Abstract
Background and Objectives
With recent advances in equipment and techniques, infrapopliteal angioplasty has shown results that are comparable to those of surgical bypass in patients with critical limb ischemia (CLI). In this study, we evaluated the efficacy and the feasibility of infrapopliteal angioplasty in patients with CLI.
Subjects and Methods
Between March 2002 and May 2008, infrapopliteal angioplasty was performed on 118 limbs of 101 patients (79 males; mean age 66 years) with CLI (Rutherford category 4, 5 or 6). Freedom from reintervention, limb salvage, and overall survival were analyzed.
Results
The median follow-up duration was 30 months. Initial technical and clinical success rates were 69.5% and 83.1%, respectively. No major complication requiring surgical intervention developed after angioplasty. Among 82 limbs with initial technical success, the rate of freedom from any reintervention at 2 years was 70.7% and that from limb salvage was 97.6%. Young age and Rutherford category 6 at initial presentation were independent predictors associated with poor 2 year primary patency in these patients with CLI. Overall survival at 1 year was 86.4% and that at 2 years 76.3%. A history of cerebrovascular accident was an independent predictor associated with poor 2 year survival in these patients.
Conclusion
Infrapopliteal angioplasty as a primary choice of treatment in CLI patients showed favorable clinical outcomes and feasibility.
Keywords: Ischemia, Limb salvage, Tibial arteries, Peroneal arteries, Angioplasty
Introduction
Among revascularization methods for critical lower limb ischemia (CLI), surgical bypass is regarded as the gold standard, with better anatomical and clinical durabilities.1-3) However, patients with CLI are often aged and not optimal candidates for surgical bypass due to medical co-morbidities with increasing perioperative mortality rates and a poor autogeneous conduit.4),5) As an alternative method of revascularization, infrapopliteal angioplasty is preferred by patients with visible stumps, good runoff of distal outflow vessels or high surgical risk. However, it had been regarded as an inferior treatment option compared to surgical bypass due to bulky catheters, lack of low caliber wires and general lack of clinical experience which frequently resulted in periprocedural complications.4),6-8) Recently, newly developed devices and technical advances have widened the therapeutic spectrum of angioplasty to more distal and complex lesions with lower complication rates.9),10) Also, it rarely compromises the option of future surgical procedures because it preserves the saphenous vein for lower extremity distal bypass surgery while at the same time it reduces the cost and shortens the hospital stay.10)
In this study, we evaluated the feasibility and the efficacy of infrapopliteal angioplasty in terms of primary patency and limb salvage as a primary choice of treatment in patients with CLI. Moreover, we evaluated predictors associated with poor primary patency and poor survival in these patients.
Subjects and Methods
Study subjects
Between April 2002 and May 2008, we retrospectively analyzed 118 limbs of 101 patients with CLI (Rutherford-Becker grades11) 4, 5, or 6) who had been treated by infrapopliteal angioplasty. Demographic, laboratory, technical, and clinical characteristics were retrospectively collected from their medical records. Blood samples were collected one day before or immediately before the index procedure.
Percutaneous transluminal angioplasty procedure
Vascular access for infrapopliteal angioplasty was gained via ipsilateral or contralateral puncture of the common femoral artery. An antegrade approach was preferred when there were no combined lesions requiring proximal iliac or femoral intervention and in patients without obesity, due to better maneuverability of the catheters and better control of the wiring in case of total occlusion. After placement of the 6 to 7 French sheath under local anesthesia, an intra-arterial bolus of 5000 IU of heparin was given, and additional heparin was administered to maintain an activated clotting time between 250 to 300 seconds, if procedure time was lengthened. Infrapopliteal lesions were passed with a 0.014" to 0.035" guide wire. If the initial transluminal recanalization failed, total occlusions were recanalized through the subintimal dissection plane with reentrance into the true lumen using 0.035" hydrophilic guide wires (Terumo, Tokyo, Japan). After crossing the lesion, a 4 Fr to 5 Fr multipurpose catheter was advanced to the distal patent segment in exchange with a 0.014" wire. Percutaneous transluminal angioplasty (PTA) was then performed with adequate sized balloons (2.25 to 4.0 mm) at 6-10 atmospheres. In case of elastic recoil or a flow limiting dissection after balloon dilatation, stents were implanted for bailout purposes. When proximal lesions existed, including ipsilateral iliac, femoral or popliteal artery lesions, concomitant procedures were performed.
