was not altered in VILI.
In non ventilated mice (A) and individuals ventilated with a tidal volume of 12 ml/kg for 6 h (6h vent.) (B) IMD- and CRLR-immunolabelling colocalized with CD31-immunoreactivity (arrows), a marker for endothelial cells. In addition, alveolar macrophages were IMD- and CRLR-positive (arrowheads). (n = 5, representative images shown).
Exogenous IMD Reduced Pulmonary Hyperpermeability in VILI
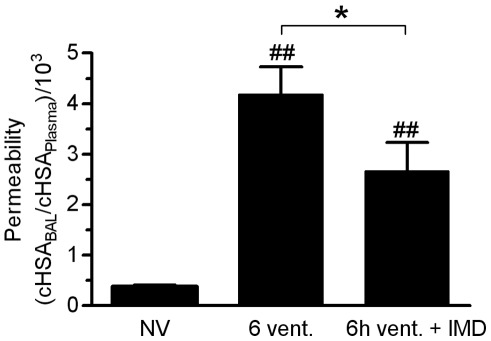
As we had been observing a robust barrier stabilizing effect of IMD in vitro, and after having characterized the impact of MV on the endogenous IMD system we aimed to investigate whether continuously infused IMD protects against VILI induced pulmonary hyperpermeability in mechanically ventilated mice. Translocation of human serum albumin (HSA) from the vascular into the alveolar compartment was quantified after 6h of MV and served as a marker for lung permeability. MV provoked a marked increase of lung vascular permeability, thereby indicating VILI. Treatment with exogenous IMD significantly reduced VILI-evoked pulmonary hyperpermeability (Fig. 4). As increased permeability is the crucial mechanism for the development of high permeability lung edema in ALI/VILI, pharmacologic targeting of this major pathomechanism by IMD is promising.
Figure 4. IMD reduced pulmonary vascular hyperpermeability in VILI.
Mice were ventilated with a tidal volume of 12 ml/kg for 6 h and treated with IMD 0.025 mg/kg*h (6h vent.+IMD) or solvent (6h vent). NV = non ventilated mice. Human serum albumin (HSA) was infused 90 min prior to termination of the experiment. HSA concentration (cHSA) in plasma and BAL were determined. Increased HSA BAL/plasma ratio indicated microvascular leakage. (*p<0.05, ## p<0.01 vs. NV, NV n = 5, 6h vent. n = 7, 6h vent. + IMD n = 8)
IMD Infusion Lowered Systemic Arterial Blood Pressure
IMD is a vasoactive peptide causing vasodilation in rodents. Arterial hypotension and consecutive disturbance of the microcirculation thus had to be excluded to avoid interference of shock with VILI and IMD treatment. Mean systemic arterial blood pressure (MAP) was measured continuously by means of a catheter in the carotid artery of each ventilated individual. Blood lactate was chosen as an indicator for disturbance of the microcirculation and urine output during the last two hours of the experiment served as an indicator for parenchymal perfusion pressure and renal function. IMD treatment resulted in a significant reduction of MAP. Notably, this decrease in blood pressure did not impact blood lactate levels and urinary output, suggesting that systemic microcirculation and renal perfusion were not relevantly compromised (Fig S3). In additional experiments, an increased IMD dose (0.075 mg/kg/h) resulted in blood lactate levels of 56 +/- 13 mg/dl (n = 4) indicating a significant deterioration of the microcirculation despite a constant mean arterial blood pressure between 60–65 mmHg throughout the experiment (data not shown). Blood lactate levels in the control group were 37 +/- 5 mg/dl respectively. Thus, the dose of 0.025 mg/kg/h IMD was chosen, which reduced hyperpermeability while severe side effects due to haemodynamic alterations were not evident.
Peripheral Oxygen Saturation in VILI was Slightly Improved by IMD Treatment
Improvement of oxygenation is not necessarily associated with improved survival in ARDS. However, loss of oxygenation capacity is a key marker of lung injury, and improvement of oxygenation would provide evidence for the efficacy of IMD associated reduction of VILI. Therefore, peripheral oxygen saturation was measured continuously throughout the experiments by pulse oxymetry, and pO2 was determined in arterial blood by a routine blood gas analyzer.
