SUMMARY
Integrin α3 is a transmembrane integrin receptor subunit that mediates signals between the cells and their microenvironment. We identified three patients with homozygous mutations in the integrin α3 gene that were associated with disrupted basement-membrane structures and compromised barrier functions in kidney, lung, and skin. The patients had a multiorgan disorder that included congenital nephrotic syndrome, interstitial lung disease, and epidermolysis bullosa. The renal and respiratory features predominated, and the lung involvement accounted for the lethal course of the disease. Although skin fragility was mild, it provided clues to the diagnosis.
Epithelial–mesenchymal interactions are important in the development and tissue homeostasis of many multicompartment organs, such as the kidneys, lungs, and skin.1 Adhesion of epithelial cells to basement membranes provides the structural and functional integrity of the organs. Cues from the extracellular environment that are transduced to the cell and vice versa regulate adhesion, which is partially dependent on integrins.2 Mutations in integrin genes are associated with various human disorders, including epidermolysis bullosa with pyloric atresia, congenital muscular dystrophy, leukocyte adhesion deficiency, and Glanzmann’s thrombasthenia.3,4 Integrin α3, which forms heterodimers with integrin β1, is widely expressed in both fetal and adult tissues5 and has complex functions, as shown in mouse models and in vitro studies.6–17
Here we report on three infants with congenital nephrotic syndrome, interstitial lung disease, and skin fragility who were homozygous for mutations in the integrin α3 gene (ITGA3). Although the renal and respiratory features predominated clinically, it was the investigation of the skin fragility that first suggested the molecular defect.
CASE REPORTS
Patient 1 was the index patient in whom the genetic defect was discovered and the complex phenotype was characterized. Subsequently, two other infants, Patients 2 and 3, were found to have similar clinical features and ITGA3 mutations (Table 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
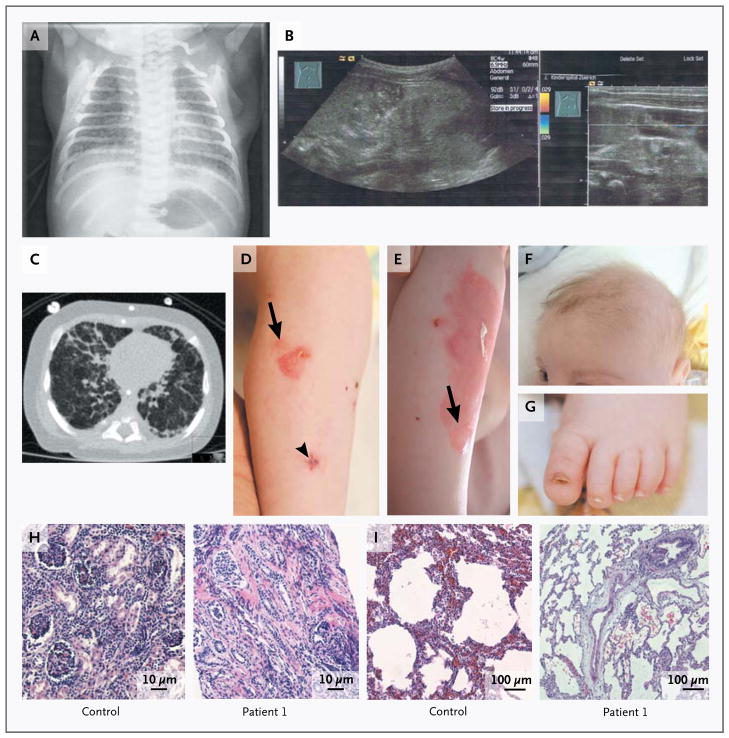
Patient 1 was a boy born to a 41-year-old mother (gravida 2, para 2) at a gestation of 41 weeks 4 days, after an uneventful pregnancy. The parents were reportedly unrelated, and the family history was unremarkable, with no history of renal disease. The mother’s kidney function was normal at the time of delivery. Labor was induced because of fetal postmaturity and oligohydramnios. Immediately after birth, severe tachypnea and respiratory distress requiring high-flow supplemental oxygen developed in the infant. On the first day of life, chest radiography showed severe reticulonodular changes (Fig. 1A). On day 13, laboratory investigations revealed renal failure (glomerular filtration rate [GFR], <10 ml per minute per 1.73 m2 of body-surface area) and the nephrotic syndrome (ratio of urinary protein to creatinine, 12.5) (Table 2 in the Supplementary Appendix), and peritoneal dialysis was initiated on day 14. Abdominal ultrasonography showed small, hyperechoic kidneys (Fig. 1B). Respiratory distress persisted, and recurrent vomiting episodes with secondary aspiration associated with life-threatening events and respiratory tract infections complicated the course. High-resolution computed tomography (CT) of the chest showed nonspecific diffuse distortion of the pulmonary architecture (Fig. 1C). From the age of 3 months, the infant had increasing skin fragility, with small blisters and erosions after mechanical manipulation. The lesions healed slowly, without scarring but with residual erythema (Fig. 1D and 1E). There was no mucosal involvement. The scalp hair, eyebrows, and eyelashes were fine and sparse (Fig. 1F). The big toenails became dystrophic (Fig. 1G), and distal onycholysis followed mild trauma to the fingernails.