Follow-up
Prior to intervention, patients were premedicated with aspirin (100 mg, daily) and maintained on this dose indefinitely after PTA. Follow-up included a clinical examination during the hospital stay and 1 month after the intervention to document hemodynamic improvement. Subsequent follow-up was considered when there was a question of worsening clinical status. If necessary, repeat peripheral angiography or a computed tomography angiogram was scheduled on their visit. The causes and date of death were examined by chart review, telephone contact with family members, or checking with the national statistical office.
Angiographic findings and clinical outcomes
Technical success was defined as PTA resulting in less than 30% residual stenosis with sufficient antegrade flow; a suboptimal result was defined as sluggish flow and/or residual stenosis 30% to 50% after repeated dilatation. Primary clinical success was defined as an improvement of at least one clinical category in the Rutherford-Becker classification.11) Primary patency was defined as persistent patency without any re-intervention including angioplasty, surgical procedures performed on or at the margins of the treated lesion, or amputation. Limb salvage was defined as prevention of major amputation. Major amputation was defined as limb loss below or above the knee level, while minor amputation was defined as a transmetatarsal or more distal level amputation of the lower extremity.
Statistical analysis
We analyzed data using the Statistical Package for the Social Sciences (SPSS) software (version 15.0; SPSS Inc., Chicago, IL, USA). Data are presented as the mean±standard deviation for continuous variables and as number (percentage) for categorical variables. Student's t-test was used for comparison of continuous variables, and chi-square analysis was used for comparison of categorical variables. Event-free survival curves were constructed by the Kaplan-Meier method. All significant univariate predictors of poor primary patency and poor survival were included in the multivariate logistic regression analyses. A p less than 0.05 was considered significant.
Results
Demographic characteristics of the study patients
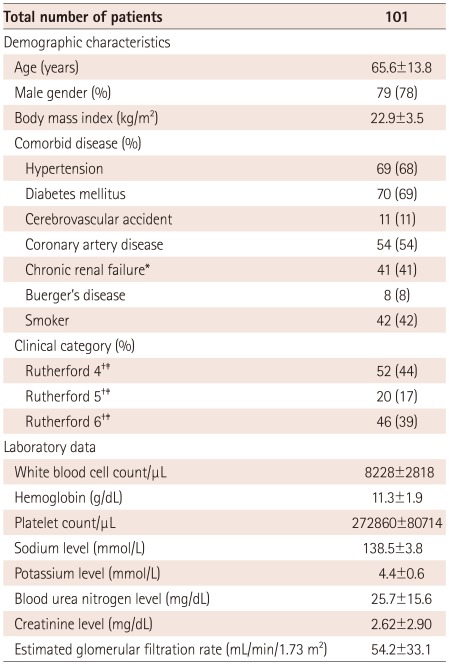
Demographic characteristics of the patients are listed in Table 1. The mean age of the study population was 65.6±13.8 years, with 79 (78%) men and 22 (22%) women. Many patients had significant co-morbidities including hypertension (68%), diabetes mellitus (69%), coronary artery disease (54%), chronic renal failure (41%), and a history of a cerebrovascular accident (11%). Also, six patients were smokers among eight patients who were diagnosed with Buerger's disease (mean age 42 years).12) Fifty-two limbs were identified as Rutherford category 4 (44%), 20 limbs as Rutherford category 5 (17%), and 46 limbs as Rutherford category 6 (39%).