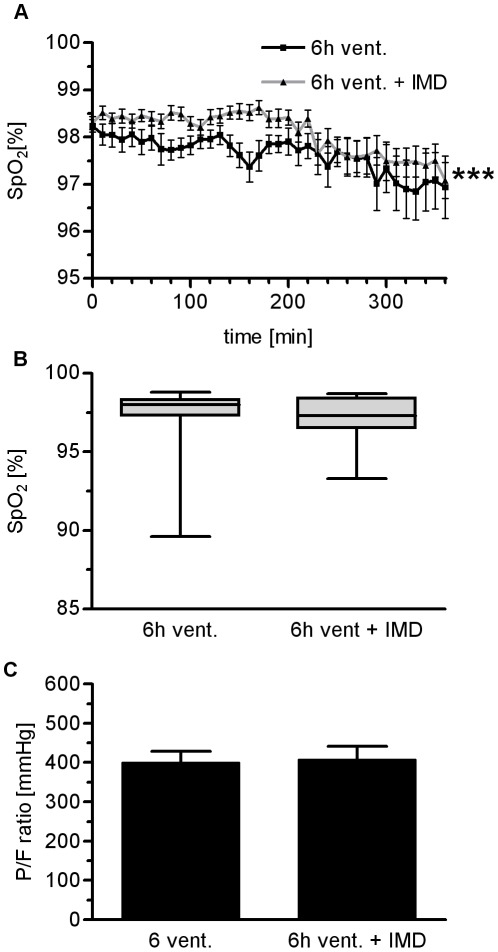
IMD treated individuals showed significantly (p<0.001) but only slightly improved peripheral oxygen saturation as compared to untreated ventilated mice throughout the observation period (Fig. 5A). However, following a final recruitment manoeuvre there was no detectable difference between groups regarding SpO2 and P/F ratio (Fig. 5B; Fig. 5C).
Figure 5. IMD had no impact on oxygenation in VILI.
Mice were ventilated with a tidal volume of 12 ml/kg for 6 h and treated with IMD 0.025 mg/kg*h (6h vent.+IMD) or solvent (6h vent). A) Peripheral SpO2 was monitored. B) At the end of the 6h ventilation period, SpO2 was not relevantly different between groups. C) After 6h of MV, the P/F ratio was not different between groups. (***p<0.001, n = 15)
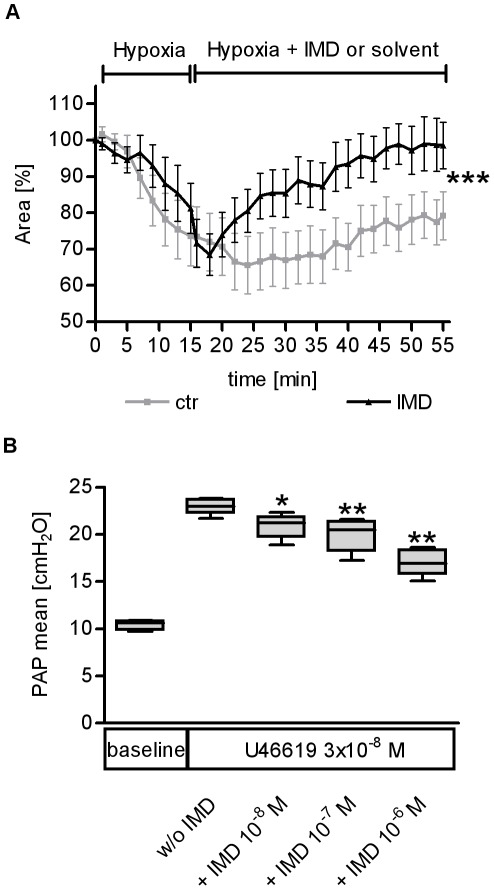
IMD Reduced Pulmonary Vasoconstriction
With regard to the missing improvement in oxygenation despite barrier-stabilizing properties of IMD, we hypothesized that vasodilatory properties known for IMD might counteract reduction of lung injury by an increase of ventilation/perfusion mismatch. Indeed, hypoxic pulmonary vasoconstriction (HPV) was reduced by IMD in murine precision cut lung slices of mice (Fig. 6A). Further, IMD dose dependently reduced pulmonary vasoconstriction evoked by the thromboxane receptor agonist U46619 in isolated ventilated and perfused mouse lungs (Fig. 6B). Thus, an IMD related increase of the ventilation/perfusion mismatch possibly overrode improvement of barrier function due to IMD treatment.