Figure 1. Clinical and Morphologic Features of Patient 1.
Panel A shows a chest radiograph obtained on the first day of life. Panel B shows the results of neonatal ultrasonography, revealing bilateral, orthotopic small kidneys with hyperechogenic parenchyma, without corticomedullary differentiation, suggesting renal dysplasia. Panel C shows a computed tomographic scan of the chest obtained at the age of 4 months, revealing diffuse distortion of the pulmonary architecture, coarsened interstitial changes in the lung with thickened interlobular and intralobular septa in all segments; other images showed consolidation in the right posterior upper lobe. Panels D and E show progressive skin blisters and erosions on the leg (arrows), with an interval of 30 days between the two photographs. The arrowhead indicates the biopsy site. Panel F shows very fine, sparse scalp and eyebrow hair, and Panel G shows a dystrophic toenail. Panel H shows kidney-biopsy samples obtained from a control subject and Patient 1, revealing atrophic glomeruli, single collapsed loops, segmental sclerosis, occasionally fibrous obliteration of Bowman’s capsule space, and focal tubular atrophy in the patient. Diffuse interstitial fibrosis is associated with lymphocytic infiltrates. Panel I shows lung-biopsy specimens obtained from a control subject and Patient 1, revealing well-expanded lung parenchyma with irregular air-space expansion, areas of alveolar duct distention, and zones of mild-to-moderate peripheral air-space distention and simplification, with intact respiratory epithelium, in the patient (hematoxylin and eosin staining in Panels H and I).
At 1 month of age, a renal specimen obtained by percutaneous needle biopsy with ultrasonographic guidance revealed globally atrophic glomeruli, focal segmental glomerulosclerosis, diffuse interstitial fibrosis, tubular atrophy, and loss and immaturity of the tubules (Fig. 1H). At the age of 5.5 months, a lung specimen obtained by open biopsy from the middle lobe, which was markedly abnormal on CT, showed hyperinflation and mild-to-moderate simplification of air spaces, suggesting impaired alveolarization or lobular remodeling (Fig. 1I). Mild reactive changes in the bronchioles included intraluminal mucus stasis, focal neutrophils, and focal bronchiolar fibrosis. (For a description of other clinical features and investigations, see Tables 1 and 2 in the Supplementary Appendix.) The infant died at the age of 7.5 months during an episode of pulmonary infection.
Patient 2, a girl born to healthy consanguineous parents, was the only affected child among nine siblings. Two days after delivery, respiratory distress and episodes of cyanosis developed. At the age of 6 weeks, chest radiography showed bilateral infiltrates, and the findings on chest CT were consistent with diffuse interstitial lung disease (Fig. 1 in the Supplementary Appendix). Laboratory tests at 6 weeks of age indicated proteinuria in the nephrotic range, and peritoneal dialysis was initiated. In spite of intensive care, the child died from multiorgan failure at 2 months of age.
Patient 3, a girl born at term, was the first child of healthy consanguineous parents. At 2 months of age, fever and respiratory distress developed. The findings on chest radiography were consistent with pneumonia in the right upper and middle lobes, which was considered to be due to aspiration. Proteinuria in the nephrotic range was noted, and a renal-biopsy specimen obtained at 5 months of age showed focal segmental glomerulosclerosis. Renal function deteriorated progressively and necessitated peritoneal dialysis at 17 months of age, although control of the edema was unsuccessful, even while the infant was receiving dialysis. She continued to have recurrent respiratory infections and required supplemental oxygen to maintain peripheral oxygen saturation values at a level above 90%. At 4 months, annular erythematous lesions were noted on the infant’s shins; these later developed into blisters that were consistent with epidermolysis bullosa. She died at the age of 19 months from multiorgan failure related to infection.
