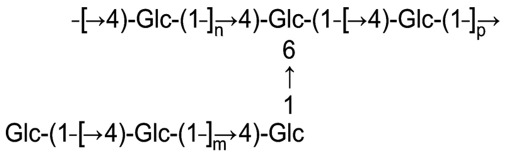Abstract
We previously reported that A. hydrophila GalU mutants were still able to produce UDP-glucose introduced as a glucose residue in their lipopolysaccharide core. In this study, we found the unique origin of this UDP-glucose from a branched α-glucan surface polysaccharide. This glucan, surface attached through the O-antigen ligase (WaaL), is common to the mesophilic Aeromonas strains tested. The Aeromonas glucan is produced by the action of the glycogen synthase (GlgA) and the UDP-Glc pyrophosphorylase (GlgC), the latter wrongly indicated as an ADP-Glc pyrophosphorylase in the Aeromonas genomes available. The Aeromonas glycogen synthase is able to react with UDP or ADP-glucose, which is not the case of E. coli glycogen synthase only reacting with ADP-glucose. The Aeromonas surface glucan has a role enhancing biofilm formation. Finally, for the first time to our knowledge, a clear preference on behalf of bacterial survival and pathogenesis is observed when choosing to produce one or other surface saccharide molecules to produce (lipopolysaccharide core or glucan).
Introduction
Mechanisms of glycogen formation have been largely studied for Escherichia coli [1]. Glucose-1-phosphate is the early precursor for glycogen synthesis, and it is first converted to ADP-glucose (ADP-Glc) in a reversible reaction catalyzed by ADP-glucose pyrophosphorylase (EC 2.7.7.27). The glucosyl unit of ADP-Glc is transferred to the nonreducing end of an α-1,4-glucan chain by glycogen synthase (EC 2.4.1.21), and α-1,6 linkages are generated by a branching enzyme (EC 2.4.1.18). UDP-glucose (UDP-Glc) is considered to be the glucosyl donor for glycogen synthesis in mammalian cells. Either ADP-Glc or UDP-Glc can serve as glucosyl donors in eukaryotic microorganisms and Plants [2].
The biosynthesis of UDP-Glc in E. coli involves the following enzymatic reactions:
1. through GalU, Glucose-1 phosphate + UTP → UDP-Glc + PPi.
2. through Gal E, UDP-Glc ↔ UDP-Gal (UDP-galactose).
3. through GalT, Glucose-1 phosphate + UDP-Gal → Galactose-1 phosphate + UDP-Glc.
Reactions 2 and 3 need the bacterial growth in galactose as a single carbon source.
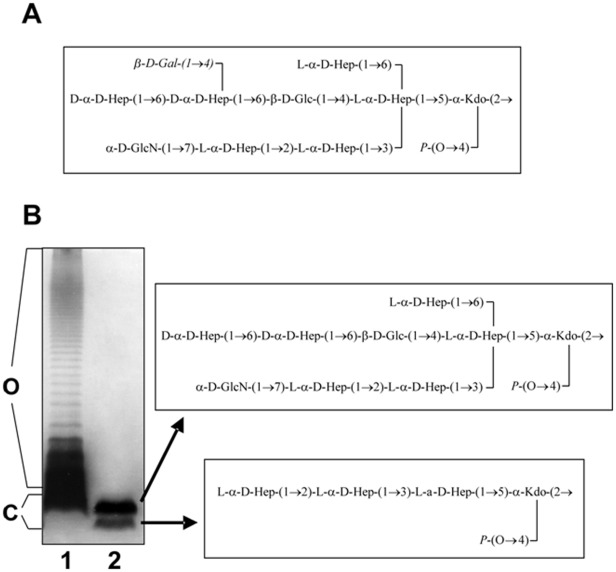
A. hydrophyla galU mutants are unable to synthesize UDP-Glc from glucose-1-phosphate and UTP [3]. Besides its function as a substrate for glucosyltransferases resulting in glucosylated surface structures, UDP-Glc plays a well-established biochemical role as a glycosyl donor in the enzymatic biosynthesis of carbohydrates. The LPS from several Aeromonas galU mutants, either growing in rich or minimal media with glucose as a single carbon source, showed the absence of the O-antigen LPS and a LPS-core with higher electrophoretic mobility (two bands) as compared with the corresponding unique band of their respective wild type strains LPS-core [3]. Taking advantage of the complete determined LPS structure of strain AH-3 (serotype O34) [4], [5] ( Figure 1A), it appears that the two LPS bands from AH-3 galU mutant correspond to one (the major product represented by the slow-migrating LPS band on the gels) which is the complete LPS-core but devoid of Gal residue in the outer core where the O34-antigen LPS is attached; and the other (the minor product represented by the fast-migrating LPS band on the gels) with a deeply truncated LPS-core structure restricted to one Kdo and and three l-glycero-d-manno-heptose (ld-Hep) residues (see Figure 1B). The low amount of UDP-Glc in the mutant respect to the wild type explains its inability to complete the LPS-core structure. In a reduced number of the LPS molecules the mutant is unable to transfer the Glc residue to the inner-core, and the LPS-core is not further extended (Figure 1B). In the majority of the LPS-core molecules, the mutant is able to incorporate the Glc residue to the inner core and part of the outer core is added (major LPS-core product, see Figure 1B). However, even in this case the mutant is unable to complete the LPS-core because it can not incorporate the terminal Gal residue, probably because as mentioned before in the mutant the amount of UDP-Glc is too low [3]. The lack of Gal residue explains why the mutant is unable to express the O34-antigen LPS, because this residue links the O:34-antigen LPS [3]. Complementation with the single A. hydrophila galU gene completely restored all the LPS defects [3].
Figure 1. LPS of Aeromonas hydrophila AH-3.
(A) Chemical structure of the LPS-core of A. hydrophila strain AH-3 [36]. The O34-antigen is linked to the Gal residue (shown in italics) of the LPS core [36]. (B) Silver-stained SDS-PAGE of purified LPS from A. hydrophila strain AH-3 (lane 1) and GalU mutant AH-2886 (lane 2) and structures of the two LPS-core bands of AH-2886 mutant [3]. All monosaccharides are in the pyranose form. Kdo, 3-deoxy-d-manno-oct-2-ulosonic acid; l-α-d-Hep, d-α-d-Hep, l-glycero- and d-glycero-α-d-manno-heptose; Glc, glucose; GlcN, glucosamine; Gal, galactose. C = LPS core, O = O34-antigen LPS.
In this work we show the origin of the UDP-Glc obtained by the A. hydrophila strain AH-3 GalU mutant through the presence of a surface polysaccharide (glucan), which represents a new way to obtain UDP-Glc in Aeromonas strains. This surface Aeromonas glucan seems to play a role in biofilm formation.
