Abstract
Background
Although early interventions in individuals with bipolar disorder may reduce the associated personal and economic burden, the neurobiologic markers of enhanced risk are unknown.
Methods
Neuroimaging studies involving individuals at enhanced genetic risk for bipolar disorder (HR) were included in a systematic review. We then performed a region of interest (ROI) analysis and a whole-brain meta-analysis combined with a formal effect-sizes meta-analysis in a subset of studies.
Results
There were 37 studies included in our systematic review. The overall sample for the systematic review included 1258 controls and 996 HR individuals. No significant differences were detected between HR individuals and controls in the selected ROIs: striatum, amygdala, hippocampus, pituitary and frontal lobe. The HR group showed increased grey matter volume compared with patients with established bipolar disorder. The HR individuals showed increased neural response in the left superior frontal gyrus, medial frontal gyrus and left insula compared with controls, independent from the functional magnetic resonance imaging task used. There were no publication biases. Sensitivity analysis confirmed the robustness of these results.
Limitations
As the included studies were cross-sectional, it remains to be determined whether the observed neurofunctional and structural alterations represent risk factors that can be clinically used in preventive interventions for prodromal bipolar disorder.
Conclusion
Accumulating structural and functional imaging evidence supports the existence of neurobiologic trait abnormalities in individuals at genetic risk for bipolar disorder at various scales of investigation.
Introduction
Bipolar disorder has a lifetime incidence of about 0.5%–1% in both male and female individuals and presents a tremendous emotional and financial burden to affected patients and their families owing to its potential for psychosis, suicide, chronicity and recurrence.1 Clinicians and researchers have recently suggested that intervening early in the course of bipolar disorder may reduce this burden, as this strategy may have the potential to delay, lessen the severity of or even prevent full-blown disorder.2 Such an approach parallels that developed to identify troubled youth who are seeking help, have manifest symptoms and impaired functioning and demonstrate a substantially increased risk of psychosis onset, and for whom indicated prevention efforts might be justified.3 A recent meta-analysis confirmed that sensitivity of psychopathologic criteria for a prodromal phase of bipolar disorder was generally low.4 Nevertheless, a pilot study of individuals in a clinical at-risk state of bipolar disorder is now available.2 However, the predictive validity of the criteria could still be enhanced by adding reliable neurobiologic markers of the risk for bipolar disorder.
There is a strong genetic component to susceptibility to bipolar disorder. The lifetime risk for bipolar affective disorder is 15%–30% in individuals with 1 first-degree relative with bipolar disorder and up to 75% in those with 2 affected first-degree relatives, and the concordance rate for monozygotic twins is around 70%. The concordance rate among monozygotic twins is higher for bipolar disorder than unipolar affective disorder, suggesting a relatively greater contribution from genetic factors in the etiology of bipolar disorder compared with schizophrenia and unipolar depression.1 Genetically at-risk yet healthy relatives or twins of patients with bipolar disorder are an excellent population in whom to study premorbid neurophysiologic markers of bipolar disorder.5 Examination of neurophysiologic markers of genetic risk for bipolar disorder is currently possible using neuroimaging techniques. Neuroimaging investigations of structural and functional brain abnormalities in individuals at genetic risk for bipolar disorder (HR) offer several advantages. These include the possibility of identifying brain abnormalities that potentially predate the onset of bipolar disorder and that are not confounded by the presence of illness duration or medication; identifying brain abnormalities that may confer risk for or protect against bipolar disorder to inform evaluation of risk for its development and the subsequent therapeutic intervention; and increasing understanding of the developmental course of bipolar disorder.5 Neuroimaging abnormalities in HR individuals may underlie the modest neurocognitive impairments observed in relatives of patients with bipolar disorder, which point to executive function and working memory deficits.6 A number of imaging studies in individuals at genetic risk for bipolar disorder are available, including functional magnetic resonance imaging (fMRI), positron emission tomography (PET), magnetic resonance spectroscopy (MRS), diffusion tensor imaging (DTI) and structural magnetic resonance imaging studies, which investigate grey or white matter volumes. Despite the number of studies published, the results are conflicting, and no reliable structural or functional markers of genetic liability to bipolar disorder have been proposed.
To address heterogeneity across studies, we have systematically reviewed published imaging studies involving HR individuals at genetic risk for bipolar disorder. This group was compared with both healthy controls and patients with established bipolar disorder. With the combination of structural and functional meta-analytic results, we sought to characterize core neuroanatomical and neurofunctional abnormalities underlying genetic risk for bipolar disorder. To provide robust results, we combined traditional voxel-based meta-analysis with formal meta-analysis of effect sizes. Identifying brain abnormalities specifically related to bipolar disorder is critical to understanding its etiopathophysiology and the rationale for the development of new treatments.
Methods
Selection procedures
Search strategies
We used a systematic search strategy to identify relevant studies. Two independent researchers conducted a 2-step literature search. First, we searched MEDLINE to identify putative studies employing neuroimaging techniques that reported data on individuals at high risk for bipolar disorder. The search was conducted between November and December 2010, and no time span was specified for date of publication. We used the following keywords: “MRI,” “fMRI,” “PET,” “MRS,” “DTI,” “bipolar disorder” and “high risk.” Two reviewers independently reviewed the database and extracted the data to avoid bias or error in the selection of articles and the extraction of data from studies. Discrepancies were resolved through discussion and consensus. Second, the reference lists of the articles included in the review were checked for relevant studies not identified by computerized literature searching. Our search included all reports published up to December 2010 without any language restriction.
Inclusion criteria
To qualify for inclusion in our review, studies must have been an original paper appearing in a peer-reviewed journal; recruited participants at genetic risk for bipolar disorder; and employed functional, structural or neurochemical imaging techniques. Studies were independently assessed by the 2 researchers and evaluated against inclusion and exclusion criteria by consensus. Almost all of the included studies were case–control studies, involving either a group of healthy controls or a group of patients with established bipolar disorder matched for age and sex. To achieve a high standard of reporting, we adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines7 and the revised Quality Of Reporting Of Meta-analyses (QUOROM) statement.8
Systematic review
The recorded variables for each article were imaging technique (fMRI, PET, MRS, MRI, DTI), imaging analysis (whole-brain/region of interest [ROI]), stimulus, field strength, sex, mean age, exposure to medication, brain regions analyzed and principal findings (HR v. controls, HR v. bipolar disorder).
Meta-analyses
Meta-analysis of regions of interest
The primary outcomes of interest were global/regional volumes for structural and regional activity for functional imaging studies, as well as metabolic ratios of cerebral tissue compounds for MRS and binding potential of cerebral receptors for PET studies. The intracerebral volume was defined as a sum of the volume of all voxels designated as grey matter volume, white matter volume plus cerebrospinal fluid (CSF) and the whole brain volume as a sum of grey matter plus white matter volume.9 Meta-analyses were conducted when at least 3 studies employing a similar imaging technique were available for a particular ROI. When there were 2 or more studies from the same centre, we carefully checked putative overlapping samples by directly contacting the authors to verify that there was not a substantial overlap. Statistical analysis was carried out using Comprehensive Meta-Analysis Software version 2 (Biostat Inc.).10 This package employs the same computational algorithms used by the Cochrane Collaborators to weight studies. First, we calculated the effect size for each study included in the meta-analyses. As a measure of effect size, we adopted the Hedges’ g (i.e., the difference between the means of the HR and control groups divided by the standard deviation [SD] and weighted for sample size) to correct for bias from small sample sizes.11 This metric is normally computed using the square root of the mean square error from the analysis of variance testing for differences between the 2 groups, as indicated by the following formula (where X is the raw score, M is the mean, and N is the number of cases):11
where
and
Second, we applied random effects models, which are more conservative than fixed-effect models and considered to better address heterogeneity between studies and study populations, allowing for greater flexibility in parsing effect size variability. Moreover, they are less influenced by extreme variations in sample size.12 Heterogeneity among studies was assessed with the Q statistic, with magnitude of heterogeneity being evaluated with the I2 index.13 As studies with negative results are less likely to be published than studies with significant results, we examined the possibility of publication bias by visually inspecting funnel plots and applying the regression intercept of Egger and colleagues.14 In this way, we assessed whether there was a tendency for selective publication of studies based on the nature and direction of their results. In addition, we used the fail-safe procedure15 to generate a number of unpublished studies that would be needed to move estimates to a nonsignificant threshold. To assess the robustness of the results, we performed sensitivity analyses by sequentially removing each study and rerunning the analysis.
Whole brain voxel-based meta-analysis
Whole brain voxel-based meta-analyses were carried out using the activation likelihood estimation (ALE) technique16 implemented in Ginger ALE (www.brainmap.org/ale/). We performed meta-analyses when at least 3 studies (functional or structural) providing coordinates suitable for meta-analysis were available. In cases when 2 or more studies from the same centre existed, we carefully checked putative overlapping samples by directly contacting the authors to verify that there was not a substantial overlap.
Although the robustness of the results depends on the size of the meta-analysis, and there are no universally accepted criteria for assessing this, for a study of this size, if 6 or more foci contribute to a cluster, it is considered to be very robust, and if 3–5 foci contribute to a cluster, it is considered acceptable (see www.brainmap.org/forum/).17 The equally weighted coordinates were used to form ALEs for each voxel in the brain, as described by Turkeltaub and colleagues.16 In brief, to allow for error in spatial localization related to intersubject variation in functional anatomy and interstudy differences in data-smoothing and registration, the reported loci of maximal activation were modelled as the peaks of 3-dimensional (3-D) Gaussian probability density functions with a full-width at half-maximum of 10 mm. The probabilities of each voxel in standard space representing each primary locus of activation were combined to form a map of the ALE score at each voxel. Statistical significance was assessed using a permutation test with 5000 permutations, corrected for multiple comparisons (the false discovery rate was set at p = 0.001). Clusters of suprathreshold voxels exceeding 400 mm3 in volume were defined as loci of brain activation in common across all studies included in the meta-analysis.18 The resulting ALE maps were thresholded at p = 0.005, in line with previous studies.19 This overall meta-analytical approach18 has been widely used in a number of functional and structural reviews.19–26 Whole brain maps of the ALE values were imported into the MRIcron software program (www.sph.sc.edu/comd/rorden/mricron) and overlaid onto the brain template for presentation purposes.