METHODS
GENETIC ANALYSES
After the infants’ parents had provided written informed consent, EDTA-treated blood samples were obtained from the three patients and their parents. Genomic DNA was extracted from peripheral-blood leukocytes, and the coding region and exon–intron boundaries of ITGA3, as well as other candidate genes, were analyzed (see the Methods section and Table 3 in the Supplementary Appendix).
MORPHOLOGIC ANALYSES
Tissue samples were studied with conventional light microscopy, transmission electron microscopy, immunohistochemical analysis, and fluorescence microscopy (see the Methods section and Table 4 in the Supplementary Appendix).
IN VITRO STUDIES OF KERATINOCYTES
Primary keratinocytes from Patient 1 were used to assess integrin α3 in vitro. The cells were isolated from skin samples obtained from a control subject and the patient, expanded in culture, and submitted to flow cytometry (see the Methods section in the Supplementary Appendix).
RESULTS
MORPHOLOGIC ANALYSES OF SKIN AND ITGA3 MUTATIONS
In Patient 1, a candidate-gene approach led to identification of the genetic defect. We first ruled out mutations in NPHS2 and WT1, which are known to cause congenital nephrotic syndrome.18 The most frequent mutations in CFTR and ABCA3 were ruled out as a cause of the interstitial lung disease.19
Morphologic analyses of skin samples were performed in order to classify the subtype of epidermolysis bullosa. Light microscopy showed a flat epidermis without rete ridges, and subepidermal blisters were evident (Fig. 2A in the Supplementary Appendix). Transmission electron microscopy revealed pronounced abnormalities of the basement membrane, with a thin lamina densa, discontinuous between the hemidesmosomes (Fig. 2B in the Supplementary Appendix). In contrast, keratin filaments, desmosomes, hemidesmosomes, and anchoring fibrils appeared to be normal. In blistered areas, the lamina densa was located at the blister floor, and occasionally, intact hemidesmosomes and cell fragments adhered to it, indicating multiple split levels. Immunofluorescence mapping revealed focal disruption of the dermal–epidermal junction with cleavage within the basement membrane. These abnormalities did not correspond to any known type of epidermolysis bullosa in humans20 but had striking similarities to the findings described in the integrin α3 knockout mouse.14
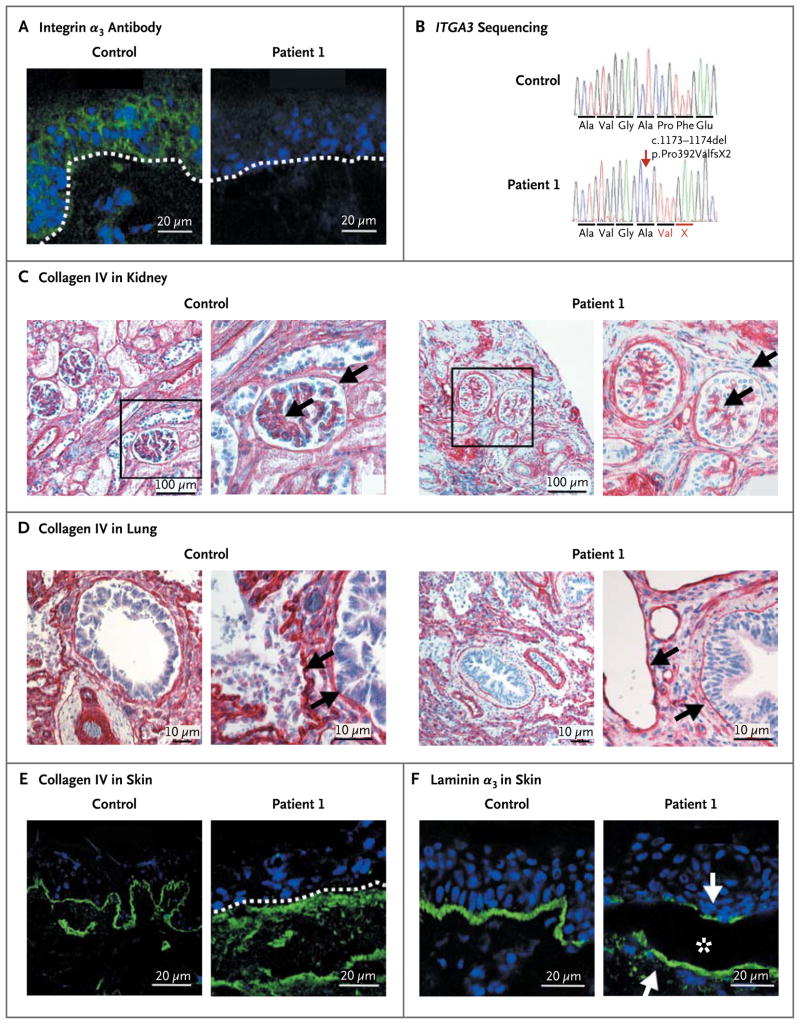
The lack of integrin α3 immunoreactivity in the skin of Patient 1 was the pivotal diagnostic feature, which suggested loss of this protein (Fig. 2A). Direct sequencing of the patient’s DNA disclosed the homozygous mutation c.1173_1174del in exon 8 in ITGA3 (Fig. 2B). Both parents were unaffected, heterozygous carriers of this mutation, which is predicted to lead to a frameshift starting with codon 392 and to premature termination of translation two codons downstream, p.Pro392ValfsX2.