Results
Aeromonas Hydrophila Strains Produce a Cell-surface Polysaccharide (Glucan)
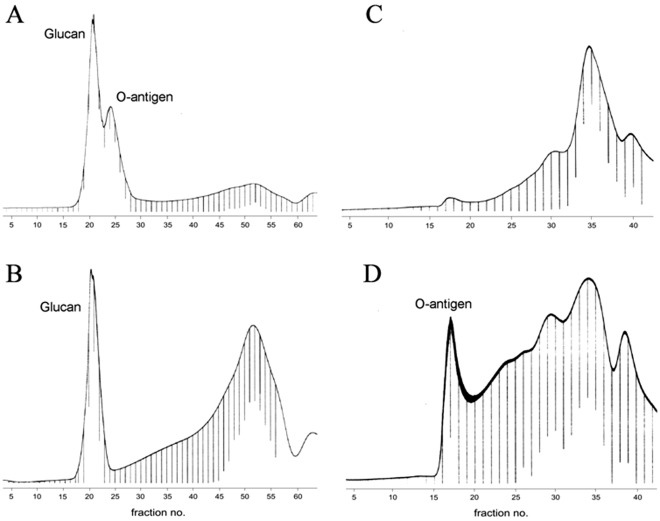
A material containing cell-surface carbohydrates (LPS and a polysaccharide) was isolated from A. hydrophila strain AH-3 by the phenol-water extraction following digestion of cells with RNAse, DNAse and Proteinase K as described [4]. It was degraded with 1% acetic acid to cleave the lipid moiety of the LPS, and the resultant water-soluble (carbohydrate) material was fractionated by gel-permeation chromatography on Sephadex G-50 to yield glucan (eluted first) and then parts of the LPS: O-polysaccharide (O-antigen) and core oligosaccharide (Figure 2A).
Figure 2. Sephadex G-50 gel permeation chromatography elution profiles of the water soluble (carbohydrate) material isolated by mild acid degradation of the LPS preparations from A. hydrophyla AH-3 (wild type) (A), AH-3ΔrmlB (B), AH-3ΔwecP (C) and AH-3ΔglgA (D) mutants.
Sugar analysis of glucan showed the presence of D-glucose (Glc) only. The D configuration of glucose was established by GLC analysis of the acetylated (+)-2-octyl glycosides as described [5]. GLC was performed using an Agilent 7820A GC system and a temperature program from 160°C (1 min) to 290°C at 7°C min–1. Methylation analysis of glucan revealed terminal Glc, 4-substituted Glc and 4,6-disubstituted Glc in a ratio of 1∶5:1. Therefore, glucan is a branched polysaccharide. The 1H- and 13C-NMR spectra of glucan were assigned using two-dimensional 1H,1H homonuclear and 1H,13C heteronuclear correlation spectroscopy (COSY, TOCSY, HSQC, HSQC-TOCSY). They indicated that all glucose residues are α-linked; particularly, this followed from chemical shifts 4.95–5.35 ppm for H-1 and 99.9–101.3 ppm for C-1. The 13C-NMR spectrum showed also that ∼20% Glc residues are terminal (characteristic chemical shifts 71.0 ppm for C-4 and 62.4 ppm for C-6) and the rest are either 4-substituted (chemical shifts 79.2 ppm for C-4 and 62.3 ppm for C-6) or 4,6-disubstituted (chemical shifts 79.2 ppm for C-4 and 69.2 ppm for C-6). These data are in full agreement with methylation analysis data. A two-dimensional nuclear Overhauser effect NMR experiment showed that all terminal Glc residues are linked to position 4 of 4-substituted (or 4,6-disubstituted) Glc residues and none of them is linked to position 6 of 4,6-disubstituted Glc residues. 4-Substituted Glc residues are linked either to position 4 of 4-substituted (or 4,6-disubstituted) Glc residues or to position 6 of 4,6-disubstituted Glc residues. Based on these data together, the glucan structure can be represented as shown in Figure 3.
Figure 3. Structure representation of the branched glucan from A. hydrophyla AH-3 (wild type).
A RmlB mutant (AH-3ΔrmlB) devoid of O34-antigen LPS because the absence of 6-deoxy-L-talose [4] showed the presence of glucan in the soluble fraction after chromatography on Sephadex G-50 like the wild type strain AH-3, but not the O34-antigen LPS (Figure 2B). WecP is the enzyme that catalyzes the transfer of N- acetylgalactosamine to undecaprenyl phosphate to initiate the O-antigen lipopolysaccharide (LPS) biosynthesis [6]. No glucan or O34-antigen LPS [7] was obtained from the AH-3ΔwecP mutant cell surface (Figure 2C). WaaL is the enzyme that ligates the O-antigen LPS to the LPS-core [8]. The AH-3ΔwaaL mutant (AH-3Δ3.1 in [8]) showed no glucan or O34-antigen LPS.
When we studied different A. hydrophila wild type strains from different O-antigen serotypes, like for instance strain AH-1 (O11) or PPD134/91 (O18), we isolated a glucan polysaccharide identical to the one obtained from strain AH-3. In all these cases this glucan eluted before the LPS domains (O-polysaccharide and core oligosaccharide), independently of the O-antigen type (data not shown).
The Glucan and the GalU Mutants
The AH-3 galU mutant (AH-2886) was devoid of the O34-antigen and showed two types of LPS structures corresponding to the two LPS bands on gels (Figure 1) [3]. When we tested for the presence of the glucan in the cell surface of AH-2886 mutant, we were unable to detect any polysaccharide by chromatography on Sephadex G-50 of the soluble fraction from the acid hydrolyzed phenol-water extract.
The AH-3ΔglgA and AH-3ΔglgC Mutants: Glucan and LPS
The genome sequence of A. hydrophila ATCC7966T [9] allow us to design primers to sequence the glgA and C region of A. hydrophila AH-3 (GenBank accession number JQ085284). The AH-3ΔglgA and AH-3ΔglgC mutants grown in a rich medium like LB were unable to produce glucan on the bacterial surface after the treatment with phenol-water and the chromatography on Sephadex G-50 of the soluble fraction, but were able to produce O34-antigen LPS (Figure 2D show the result for AH-3ΔglgA).