Results
Studies found
Thirty-seven studies met the inclusion criteria for the current study. Tables 1–4 summarize all reviewed structural and functional studies. The sample included 27 structural and 10 functional imaging studies (Fig. 1). The overall sample included 1046 controls (mean age 29.8 [SD 10.5] yr) and 745 HR individuals (mean age 30.6 [SD 11.3] yr) for the structural studies and 212 controls (mean age 29.3 [SD 10.5] yr) and 251 HR individuals (mean age 29.8 [SD 10.5] yr) for the functional studies. The HR and control group participants were well matched with respect to age and sex (all p > 0.05). Most studies were performed using a 1.5-T MRI scanner. Sociodemographic characteristics of the sample are reported in Tables 1–4.
Table 1.
Structural imaging studies of individuals at risk for bipolar disorder: study characteristics
| Group; no. (% female) [age, yr] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Year | Technique | Type of risk | Assessment instruments | Lifetime/current diagnoses HR | Controls | HR | BD |
| Noga et al.1 | 2001 | MRI (ROI) | MZ twins | SCID | None | 22 (54) [30] | 6 (83) [34] | 6 (83) [34] |
| Kieseppä et al.27 | 2003 | MRI (ROI) | MZ and DZ twins | SCID | Anxiety disorder, substance use disorder (alcohol dependence) | 27 (48) [47] | 15 (60) [44] | 24 (46) [44] |
| Ahearn et al.28 | 2002 | MRI (qualitative) | Relatives of BD patients | SCID, DDES | None | 10 [38] | 8 [50] | |
| Connor et al.29 | 2004 | MRI (ROI) | First-degree relatives | SADS-L, FHRDC, FIGS | Nonpsychotic Axis-1 disorders (mostly MDD) | 219 (47) [34] | 54 (52) [44] | 39 (61) [41] |
| McDonald et al.30 | 2004 | MRI (VBM) | First-degree relatives | SADS-L, FIGS, SSP | Axis-1 disorders (mostly MDD) | 50 (52) [44] | 37 (59) [41] | |
| McIntosh et al.31 | 2004 | MRI (VBM+SVC) | First- or second-degree relatives | SADS-L, HAM-D, YMRS, PANSS | None | 49 (53) [35] | 22 (59) [35] | 26 (46) [34] |
| McIntosh et al.32 | 2005 | MRI (VBM+SVC) | First- or second-degree relatives | SADS-L, HAM-D, YMRS, PANSS | None | 49 (53) [35] | 22 (59) [35] | 26 (46) [34] |
| Gulseren et al.33 | 2006 | MRI (qualitative) | Sibling of BD patients | SCID | None | 12 (33) [30] | 12 (50) [29] | 12 (33) [31] |
| McDonald et al.34 | 2006 | MRI (ROI) | First-degree relatives | SADS-L, FIGS, SSP | MDD, panic disorder without agoraphobia | 54 (54) [40] | 52 (52) [44] | |
| McIntosh et al.35 | 2006 | MRI (VBM+SVC) | First- or second-degree relatives | SADS-L, HAM-D, YMRS, PANSS | None | 49 (53) [35] | 22 (59) [35] | |
| Frazier et al.36 | 2007 | DTI | First-degree relative | K-SADS-E, YMRS, CDRS-R, GAF | Anxiety disorder, ADHD, conduct/ODD, pervasive developmental disorder, PTSD | 8 (37) [9] | 7 (43) [9] | |
| Hajek et al.37 | 2008 | MRI (ROI) | Offspring of BD patients | K-SADS-PL/L, HAM-D, YMRS | None | 31 (64) [21] | 24 (62) [20] | 19 (73) [21] |
| Hajek et al.38 | 2008 | MRI (ROI) | First- or second-degree relatives | K-SADS-PL/L | None | 31 (64) [21] | 24 (62) [20] | 19 (73) [21] |
| Ladouceur et al.5 | 2008 | MRI (VBM+SVC) | Healthy bipolar offspring | K-SADS-PL, SCID | Anxiety disorder | 22 (68) [14] | 20 (55) [13] | |
| Mondelli et al.39 | 2008 | MRI (ROI) | First-degree relatives | SADS-L, FIGS, PANSS | None | 46 (52) [40] | 38 (50) [42] | 29 (62) [40] |
| Singh et al.40 | 2008 | MRI (ROI) | Children of BD patients | K-SADS-PL, C-GAS, FIGS | Anxiety disorder, ADHD, cyclothymia, dysthymia, MDD, ODD | 24 (54) [10] | 21 (43) [10] | |
| Chaddock et al.41 | 2009 | DTI | First-degree relatives | SADS-L, BDI, ASRM | MDD, substance-induced mood disorder | 18 (44) [42] | 21 (43) [42] | 19 (53) [43] |
| Kempton et al.42 | 2009 | MRI (VBM–ROI) | First-degree relatives | SCID, HAM-D, YMRS, FIGS, BPRS | MDD | 52 (48) [35] | 50 (52) [34] | 30 (50) [39] |
| Hajek et al.43 | 2009 | MRI (ROI) | Offspring of parents with mood disorder | K-SADS-PL, SADS-L | None | 31 (64) [21] | 26 (65) [20] | 20 (75) [21] |
| Hajek et al.44 | 2010 | MRI (ROI) | Offspring of BD parents | K-SADS-PL, SADS-L | None | 18 (61) [23] | 20 (55) [20] | 15 (73) [22] |
| Hajek et al.45 | 2009 | MRI (ROI) | Relatives of BD patients | K-SADS-PL, SADS-L | None | 31 (64) [21] | 26 (65) [20] | 20 (75) [21] |
| van der Schot et al.46 | 2009 | MRI (ROI) | MZ or DZ twins | SCID, IDS, YMRS, FIGS | Alcohol use disorder, BPD, depressive disorder NOS, dissociative disorder NOS, MDD, mood disorder due to hyperthyroidism, OCD, panic disorder without agoraphobia, personality disorder NOS, schizophrenia (paranoid type) | 134 (57) [39] | 37 [41] | 63 [41] |
| Walterfang et al.47 | 2009 | MRI (ROI) | Relatives of BD patients | SCID, HAM-D, YMRS, BPRS, GAF, FIGS | None | 75 (48) [36] | 45 (51) [35] | 70 (53) [43] |
| Gunde et al.48 | 2010 | MRI (qualitative) | Relatives of BD patients | SADS-L, K-SADS-PL | None | 49 (63) [22] | 79 (68) [21] | 35 (74) [21] |
| Takahashi et al.49 | 2010 | MRI (ROI) | First-degree relatives | SCID, HAM-D, YMRS, FIGS | MDD | 52 (46) [36] | 49 (53) [34] | 29 (48) [40] |
| Forcada et al.50 | 2010 | MRI (VBM) | Relatives of BD patients | SCID, HAM-D, YMRS, BPRS | MDD, anxiety disorder, substance abuse | 27 [21] | 41 [25] | |
| van der Schot et al.51 | 2010 | MRI | MZ or DZ twins | SCID, IDS, YMRS, FIGS | Alcohol use disorder, BPD, depressive disorder NOS, dissociative disorder NOS, MDD, mood disorder due to hyperthyroidism, OCD, panic disorder without agoraphobia, personality disorder NOS, schizophrenia (paranoid type) | 134 (57) [39] | 37 [41] | 63 [41] |
| Versace et al.52 | 2010 | DTI | Offspring of BD parents | SCID, K-SADS-PL, MFQ, CALS, SCARED | None | 25 (72) [14] | 20 (55) [13] | |
ADHD = attention-deficit/hyperactivity disorder; ASRM = Altman Self-Rated Mania Scale; BD = bipolar disorder; BDI = Beck Depression Inventory; BPD = borderline personality disorder; BPRS = Brief Psychiatric Rating Scale; CALS = Child Affect Lability Scale; CDRS-R = Children’s Depression Rating Scale-Revised; C-GAS = Child Global Assessment Scale; DDES = Duke Depression Evaluation Schedule; DTI = diffusion tensor imaging; DZ = dizygotic; E-YMRS = Young Mania Rating Scale; FHRDC = Family History Research Diagnostic Criteria; FIGS = Family Interview for Genetic Studies; GAF = Global Assessment of Functioning; HAM-D = Hamilton Rating Scale for Depression; HR = high risk for bipolar disorder; IDS = Inventory for Depressive Symptomatology; K-SADS-E = Kiddie-Schedule for Affective Disorders and Schizophrenia; MDD = major depressive disorder; MFQ = Mood and Feelings Questionnaire; MRI = magnetic resonance imaging; MZ = monozygotic; NOS = not otherwise specified; OCD = obsessive–compulsive personality disorder; ODD = oppositional defiant disorder; PANSS = Positive and Negative Syndrome Scale; PTSD = posttraumatic stress disorder; ROI = region of interest; SADS-PL/P/L = Schedule of Affective Disorders and Schizophrenia-Present and Lifetime version; SADS-E = Schedule of Affective Disorders and Schizophrenia-Epidemiologic version; SCARED = Screen for Childhood Anxiety and Related Disorders; SCID = Structured Structured Clinical Interview for DSM-III/IV; SSP = Schedule of Schizotypal Personalities; SVC = small volume correction; VBM = voxel-based morphometry; YMRS = Young Mania Rating Scale.