Figure 6. IMD mediated pulmonary vasodilation and reversed hypoxic pulmonary vasoconstriction.
A) In precision cut lung slices (PCLS) hypoxic pulmonary vasoconstriction (HPV) was induced, and the luminal areas of single intra-acinar pulmonary arteries were continuously analyzed by planimetry. HPV was markedly reduced by 500 nM IMD (***p<0.001; n = 10). B) In isolated ventilated and perfused mouse lungs the thromboxane agonist U46619 induced a marked elevation of pulmonary artery pressure (PAP) during constant perfusion flow. PAP was lowered dose dependently by IMD bolus injection (* p<0.05, ** p<0.01, n = 5)
IMD did not Reduce Inflammation in VILI
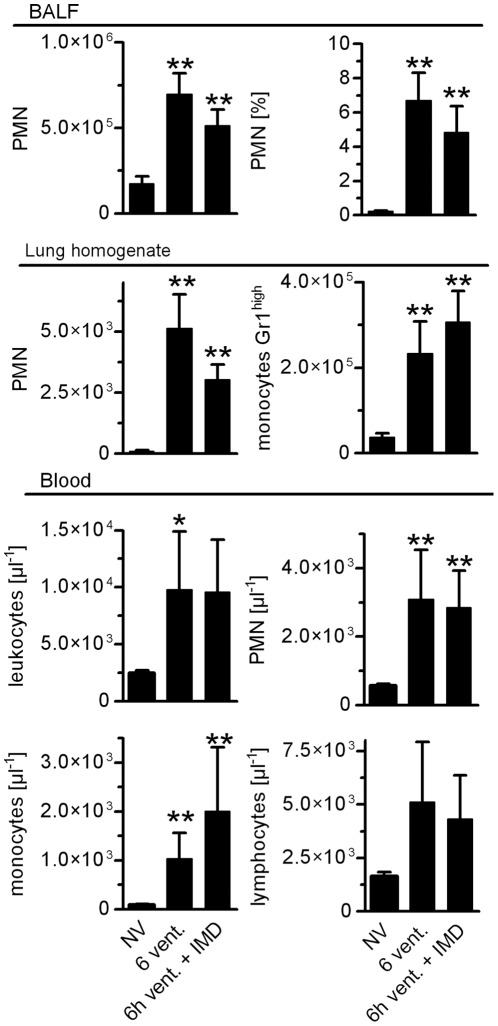
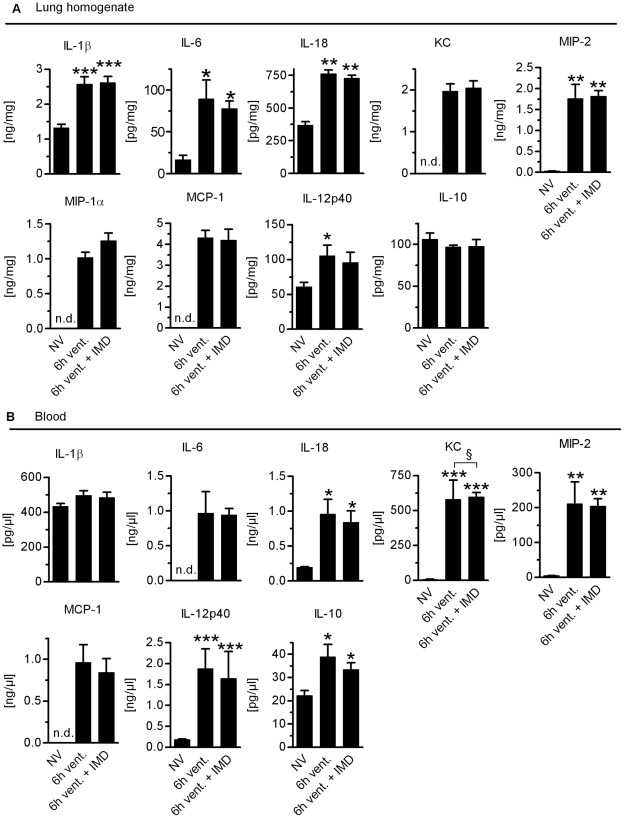
Activation of the innate immune system plays a key role in the pathophysiology of VILI, and dampening this response can ameliorate VILI. However, interference with pathways of the innate immune system may render ARDS patients even more susceptible towards infections and cause unfavorable outcomes. Thus, we aimed to determine the impact of IMD on crucial innate immune responses in VILI. We analyzed leukocyte counts in the BAL fluid, lung homogenate and blood and quantified cytokines in lungs and blood. VILI evoked recruitment of neutrophils and Gr-1high monocytes as detected in BAL fluid and in lung homogenates. IMD did not impact pulmonary recruitment of neutrophils or Gr-1high monocytes in VILI (Fig. 7). VILI provoked blood leukocytosis with elevated numbers of monocytes and neutrophils, which was not modified by IMD treatment (Fig. 7). VILI increased pulmonary levels of IL-1β, IL-18, IL-6, KC, MIP-1α, MIP-2, MCP-1 and IL12p40, while IL-10 levels remained at baseline. IMD treatment did not alter pulmonary cytokine profiles (Fig. 8A). Also, plasma levels of IL-18, IL-6, KC, MIP-2, MCP-1, IL-12p40 