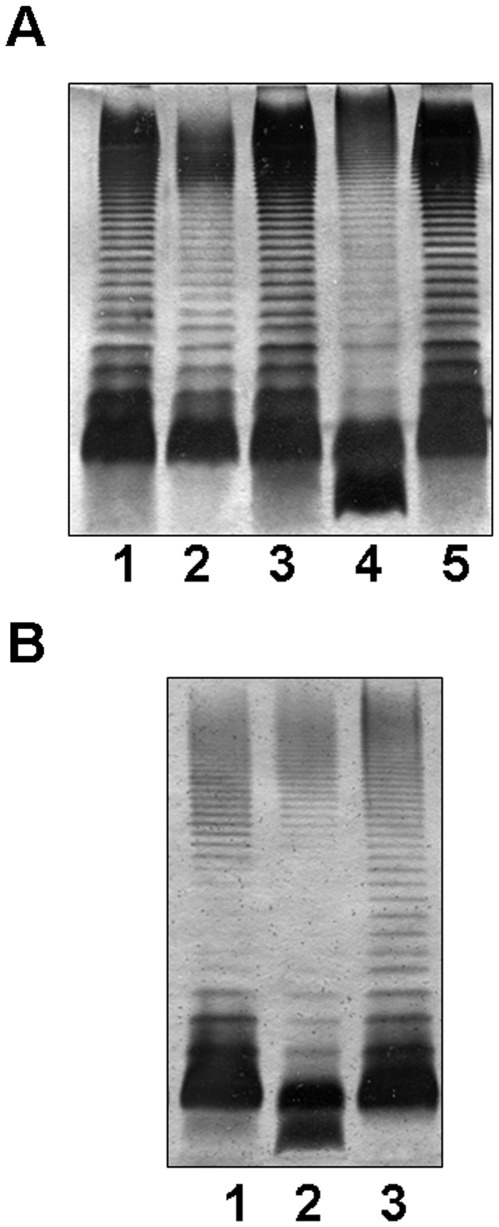
The LPS profiles of both mutants, grown under the same conditions, analyzed on gels showed the presence of O34-antigen LPS with no significant differences with that of the wild type strain (Figure 4A lanes 2 and 3 show the results for AH-3ΔglgA mutant and wild type). However, when the mutant strains were grown on minimal medium with glucose as a unique carbon source, the LPS profiles of both mutants showed a reduced number of O34-antigen LPS molecules in comparison with the wild type strain and the appearance of two bands for the LPS-core (Figures 4A lane 4 and 4B lane 2). All these characteristics were rescued by the reintroduction of the A. hydrophila AH-3 glgA or glgC in a vector (pBAD33-GlgA or pBAD33-GlgC) (Figures 4A lane 5 and 4B lane 3), but not with the plasmid vector alone.
Figure 4. LPS of wild type strain and glgA and C mutants.
(A) Silver-stained SDS-PAGE of the LPS from A. hydrophyla AH-3 (1), AH-3ΔglgA (2), and AH-3ΔglgA plus pBAD33-GlgA (3) when the strains were grown in LB. Lanes 4 and 5 show LPS from the same strains as 2 and 3, respectively, grown in minimal medium with glucose as a unique carbon source. (B) SDS-PAGE of the LPS from A. hydrophyla AH-3 (1), AH-3ΔglgC (2), and AH-3ΔglgC plus pBAD33-GlgC (3) strains grown in minimal medium with glucose as a unique carbon source.
The AH-3ΔgalU:glgA and AH-3ΔgalU:glgC Double Mutants
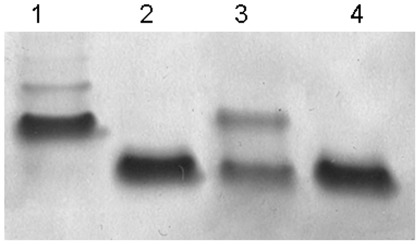
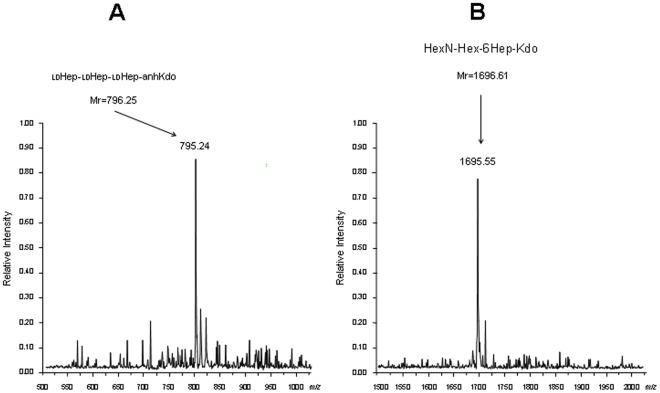
From the AH-3 galU mutant (AH-2886) we obtained double mutants AH3ΔgalU:glgA and AH3ΔgalU:glgC as described in Materials and Methods. We were unable to detect the presence of glucan in the cell surface because they are galU mutants, but only a single LPS band (corresponding to the the one with faster migration in AH-3 galU mutant) could be detected on gels (Figure 5, lane 2, data for AH3ΔgalU:glgA). LPS was extracted and purified from strain AH-3ΔgalU:glgA, and the oligosaccharide fraction was obtained by mild acid hydrolysis (see Materials and Methods), and this fraction analyzed by Electrospray mass spectrum. A major ion peak for a Hep3Kdo compound with the molecular mass 795.24 Da was obtained (Figure 6A), in agreement with the LPS-core structure:
Figure 5. Silver-stained Tricine SDS-PAGE of the LPS from A. hydrophyla AH-3 (1), AH-3ΔgalU:glgA double mutant (2), AH-3ΔgalU:glgA plus pBAD33-GlgA from AH-3 (3), and AH-3ΔgalU:glgA plus pBAD33-GlgAEc from E. coli (4).
Figure 6. Negative ions ESI-MS of the core oligosaccharide LPS.
(A) Core oligosaccharide obtained by acid release from the purified LPS of AH-3ΔgalU:glgA. double mutant. (B) Core oligosaccharide obtained from the slow LPS migration band from AH-3ΔgalU:glgA harbouring the AH-3 glgA (pBAD33-GlgA). anhKdo, an anhydro form of Kdo; Mr, calculated molecular mass (Da).
L-α-D-Hep-(1→2)-L-α-D-Hep-(1→3)-L-α-D-Hep-(1→5)-Kdo.
Similar results were obtained for AH3ΔgalU:glgC double mutant.
Enzyme Activities
The results for UDP-Glc or ADP-Glc pyrophosphorylase and glycogen synthase enzyme activities, either using crude extracts or purified proteins from A. hydrophila AH-3 (wild type) or mutant strains, are summarized on Table 1. Briefly, wild type crude extracts showed UDP-Glc and ADP-Glc pyrophosphorylase activities but a reduced UDP-Glc pyrophosphorylase activity was obtained on crude extracts of the GalU mutant in comparison with the corresponding from the wild type strain. However, the UDP-Glc pyrophosphorylase enzyme activity on GalU mutant crude extracts was not abolished. The AH-3ΔglgC crude extracts showed a reduced UDP-Glc pyrophosphorylase enzyme activity, less than the ones obtained from the GalU mutant crude extracts, versus the wild type crude extracts. The crude extracts from the AH-3ΔglgA mutant showed no glycogen synthase activity while high activity was detected for purified AH-3GlgA. Purified AH-3 GlgC showed UDP-Glc but not ADP-Glc pyrophosphorylase enzyme activity. Furthermore, purified AH-3 GlgA glycogen synthase activity was largely inhibited by the presence of unlabelled UDP-Glc but not by unlabelled ADP-Glc.