Figure 2. Identification and Consequences of the ITGA3 Deletion in Patient 1.
In Panel A, immunofluorescence (green) staining of a skin specimen with the monoclonal integrin α3 antibody P1B5 shows loss of expression; a skin specimen from a control subject is shown for comparison. The position of the epidermal basement membrane is indicated by the white dashed line; nuclei are blue. In Panel B, chromatograms show the partial sequences of ITGA3. The homozygous mutation c.1173_1174del (arrow) leads to frameshift and formation of a premature termination codon, p.Pro392ValfsX2. Immunohistochemical and immunofluorescence staining of collagen IV is shown in biopsy specimens of kidney (Panel C), lung (Panel D), and skin (Panel E) obtained from Patient 1 and age-matched controls. In Panel C, the marked areas in the images on the left side are shown at higher magnification on the right side; the arrows indicate the glomerular basement membrane and Bowman’s capsule basement membrane. In addition, there is a loss of lateral cell junctions in the patient. In Panel D, arrows point to the basement membranes, which are very thin in the specimen from the patient. Panel E shows irregularly distributed collagen IV below the basement membrane (dashed white line) in the patient’s skin. Panel F shows immunofluorescence staining of the laminin α3 chain, a ligand of integrin α3. In the patient’s skin, laminin α3 appears at the blister (asterisk) roof and base (arrows).
Given our findings in Patient 1, we screened eight patients with congenital nephrotic syndrome and homozygosity at the ITGA3 locus for mutations. Patients 2 and 3 were found to be homozygous for the ITGA3 mutations c.1538-1G→A, in intron 11, and c.1883G→C, p.Arg628Pro, in exon 14, respectively (Fig. 3 in the Supplementary Appendix). The mutation c.1538-1G→A is predicted to abolish the splice acceptor site of exon 12, according to a database with predictions of splice sites (www.cbs.dtu.dk/services/NetGene2). Consequently, exon 12 could be skipped, leading to frameshift and a premature stop codon. The mis-sense mutation found in Patient 3 replaces a conserved, positively charged arginine with a neutral proline at position 628 in the extracellular domain of integrin α3. Polymorphism Phenotyping, version 2 (Polyphen-2) suggested that the mutation might be damaging; it is not classified as a single-nucleotide polymorphism. Each variant was not found in at least 100 chromosomes from ethnically matched controls.
CONSEQUENCES OF LOSS OF INTEGRIN α3
The mutation c.1173_1174del led to loss of integrin α3 in the skin, kidneys, and lungs of Patient 1 (Fig. 4 in the Supplementary Appendix). In vitro, lack of integrin α3 was shown on flow-cytometric studies of primary keratinocytes, without substantial changes in α2, β1, or α6 integrin subunits (Fig. 5 in the Supplementary Appendix). We next explored the consequences of this mutation on tissue architecture.
The basement membranes in kidney, lung, and skin specimens from Patient 1 had profound abnormalities, as visualized by collagen IV staining. In contrast to the strong, linear staining of normal basement membranes, the basement membranes in the patient’s kidney and lung had a weak and interrupted signal, and the epidermal basement membrane was clearly disorganized (Fig. 2C, 2D, and 2E).