Table 4.
Functional imaging studies of individuals at risk for bipolar disorder: imaging results
| Study | Year | Areas analyzed | FWHM | Tesla | Antip | Main findings |
|---|---|---|---|---|---|---|
| Cecil et al.53 | 2003 | Frontal cortex, frontal white matter, cerebellar vermis | 1.5 | 1.5 | Y | Lower levels of cerebellar NAA and creatine and elevated frontal mI levels for children with a mood disorder than healthy children. |
| Gallelli et al.54 | 2005 | Dorsolateral prefrontal cortex | ? | 3 | Y | No significant group differences in prefrontal NAA/creatine rations or in additional metabolites (myoinositol, choline) |
| Krüger et al.55 | 2006 | Whole brain + ROIs | 10 | ? | N | HR showed increased RBF in the medial frontal cortex while patients showed decreased RBF |
| Hajek et al.38 | 2008 | Prefrontal cortices | ? | 1.5 | N | No significant differences in choline, creatine, NAA, myoinositol of HR |
| Drapier et al.56 | 2008 | Whole brain | ? | 1.5 | N | During the 2-back task the HR showed greater activation in the left frontal pole than controls |
| Costafreda et al.57 | 2009 | Inferior frontal gyrus | 8 | 1.5 | N | No significant differences in HR individuals as compared with controls |
| Thermenos et al.58 | 2010 | Whole brain + ROIs | 8 | 1.5 | N | HR failed to suppress activation in the left anterior insula, in the orbitofrontal cortex and superior parietal cortex and showed greater activation than BD in the left frontopolar cortex |
| Allin et al.59 | 2010 | Whole brain | ? | 1.5 | N | BD and HR showed similar deficits of deactivation in retrosplenial cortex and reduced activation of left prefrontal cortex |
| Singh et al.60 | 2010 | Anterior cingulate, orbitofrontal cortex | ? | 3 | Y | No significant differences between HR and C |
| Surguladze et al.61 | 2010 | Whole brain + ROIs | ? | 1.5 | N | Activity in medial prefrontal cortex, left putamen and amygdala is greater in BD and in HR as compared with controls |
Antip = antipsychotic exposure; BD = bipolar disorder; C = controls; FWHM = full-width at half-maximum; HR = high risk for bipolar disorder; N = no; NAA = N-acetylaspartate; RBF = regional blood flow; ROI = region of interest; SVC = small volume correction; Y = yes.
Fig. 1.
Selection of studies identified through database searching for inclusion in the review and meta-analysis.
Genetic risk for bipolar disorder
The studies included in the present review employed different genetic HR groups: monozygotic twins,1,57 monozygotic5 and dizygotic twins,27,46 offspring,40,43,44,53,54,60,62 siblings,33,55 first-degree relatives,29,30,34,36,39,41,42,49,56,58,59 first- or second-degree relatives31,32,35,37,38,44 and undefined relatives of patients with bipolar disorder.28,45,47,48,50,61 The risk of the disorder developing across such samples is not the same. First-degree relatives of affected individuals have about a 10-fold increased risk for the disorder compared with unaffected controls.62 The risk for bipolar disorder varies from about 5%–6% when there is only 1 affected first-degree relative to more than 50% when there are 4.62 Other authors have indicated that children of 1 parent with bipolar disorder have a 30% chance of a mood disorder developing; the likelihood increases to 70% if the second parent also has a mood disorder.40 Twin studies, by essentially comparing groups of twin pairs matched for shared environment but differing in degree of genetic relatedness, can help parse genetic and environmental contributions. Twin studies typically involve monozygotic twins (who are essentially genetically identical) and dizygotic twins (who share on average half of their genes) and have concordance rates of 38%–44% and 79%–87%, respectively.62 The presence of qualitatively similar deficits in the nonaffected co-twins, siblings and offspring of patients with bipolar disorder suggests that at least some of these abnormalities are related to the familial (and in the main genetic) risk for the disorder, independent of environmental or illness effects.
Structural imaging studies
Magnetic resonance imaging
Earlier MRI studies involving monozygotic twins have explored the involvement of the striatum, amygdala and hippocampus in genetic liability to bipolar disorder. Caudate nuclei were larger in both affected (bipolar disorder) and unaffected (HR) twins than in healthy twins, suggesting the possibility of structural risk factors shared by both bipolar twins of the discordant pairs.1 Conversely, the affected twins showed a consistently smaller region of the right hippocampus, suggesting a structural marker for the presence of disease.1 However, subsequent studies involving monozygotic and dizygotic twins found no decrease in hemispheric grey matter volume but decreased white matter volume in patients with bipolar disorder and co-twins (HR) compared with unaffected twins,27 reflecting genetic factors predisposing to bipolar disorder. Another twin study, by applying structural equation modelling, demonstrated that at least 38% of the covariance between white matter volume and bipolar disorder could be explained by genetic factors that influence both the volume of white matter and the risk for the disease.46 This indicates that genes involved in the etiology of bipolar disorder may contribute to the white matter decreases found in patients with the disorder and in their co-twins (HR). In a follow-up study, the same authors clarified that widespread grey matter density decreases were predominantly associated with unique environmental factors related to bipolar disorder. In contrast, the brain abnormalities associated with the genetic risk for bipolar disorder were much more circumscribed and limited to white matter decreases bilaterally in the superior longitudinal fasciculi and grey matter loss in the right medial frontal gyrus, precentral gyrus and insula.51 These results suggest that white matter pathology in the frontal lobe may be central to the genetic risk for bipolar disorder, whereas most of the widespread grey matter abnormalities may be related to environmental effects and the illness itself.51
The other MRI studies in our meta-analysis investigated relatives (first- and second-degree, children) of patients with bipolar disorder. McDonald and colleagues30 first employed structural equation modelling to distinguish the neuroanatomical correlates of a genetic risk for schizophrenia or bipolar disorder. Genetic risk for schizophrenia was specifically associated with distributed grey matter volume deficits in the bilateral fronto–striato–thalamic and left lateral temporal regions, whereas genetic risk for bipolar disorder was specifically associated with grey matter deficits only in the right anterior cingulate gyrus and ventral striatum. In a follow-up study, the same authors found that whereas relatives of patients with schizophrenia had enlarged lateral ventricles, relatives of patients with bipolar disorder showed preservation of ventricular volume.34 Ladouceur and colleagues5 were the only authors who reported significant grey matter differences in the hippocampus of HR individuals, with larger hippocampal volume in the HR group compared with controls. As this is the opposite to findings of reduced hippocampal volume in patients with established bipolar disorder, the authors speculated that larger hippocampi may have a role in protecting against or delaying subsequent development of the disease.5 However, other studies have not replicated this finding, reporting no hippocampal volume45 or shape29 abnormalities in the HR group. Hajek and colleagues43 have investigated other brain regions, showing significant caudate increases in the HR group compared with controls, with comparable caudate volumes among patients with bipolar disorder and controls. The pattern of changes observed with the largest changes in HR individuals and comparable volumes between patients with bipolar disorder and controls did not meet criteria for an endophenotype and was interpreted as reflecting a compensatory/protective change. Other authors have uncovered contrasting findings, with caudate reduction in HR individuals compared with controls.31 McIntosh and colleagues35 related grey and white matter volume to a continuous measure of genetic liability and found no findings specific to a genetic liability to bipolar disorder; this finding was contrary to that of another study.30 No significant abnormalities were observed in the subgenual cingulate cortical volumes of HR individuals.37,44 Independent studies confirmed that pituitary volume abnormalities are not a consistent hallmark of genetic liability to bipolar disorder, but rather may be related to clinical symptoms, treatment or comorbidities.39,49,63 A study involving children of patients with bipolar disorder found no statistically significant differences in prefrontal striatal, thalamic and amygdala volumes between HR individuals and healthy controls.40 In a recent study, neither cognition nor white matter volume was associated with the level of functioning in the HR group, and the authors speculated that genetic risk for bipolar disorder may not translate into uniform changes in brain structure.50 Another study found increased insular and cerebellar volume in HR individuals compared with controls. However, whereas insular changes were associated with familial predisposition to bipolar disorder regardless of clinical phenotype (i.e., present in patients with bipolar disorder and HR individuals but not in controls), cerebellar abnormalities were associated with resilience (present in HR individuals but not in patients with bipolar disorder or controls).42 Two MRI studies have investigated the corpus callosum size47 and shape and whole-brain white matter potential abnormalities32 in HR individuals and yielded nonsignificant results. Finally, 3 independent studies have qualitatively addressed white matter hyperintensities in HR individuals. Ahearn and colleagues28 uncovered white matter hyperintensities in 60% of HR individuals and 100% of patients with bipolar disorder, suggesting that MRI hyperintensities cosegregate with bipolar disorder. Gulseren and colleagues33 showed more hyperintensities in patients with bipolar disorder than in their siblings or in healthy controls. Lesions were detected in 67% of patients, 17% of their siblings and 33% of controls. However, a recent study with a large sample found comparable proportions of white matter hyperintensities in affected familial, unaffected HR and control groups.48 After eliminating the effects of illness burden, comorbid conditions and older age, the proportions of patients with white matter hyperintensities were comparable among HR individuals and healthy controls, suggesting that they may not be directly related to bipolar disorder.48
Diffusion tensor imaging
Diffusion tensor imaging is an MRI technique that enables the measurement of the restricted diffusion of water in tissue and allows for the study of axonal structure and white matter bundle coherence by measuring across white matter fibres. White matter abnormalities are one of the most consistently reported neuroimaging findings in patients with bipolar disorder.64 Two studies included in our analysis used DTI to assess the integrity of white matter tracts in the brain. Frazier and colleagues36 showed reduced fractional anisotropy in the superior longitudinal fasciculus of HR individuals compared with controls. The second study found that genetic liability to bipolar disorder was correlated with lower fractional anisotropy in several major white matter tracts of the brain. Of interest, no significant differences in fractional anisotropy were observed between HR individuals and controls, but the HR individuals showed intermediate alterations compared with controls and patients with bipolar disorder.41 Detailed summaries of the main findings of the DTI studies are reported in Tables 1 and 2.