Table 1. Enzymatic average values for A. hydrophila crude extracts and purified proteins.
| Strain | UDP-Glca pyrophosphorylase | Sp act (nmol/min/mg of protein) | |
| ADP-Glca pyrophosphorylase | Glycogen synthase | ||
| AH-3 (wild type) | |||
| Crude extract | 1.326+/–28 | 3.189+/–42 | 12+/–3 |
| purified GlgA | ND | ND | 52+/–14 |
| purified GlgC | 9,324+/–144 | <1,0 | ND |
| AH-2286 (AH-3 galU mutant) | |||
| Crude extract | 0.203+/–8 | 3.098+/–39 | 11+/–2 |
| AH-3ΔglgA | |||
| Crude extract | 0.973+/–28 | 2.479+/–37 | <0.2 |
| AH-3ΔglgC | |||
| Crude extract | 0.935+/–21 | 2.971+/–40 | 11+/–3 |
A. hydrophila strains were grown under the conditions mentioned in Materials and Methods.
Activity was measured in the pyrophosphorolysis direction.
Values are the average of three independent experiments +/– standard deviation.
ND, not determined.
Complementation Studies Using AH-3ΔgalU:glgA Mutant
The enzyme activities results previously obtained prompted us to study the complementation of AH-3ΔgalU:glgA mutant with glgA from A. hydrophila AH-3 or E. coli through LPS studies. When plasmid vector carrying the AH-3 glgA (pBAD33-GlgA) was introduced into the AH-3ΔgalU:glgA mutant, two LPS-core bands without O34-antigen LPS were found (Figure 5, lane 3). The LPS migration profile in gel of this complemented mutant, was identical to that of GalU mutant, and the new band with slow migration on gel showed a major ion peak corresponding to a HexNHexHep6Kdo compound with a molecular mass 1696.61 (Figure 6B) in agreement with the LPS structure found in the single galU mutant [3] (see Figure 1B lane 2 upper band).
However, when the AH-3ΔgalU:glgA mutant was complemented with the same plasmid vector but carrying the E. coli DH5α glgA (pBAD33-GlgAEc) no changes in its LPS profile was observed (Figure 5, lane 4). The only LPS-core band observed was the unique band for AH-3ΔgalU:glgA mutant according to its migration in the gel which correspond to the LPS chemical structure Hep3Kdo (Figure 6A).
Adhesion to HEp-2 Cells and Biofilm Formation
No significant differences in adherence to HEp-2 cells were found between glucan producing or non-producing strains. Also, we compared the ability of the wild-type and mutant strains to form biofilms in microtiter plates (Table 2). The A. hydrophila wild-type strain, AH-3, exhibited a biofilm formation ability with an OD570 value of 1.32 ± 0.11. The mutant lacking only the O34-antigen LPS tested.
Table 2. Biofilm values of several Aeromonas strains using the method of Pratt and Kolter [35].
| Strain | Value |
| AH-3 (wild type) | 1.32+/–0.11 |
| AH-3ΔrmlB (O–; glucan+) | 0.65+/–0.07 |
| AH-3ΔwecP (O–; glucan–) | 0.47+/–0.05 |
| AH-3ΔwecP + pBAD-WecP | 1.29+/–0.13 |
| AH-3ΔwaaL (O–; glucan–) | 0.48+/–0.06 |
| AH-3ΔwaaL + pBAD-WaaL | 1.33+/–0.09 |
| AH-3ΔglgA (O+; glucan–) | 0.98+/–0.07 |
| AH-3ΔglgA + pBAD33-GlgA | 1.30+/–0.15 |
| AH-3ΔglgA + pBAD33-GlgAEc | 0.96+/–0.10 |
| AH-3ΔglgC(O+; glucan–) | 0.99+/–0.08 |
| AH-3ΔglgC + pBAD33-GlgC | 1.30+/–0.14 |
(AH-3ΔrmlB) showed approximately a 55% reduction in their biofilm formation ability, while the mutants lacking both the O34-antigen LPS and surface glucan (AH-405ΔwecP or AH-3ΔwaaL) showed a reduction of 70%. Finally, the mutants with the O34-antigen LPS but unable to produce surface glucan (AH-3ΔglgA and AH-3ΔglgC) showed approximately a 25% reduction in their biofilm formation ability. The recovery of the O34-antigen LPS or surface glucan production in the mutants rescues the wild type strain value. This assay showed that surface glucan production has a role in biofilm formation, as well as O34-antigen LPS previously described by us as an adhesin [10].
AH-3ΔgalU:glgA double mutant (unable to produce O34-antigen LPS and the complete external LPS core, as well as glucan) was practically unable to form biofilm. It shows a drastic reduction (>85%) in their ability to form biofilm in comparison with the wild type strain using the same technique.
Discussion
A. hydrophyla strain AH-3 GalU (UDP-Glc pyrophosphorylase) is responsible for synthesis of UDP-Glc from glucose-1-phosphate and UTP and galU mutants are unable to synthesize UDP-Glc by this reaction [3]. Chemical analysis of the LPS from these mutants showed the presence of glucose in a high percentage of the molecules isolated, which is not provided by GalU. This glucose residue is transferred by a β1,4 UDP-glucosyltransferase to the l-glycero-d-manno-heptoseII in the LPS inner-core. A second UDP-Glc pyrophosphorylase was found (GlgC), which together with the glycogen synthase GlgA is responsible for the biosynthesis of a branched α-glucan in strain AH-3. The UDP-Glc pyrophosphorylase GlgC was wrongly indicated as an ADP-Glc pyrophosphorylase in the complete Aeromonas genomes available [9], [11]. Therefore, in strain AH-3 the UDP-Glc can be synthesized via both GalU and GlgC. This situation is similar to the one described in some bacterial species, were there is a second UTP-glucose-1-phosphate uridyltransferase dedicated to the additional supply of UDP-Glc for specific purposes. Examples of this are the Streptococcus hasC supplying extra UDP-Glc for hyaluronic acid biosynthesis or the Sphingomonas paucimobilis ugpG performing the same role in gellan biosynthesis.
The A. hydrophila AH-3 α-glucan is a surface polysaccharide exported via the WecP which is also used by the O34-antigen LPS, and ligated to the surface through the O34-antigen polysaccharide ligase (WaaL). Nevertheless, it is an independent polysaccharide versus the O34-antigen LPS despite the common use of the export and ligation system, because we could find mutants devoid of either polysaccharide or both. The surface glucan is common to the mesophilic Aeromonas strains tested.
Bacteria, such as Escherichia coli, could show up to six distinct saccharide polymers simultaneously present within the glycocalyx. At present, the known components of the saccharide matrix include LPS O-antigens [12], enterobacterial common antigen (ECA) [13], capsular polysaccharides (K-antigen) [14], colanic acid (CA or M-antigen) [15], poly β-1,6-N-acetyl-D-glucosamine (PNAG) [16], and the β-1,4-glucan bacterial cellulose [17].