Table 1.
Demographic and laboratory characteristics of the study population
Data are expressed as the mean value±SD or percentage of patients. *Serum creatinine >1.5 mg/dL, †Among 118 limbs, ‡Rutherford classification can be found in reference 11
Laboratory data of the study patients
Laboratory data of the patients are listed in Table 1. The mean blood urea nitrogen level was 25.7±15.6 mg/dL, and the mean creatinine level 2.62±2.90 mg/dL. The mean estimated glomerular filtration rate was 54.2±33.1 mL/min/1.73 m2.
Angiographic findings of the study patients
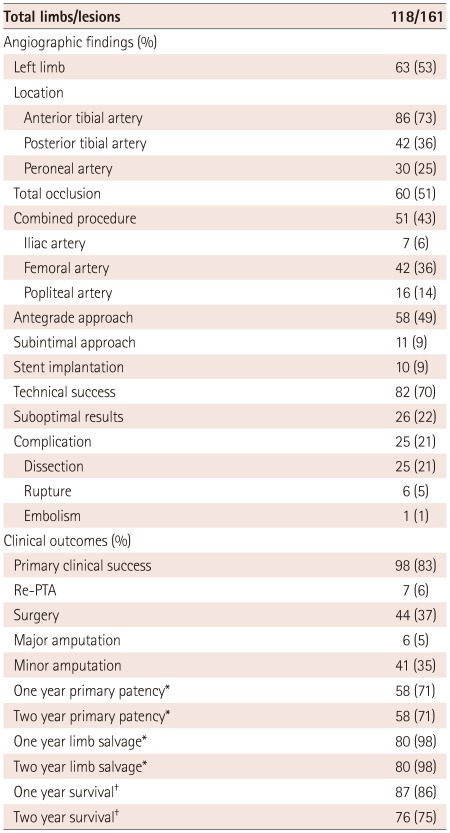
Angiographic findings of the study patients are shown in Table 2. A total of 161 lesions were treated in 118 limbs of 101 patients with CLI. Anterior tibial artery was the most commonly treated location (53%), and totally occluded arteries were observed in 60 limbs (51%). Concomitant procedures in the proximal lesion as ipsilateral iliac or superficial femoral artery were performed in 51 limbs (43%). Among them, a superficial femoral artery procedure was most commonly performed (36%). Antegrade access was done in 58 limbs (49%), and subintimal angioplasty was done in 11 limbs (9%). Initial technical success was achieved in 82 limbs (69.5%). On final angiography, a suboptimal result was obtained in 26 limbs (22%), which showed diffuse and heavily calcified lesions at initial presentation. There were 10 technical failures due to 8 unsuccessful placements of guide wires, 1 heavy calcified lesion, and 1 arterial rupture. No major complication requiring surgical intervention occurred after PTA. Minor procedural complications such as flow limiting dissection, rupture, and embolization occurred in 25 limbs (21%), which were treated by conservative treatment or stent implantation. In 10 cases of elastic recoil or flow limiting dissection, 11 stents were implanted for bailout purpose. Types of stents included 8 bare-metal coronary stents, 2 drug-eluting coronary stents, and 1 balloon-expandable peripheral stent. Among 11 stents, 3 were implanted in the anterior tibial artery, 5 in the posterior tibial artery, and 3 in the peroneal artery.
Table 2.
Angiographic findings and clinical outcomes of the study population
Data are expressed as percentage of limbs. *Among 82 limbs with initial technical success, †Among 101 patients. PTA: percutaneous transluminal angioplasty
There were no significant differences in terms of technical success (82% vs. 74%, p=0.726) irrespective of the presence or absence of subintimal angioplasty.