Table 2.
Structural imaging studies of individuals at risk for bipolar disorder: imaging results
| Study | Year | Areas analyzed | Tesla | Antip | HR versus controls | HR versus BD |
|---|---|---|---|---|---|---|
| Noga et al.1 | 2001 | Striatum (bilateral caudate nuclei, putamen, globus pallidus), amygdala– hippocampus (bilateral), cerebral hemispheric volumes | 1.5 | N | ↑ bilateral caudate (HR > C and BD > C) no differences in hemispheric volumes | ↑ right hippocampus (HR > BD) no differences in hemispheric volumes |
| Kieseppä et al.27 | 2003 | Bilateral ventricles, frontal and temporal lobes | 1 | N | ↓ left hemispheric white matter (HR < C) no changes in grey matter or ventricular volumes | No white or grey matter differences between HR and BD. |
| Ahearn et al.28 | 2002 | Whole brain | 1.5 | ? | White matter hyperintensities in 60% of HR | White matter hyperintensities in 100% of BD |
| Connor et al.29 | 2004 | Hippocampus | 1.5 | N | No significant differences | No significant differences |
| McDonald et al.30 | 2004 | Whole brain | 1.5 | N | Vulnerability to bipolar disorder is associated with: ↓ grey matter in right anterior cingulate gyrus and ventral striatum and ↓ white matter in the left prefrontal, left temporoparietal, right frontal and parietal regions and in the anterior corpus callosum | |
| McIntosh et al.31 | 2004 | Whole brain (SVC: amygdala- hippocampus, thalamus) | 1.5 | N | ↓ bilateral thalamus and caudate (HR < C) | No significant differences |
| McIntosh et al.32 | 2005 | Whole brain (SVC: frontal white matter and anterior limb of the internal capsule) | 1.5 | N | No significant white matter differences (HR = C) | Not tested |
| Gulseren et al.33 | 2006 | Frontal, parietal, temporal, occipital lobe, internal capsule | 0.5 | N | Hyperintensities in the right cerebral hemisphere of HR | BD have more hyperintensity than HR in the right cerebral hemisphere |
| Mc Donald et al.34 | 2006 | Brain volume, lateral ventricular volume, third ventricular volume, bilateral hippocampus | 1.5 | N | No differences in ventricular or hippocampal volumes. Trend toward larger cerebral volume (HR > C) | No significant differences |
| McIntosh et al.35 | 2006 | Whole brain (SVC: prefrontal cortex, temporal lobe, amygdala– hippocampal complex, thalamus) | 1.5 | N | No significant structural alterations related with an increased liability to bipolar disorder | |
| Frazier et al.36 | 2007 | Superior longitudinal fasciculus and cingulate-paracingulate white matter | 1.5 | N | ↓ FA in bilateral superior longitudinal fasciculus | — |
| Hajek et al.37 | 2008 | Pituitary | 1.5 | N | No difference in pituitary volume (HR = C) | No significant differences (HR = BD) |
| Hajek et al.38 | 2008 | Subgenual cingulate | 1.5 | N | No significant differences (HR = C) | No significant differences (HR = BD) |
| Ladouceur et al.5 | 2008 | Whole brain (SVC: amygdala– orbitomedial prefrontal cortex volumes) | 3 | N | ↑ left parahippocampal gyrus extending into left hippocampus | — |
| Mondelli et al.39 | 2008 | Pituitary | 1.5 | N | No significant difference in pituitary volumes (HR = C) | No significant differences (HR = BD) |
| Singh et al.40 | 2008 | Striatum, amygdala, prefrontal cortex, thalamus | 1.5 | Y | No significant differences in the selected ROIs | — |
| Chaddock et al.41 | 2009 | whole brain | 1.5 | N | No significant differences in fractional anisotropy (HR = C); the HR showed intermediate alterations to C and BD | Genetic liability is correlated with lower FA in several major white matter tracts of the brain |
| Kempton et al.42 | 2009 | Whole brain (ROI: amygdala, anterior and posterior cingulate, prefrontal cortex, hippocampus) | 1.5 | N | ↑ left insula (HR > C), ↑ left cerebellum (HR > C) | ↑ left cerebellum (HR > BD) |
| Hajek et al.43 | 2009 | Caudate and putamen | 1.5 | N | ↑ caudate volume (HR > C) | No significant differences in caudate and putamen (HR = BD) |
| Hajek et al.44 | 2010 | Subgenual cingulate | 1.5 | N | No significant differences (HR = C) | No significant differences (HR = BD) |
| Hajek et al.45 | 2009 | Hippocampus, amygdala | 1.5 | N | No significant differences (HR = C) | No significant differences (HR = BD) |
| van der Schot et al.46 | 2009 | Cerebellum, frontal, temporal, parietal, occipital lobe, ventricles | 1.5 | N | Genetic risk to bipolar disorder is associated with decreased white matter volume | |
| Walterfang et al.47 | 2009 | Corpus callosum | 1.5 | N | No significant differences (HR = C) | Callosal area smaller in BD (BD < HR) |
| Gunde et al.48 | 2010 | Subcortical, deep, periventricular white matter hyperintensity | 1.5 | N | No significant differences in white matter hyperintensities (HR = C) | No significant differences in white matter hyperintensities (HR = BD) |
| Takahashi et al.49 | 2010 | Pituitary | 1.5 | N | No difference in pituitary volume | Significant ↑ pituitary volume (BD > HR) |
| Forcada et al.50 | 2010 | Whole brain | 1.5 | N | — | No significant differences in white or grey matter volumes (HR = BD) |
| van der Schot et al.51 | 2010 | Whole brain | 1.5 | N | The genetic risk to develop bipolar disorder was related to decreased grey matter density in the right medial frontal gyrus, precentral gyrus and insula and with decreased white matter density in the superior longitudinal fasciculi bilaterally. | |
| Versace et al.52 | 2010 | Whole brain | 3 | N | ↑ fractional anisotropy (HR > C) and ↓ radial diffusivity (HR < C) in left corpus callosum and in right inferior longitudinal fasciculus | — |
Antip = antipsychotic exposure; BD = bipolar disorder; C = controls; HR = high risk for bipolar disorder; N = no; ROI = region of interest; SVC = small volume correction;Y = yes.
Functional imaging studies
Positron emission tomography
There was 1 study included in our analysis that measured regional cerebral blood flow (rCBF) using PET and a previously validated sample of patients with bipolar disorder with acute mood challenge treated with lithium and who had remained stable for a long period as well as their unaffected, healthy siblings.55 When emotionally provoked, the HR individuals showed changes in brain activity consistent with those of their family members with bipolar disorder. Specifically, high baseline rCBF in the ventral medial frontal cortex that increased further with provoked sadness distinguished the HR individuals from the patients with bipolar disorder treated with lithium, in whom rCBF was decreased.55 This increase in the medial frontal cortex in the HR group was also not seen in the healthy controls who were not at risk, suggesting that it could be a compensatory change, making it a good candidate for future research into the factors mediating resilience and vulnerability.
Magnetic resonance spectroscopy
Proton MRS (H-MRS) is a noninvasive procedure using magnetic resonance technology to determine levels of specific neuronal substrates such as N-acetylaspartate (NAA), choline (Cho), myoinositol (mI) and creatine + phosphocreatine. Four studies included in our analysis used MRS to evaluate several brain regions in individuals at enhanced genetic risk for bipolar disorder. Cecil and colleagues53 found a trend for the levels of NAA and Cr to be lower, by about 8%, within the cerebellar vermis in children of patients with bipolar disorder than in children of parents without a psychiatric disorder. In addition, the frontal cortex in children of patients with bipolar disorder revealed elevated mI concentrations, about a 16% increase, compared with children of parents without a psychiatric disorder.53 Another MRS study, however, found no significant group differences in metabolite indices (NAA:Cr ratios or levels of mI or Cho) in the dorsolateral prefrontal cortex in HR individuals compared with controls.54 Another independent study with a large sample also found no significant pre-frontal differences in Cho, creatine, NAA or mI levels in HR individuals.38 Singh and colleagues60 reported that the offspring of parents with bipolar disorder showed decreases in anterior cingulate glutamate absolute concentrations and trends toward decreases in glutamate relative to creatine after fully syndromal mania developed, but not at baseline. This suggests that for HR individuals, altered glutamatergic functioning is connected to the development of bipolar disorder rather than associated with familial risk.