According to our results, the Aeromonas glucan production is via the glycogen synthase (GlgA) and the UDP-Glc pyrophosphorylase (GlgC). A. hydrophila AH-3 or ATCC7966T (AHA_3804) GlgA showed a limited homology with E. coli GlgA, 38% identity and 52% similarity as the best result, and is the unique GlgA protein found in the Aeromonas complete genomes [9], [11]. A. hydrophila AH-3 or ATCC7966T (AHA_3803) GlgC showed also a rather limited homology with E. coli GlgC, 45% identity and 63% similarity as the best result, however two more A. hydrophila ATCC7966T GlgC homologues named GlgC1 and GlgC2 were found in the Aeromonas ATCC7966Tgenome. These two A. hydrophila ATCC7966T proteins GlgC1 (AHA_2482) and GlgC2 (AHA_2740) showed a higher homology (65% identity/82% similarity as the worse result) with E. coli GlgC than A. hydrophila GlgC.
The combination of the enzyme activities altogether with the LPS structures of the AH-3ΔgalU:glgA mutant and their complementation with A. hydrophila or E. coli glgA, supported the following considerations:
-
-
A. hydrophila AH-3GlgA is a glycogen synthase able to produce glucan through the addition of UDP-Glc and their analogue in E. coli is unable to do it. While E. coli GlgA only reacts with ADP-Glc in the glycogen synthesis, A. hydrophila GlgA reacts both with UDP-Glc (glucan synthesis) and probably with ADP-Glc (glycogen synthesis).
-
-
A. hydrophila AH-3 GlgC is an UDP-Glc pyrophosphorylase and should be renamed as this in the genome of strain ATCC7966T, because it is not the homologue for E. coli GlgC (ADP-Glc pyrophosphorylase). It seems that the E. coli GlgC homologues in Aeromonas strain ATCC7966T are GlgC1 and GlgC2.
-
-
It is clear that Aeromonas is able to sense the drop of UDP-Glc generate by GalU mutation. When it happens, Aeromonas establish a preference for survival, abolish the production of surface glucan polysaccharide and produces a rather complete LPS-core by the incorporation of UDP-Glc molecules synthesized via GlgC (UDP-Glc pyrophosphorylase). To our knowledge it is the first report of a clear preference in choosing one or other surface saccharide molecule to produce on behalf of bacterial survival.
The results obtained indicated that surface glucan production may not have a significant role in cell adhesion but clearly has a role in biofilm formation. However, the O34-antigen LPS has a predominant role in both pathogenic characteristics: cell adhesion and biofilm formation. All of these surface polysaccharides characteristics also support the preference of the microorganism to produce LPS rather than surface glucan. Some E. coli exopolysaccharides (in particular CA and PNAG [18]) are integral components of biofilms, acting as the “cement,” which holds together the various protein, lipid, and polysaccharide components [19]. We suggested that a similar role may be played by Aeromonas surface α-glucan polysaccharide.
Several published reports indicate that the use of β-glucans enhances Aeromonas disease resistance in fish by potentiating innate immunity [20], [21]. The β-glucans used are from yeast representing a heterogeneous group of glucose polymers, consisting of a backbone of β-(1→3)-linked β-d-glucopyranosyl units with β-(1→6)-linked side chains of varying length and distribution. However, in no case the authors were able to show the scientific reason for this Aeromonas resistance. The fact that Aeromonas produces a surface α-glucan may explain these results, and also suggests that the use of α-glucans instead of β-glucans could be more helpful to enhance the fish resistance to Aeromonas disease.
Materials and Methods
Bacterial Strains, Plasmids and Growth Conditions
Bacterial strains and plasmids used in this study are shown in Table 3. Aeromonas were grown either in tryptic soy broth (TSB) or tryptic soy agar (TSA) and E. coli in Luria-Bertani (LB) Miller broth and LB Miller agar. Kanamycin (50 µg/ml), chloranphenicol (25 µg/ml) or ampicillin (100 µg/ml) were added to the different media when needed. When required the strains were cultured in Davis minimal medium [22] with glucose or galactose as the unique carbon source.
Table 3. Bacterial strains, and plasmids used.
| Strain or plasmid | Relevant characteristics | Source or reference |
| E. coli strains | ||
| DH5α | F– end A hsdR17 (rK – mK +) supE44 thi-1 recA1 gyr-A96 ϕ80lacZM15 | [37] |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac (F- proAB lacI q ZM15 Tn10) | Stratagene |
| S17-1 | hsdR pro recA, RP4-2 in chromosome Km::Tn7 (Tc::Mu) | [7] |
| BL21(λD3) | F– ompT hsdSB (rB – mB –) gal dcm(λD3) | Novagen |
| A. hydrophila strains | ||
| AH-3 | O34, Wild type | [7] |
| AH-405 | AH-3, spontaneous RifR | [7] |
| AH-2886 | AH-3 galU mutant, KmR | [3] |
| AH-3ΔrmlB | AH-3 rmlB mutant in frame with pDM4 | This study |
| AH-3ΔwecP | AH-3 wecP mutant in frame with pDM4 | [6] |
| AH-3ΔwaaL | AH-3 waaL mutant in frame with pDM4 | [8] |
| AH-3ΔglgA | AH-3 glgA mutant in frame with pDM4 | This study |
| AH-3ΔglgC | AH-3 glgC mutant in frame with pDM4 | This study |
| AH-3ΔgalU glgA | AH-2886 glgA mutant in frame with pDM4 | This study |
| AH-3ΔgalU glgC | AH-2886 glgC mutant in frame with pDM4 | This study |
| Plasmids | ||
| pRK2073 | Helper plasmid, SpcR | [7] |
| pBAD33 | arabinose inducible expression vector, CmR | ATCC |
| pBAD33-GlgA | pBAD33 with A. hydrophila AH-3 glgA | This study |
| pBAD33-GlgC | pBAD33 with A. hydrophila AH-3 glgC | This study |
| pBAD33-GlgAEc | pBAD33 with E. coli DH5α glgA | This study |
| pDM4 | pir dependent with sacAB genes, oriR6K, CmR | [27] |
| pDM4ΔrmlB | pDM4 with A. hydrophila rmlB fragment, CmR | This study |
| pDM4ΔglgA | pDM4 with A. hydrophila glgA fragment, CmR | This study |
| pDM4ΔglgC | pDM4 with A. hydrophila glgC fragment, CmR | This study |
| pET30 Xa/LIC | Vector for overexpressing His-tagged proteins | Novagen |
| pET30-glgA | pET30 overexpressing His6-GlgA strain AH-3 | This study |
| pET30-glgC | pET30 overexpressing His6-GlgC strain AH-3 | This study |
DNA Techniques
DNA manipulations were carried out essentially according to standard procedures [23]. DNA restriction endonucleases and E. coli DNA polymerase Klenow fragment were obtained from Promega. T4 DNA ligase and alkaline phosphatase were obtained from Invitrogen and GE Healthcare, respectively. PCR was performed using BioTaq DNA polymerase (Ecogen) in a Gene Amplifier PCR System 2400 Perkin Elmer Thermal Cycler.