and IL-10 were increased in mice ventilated for 6 h. IL-1β levels remained at baseline. IMD did not alter levels of measured plasma cytokines (Fig. 8B). Notably, in additional experiments with a higher dosage of IMD (0.075 mg/kg/h) we observed a trend towards reduced neutrophil counts in the BAL fluid (p = 0.0571, n = 4) and reduced monocyte counts in blood (p = 0.0286 n = 4) due to IMD treatment. As outlined above, 0.075 mg/kg/h IMD probably tended to cause deterioration of microcirculation and was therefore not used for further investigations.
Figure 7. VILI induced pulmonary leukocyte recruitment and blood leukocytosis were unaffected by IMD treatment.
Mice were ventilated with a tidal volume of 12 ml/kg for 6 h and treated with IMD 0.025 mg/kg*h (6h vent.+IMD) or solvent (6h vent). NV = non ventilated mice. Leukocytes isolated from bronchoalveolar lavage fluid (BALF), lung homogenate and blood, respectively, were quantified and differentiated by flow cytometry (*p<0.05 vs. NV, **p<0.01 vs. NV, NV n = 5, 6h vent. n = 7, 6h vent. + IMD n = 8)
Figure 8. VILI-induced cytokine production was not altered by IMD.
Mice were ventilated with a tidal volume of 12 ml/kg for 6 h and treated with IMD 0.025 mg/kg*h (6h vent.+IMD) or solvent (6h vent). NV = non-ventilated mice. A) IL-1β, IL-18, IL-6, KC, MIP-1α, MIP-2, MCP-1, IL12p40 were detemined in lung homogenate by multiplex assay technique. (*p<0.05 vs. NV, **p<0.01 vs. NV n = 8) B) IL-1β, IL-18, IL-6, KC, MIP-1α, MIP-2, MCP-1, IL12p40 were determined in plasma by multiplex assay technique. (*p<0.05 vs. NV, **p<0.01 vs. NV n = 8).
Discussion
In the current study, IMD enhanced endothelial barrier function in vitro and diminished lung hyperpermeability, but not pulmonary and systemic inflammation in a clinically relevant mouse model of VILI.