Functional MRI
There were 5 studies employing fMRI techniques to address the neural activation during different tasks in individuals at genetic risk for bipolar disorder. Allin and colleagues59 used a verbal fluency task to show that patients with bipolar disorder and their relatives shared similar deficits of deactivation in the retrosplenial cortex. Failure to deactivate this area is expected to impair task performance, consistent with an inherited deficit, either as part of the “evaluative” system in its role as a regulator of emotion and affect or overactivity of the default mode, which interferes with task performance.59 An fMRI study of working memory processing in the same participants demonstrated increased prefrontal activation in the HR group.56 This suggests that prefrontal hyperactivation during working memory may represent a potential endophenotype for bipolar disorder. An independent fMRI study confirmed a trend toward hyperactivation during working memory in HR individuals. This group failed to suppress activation in the left anterior insula, orbitofrontal cortex and parietal lobule.58 Surguladze and colleagues61 have further identified exaggerated regional cerebral activation in response to facial emotional expressions of fear and happiness both in patients with bipolar disorder and in their unaffected first-degree relatives. The authors propose that overactivation of the medial prefrontal cortex and subcortical structures in response to facial emotion processing tasks may represent a neurobiologic abnormality associated with genotypic variation conferring susceptibility for bipolar disorder. However, another verbal fluency study reported there were no significant differences between the HR and control groups, suggesting that hyperactivity during cognitive functioning is not a marker of genetic risk for bipolar disorder.57
Meta-analysis
Meta-analysis of regions of interest
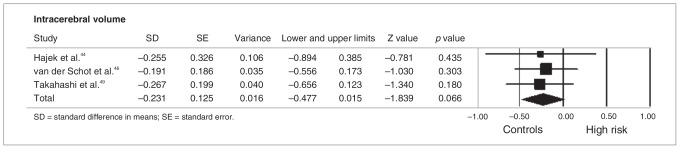
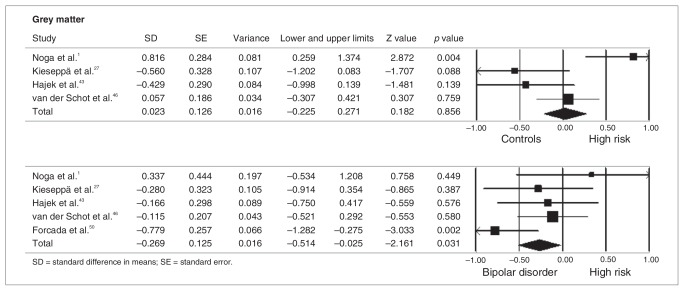
As stated in the Methods section, meta-analyses were performed when at least 3 studies were available for a given ROI. There were not enough functional studies of the same ROIs to allow meta-analysis; however, there were sufficient structural studies to perform meta-analyses for the following regions: thalamus (n = 3),31,35,40 striatum (n = 4),1,31,40,43 amygdala (n = 7),1,5,31,35,40,42,45 hippocampus (n = 8),1,5,29,31,34,35,42,45 pituitary gland (n = 3)39,49,63 and frontal lobe (n = 4).5,40,42,65 In all of these brain regions there were no meta-analytical significant differences between HR individuals and controls. Conversely, we detected a trend toward larger intracerebral volumes in HR individuals compared with controls44,46,49 (p = 0.07; Fig. 2). The HR group showed similar grey matter volumes compared with the control group1,27,43,46 (p = 0.86) but increased grey matter volumes compared with patients with bipolar disorder (p = 0.031, Fig. 3).1,27,43,46,50
Fig. 2.
Meta-analysis of intracerebral volume in controls and individuals at genetic risk for bipolar disorder. Lower and upper limits indicate 95% confidence intervals. Negative values indicate reduced volumes.
Fig. 3.
Meta-analysis of grey matter volume in controls, individuals at genetic risk for bipolar disorder (HR) and patients with the disorder. (Top) controls versus HR, (bottom) patients with bipolar disorder versus HR. Lower and upper limits indicate 95% confidence intervals. Negative values indicate reduced volumes.
Whole brain meta-analysis
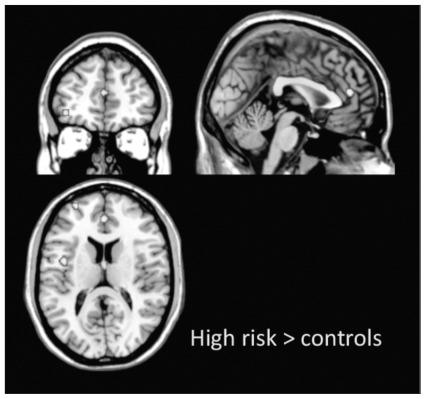
As stated in the Methods section, meta-analyses were performed when at least 3 whole brain studies (functional or structural) providing spatial coordinates suitable for computation were available. There were insufficient whole brain structural studies (grey matter or white matter) for meta-analysis; however, we found 4 fMRI studies suitable for an ALE meta-analytical approach.56,58,59,61 Two of them explored cortical response during working memory,56,58 1 during verbal fluency59 and 1 during emotional processing.61 Independent of task, HR individuals showed increased neural response in the left superior frontal gyrus (Talairach coordinates x, y, z = −27, 59, 14), in the medial frontal gyrus (Talairach coordinates x, y, z = 0, 44, 14) and in the left insula (Talairach coordinates x, y, z = −43, −1, −15; Fig. 4). Conversely, controls did not show increased neural activation in any brain regions compared with HR individuals.
Fig. 4.
Activation likelihood estimate voxel-based meta-analysis of functional magnetic resonance imaging studies of individuals at enhanced genetic risk for bipolar disorder (HR > controls, p < 0.001), independent of task. The coordinates are displayed in Talairach space.
Publication bias, heterogeneity, sensitivity analysis
Visual inspection of funnel plots revealed no evidence of publication bias. Quantitative evaluation of publication bias, as measured by the Egger intercept, was nonsignificant (p = 0.34). The fail-safe procedure determined that 113 unpublished studies would be needed to bring the overall meta-analytic estimate to a nonsignificant threshold. Robustness of meta-analytic findings was examined by sequentially removing each study and reanalyzing the remaining dataset (producing a new analysis for each study removed). No study affected the overall meta-analytic estimates of Hedges’ g by more than 0.1%. The pattern of differences across the subanalyses remained essentially unchanged in direction and magnitude. According to the criteria set by Higgins and Thompson,66 heterogeneity across published studies was small in magnitude and statistically nonsignificant (p = 0.10, I2 = 5.467).
Discussion
We present here a comprehensive systematic review and meta-analysis of structural and functional neuroimaging markers of genetic risk for bipolar disorder. To better understand our results we will first summarize the core imaging findings in patients with bipolar disorder and will then relate them to individuals at genetic risk for the disease.
Neuroimaging correlates of established bipolar disorder
The pathophysiology of bipolar disorder remains poorly understood, although reviews and meta-analyses of structural imaging studies67 suggest the presence of subtle abnormalities in the brains of patients with the disorder. The most consistent structural findings include preservation of total cerebral volume with regional grey and white matter changes in prefrontal, midline and limbic networks, noncontingent ventriculomegaly and increased rates of white matter hyper-intensities (for a review, see Emsell and McDonald68). Functional alterations have been reported in the neural systems for emotion processing and executive control (for a review, see Pan and colleagues69). However, these findings are not consistent. For example, a meta-analysis of regional morphometry studies in adults with bipolar disorder suggests significant heterogeneity across studies and brain structures.70 It was not possible to account for differences in medication in studies because of the limited information reported. Some of the reported discrepancies may be owing to effects of medication (especially lithium67), the effect of illness duration or small sample sizes. Despite the pronounced genetic effects on patients with bipolar disorder, the question of whether the genetic risk for the disorder is associated with some of the reported functional or structural brain abnormalities has hardly been addressed.46
Genetic studies in patients with bipolar disorder
The genetic inheritance of the disorder is complex; it is most likely to be polygenic rather than due to genes of major effect. Since unaffected relatives of patients with bipolar disorder are likely to share some susceptibility genes with affected patients, without overt expression of the clinical phenotype, abnormalities of structure or functioning detectable in HR individuals but not in controls may reflect genetically driven trait-related deficits (trait markers) for the disorder. Sometimes the same abnormalities may also be evident both in the HR and in the bipolar disorder groups, suggesting the possibility of genetic risk factors shared by both groups. Conversely, neuroimaging alterations observed in patients with bipolar disorder but not in HR individuals and controls can be interpreted as relating to the clinical status of the patients (state markers). Structural or functional abnormalities associated with resilience (resilience markers) are present in HR individuals but not in patients with bipolar disorder or controls. Overall, these alterations represent specific endophenotypes and can be conceptualized as neurobiologic markers that are intermediate between the underlying susceptibility genes for an illness and the clinical expression of the illness. An advantage of studying unaffected relatives is that abnormalities detected cannot be confounded by medication or other illness-related factors.56 Thus, examination of the unaffected relatives of patients is used to study the relation between increased genetic risk and brain abnormalities because these people carry the genetic risk for the disease but not the disease itself. However, these studies are limited by the fact that they cannot discriminate between genetic and shared environmental influence. In contrast, studying monozygotic and dizygotic twin pairs in which 1 twin is affected by bipolar disorder is a powerful approach to determine the relative contribution of genetic and environmental influence.51
Gray matter alterations and genetic risk for bipolar disorder
Our whole-brain meta-analysis showed grey matter volume reductions in the bipolar disorder group compared with the HR group,1,27,43,46,50 with grey matter volume in HR individuals being similar to that in controls.1,27,43,46 In line with the latter finding, no significant grey matter volume reductions in the frontal lobe were observed in HR individuals compared with controls.5,40,42,65 However, the nonsignificant trend toward larger intracerebral volumes in HR individuals compared with controls might indicate a dynamic process during the transition to bipolar disorder, presumably affecting various cortical areas at approximately identical time points. It could reflect either an effort to engage other, volumetrically larger regions aiming to compensate commencing pathologic processes or a resilience marker, since not all HR individuals will transition to bipolar disorder. These findings of larger whole-brain volumes can simply signify differences in tissue behaviour (i.e., tissue swelling or shrinkage) or involve changes in the cell density or composition of the neuropil and/or the myelinated sheets of neurons.