The distribution of basement-membrane–associated molecules was altered in the patient’s skin. Instead of the normal, linear pattern at the dermal–epidermal junction, the laminin α3 chain (a component of laminin-332) was detected in blistered areas both at the roof and on the floor of the split (Fig. 2F) and in nonblistered areas below the plane of the basement membrane. Similarly, collagen VII was widely distributed in the upper dermis together with increased tenascin C and fibronectin (Fig. 6 in the Supplementary Appendix). The hemidesmosomal integrin α6β4 showed interrupted staining, whereas β1 integrin appeared to be unchanged (Fig. 7 in the Supplementary Appendix). These observations clearly indicate that loss of integrin α3 impairs not only epidermal adhesion but also basement-membrane assembly and remodeling.
DISCUSSION
The ITGA3 mutations in the three patients we describe were associated with a complex phenotype comprising congenital nephrotic syndrome, interstitial lung disease, and epidermolysis bullosa. The deletion mutation, which abolished integrin α3, and the splice-site and missense mutations all led to similar clinical features. Both full knockout mouse models11,14 and organ-specific α3 knockout models12,13,16,17 support this conclusion.
Integrin α3 knockout mice have defects in kidney and lung organogenesis and skin fragility, and they die within 24 hours after birth.11,14 Abnormal nephrogenesis, disorganized and widened glomerular basement membranes, and the absence of podocyte foot processes11 support the roles of integrin α3 during development, as well as in organization of the extracellular matrix, modulation of the actin cytoskeleton, and cell shape.3 Podocyte-specific integrin α3 knockout mice have massive postnatal proteinuria,12 as seen in our patients.
In the three patients, the lung findings were surprising and were not predicted by the animal models.11,13 In contrast to the severe clinical course and abnormal imaging findings, routine staining of lung-biopsy samples showed nonspecific changes. We speculate that the observed lung disease probably reflects the consequences of the basic genetic defect, since respiratory distress began in the first days of life, when severe abnormalities were already evident on chest radiography. Defects in bronchial development, as described in the integrin α3 knockout mouse model, were not documented. Surprisingly, mice with specific loss of integrin α3 in lung epithelial cells have no abnormalities of the alveolar architecture or respiratory function, but integrin α3 has been identified as a critical regulator of epithelial–mesenchymal transition and tissue remodeling in response to injury.13 We speculate that perturbed morphogenesis and abnormal functioning of the blood–air barrier accounted for reduced gas diffusion, resulting in respiratory failure in our patients.
Skin was mildly affected in our patients. Blisters and erosions began at the age of 2 to 4 months. Disorganization of the basement membrane diminished dermal–epidermal adhesion, and on exposure to external shearing stress, the basement membrane ruptured and blisters arose. The anomalies that are caused by loss of integrin α3 are unique and represent a new form of epidermolysis bullosa. In the context of the complex disorder, reepithelialization of the wounds was somewhat delayed in Patient 1, in contrast to the faster wound healing reported in keratinocyte-specific knockout mice.16,17
These three children with integrin α3 mutations had multiorgan involvement and survived the neonatal period. It is possible that the loss of integrin α3 has additional direct consequences (e.g., the involvement of the nervous system, since in the integrin α3 knockout mice the architecture of the cerebral cortex was perturbed, probably because of abnormal neuron–glia interactions during development).21,22
Taken together, this phenotype, consisting of congenital nephrotic syndrome, interstitial lung disease, and epidermolysis bullosa, reflects the developmental and postnatal pleiotropic functions of integrin α3, showing that this integrin is indispensable for basement-membrane organization. The exquisite interdependence between the structural assembly of basement membranes and the modulation of the cytoskeleton in vivo is pivotal for adequate barrier functions in the kidney, lung, and skin. In contrast to in vitro systems, in which compensatory mechanisms have been described,17 these functions are insufficient in vivo.
Supplementary Material
Acknowledgments
Supported by a grant from the Federal Ministry for Education and Research (to Dr. Bruckner-Tuderman), a grant from the Excellence Initiative of the German Federal Government and the Freiburg Institute for Advanced Studies School of Life Sciences–LifeNet (to Dr. Bruckner-Tuderman), and grants from the National Institutes of Health (DK076683 and DK086542, to Dr. Hildebrandt).