Nucleotide Sequencing and Computer Sequence Analysis
Double-stranded DNA sequencing was performed by using the dideoxy-chain termination method [24] with the Big Dye Terminator v3.1 cycle sequencing kit (Applied Biosystem). Oligonucleotides used for genomic DNA amplifications and DNA sequencing were purchased from Sigma-Aldrich. The DNA sequences were inspected in the GenBank and EMBL databases at the National Center for Biotechnology Information (NCBI) [25]. The Terminator search program in the GCG Wisconsin package was used to search for factor independent transcriptional terminators. ClustalW was used for multiple-sequence alignments [26].
Construction of Defined Mutants
The chromosomal in-frame rmlB, glgA, and glgC deletion mutants, AH-3ΔrmlB, AH-3ΔglgA and AH-3glgC, respectively, were constructed by allelic exchange as described Milton et al. [27]. The primers used to obtain the mutants are listed in Table 4. Two asymmetric PCRs were carried out to obtain two DNA fragments (A-B and C-D) that were annealed at their overlapping region, and PCR amplified as a single DNA fragment using primers A and D. DNA fragments AB and CD of each gene were annealed at the overlapping regions provided by the primers B and C and amplified as a single fragment using primers A and D (Table 2). The fusion products were purified, BglII digested (the BglII site is present in primers A and D), ligated into BglII-digested and phosphatase-treated pDM4 vector, electroporated into E. coli MC1061 (λpir), and plated on chloramphenicol plates at 30°C to obtain pDM4ΔrmlB, pDM4ΔglgA and pDM4ΔglgC plasmids. Plasmids with mutated genes were transferred into A. hydrophila AH-405 rifampicin-resistant (Rifr) by triparental matings using the E. coli MC1061 (λpir) containing the insertion constructs and the mobilizing strain HB101/pRK2073. Transconjugants were selected on plates containing chloramphenicol and rifampicin. PCR analysis confirmed that the vector had integrated correctly into the chromosomal DNA. After sucrose treatment, transconjugants that were rifampicin resistant (RifR) and chloramphenicol sensitive (CmS) were chosen and confirmed by PCR.
Table 4. Primers used in the construction of chromosomal in-frame deletion mutants.
| Primersa,b | Amplified Fragment |
| rmlB | |
| A: 5′- GAAGATCTCTTAGCACTCAACCCCTGT-3′ | |
| B: 5′-TGTTTAAGTTTAGTGGATGGGATCCCAGATGATATGACGAA3’ | AB (595 bp) |
| C: 5′-CCCATCCACTAAACTTAAACAAAGACGGTGCAGTGGTATC-3′ | |
| D: 5′- GAAGATCTACTGGTGATTTCCAACTCG-3′ | CD (698 bp) |
| glgA | |
| A: 5′- GAAGATCTGCTCTATCGCGAGCTCAA-3′ | |
| B: 5′- TGTTTAAGTTTAGTGGATGGGTTCCGAGGCAACAAACAG-3′ | AB (638 bp) |
| C: 5′- CCCATCCACTAAACTTAAACAATGAACACCCGCTTCAACT-3′ | |
| D: 5′- GAAGATCTGGAACGCATTGCTCTGTTT-3′ | CD (714 bp) |
| glgC | |
| A: 5′- GAAGATCTGGGTCATTGGATCTGTGCT-3′ | |
| B: 5′- TGTTTAAGTTTAGTGGATGGGCTGAAGACGGGTACCTTCG-3′ | AB (594 bp) |
| C: 5′- CCCATCCACTAAACTTAAACAGTGATCGGTGAAAATCTGGA-3′ | |
| D: 5′- GAAGATCTGCATTTGAGCAGATTGACG-3′ | CD (718 bp) |
Italic letters show overlapping regions.
Underlined letters show BglII restriction site.
A double mutant galU and glgA (AH-3ΔgalU:glgA) was obtained using AH-3 galU mutant (AH-2886) and pDM4ΔglgA plasmid as mentioned above.
Plasmid Constructions and Mutant Complementation Studies
For complementation studies, the A. hydrophila AH-3 genes rmlB, glgA and C, and E. coli glgA were PCR-amplified by using specific primer pairs and ligated to the plasmid pBAD33 (see the list of primers in Table 5). The plasmid constructions were transformed into E. coli LMG194 by electroporation, plated on chloramphenicol LB agar plates and incubated at 30°C. Plasmids with the amplified genes were independently transferred into the corresponding mutants by triparental mating using the mobilizing strain HB101/pRK2073. Transconjugants were selected on plates containing chloramphenicol and rifampicin and confirmed by PCR. Each gene was expressed from the arabinose-inducible and glucose-repressible promoter (pBAD) on pBAD33. Repression from the araC promoter was achieved by growth in medium containing 0.2% (w/v) D-glucose, and induction was obtained by adding l-arabinose to a final concentration of 0.2% (w/v). The cultures were grown for 18 h at 30°C in TSB medium supplemented with chloramphenicol and 0.2% glucose, diluted 1∶100 in fresh medium (without glucose) and grown until they reached A 600 nm of about 0.2. Then l-arabinose was added, and the cultures were grown for another 2 h. Repressed controls were maintained in glucose-containing medium.
Table 5. Primers used for mutant complementation using vector pBAD33.
| Plasmid | Primer |
| pBAD33-GlgA a | |
| A3glgAF1: 5′- TCCCCCGGGCTCGGGGATGTGAAGATTG -3′ | |
| A3glgAR1: 5′- CCCAAGCTTGTTCAAAGCCGTGCTAAGG -3′ | |
| pBAD33-GlgC b | |
| PRIMER FOR: 5′-GATATCTTCCGGGATCTGATAAGG-3′ | |
| PRIMER REV: 5′- CCCAAGCTTAGATGCGATAGCCGATGT-3′ | |
| pBAD33-GlgAEc c | |
| w3310 glgA F1: 5′ TCCCCCGGGAAAACGCAGAGGAAGATGC-3′ | |
| w3310 glgA R1: 5′- CCCAAGCTTCGTGGGCGATGAATATGTA-3′ |
Primers contain SmaI(bold) and HindIII(underlined), the PCR amplified product (1668 bp) was ligated to SmaI- HindIII digested pBAD33.
Primers contain EcoRV(bold) and HindIII(underlined), the PCR amplified product (1559 bp) was ligated to SmaI- HindIII digested pBAD33.
Primers contain SmaI(bold) and HindIII(underlined), the PCR amplified product (1267 bp) was ligated to SmaI- HindIII digested pBAD33.
LPS Isolation and Electrophoresis
LPS was extracted from dry cells grown in LB. The phenol/chloroform/light petroleum ether method [28] was used for strains producing rough LPS, while the phenol/water procedure [29] was used for the strains producing the O antigen domain (smooth LPS). For screening purposes LPS was obtained after proteinase K digestion of whole cells [30]. LPS samples were separated by SDS-PAGE or N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine (Tricine)-SDS-PAGE and visualized by silver staining as previously described [31].