There has been an interest in the possibility of basal ganglia involvement in the genetic risk for bipolar disorder because of the increased prevalence of bipolar disorder in patients with diseases affecting these regions, including Huntington disease and cerebrovascular disease.1 In particular, we found a number of structural imaging studies of HR individuals focusing on the striatum, including the caudate, putamen and nucleus accumbens.1,31,40,43 Considering the functional heterogeneity of the striatum, it is not surprising that it has been associated with a wide range of behavioural, cognitive and emotional disorders, including mood disorders.43 Other studies have focused on putative thalamic structural alterations in HR individuals,31,35,40 as the thalamus plays a key role in cognitive functioning, and prefrontal–thalamic interactions mediate executive functions.71 However, our meta-analysis found no significant differences between HR individuals and controls in such regions, suggesting that striatal or thalamic structural abnormalities are not trait markers of bipolar disorder.
The theory that neuroanatomical circuitries are implicated in the pathology of bipolar disorder would imply the involvement of the temporal lobe, particularly the hippocampus and amygdala.1 In particular, a dynamic relation between the amygdala and parahippocampal gyrus may regulate emotional appraisal5 in patients with affective disorders. Consistent with these notions, we uncovered a number of structural studies addressing hippocampal regions and the amygdala in individuals at genetic risk for bipolar disorder.1,5,29,31,34,35,42,45 Most of these studies revealed inconsistent findings and nonsignificant differences between HR individuals and controls. The present meta-analysis confirmed that hippocampal and amygdala structural alterations are not trait markers for bipolar disorder. This is somewhat consistent with evidence from twin studies of schizophrenia for nongenetic factors affecting hippocampal volume and the susceptibility of the hippocampus to environmental risk factors, such as obstetric complications and stress-induced glucocorticoid excess.34 The preservation of hippocampal/amygdalar volume in HR individuals is contrary to reports of decreased volumes in children or adolescents with bipolar disorder. This discrepancy is likely related to clinical heterogeneity and diagnostic controversies around pediatric bipolar disorder. The cluster of mood liability, irritability, impulsivity and attention deficits, which constitutes the phenotype of pediatric bipolar disorder, as diagnosed in these studies, may not be continuous with adult bipolar disorder, which presents with different behavioural symptoms.45
Studies in patients with bipolar disorder often reported increased, decreased and unchanged pituitary volumes. As it is unclear whether pituitary volume changes are secondary to burden of illness or primary, increasing vulnerability for mood disorders, we reviewed some MRI studies addressing this point.39,49,63 If pituitary volume abnormalities are secondary, they should be present only in patients with longer history of illness. If, however, pituitary volume abnormalities are primary and causative of episodic hormonal dysregulations or increased vulnerability to mood disorders, they should already be present before or early in the course of illness in individuals at risk for bipolar disorder.39 Our meta-analysis uncovered no significant pituitary difference between the HR individuals and matched controls, suggesting that pituitary volume abnormalities are not a consistent trait marker of bipolar disorder, but rather may be related to clinical symptoms. This finding is in line with evidence indicating that cortisol abnormalities are mostly present only during episodes of mood dysregulation. The pituitary volume could then represent a state marker. This hypothesis is supported by association between pituitary volumes and postdexamethasone cortisol levels.39
The present meta-analysis suggests that grey matter volume alterations can be interpreted as state markers of bipolar disorder, reflecting the clinical presentation of symptoms, duration of illness, exposure to treatment46 or a combination of these factors.
White matter alterations and genetic risk for bipolar disorder
Although the first MRI twin studies found decreased white matter volume in patients with bipolar disorder and co-twins (HR) compared with control twins,27 subsequent studies have not found significant white matter volumetric alterations in HR individuals compared with controls32,47 or patients with bipolar disorder.50 However, all MRI studies using structural equation modelling uncovered significant correlations between genetic liability to bipolar disorder and white matter alterations,30,46,51 suggesting that white matter alterations are trait markers for the genes involved in the etiology of bipolar disorder.29 These authors speculated that white matter pathology may be central to the genetic risk for bipolar disorder, particularly circumscribed pathology limited to alterations in the principal white matter tracts of the brain.51 This is supported by the evidence that there is an increased prevalence of white matter hyperintensities in patients with established bipolar disorder, one of the most consistently replicated findings in structural MRI studies of bipolar disorder.36 Hyperintensities are reported to localize mostly on frontal white matter and the subcortical grey nucleus (thalamus, basal ganglia), and there is evidence that they localize in the deep white matter rather than in the periventricular area. Several other lines of evidence, such as those derived from gene expression and genetic association studies, also suggest the involvement of white matter pathology in patients with established bipolar disorder. For instance, reductions in the number, size and density of glial cells as well as downregulation of key oligodendrocyte and myelination genes have been reported in postmortem studies of individuals with bipolar disorder. Significant reductions in the number, density and size of glial cells could be reflected in reduced white matter tissue, as well as signal hyperintensities. Interestingly, white matter pathology has also been suggested to be central to the genetic risk for schizophrenia.46
Whereas the findings from studies of white matter hyperintensities in patients with bipolar disorder have been consistent, only 2 studies have examined white matter alterations in HR individuals, and their results are conflicting. The first study found evidence of an increased prevalence of hyper-intensities in HR individuals compared with controls,28 whereas a subsequent study found no significant differences.48 Further studies are clearly needed before firm conclusions can be drawn. There is a little more evidence comparing HR individuals to patients with bipolar disorder: 2 studies found more hyperintensities in the bipolar disorder compared with the HR group,28,33 whereas a subsequent study failed to replicate the result.48 Differences in imaging methods, small sample sizes and sociodemographic characteristics of the HR individuals may account for high heterogeneity across studies and for inconclusive findings.
Functional alterations and genetic risk for bipolar disorder
There were fewer functional (fMRI and PET) than structural studies investigating individuals at genetic risk for bipolar disorder. Nevertheless, our voxel-based meta-analysis uncovered a consistent network of neurofunctional abnormalities in the superior and medial frontal cortex and in the insula in HR individuals; in these regions the neural response was greater in HR individuals compared with controls, independent of the cognitive task employed. Studies of neuropsychologic function in HR individuals show there are similar impairments to those seen in patients with bipolar disorder.6 A recent meta-analysis in HR individuals and patients with bipolar disorder confirmed that executive function and working memory impairments are state independent and present in unaffected relatives who are likely to be carrying susceptibility genes for the illness.6,59 Taken with the result of our meta-analysis of functional studies, increased activation in regions involved in cognitive functions that are impaired in HR individuals and in patients with bipolar disorder can be interpreted as a shared trait marker of illness. Increased activation can compensate for functional deficits elsewhere in the cognitive network of HR individuals, enabling them to maintain performance, although performance becomes impaired when demands increase.56 In other words, the greater activation in the prefrontal cortex demonstrated by HR individuals does not reflect better or worse performance but rather the optimal level of activation to achieve that level of performance. With respect to PET studies, the siblings’ unique increases in the medial frontal cortex appear to identify a compensatory response in this HR group, as this pattern was not seen previously in healthy individuals without depression risk factors. Similarly, studies of patients with schizophrenia demonstrated that the increasing blood oxygen level–dependent response was compensatory in nature and an indicator of inefficiency of frontal cortical structures during cognitive tasks.56 This has been termed “functional inefficiency,” as this increase in fMRI response did not produce greater performance accuracy. Our findings can be interpreted in a similar fashion.57 Abnormalities in prefrontal regions can be associated with suppression of task-induced negative emotion, leading to abnormal activation in regions associated with emotional arousal (e.g., insula), and further interfere with cognition in the HR group.58 However, the neurochemical basis of the functional abnormalities in HR individuals is unclear. Whereas there have MRS studies in the HR population, the findings have been inconsistent, with 3 of 4 studies reporting no significant differences in metabolites between the HR and control groups.38,54,60 It remains unknown whether functional abnormalities precede structural ones and how reversible these abnormalities are.
Limitations
The evidence available is from cross-sectional studies where the clinical outcomes of the HR individuals are unknown. Although we are able to identify potential imaging markers of risk and resilience in HR individuals by comparison with findings in patients with bipolar disorder, this approach is not as robust as a within-subject design, and longitudinal evaluation of the markers will be needed before they can be used in clinical practice. To date, no longitudinal studies are available that allow for a separation between resilient individuals at high risk who do not transition to the disease and those who are presymptomtic. Thus, increased intracerebral volume could be interpreted as conferring resilience onto HR individuals by increased structural connectivity or a similarly protective mechanism. Longitudinal neuroimaging studies in HR individuals will be able to definitively ascertain the core neuroanatomical alterations at different time points underlying the development of bipolar disorder. Relatively few studies involving relatives of patients with bipolar disorder were available, which prevented meta-analysis in some domains and limited the robustness of imaging findings in HR individuals.72 The meta-analysis of data collected on different MRI scanners, and the use of different acquisition protocols, is complicated by the potentially confounding effects of differences between sites. Conflicting results in the field may also be a consequence of heterogeneity across studies with different analysis methods, small sample sizes and different inclusion criteria for the HR cohorts. For example, most studies included HR participants with a family history of bipolar disorder without specifying whether the patients had bipolar disorder I or II. It should also be noted that some of the reviewed studies included unaffected and/or healthy first-degree relatives or other relatives, whereas other studies included relatives who had other psychiatric conditions such as attention-deficit/hyperactivity disorder, anxiety or affective disorders. It is also important to consider the clinical characteristics of the participants, particularly the HR individuals, some of whom may have had subsyndromal symptoms when studied, whereas others may have been asymptomatic. The level of subsyndromal symptoms in HR individuals is not reported in a number of studies, but differences could contribute to the inconsistency in some of the findings.