Impairment of endothelial barrier function is a hallmark of VILI and ARDS, leading to development of lung edema, surfactant dysfunction and deterioration of pulmonary gas exchange [6]. Particularly preinjured lungs are susceptible to VILI, and VILI may contribute to lung injury in ARDS patients despite lung protective ventilation strategies [4], [5]. A pharmacologic approach to reduce pulmonary permeability in addition to lung protective ventilation strategies thus may be a promising adjuvant therapeutic strategy to minimize VILI in ARDS patients.
IMD is an endogenous CRLR ligand that mediated endothelial barrier stabilizing effects in vitro and in an isolated lung model of hydrostatic lung edema [10], [11]. In mechanically ventilated mice, we investigated the regulation of IMD and its receptor complex composed of CRLR and one of the auxiliary proteins RAMP1, 2 and 3, which are mandatory for efficient CRLR signalling [8], [9]. Expression of IMD, CRLR and RAMP1 and 2 were not affected by MV, whereas RAMP3 expression was significantly reduced. Previous studies suggested that IMD predominantly signals through receptor complexes composed of CRLR and RAMP2 or 3 [8], [9]. Thus, the observed down regulation of RAMP3 may indicate reduced efficacy of endogenous IMD signalling in VILI, and treatment with exogenous IMD may restore or further amplify endogenous barrier stabilizing IMD effects in VILI.
Indeed, in the currently employed mouse model, continuously infused IMD markedly reduced lung permeability in VILI. To the best knowledge of the authors this study is the first providing evidence for pulmonary permeability reduction due to exogenous IMD in VILI. Mechanisms suggested to be responsible for the barrier stabilizing properties of CRLR signalling include increase of intracellular cAMP, leading to a protein kinase A dependent inhibition of myosin light chain phosphorylation and stabilization of intercellular junctions, finally leading to reduced endothelial intercellular gap formation [10], [11], [24], [25]. This mechanism, originally studied by CRLR stimulation with AM has been confirmed in a murine VILI model, in models of ALI and in isolated rat intestine exposed to bacterial toxin [7], [24], [26], [27].
Despite marked reduction of VILI-induced pulmonary permeability in IMD treated individuals, oxygenation was not improved. This contradictory finding may be partly explained by vasodilatory effects displayed by IMD. Specifically in the current study, increased pulmonary vascular resistance due to thromboxane receptor stimulation was reduced by IMD in isolated perfused and ventilated mouse lungs, and HPV in murine lung tissue slices was diminished by IMD. Although direct evidence is not provided by the current study, it is tempting to speculate that reduction of pulmonary vascular resistance by IMD resulted in ventilation/perfusion (V/Q) mismatch and increment of pulmonary shunt perfusion, probably masking improved oxygenation capacity due to reduced oedema formation in IMD treated individuals.
Pulmonary hyperinflammation is a central feature of VILI and contributes to lung permeability [6]. Previously, the CRLR ligand adrenomedullin ameliorated inflammatory responses in polymicrobial sepsis suggesting that CRLR signalling may mediate immunomodulating effects [28], [29]. In hyperoxic VILI, we observed ameliorated leukocyte infiltration into the alveolar space due to AM treatment [7], which was not evident in the current study using 0.025 mg/kg/h IMD. Also, cytokine production was unaltered by IMD. Notably in preliminary experiments, IMD in a higher dosage (0.075 mg/kg/h) reduced neutrophil counts in the BAL fluid of IMD treated mice subjected to VILI as compared to solvent treated controls by trend (p = 0.057; n = 4; data not shown in detail). Concurrently, neutrophil counts were significantly reduced in the blood and increased in lung tissue (p<0.05; n = 4; data not shown in detail), probably as a result of leukocyte sequestration due to deterioration of microcirculation, as lactate levels were elevated in these mice. In line, IMD has been reported to lower systemic blood pressure in rodents [8], [13], [30]. In preliminary experiments (n = 4), 0.075 mg/kg/h IMD lowered mean arterial blood pressure and compromised systemic microcirculation. However, treatment with 0.025 mg/kg/h IMD reduced MAP only moderately and did not result in compromised systemic microcirculation or relevant reduction of end organ perfusion pressure as indicated by comparable blood lactate levels and urine output in both groups. Thus, IMD most likely reduced endothelial gap formation in VILI by inflammation-independent mechanisms in the current study, which may hold relevant clinical benefits. First, in ventilated ARDS patients with sepsis or severe pneumonia, hyperinflammation may override anti-inflammatory properties of pharmacologic therapies. Second, limiting central mechanisms of the innate immune response may compromise host defence against invading pathogens and thereby pave the way for secondary bacterial infections.