Large-scale Isolation and Mild Acid Degradation of LPS
Dry bacterial cells of each mutant in 25 mM Tris·HCl buffer containing 2 mM CaCl2 pH 7.63 (10 mL g–1) were treated at 37°C with RNAse, DNAse (24 h, 1 mg g–1 each), and then with Proteinase K (36 h, 1 mg g–1). The suspension was dialyzed, lyophilized, and the LPS extracted by the phenol-water procedure [29]. A portion of the LPS (∼50 mg) from each strain was heated with aqueous 2% HOAc (6 mL) at 100°C for 45 min. The precipitate was removed by centrifugation (13,000 g, 20 min) and the supernatant fractionated on a column (56 × 2.6 cm) of Sephadex G-50 Superfine in 0.05 M pyridinium acetate buffer pH 4.5 with monitoring using a differential refractometer (Knauer, Germany).
Methylation Analysis
A sample of each oligosaccharide (0.5 mg) was dissolved in 1 mL dimethyl sulfoxide. An excess of powdered NaOH was added, the reaction glass flushed with dry N2, and sealed. After stirring at 20°C for 1 h, 0.5 mL of cold CH3I was added and the mixture stirred at 20°C for 1 h. Then water was added, the methylated product extracted with CHCl3, the extract washed with water, and evaporated with a stream of dry nitrogen. The methylated oligosaccharide was hydrolysed with 2 M CF3CO2H. (120°C, 2 h) and acid was removed with a stream of nitrogen. The methylated monosaccharides were conventionally reduced with NaBH4, acetylated with Ac2O in pyridine, and analysed by GLC on a Hewlett-Packard 5880 chromatograph and GLC-MS on a Hewlett-Packard HP 5989A instrument using a HP-5ms capillary column and a temperature gradient of 150°C (3 min) to 290°C at 7°C min–1.
Mass Spectrometry
Electrospray MS was performed on a Micromass ZQ mass spectrometer (Waters) The sample (100 pmol) was deionized on Dowex H+ resin (Fluka) and dissolved in 2% triethylamined in 50% acetonitrile and injected in the ion source at a flow rate of 5 µl/min. The spectrum was acquired in the negative mode.
Purification of A. hydrophila AH-3 His6-GlgA and GlgC
For glgA and glgC overexpression the pET-30 Xa/LIC vector (Novagen) and AccuPrime (Invitrogene, High-fidelity) polymerase were used. The A. hydrophila AH-3 glgA was amplified from genomic DNA using primers PET-A3glgA-for 5′- GGTATTGAGGGTC GCTTGGCTATTAACCCACTGA-3′and PET-A3glgA-rev 5′- AGAGGAGAGTTA GAGCCGTTCAAAGCCGTGCTAAG-3′, and for A. hydrophila AH-3 glgC primers PET-A3glgC-for 5′-GGTATTGAGGGTCGCATGGCAGGCA TATTCACCT-3′and PET-A3glgC-rev 5′- AGAGGAGAGTTAGAGCCCTTCAC AAGCCCCTCAACT-3′, and the PCR products independently ligated into pET-30 Xa/LIC (Novagen), and electroporated into E. coli BL21(λDE3). The His6-GlgA and His6-GlgC proteins were overexpressed and cell lysates obtained as previously reported for other proteins [6]. The total membrane fraction was obtained by ultracentrifugation (200.000 × g 30 min at 10°C), the His6-GlgA and His6-GlgC proteins were solubilized and purified with a Ni2+-NTA agarose (Quiagen) as previously reported [6]. When needed the His6-GlgA and His6-GlgC proteins were concentrated using a Centriplus 10-ml YM-30 centrifugal filter device (Amicon Bioseparations), and typical protein preparations contained yields of 0.07–0.08 g/ml as determined by the Bio-Rad Bradford assay.
Crude Extracts Production and Enzymatic Activity Measurements
Suspensions of bacteria (25% weight per volume), washed in 25 mM Tris-HCl buffer (pH 7.5) containing 1 mM MgCl2, and crude extracts obtained by passing the washed cells through a French pressure cell (1,120 kg/cm2) twice, and cellular debris removed by centrifugation (32,000 × g for 15 min at 4°C). Permeabilized cells were prepared by adding 25 ml of a toluene-ethanol mixture (1∶9 [vol/vol]) to 1 ml of cell suspension (approximately 3 mg of protein) as described previously [32]. Permeabilized cells were then pelleted by centrifugation (15,000 × g for 8 min at 4°C) and resuspended in 50 mM of Tris-HCl (pH 7.2). Protein concentrations of extracts were determined by using the Bio-Rad Bradford assay as directed by the manufacturer with bovine serum albumin as the standard.
NDP-glucose (ADP-glucose or UDP-glucose) pyrophosphorylase activities were determined spectrophotometrically by monitoring NADPH formation at 340 nm. Briefly, the reaction mixture (500 µl) contained 100 mM Tris-HCl (pH 7.2), 2.5 mM MgCl2, 2 mM fructose-1,6-bis phosphate, 10 mM sodium fluoride, 5 mM tetrasodium pyrophosphate, 1.5 U of phosphoglucomutase, 2 U of glucose-6-phosphate dehydrogenase, and 100 µl of crude extract (approximately 1 mg of protein) or purified AH-3 GlgC (50 µg ). The reaction was started by addition of 2 mM (final concentration) of ADP-glucose or UDP-glucose.
Glycogen synthase activity was determined by the incorporation of glucosyl units from UDP-[3H] glucose into glycogen [33]. The reaction mixture (600 µl) at 37°C included 50 mM Tris-HCl (pH 7.2), 3 mM MgCl2, 6 mM glucose-6-phosphate, 3.3 mM UDP-glucose, 10 µl of UDP-[3H]glucose (0.067 mCi/ml; 15.3 Ci/mmol), and permeabilized cells (approximately 1 mg of protein) or purified AH-3 GlgA (50 µg ). Aliquots (100 µl) were withdrawn at different times and immediately added to tubes containing 300 µl of 67.5% KOH to stop the reaction. Glycogen was isolated from this mixture by the ethanol precipitation. Dried glycogen was dissolved in 150 µl of H2O, and radioactivity was determined by liquid scintillation.
Adherence Assay to HEp-2 Cell
The adherence assay was previously described [34]. Twenty HEp-2 cells/coverslips were randomly chosen, and the number of bacteria adhering/HEp-2 cell was recorded. Assays were carried out in triplicates.
Biofilm Formation
Quantitative biofilm formation was performed in a microtiter plate as described previously [35], with minor modifications. Briefly, bacteria were grown on TSA and several colonies were gently resuspended in TSB (with or without the appropriated antibiotic); 100 µl aliquots were place in a microtiter plate (polystyrene) and incubated 48 h at 30°C without shaking. After the bacterial cultures were poured out, the plate was washed extensively with water, fixed with 2,5% glutaraldehyde, washed once with water and stained with 0,4% crystal violet solution. After solubilization of the crystal violet with ethanol-acetone (80/20, v/v) the absorbance was determined at 570 nm.
Statistical Analysis
The data obtained in several assays were analysed by the t-test using Microsoft Excel software.