In addition, bipolar disorder is likely to be influenced by many genetic and epigenetic factors, and the assumption of a simple linear genetic relation (i.e., controls > HR > bipolar disorder or controls < HR < bipolar disorder) may be naive. In fact, familial genetic effects may also be confounded by influences specific to the patient group, such as lifetime episodes of mania and depression or medication (including lithium), which may be associated with neurophysiologic changes that are likely to further confound differences in neural activation patterns or structural changes.59 A further limitation was that there was not enough statistical power to compare different HR groups (i.e., first-degree relatives v. twins or clinically symptomatic HR v. nonsymptomatic HR). Finally, because of heterogeneity of the assessment instruments used across studies, it was not possible to correlate imaging modalities with psychopathologic measures or clinical outcomes. The overall sample size of our meta-analysis was small, and consequently the chance of finding significant p values for tests of heterogeneity and bias were remote. Moreover, owing to heterogeneity of the phenotype of bipolar disorder and the HR status, it will be important to identify markers of risk for psychopathology in individuals at high familial risk as well as those at increased risk of bipolar disorder per se.
Implications
Currently, there are only preliminary data3 distinguishing between individuals presenting with possible prodromal features of bipolar disorder who will transition to bipolar disorder and those who will not. This limits clinicians’ capacity to offer accurate prognoses and preventive interventions. Thus, biomarkers, either of risk or resilience, that differentiate those who will transition to bipolar disorder from those who will not would be invaluable. Our finding that grey matter decrease may be a state-related factor for bipolar disorder suggests that structural imaging may be a useful biomarker to distinguish between individuals who will transition to bipolar disorder from those who will not. Prospective studies of grey matter volume in HR individuals are needed to evaluate this. A further implication of our meta-analysis is that hyperactivation in the frontal and insular cortex is the neural substrate for the executive function impairments seen in HR individuals and patients with bipolar disorder. The similarity between our findings in HR individuals and previous findings in patients with bipolar disorder suggests that hyperactivation in these regions is a good candidate as a trait endophenotype for bipolar disorder. However, whereas it indicates that bipolar disorder develops in the context of a dysfunctional brain, further work is needed to determine how this relates to the affective disturbance and other clinical features that develop with the onset of bipolar disorder. Finally, it is clear that there have been few studies of white matter hyperintensities in HR individuals. This is surprising given the number of studies indicating that these are increased in patients with bipolar disorder and warrant further investigation in HR individuals.
Conclusion
Despite heterogeneous imaging methods, samples and conflicting findings pervading the literature, there is accumulating evidence for the existence of neurobiologic abnormalities in individuals at genetic risk for bipolar disorder at various scales of investigation. The etiopathogenesis of bipolar disorder will be better elucidated by future imaging studies investigating larger and more homogeneous samples and using longitudinal designs to dissect neurobiologic abnormalities that are underlying traits of the illness from those related to psychopathologic states, such as episodes of mood exacerbation or pharmacologic treatment.
Table 3.
Functional imaging studies of individuals at risk for bipolar disorder: study characteristics
| Study | Year | Technique | Type of risk | Assessment instruments | Task | Lifetime/current diagnoses HR | Group; no. (% female) [age, yr] | ||
|---|---|---|---|---|---|---|---|---|---|
| Controls | HR | BD | |||||||
| Cecil et al.53 | 2003 | MRS | Children of BD patient | SCID, K-SADS, C-GAS, YMRS, IDS | — | BD, ADHD, MDD | 9 (44) [11] | 9 (44) [9] | |
| Gallelli et al. 54 | 2005 | MRS | Offspring of BD parent | K-SADS-PL, SCID, YRMS, CDRS-R | — | Anxiety disorders, ADHD, ODD | 26 (35) [14] | 28 (32) [12] | 32 (31) [14] |
| Krüger et al. 55 | 2006 | PET | Sibling of BD patients | SCID | Mood induction | None | — | 9 (67) [40] | 9 (56) [38] |
| Hajek et al. 38 | 2008 | MRS | First- or second- degree relatives | K-SADS PL/L | — | None | 31 (64) [21] | 36 (61) [20] | 33 (63) [21] |
| Drapier et al.56 | 2008 | fMRI | First-degree relatives | SADS-L, BDI, FIGS, ASRM | N-back working memory | MDD, substance-induced mood disorder | 20 (50) [50] | 20 (40) [43] | 20 (55) [43] |
| Costafreda et al.57 | 2009 | fMRI | MZ cotwins | BDI, ASRM, HAM-D, YMRS | Verbal fluency task | None | 48 (48) [37] | 7 (86) [39] | 28 (57) [40] |
| Thermenos et al.58 | 2010 | fMRI | First-degree relatives | SCID, SANS/SAPS | N-back working memory | Alcohol and drugs abuse or dependence, MDD | 19 (53) [39] | 18 (56) [36] | 19 (42) [41] |
| Allin et al.59 | 2010 | fMRI | First-degree relatives | SADS-L, ASRM, BDI | Verbal fluency task | MDD, substance- induced mood disorder | 19 (47) [40] | 19 (42) [40] | 18 (61) [39] |
| Singh et al. 60 | 2010 | MRS | Offspring of BD patients | SCID, KSADS/PL, YMRS, CDRS-R | — | None | 20 (25) [15] | 20 (50) [13] | 20 (35) [16] |
| Surguladze et al.61 | 2010 | fMRI | Relatives of BD patients | SADS-L, BDI, ASRM | Emotional faces | None | 20 (50) [50] | 20 (40) [43] | 20 (55) [43] |
ADHD = attention-deficit/hyperactivity disorder; ASRM = Altman Self-Rated Mania Scale; BD = bipolar disorder; BDI = Beck Depression Inventory; CDRS-R = Children’s Depression Rating Scale-Revised; C-GAS = Child Global Assessment Scale; FIGS = Family Interview for Genetic Studies; fMRI = functional magnetic resonance imaging; HAM-D = Hamilton Rating Scale for Depression; HR = high risk for bipolar disorder; IDS = Inventory for Depressive Symptomatology; K-SADS-E = Kiddie-Schedule for Affective Disorders and Schizophrenia; MDD = major depressive disorder; MRS = magnetic resonance spectroscopy; MZ = monozygotic; ODD = oppositional defiant disorder; PET = positron emission tomography; SADS-PL/P/L/ = Schedule of Affective Disorders and Schizophrenia-Present and Lifetime version; SANS/SAPS = Scale for the Assessment of Negative Symptoms/Scale for the Assessment of Positive Symptoms; SCID = Structured Structured Clinical Interview for DSM-III/IV; YMRS = Young Mania Rating Scale.
Footnotes
Competing interests: None declared for P. Fusar-Poli, O. Howes and S. Borgwardt. A. Bechdolf declares having received speaker fees from Bristol Meyers Squibb, Lilly and Wyeth.
Contributors: P. Fusar-Poli designed the study, acquired the data and wrote the article. All authors analyzed the data, reviewed the article and approved its publication.
References
- 1.Noga JT, Vladar K, Torrey EF. A volumetric magnetic resonance imaging study of monozygotic twins discordant for bipolar disorder. Psychiatry Res. 2001;106:25–34. doi: 10.1016/s0925-4927(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 2.Bechdolf A, Nelson B, Cotton SM, et al. A preliminary evaluation of the validity of at-risk criteria for bipolar disorders in help-seeking adolescents and young adults. J Affect Disord. 2010;127:316–20. doi: 10.1016/j.jad.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Yung AR, Nelson B, Stanford C, et al. Validation of “prodromal” criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr Res. 2008;105:10–7. doi: 10.1016/j.schres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Howes OD, Lim S, Theologos G, et al. A comprehensive review and model of putative prodromal features of bipolar affective disorder. Psychol Med. 2011;41:1567–77. doi: 10.1017/S0033291710001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladouceur CD, Almeida JR, Birmaher B, et al. Subcortical gray matter volume abnormalities in healthy bipolar offspring: Potential neuroanatomical risk marker for bipolar disorder? J Am Acad Child Adolesc Psychiatry. 2008;47:532–9. doi: 10.1097/CHI.0b013e318167656e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arts B, Jabben N, Krabbendam L, et al. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38:771–85. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. QUOROM Group. Br J Surg. 2000;87:1448–54. doi: 10.1046/j.1365-2168.2000.01610.x. [DOI] [PubMed] [Google Scholar]
- 9.Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–82. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 10.Borenstein MHL, Higgins J, Rothstein H. Comprehensive meta-analysis version 2. Englewood (NJ): Biostat; 2005. [Google Scholar]
- 11.Hedges L, Holkin I. Statistical methods for meta-analysis. New York: Academic Press; 1985. [Google Scholar]
- 12.Cooper H, Hedges L, Valentine J. Handbook of research synthesis and meta-analysis. New York: Russell Sage Foundation; 2009. [Google Scholar]
- 13.Lipsey M, Wilson D. Practical meta-analysis. Thousand Oaks (CA): Sage Publications; 2000. [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orwin R. A fail-safe N for effect size in meta-analysis. J Educ Stat. 1983;8:157–9. [Google Scholar]
- 16.Turkeltaub PE, Eden GF, Jones KM, et al. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Chan RC, McAlonan GM, et al. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull. 2010;36:1029–39. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laird AR, Fox PM, Price CJ, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–64. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellison-Wright I, Glahn DC, Laird AR, et al. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–23. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chikazoe J, Konishi S, Asari T, et al. Activation of right inferior frontal gyrus during response inhibition across response modalities. J Cogn Neurosci. 2007;19:69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- 21.Dickstein SG, Bannon K, Castellanos FX, et al. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–62. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 22.Owen AM, McMillan KM, Laird AR, et al. N-back working memory paradigm: a meta-analysis of normative functional neuroim-aging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMillan KM, Laird AR, Witt ST, et al. Self-paced working memory: validation of verbal variations of the n-back paradigm. Brain Res. 2007;1139:133–42. doi: 10.1016/j.brainres.2006.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage. 2008;42:343–56. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–85. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Fusar-Poli P, Radua J, McGuire P, et al. Neuroanatomical maps of psychosis onset: voxelwise meta-analysis of antipschotic-naïve VBM studies. Schizophr Bull. 2011 Nov 17; doi: 10.1093/schbul/sbr134. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieseppä T, van Erp TG, Haukka J, et al. Reduced left hemispheric white matter volume in twins with bipolar I disorder. Biol Psychiatry. 2003;54:896–905. doi: 10.1016/s0006-3223(03)00373-1. [DOI] [PubMed] [Google Scholar]