We thank V. Morand, K. Thoma, G. Grüninger, and Z. Antoni for expert technical assistance; Dr. A. Gaspert and Prof. R. Caduff (Department of Pathology, University Hospital Zurich) for microscopical analysis of kidney and lung specimens; Prof. G. Paret for technical assistance; the Pathology Department at Sheba Medical Center for histologic analysis; and Dr. C. Monoranu (Department of Pathology, University of Würzburg) and Dr. G. Zambruno (Istituto Dermopatico dell’Immacolata, Rome) for tissue and DNA samples.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivaska J, Heino J. Interplay between cell adhesion and growth factor receptors: from the plasma membrane to the endosomes. Cell Tissue Res. 2010;339:111–20. doi: 10.1007/s00441-009-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;200:471–80. doi: 10.1002/path.1416. [Corrected and republished, J Pathol 2003;201: 632–41.] [DOI] [PubMed] [Google Scholar]
- 4.Kambham N, Tanji N, Seigle RL, et al. Congenital focal segmental glomerulosclerosis associated with beta4 integrin mutation and epidermolysis bullosa. Am J Kidney Dis. 2000;36:190–6. doi: 10.1053/ajkd.2000.8293. [DOI] [PubMed] [Google Scholar]
- 5.Margadant C, Charafeddine RA, Sonnenberg A. Unique and redundant functions of integrins in the epidermis. FASEB J. 2010;24:4133–52. doi: 10.1096/fj.09-151449. [DOI] [PubMed] [Google Scholar]
- 6.Reiser J, Oh J, Shirato I, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279:34827–32. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 7.Lubman RL, Zhang XL, Zheng J, et al. Integrin alpha(3)-subunit expression modulates alveolar epithelial cell monolayer formation. Am J Physiol Lung Cell Mol Physiol. 2000;279:L183–L193. doi: 10.1152/ajplung.2000.279.1.L183. [DOI] [PubMed] [Google Scholar]
- 8.Hodivala-Dilke KM, DiPersio CM, Kreidberg JA, Hynes RO. Novel roles for alpha3beta1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J Cell Biol. 1998;142:1357–69. doi: 10.1083/jcb.142.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Symons JM, Goldstein SL, McDonald A, Miner JH, Kreidberg JA. (Alpha)3(beta)1 integrin regulates epithelial cytoskeletal organization. J Cell Sci. 1999;112:2925–35. doi: 10.1242/jcs.112.17.2925. [DOI] [PubMed] [Google Scholar]
- 10.Wen T, Zhang Z, Yu Y, Qu H, Koch M, Aumailley M. Integrin alpha3 subunit regulates events linked to epithelial repair, including keratinocyte migration and protein expression. Wound Repair Regen. 2010;18:325–34. doi: 10.1111/j.1524-475X.2010.00590.x. [DOI] [PubMed] [Google Scholar]
- 11.Kreidberg JA, Donovan MJ, Goldstein SL, et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–47. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 12.Sachs N, Kreft M, van den Bergh Weerman MA, et al. Kidney failure in mice lacking the tetraspanin CD15. J Cell Biol. 2006;175:33–9. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KK, Wei Y, Szekeres C, et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–24. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. Alpha-3beta1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–42. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti FJ, Rudling RJ, Robson A, Hodivala-Dilke KM. Alpha3beta1-integrin regulates hair follicle but not interfollicular morphogenesis in adult epidermis. J Cell Sci. 2003;116:2737–47. doi: 10.1242/jcs.00475. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell K, Szekeres C, Milano V, et al. Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J Cell Sci. 2009;122:1778–87. doi: 10.1242/jcs.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margadant C, Raymond K, Kreft M, Sachs N, Janssen H, Sonnenberg A. Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci. 2009;122:278–88. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- 18.Hildebrandt F. Genetic kidney diseases. Lancet. 2010;375:1287–95. doi: 10.1016/S0140-6736(10)60236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clement A, Nathan N, Epaud R, Fauroux B, Corvol H. Interstitial lung diseases in children. Orphanet J Rare Dis. 2010;5:22. doi: 10.1186/1750-1172-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Has C, Bruckner-Tuderman L. Molecular and diagnostic aspects of genetic skin fragility. J Dermatol Sci. 2006;44:129–44. doi: 10.1016/j.jdermsci.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–89. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- 22.Schmid RS, Anton ES. Role of integrins in the development of the cerebral cortex. Cereb Cortex. 2003;13:219–24. doi: 10.1093/cercor/13.3.219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.