In conclusion, the endogenous CRLR ligand peptide IMD reduced pulmonary vascular leakage in a clinically relevant mouse model of VILI. Reduction of lung permeability was not accompanied by reduced pulmonary or systemic inflammation. However, regarding oxygenation capacity IMD may counteract reduction of lung edema by increasing V/Q mismatch.
Supporting Information
Preabsorption of the IMD antibody with mouse IMD (1–47) resulted in almost complete absence of labelling, suggesting high specificity of the primary antibody. Both tissue sections were cut from the same specimen and processed simultaneously. Images were taken at the same exposure time (190 ms).
(TIF)
Double labelling with LEA to depict the epithelial lining of the alveolus in non ventilated lungs. The merged image also includes DNA labelling with DAPI to highlight cellular nuclei. There is little overlap of LEA binding to alveolar type I cells with IMD- or CRLR-immunoreactivity. Arrows in the merged image point to LEA-binding epithelial stretches without IMD- or CRLR-immunoreactivity.
(TIF)
Mice were ventilated with a tidal volume of 12 ml/kg for 6 h and treated with IMD 0.025 mg/kg*h (6 h vent.+IMD) or solvent (6 h vent). A) Mean arterial blood pressure (MAP) was monitored. MAP was lower in IMD treated individuals B) Lactate levels quantified at the end of the experiment and C) urine output were not different between groups (***p<0.001; n = 15).
(TIF)
(DOC)
Acknowledgments
We thank Dr. G. P. McGregor for kindly providing CRLR antiserum and Annika Kühn, Doris Stoll and Anna Goldenberg for excellent technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study is supported in part by grants from the German Research Foundation (http://www.dfg.de/index.jsp) to ACH, SH, NS, MW (SFB/TR84) and SH (HI-789/6-1) and MW (OP-87/1), the Excellence Cluster Cardio-Pulmonary System to UP and WK, and the German Federal Ministry of Education and Research (http://www.bmbf.de/)to SH, NS (Pneumonia Research Network on Genetic Resistance and Susceptibility for the Evolution of Severe Sepsis PROGRESS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287:345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 2.Wolthuis EK, Vlaar AP, Choi G, Roelofs JJ, Juffermans NP, et al. Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care. 2009;13:R1. doi: 10.1186/cc7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.Dhanireddy S, Altemeier WA, Matute-Bello G, O’Mahony DS, Glenny RW, et al. Mechanical ventilation induces inflammation, lung injury, and extra-pulmonary organ dysfunction in experimental pneumonia. Lab Invest. 2006;86:790–799. doi: 10.1038/labinvest.3700440. [DOI] [PubMed] [Google Scholar]
- 5.O’Mahony DS, Liles WC, Altemeier WA, Dhanireddy S, Frevert CW, et al. Mechanical ventilation interacts with endotoxemia to induce extrapulmonary organ dysfunction. Crit Care. 2006;10:R136. doi: 10.1186/cc5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verbrugge SJ, Lachmann B, Kesecioglu J. Lung protective ventilatory strategies in acute lung injury and acute respiratory distress syndrome: from experimental findings to clinical application. Clin Physiol Funct Imaging. 2007;27:67–90. doi: 10.1111/j.1475-097X.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 7.Müller HC, Witzenrath M, Tschernig T, Gutbier B, Hippenstiel S, et al. Adrenomedullin attenuates ventilator-induced lung injury in mice. Thorax. 2010;65:1077–1084. doi: 10.1136/thx.2010.135996. [DOI] [PubMed] [Google Scholar]