Clinical outcomes of the study patients
Clinical outcomes of the patients are shown in Table 2. Primary clinical success was obtained in 98 limbs (83.1%); 18 limbs showed persistent gangrene and 2 other limbs persistent pain. The mean follow-up duration was 34 months (range 1-94 months). During follow-up, re-PTA was performed in 7 limbs (6%), surgery in 44 limbs (37%), major amputation in 6 limbs (5%), and minor amputation in 41 (35%).
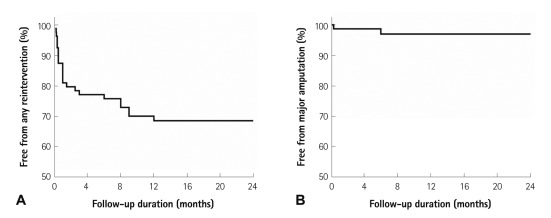
Among 82 limbs with initial technical success, primary patency was 70.7% at 1 year and 2 years, and the limb salvage rate was 97.6% at 1 year and 2 years (Table 2). There were no significant differences in terms of 2 year primary patency (70% vs. 63%, p=0.744) and 2 year limb salvage (100% vs. 95%, p=1.000) regardless of the presence or absence of stent implantation. Moreover, there were no significant differences in terms of 2 year primary patency (82% vs. 62%, p=0.322) and 2 year limb salvage (100% vs. 95%, p=1.000) regardless of the presence or absence of subintimal angioplasty. Kaplan-Meier analysis was used to obtain primary patency (Fig. 1A) and limb salvage rates (Fig. 1B) during 2 year follow-up.
Fig. 1.
Kaplan-Meier analysis shows primary patency (A) and limb salvage rate (B) during 2 year follow-up among patients with initial technical success. Primary patency at 2 years was 70.7%, and limb salvage rate at 2 years was 97.6%.
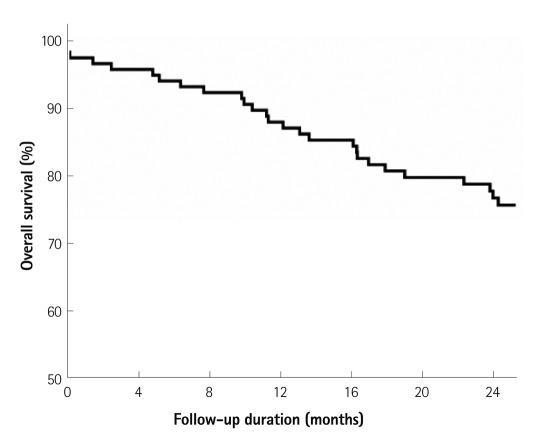
Overall survival was 86.1% at 1 year and 75.2% at 2 years (Table 2). The most common cause of death was acute myocardial infarction (n=10) and renal insufficiency (n=10). There was no significant difference in terms of 2 year survival (60% vs. 78%, p=0.243) irrespective of the presence or absence of stent implantation. Furthermore, there was no significant difference in terms of 2 year survival (55% vs 79%, p=0.126) irrespective of the presence or absence of subintimal angioplasty. Kaplan-Meier analysis was used to calculate survival during the 2 year follow-up period (Fig. 2).
Fig. 2.
Kaplan-Meier analysis shows overall survival during 2 year follow-up. Overall survival at 2 years was 76.3%.
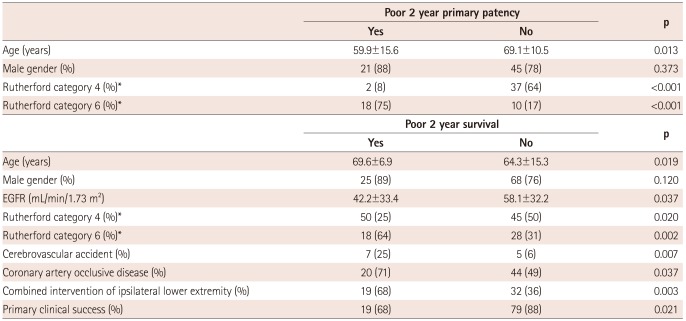
Univariate analysis associated with poor 2 year primary patency
Univariate analysis showed that younger age (59.9±15.6 years vs. 69.1±10.5 years, p=0.013), less frequent Rutherford category 4 (8% vs. 64%, p<0.001), and more frequent Rutherford category 6 (75% vs. 17%, p<0.001) were associated with poor 2 year primary patency rates (Table 3).