Acknowledgments
We thank Dr. Alexander S. Shashkov for NMR measurements and Maite Polo for her technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Plan Nacional de I + D + i (Ministerio de Educación, Ciencia y Deporte, Spain) and from Generalitat de Catalunya (Centre de Referà ¨ncia en Biotecnologia) grants. These are competitve grants from the Spanish government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev 2. Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- 2.Sowokinos JR. Pyrophosphorylases in Solanum tuberosum. I. Changes in ADP-glucose and UDP-glucose pyrophosphorylase activities associated with starch biosynthesis during tuberization, maturation, and storage of potatoes. Plant Physiol. 1976;57:63–68. doi: 10.1104/pp.57.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilches S, Canals R, Wilhelms M, Saló MT, Knirel YA, et al. Mesophilic Aeromonas UDP-glucose pyrophosphorylase (GalU) mutants showed two types of lipopolysaccharide structures and reduced virulence. Microbiol. 2007;153:2393–2404. doi: 10.1099/mic.0.2007/006437-0. [DOI] [PubMed] [Google Scholar]
- 4.KnirelYA, Shaskov AS, Senchenkova SN, Merino S, Tomás JM. Structure of the O-polysaccharide of Aeromonas hydrophila O:34: a case of random O-acetylation of 6-deoxy-L-talose. Carbohydr Res. 2002;337:1381–1386. doi: 10.1016/s0008-6215(02)00136-2. [DOI] [PubMed] [Google Scholar]
- 5.Leontein K, Lönngren J. Determination of the absolute configuration of sugars by gas-liquid chromatography of their acetylated 2-octyl glycosides. Methods Carbohydr Chem. 1993;9:87–89. [Google Scholar]
- 6.Merino S, Jimenez N, Molero R, Bouamama L, Regué M, et al. A UDP-HexNAc polyprenol-P GalNAc-1-P transferase (WecP) representing a new subgroup of this enzyme family. J Bacteriol. 2011;193:1943–1952. doi: 10.1128/JB.01441-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiménez N, Canals R, Saló MT, Vilches S, Merino S, et al. The Aeromonas hydrophila wb* O34 gene cluster: genetics and temperature regulation. J Bacteriol. 2008;190:4198–4209. doi: 10.1128/JB.00153-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiménez N, Canals R, Lacasta A, Kondakova AN, Lindner B, et al. Molecular Analysis of three Aeromonas hydrophila AH-3 (serotype O34) Lipopolysaccharide Core Biosynthesis Gene Clusters. J Bacteriol. 2008;190:3176–3184. doi: 10.1128/JB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seshadri XR, Joseph SW, Chopra AK, Sha J, Shaw J, et al. Genome sequence of Aeromonas hydrophila ATCC 7966T: Jack of All Trades. . J Bacteriol. 2006;188:8272–8882. doi: 10.1128/JB.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merino S, Rubires X, Aguilar A, Tomás JM. The O:34-antigen lipopolysaccharide as an adhesin in Aeromonas hydrophila. FEMS Microbiobiol. 1996;Lett139:97–101. doi: 10.1111/j.1574-6968.1996.tb08186.x. [DOI] [PubMed] [Google Scholar]
- 11.Reith ME, Singh RK, Curtis B, Boyd JM, Bouevitch A, et al. The genome of Aeromonas salmonicida subsp. salmonicida A449: insights into the evolution of a fish pathogen. BMC Genomics. 2008;9:427. doi: 10.1186/1471-2164-9-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raetz CR, Whitfield C. Lypopolysaccharide endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn, HM, Meier-Dieter U, Mayer H. ECA, the enterobacterial common antigen. FEMS Microbiol Rev. 1988;4:195–222. doi: 10.1111/j.1574-6968.1988.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 14.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 15.Grant WD, Sutherland IW, Wilkinson JF. Exopolysaccharide colanic acid and its occurrence in the Enterobacteriaceae. J Bacteriol. 1969;100:1187–1193. doi: 10.1128/jb.100.3.1187-1193.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Preston JF, 3rd, Romeo T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol. 2004;186:2724–2734. doi: 10.1128/JB.186.9.2724-2734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zogaj X, Nimtz M, Rohde M, Bokranz W, Romling U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol. 2001;39:1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]
- 18.Agladze K, Wang X, Romeo T. Spatial periodicity of Escherichia coli K-12 biofilm microstructure initiates during a reversible, polar attachment phase of development and requires the polysaccharide adhesin PGA. J Bacteriol. 2005;187:8237–8246. doi: 10.1128/JB.187.24.8237-8246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutherland I. Biofilm exopolysaccharides: a strong and sticky framework. Microbiol. 2001;147:3–9. doi: 10.1099/00221287-147-1-3. [DOI] [PubMed] [Google Scholar]
- 20.Kumari J, Sahoo PK. Dietary β-1,3 glucan potentiates innate immunity and disease resistance of Asian catfish, Clarias batrachus (L.).J Fish Dis. 2006;29:95–101. doi: 10.1111/j.1365-2761.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez I, Chamorro R, Novoa B, Figueras A. β-Glucan administration enhances disease resistance and some innate immune responses in zebrafish (Danio rerio). Fish Shellfish Immunol. 2009;27:369–373. doi: 10.1016/j.fsi.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Atlas RM. CRC Press, Boca Raton, USA; 1993. Handbook of Microbiological Media. [Google Scholar]
- 23.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual, second Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor New York. 1989.
- 24.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, et al. The Pfam protein families database. Nucleic Acids Res. 2002;30:276–280. doi: 10.1093/nar/30.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milton DL, O’Toole R, Horstedt P, Wolf-Watz H. Flagellin A is essential for the virulence of Vibrio anguillarum. . J Bacteriol. 1996;178:1310–1319. doi: 10.1128/jb.178.5.1310-1319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galanos C, Lüderitz O, Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969;9:245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 29.Westphal O, Jann K. Bacterial lipopolysaccharides. Methods Carbohydr Chem. 1965;5:83–89. [Google Scholar]
- 30.Hitchcock PJ, Brown TM. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai CM, Frasch CE. A sensitive silver stain for detecting l ipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 32.Strobel HJ. Evidence for catabolite inhibition in regulation of pentose utilization and transport in the ruminal bacterium. Appl Environ Microbiol. 1993;59:40–46. doi: 10.1128/aem.59.1.40-46.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lou J, Dawson KA, Strobel HJ. Glycogen Biosynthesis via UDP-Glucose in the Ruminal Bacterium Prevotella bryantii B14. Appl Environ Microbiol. 1997;63:4355–4359. doi: 10.1128/aem.63.11.4355-4359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canals R, Ramirez S, Vilches S, Horsburgh G, Shaw JG, et al. Polar flagella biogenesis in Aeromonas hydrophila. J Bacteriol. 2006;188:542–555. doi: 10.1128/JB.188.2.542-555.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: role of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 36.KnirelYA, Vinogradov E, Jimenez N, Merino S, Tomás JM. Structural studies on the R-type lipopolysaccharide of Aeromonas hydrophila. Carbohydr Res. 2004;339:787–793. doi: 10.1016/j.carres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]