- 28.Ahearn EP, Speer MC, Chen YT, et al. Investigation of Notch3 as a candidate gene for bipolar disorder using brain hyperintensities as an endophenotype. Am J Med Genet. 2002;114:652–8. doi: 10.1002/ajmg.10512. [DOI] [PubMed] [Google Scholar]
- 29.Connor SE, Ng V, McDonald C, et al. A study of hippocampal shape anomaly in schizophrenia and in families multiply affected by schizophrenia or bipolar disorder. Neuroradiology. 2004;46:523–34. doi: 10.1007/s00234-004-1224-0. [DOI] [PubMed] [Google Scholar]
- 30.McDonald C, Bullmore ET, Sham PC, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–84. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- 31.McIntosh AM, Job DE, Moorhead TW, et al. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2004;56:544–52. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 32.McIntosh AM, Job DE, Moorhead TW, et al. White matter density in patients with schizophrenia, bipolar disorder and their unaffected relatives. Biol Psychiatry. 2005;58:254–7. doi: 10.1016/j.biopsych.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 33.Gulseren S, Gurcan M, Gulseren L, et al. T2 hyperintensities in bipolar patients and their healthy siblings. Arch Med Res. 2006;37:79–85. doi: 10.1016/j.arcmed.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 34.McDonald C, Marshall N, Sham PC, et al. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry. 2006;163:478–87. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- 35.McIntosh AM, Job DE, Moorhead WJ, et al. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:76–83. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- 36.Frazier JA, Breeze JL, Papadimitriou G, et al. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disord. 2007;9:799–809. doi: 10.1111/j.1399-5618.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 37.Hajek T, Gunde E, Bernier D, et al. Subgenual cingulate volumes in affected and unaffected offspring of bipolar parents. J Affect Disord. 2008;108:263–9. doi: 10.1016/j.jad.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajek T, Bernier D, Slaney C, et al. A comparison of affected and unaffected relatives of patients with bipolar disorder using proton magnetic resonance spectroscopy. J Psychiatry Neurosci. 2008;33:531–40. [PMC free article] [PubMed] [Google Scholar]
- 39.Mondelli V, Dazzan P, Gabilondo A, et al. Pituitary volume in unaffected relatives of patients with schizophrenia and bipolar disorder. Psychoneuroendocrinology. 2008;33:1004–12. doi: 10.1016/j.psyneuen.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Singh MK, Delbello MP, Adler CM, et al. Neuroanatomical characterization of child offspring of bipolar parents. J Am Acad Child Adolesc Psychiatry. 2008;47:526–31. doi: 10.1097/CHI.0b013e318167655a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaddock CA, Barker GJ, Marshall N, et al. White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry. 2009;194:527–34. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- 42.Kempton MJ, Haldane M, Jogia J, et al. Dissociable brain structural changes associated with predisposition, resilience, and disease expression in bipolar disorder. J Neurosci. 2009;29:10863–8. doi: 10.1523/JNEUROSCI.2204-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hajek T, Gunde E, Slaney C, et al. Striatal volumes in affected and unaffected relatives of bipolar patients–high-risk study. J Psychiatr Res. 2009;43:724–9. doi: 10.1016/j.jpsychires.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Hajek T, Novak T, Kopecek M, et al. Subgenual cingulate volumes in offspring of bipolar parents and in sporadic bipolar patients. Eur Arch Psychiatry Clin Neurosci. 2010;260:297–304. doi: 10.1007/s00406-009-0077-2. [DOI] [PubMed] [Google Scholar]
- 45.Hajek T, Gunde E, Slaney C, et al. Amygdala and hippocampal volumes in relatives of patients with bipolar disorder: a high-risk study. Can J Psychiatry. 2009;54:726–33. doi: 10.1177/070674370905401102. [DOI] [PubMed] [Google Scholar]
- 46.van der Schot AC, Vonk R, Brans RG, et al. Influence of genes and environment on brain volumes in twin pairs concordant and discordant for bipolar disorder. Arch Gen Psychiatry. 2009;66:142–51. doi: 10.1001/archgenpsychiatry.2008.541. [DOI] [PubMed] [Google Scholar]
- 47.Walterfang M, Wood AG, Barton S, et al. Corpus callosum size and shape alterations in individuals with bipolar disorder and their first-degree relatives. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1050–7. doi: 10.1016/j.pnpbp.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Gunde E, Novak T, Kopecek M, et al. White matter hyperintensities in affected and unaffected late teenage and early adulthood offspring of bipolar parents: a two-center high-risk study. J Psychiatr Res. 2011;45:76–82. doi: 10.1016/j.jpsychires.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi T, Walterfang M, Wood SJ, et al. Pituitary volume in patients with bipolar disorder and their first-degree relatives. J Affect Disord. 2010;124:256–61. doi: 10.1016/j.jad.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Forcada I, Papachristou E, Mur M, et al. The impact of general intellectual ability and white matter volume on the functional outcome of patients with bipolar disorder and their relatives. J Affect Disord. 2011;130:413–20. doi: 10.1016/j.jad.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 51.van der Schot AC, Vonk R, Brouwer RM, et al. Genetic and environmental influences on focal brain density in bipolar disorder. Brain. 2010;133:3080–92. doi: 10.1093/brain/awq236. [DOI] [PubMed] [Google Scholar]
- 52.Versace A, Ladouceur CD, Romero S, et al. Altered development of white matter in youth at high familial risk for bipolar disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49:1249–59. doi: 10.1016/j.jaac.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cecil KM, DelBello MP, Sellars MC, et al. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J Child Adolesc Psychopharmacol. 2003;13:545–55. doi: 10.1089/104454603322724931. [DOI] [PubMed] [Google Scholar]
- 54.Gallelli KA, Wagner CM, Karchemskiy A, et al. N-acetylaspartate levels in bipolar offspring with and at high-risk for bipolar disorder. Bipolar Disord. 2005;7:589–97. doi: 10.1111/j.1399-5618.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 55.Krüger S, Alda M, Young LT, et al. Risk and resilience markers in bipolar disorder: brain responses to emotional challenge in bipolar patients and their healthy siblings. Am J Psychiatry. 2006;163:257–64. doi: 10.1176/appi.ajp.163.2.257. [DOI] [PubMed] [Google Scholar]
- 56.Drapier D, Surguladze S, Marshall N, et al. Genetic liability for bipolar disorder is characterized by excess frontal activation in response to a working memory task. Biol Psychiatry. 2008;64:513–20. doi: 10.1016/j.biopsych.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 57.Costafreda SG, Fu CH, Picchioni M, et al. Increased inferior frontal activation during word generation: A marker of genetic risk for schizophrenia but not bipolar disorder? Hum Brain Mapp. 2009;30:3287–98. doi: 10.1002/hbm.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thermenos HW, Goldstein JM, Milanovic SM, et al. An fMRI study of working memory in persons with bipolar disorder or at genetic risk for bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:120–31. doi: 10.1002/ajmg.b.30964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allin MP, Marshall N, Schulze K, et al. A functional MRI study of verbal fluency in adults with bipolar disorder and their unaffected relatives. Psychol Med. 2010;40:2025–35. doi: 10.1017/S0033291710000127. [DOI] [PubMed] [Google Scholar]
- 60.Singh M, Spielman D, Adleman N, et al. Brain glutamatergic characteristics of pediatric offspring of parents with bipolar disorder. Psychiatry Res. 2010;182:165–71. doi: 10.1016/j.pscychresns.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Surguladze SA, Marshall N, Schulze K, et al. Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. Neuroimage. 2010;53:58–64. doi: 10.1016/j.neuroimage.2010.05.069. [DOI] [PubMed] [Google Scholar]
- 62.Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C:48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- 63.Hajek T, Gunde E, Bernier D, et al. Pituitary volumes in relatives of bipolar patients: high-risk study. Eur Arch Psychiatry Clin Neurosci. 2008;258:357–62. doi: 10.1007/s00406-008-0804-0. [DOI] [PubMed] [Google Scholar]
- 64.Vederine FE, Wessa M, Leboyer M, et al. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1820–6. doi: 10.1016/j.pnpbp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Whalley HC, Simonotto E, Moorhead W, et al. Functional imaging as a predictor of schizophrenia. Biol Psychiatry. 2006;60:454–62. doi: 10.1016/j.biopsych.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 66.Higgins JP, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 67.Kempton MJ, Geddes JR, Ettinger U, et al. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–32. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 68.Emsell L, McDonald C. The structural neuroimaging of bipolar disorder. Int Rev Psychiatry. 2009;21:297–313. doi: 10.1080/09540260902962081. [DOI] [PubMed] [Google Scholar]
- 69.Pan L, Keener MT, Hassel S, et al. Functional neuroimaging studies of bipolar disorder: examining the wide clinical spectrum in the search for disease endophenotypes. Int Rev Psychiatry. 2009;21:368–79. doi: 10.1080/09540260902962164. [DOI] [PubMed] [Google Scholar]
- 70.McDonald C, Zanelli J, Rabe-Hesketh S, et al. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry. 2004;56:411–7. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 71.Zikopoulos B, Barbas H. Parallel driving and modulatory pathways link the prefrontal cortex and thalamus. PLoS One. 2007;2:e848. doi: 10.1371/journal.pone.0000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lawrie SM, McIntosh AM, Hall J, et al. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr Bull. 2008;34:330–40. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]