Table 3.
Univariate analysis of predictors associated with poor 2 year primary patency and poor 2 year survival
Data are expressed as the mean value±SD or percentage of patients. *Rutherford classification can be found in reference 11. EGFR: estimated glomerular filtration rate, SD: standard deviation
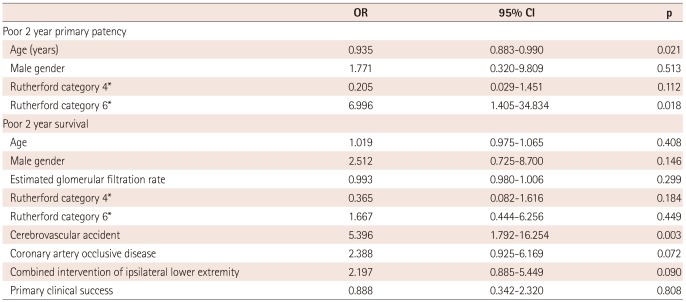
Multivariate analysis associated with poor 2 year primary patency
Multivariate analysis showed that young age {odds ratio (OR) 0.935, 95% confidence interval (CI) 0.883-0.990, p=0.021} and Rutherford category 6 at initial presentation (OR 6.996, 95% CI 1.405-34.834, p=0.018) were independent predictors associated with poor 2 year primary patency in these patients (Table 4).
Table 4.
Multivariate analysis of independent predictors associated with poor 2 year primary patency and poor 2 year survival
*Rutherford classification can be found in reference 11. CI: confidence interval, OR: odds ratio
Univariate analysis associated with poor 2 year survival
Univariate analysis showed that older age (69.6±6.9 years vs. 64.3±15.3 years, p=0.019), lower estimated glomerular filtration rate (42.2±33.4 mL/min/1.73 m2 vs. 58.1±32.2 mL/min/1.73 m2, p=0.037), less frequent Rutherford category 4 (25% vs. 50%, p=0.020), more frequent Rutherford category 6 (64% vs. 31%, p=0.002), more frequent cerebrovascular accident (25% vs. 6%, p=0.007), more frequent coronary artery disease (71% vs. 49%, p=0.037), more frequent combined intervention of ipsilateral lower extremity (68% vs. 36%, p=0.003), and worse primary clinical success (68% vs. 88%, p=0.021) were associated with poor 2 year survival (Table 3).
Multivariate analysis associated with poor 2 year survival
Multivariate analysis showed that a history of cerebrovascular accident (OR 5.396, 95% CI 1.792-16.254, p=0.003) was an independent predictor associated with poor 2 year survival in these patients (Table 4).
Discussion
The Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL) trial, a multicenter, randomized controlled trial that compared 6 month clinical outcomes after angioplasty or surgery among CLI patients, did not show a significant difference with respect to amputation-free survival, all-cause mortality and quality of life during short-term follow-up.13) However, most data about CLI included the proximal segments as femoral or femopopliteal segments, and few reports have focused on the infrapopliteal segment due to high technical failure rates and low patency rates. Recently, with technological advancements in wires and increased clinical experience, infrapopliteal angioplasty has been more frequently attempted and has shown results comparable to surgical bypass.4),5),14),15) In meta-analyses, which compared infrapopliteal angioplasty with surgical bypass, the limb salvage rates of the two strategies were similar,16) and our data showed favorable clinical outcomes after infrapopliteal angioplasty.