- 8.Bell D, McDermott BJ. Intermedin (adrenomedullin-2): a novel counter-regulatory peptide in the cardiovascular and renal systems. Br J Pharmacol. 2008;153(Suppl 1):S247–S262. doi: 10.1038/sj.bjp.0707494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roh J, Chang CL, Bhalla A, Klein C, Hsu SY. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem. 2004;279:7264–7274. doi: 10.1074/jbc.M305332200. [DOI] [PubMed] [Google Scholar]
- 10.Aslam M, Pfeil U, Gunduz D, Rafiq A, Kummer W, et al. Br J Pharmacol; 2011. Intermedin/adrenomedullin2 stabilises endothelial barrier and antagonises thrombin-induced barrier failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeil U, Aslam M, Paddenberg R, Quanz K, Chang CL, et al. Intermedin/adrenomedullin-2 is a hypoxia-induced endothelial peptide that stabilizes pulmonary microvascular permeability. Am J Physiol Lung Cell Mol Physiol. 2009;297:L837–L845. doi: 10.1152/ajplung.90608.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Körner C, Kuchenbuch T, Pfeil U, Jung K, Padberg W, et al. Intermedin-treatment of pulmonary ischemia/reperfusion injury in the living mouse. Pneumologie. 2011;65:114–122. [Google Scholar]
- 13.Fujisawa Y, Nagai Y, Miyatake A, Miura K, Nishiyama A, et al. Effects of adrenomedullin 2 on regional hemodynamics in conscious rats. Eur J Pharmacol. 2007;558:128–132. doi: 10.1016/j.ejphar.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 14.Yang JH, Jia YX, Pan CS, Zhao J, Ouyang M, et al. Effects of intermedin(1–53) on cardiac function and ischemia/reperfusion injury in isolated rat hearts. Biochem Biophys Res Commun. 2005;327:713–719. doi: 10.1016/j.bbrc.2004.12.071. [DOI] [PubMed] [Google Scholar]
- 15.Müller HC, Hocke AC, Hellwig K, Gutbier B, Peters H, et al. The Sphingosine-1 Phosphate receptor agonist FTY720 dose dependently affected endothelial integrity in vitro and aggravated ventilator-induced lung injury in mice. Pulm Pharmacol Ther. 2011;24:377–385. doi: 10.1016/j.pupt.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Müller HC, Hellwig K, Rosseau S, Tschernig T, Schmiedl A, et al. Simvastatin attenuates ventilator-induced lung injury in mice. Crit Care. 2010;14:R143. doi: 10.1186/cc9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagner S, Haberberger R, Kummer W, Springer J, Fischer A, et al. Immunohistochemical detection of calcitonin gene-related peptide receptor (CGRPR)-1 in the endothelium of human coronary artery and bronchial blood vessels. Neuropeptides. 2001;35:58–64. doi: 10.1054/npep.2000.0844. [DOI] [PubMed] [Google Scholar]
- 18.Vecchi A, Garlanda C, Lampugnani MG, Resnati M, Matteucci C, et al. Monoclonal antibodies specific for endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol. 1994;63:247–254. [PubMed] [Google Scholar]
- 19.Bankston PW, Porter GA, Milici AJ, Palade GE. Differential and specific labeling of epithelial and vascular endothelial cells of the rat lung by Lycopersicon esculentum and Griffonia simplicifolia I lectins. Eur J Cell Biol. 1991;54:187–195. [PubMed] [Google Scholar]
- 20.Paddenberg R, Konig P, Faulhammer P, Goldenberg A, Pfeil U, et al. Hypoxic vasoconstriction of partial muscular intra-acinar pulmonary arteries in murine precision cut lung slices. Respir Res. 2006;7:93. doi: 10.1186/1465-9921-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paddenberg R, Faulhammer P, Goldenberg A, Gries B, Heinl J, et al. Impact of modulators of mitochondrial ATP-sensitive potassium channel (mitoK(ATP)) on hypoxic pulmonary vasoconstriction. Adv Exp Med Biol. 2009;648:361–368. doi: 10.1007/978-90-481-2259-2_41. [DOI] [PubMed] [Google Scholar]
- 22.Witzenrath M, Ahrens B, Kube SM, Hocke AC, Rosseau S, et al. Allergic lung inflammation induces pulmonary vascular hyperresponsiveness. Eur Respir J. 2006;28:370–377. doi: 10.1183/09031936.06.00080105. [DOI] [PubMed] [Google Scholar]
- 23.Witzenrath M, Gutbier B, Hocke AC, Schmeck B, Hippenstiel S, et al. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit Care Med. 2006;34:1947–1954. doi: 10.1097/01.CCM.0000220496.48295.A9. [DOI] [PubMed] [Google Scholar]
- 24.Hippenstiel S, Witzenrath M, Schmeck B, Hocke A, Krisp M, et al. Adrenomedullin reduces endothelial hyperpermeability. Circ Res. 2002;91:618–625. doi: 10.1161/01.res.0000036603.61868.f9. [DOI] [PubMed] [Google Scholar]
- 25.Temmesfeld-Wollbruck B, Hocke AC, Suttorp N, Hippenstiel S. Adrenomedullin and endothelial barrier function. Thromb Haemost. 2007;98:944–951. doi: 10.1160/th07-02-0128. [DOI] [PubMed] [Google Scholar]
- 26.Brell B, Temmesfeld-Wollbruck B, Altzschner I, Frisch E, Schmeck B, et al. Adrenomedullin reduces Staphylococcus aureus alpha-toxin-induced rat ileum microcirculatory damage. Crit Care Med. 2005;33:819–826. doi: 10.1097/01.ccm.0000159194.53695.7a. [DOI] [PubMed] [Google Scholar]
- 27.Temmesfeld-Wollbruck B, Brell B, David I, Dorenberg M, Adolphs J, et al. Adrenomedullin reduces vascular hyperpermeability and improves survival in rat septic shock. Intensive Care Med. 2007;33:703–710. doi: 10.1007/s00134-007-0561-y. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Rey E, Chorny A, Varela N, Robledo G, Delgado M. Urocortin and adrenomedullin prevent lethal endotoxemia by down-regulating the inflammatory response. Am J Pathol. 2006;168:1921–1930. doi: 10.2353/ajpath.2006.051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itoh T, Obata H, Murakami S, Hamada K, Kangawa K, et al. Adrenomedullin ameliorates lipopolysaccharide-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2007;293:L446–L452. doi: 10.1152/ajplung.00412.2005. [DOI] [PubMed] [Google Scholar]
- 30.Takei Y, Hyodo S, Katafuchi T, Minamino N. Novel fish-derived adrenomedullin in mammals: structure and possible function. Peptides. 2004;25:1643–1656. doi: 10.1016/j.peptides.2004.06.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preabsorption of the IMD antibody with mouse IMD (1–47) resulted in almost complete absence of labelling, suggesting high specificity of the primary antibody. Both tissue sections were cut from the same specimen and processed simultaneously. Images were taken at the same exposure time (190 ms).
(TIF)
Double labelling with LEA to depict the epithelial lining of the alveolus in non ventilated lungs. The merged image also includes DNA labelling with DAPI to highlight cellular nuclei. There is little overlap of LEA binding to alveolar type I cells with IMD- or CRLR-immunoreactivity. Arrows in the merged image point to LEA-binding epithelial stretches without IMD- or CRLR-immunoreactivity.
(TIF)
Mice were ventilated with a tidal volume of 12 ml/kg for 6 h and treated with IMD 0.025 mg/kg*h (6 h vent.+IMD) or solvent (6 h vent). A) Mean arterial blood pressure (MAP) was monitored. MAP was lower in IMD treated individuals B) Lactate levels quantified at the end of the experiment and C) urine output were not different between groups (***p<0.001; n = 15).
(TIF)
(DOC)