A relatively low technical success rate (70%) in this study may be attributed to a high proportion of suboptimal results (22%). Most of the suboptimal results involved diffuse and heavily calcified lesions, and had 30% to 50% of residual stenosis despite repeated balloon dilatation. Any supplementary intervention such as stent implantation was not performed in those lesions. Stent implantation in infrapopliteal arteries have been avoided due to the diffuse, complex lesion characteristics in the majority of CLI cases, and the risk of crushed stents or thromboses due to flow stasis.6),17) Recent advancements in coronary stenting technology now allows for the use of stent implantation in more distal crural arteries. Feiring et al.14) demonstrated that stent-supported infrapopliteal angioplasty is effective, with few adverse events for patients with CLI or lifestyle-limiting claudication. Also, Siablis et al.9) demonstrated that sirolimus-eluting stents reduced the restenosis rate and a favorable midterm clinical outcome was obtained with a significant decrease in clinically driven re-interventions compared with bare metal stents in suboptimal infrapopliteal angioplasty. It is plausible that more aggressive use of stent implantations might improve angiographic and clinical results. However, our study showed no significant improvements of clinical outcomes, including 2 year primary patency and 2 year survival, in the stent implantation group. It may be explained by the small number of cases in the stent implantation group. Therefore, long-term follow-up data from multiple centers would be needed, and the potential benefit of stent implantation should be considered over the financial cost.
Although the BASIL trial13) included patients who were good candidates for either infra-inguinal bypass surgery or balloon angioplasty, many patients with CLI are not optimal candidates for surgical bypass, and most likely undergo amputation for their critical limb ischemia. Considering that the objective of catheter-based angioplasty is also to lower the rate of amputations and to support wound healing by ameliorating distal flow,14) angioplasty can be considered a first line treatment option in CLI patients who otherwise are expected to undergo major amputation.
In our study, 2 year overall survival was 75.2%, and the main causes of death were cardiovascular events, which correspond with the significant associations between CLI and cardiovascular morbidity or mortality in previous reports.18-21) Therefore, in the management of CLI patients with poor life expectancy, the maintenance of symptom relief and limb salvage is more crucial than for their quality of life.4),22),23) Hence, infrapopliteal angioplasty is a less-invasive, safe and reproducible method, and could be a better strategy for CLI patients with comorbidities who are at high surgical risk.
Several limitations of our study need to be considered. First, this was a retrospective study from a single institution, which can create referral bias. Also, extrapolation of these results to most patients with CLI has limited validity because of the small population size. Second, we did not perform follow-up angiography or other modalities for long-term patency in asymptomatic patients. Regular follow-up based on ankle-brachial index or duplex scan is needed to obtain better patency results in future prospective studies. Third, the efficacy of proximal PTA in cases with combined proximal and distal angioplasty could not be distinguished from those of distal angioplasty alone. Fourth, we did not analyze the relationship between outcomes and prescriptions such as cilostazol after the procedure because of a deficiency of long-term follow-up data.
In conclusion, infrapopliteal angioplasty showed favorable clinical outcomes in terms of primary patency and limb salvage rate, and demonstrated its feasibility as a primary choice of treatment in patients with CLI. Although it is still a technically challenging procedure, more aggressive treatment with advanced devices and more clinical experience would allow us to achieve better results.
Acknowledgments
This study was partly supported by grants (No. A085012 and A102064) from the Korea Healthcare Technology R&D Project and a grant (No. A085136) from the Korea Health 21 R&D Project, both of which are managed by the Ministry for Health, Welfare and Family Affairs of the Republic of Korea and the Cardiovascular Research Center in Seoul, Korea.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Blair JM, Gewertz BL, Moosa H, Lu CT, Zarins CK. Percutaneous transluminal angioplasty versus surgery for limb-threatening ischemia. J Vasc Surg. 1989;9:698–703. doi: 10.1067/mva.1989.vs0090698. [DOI] [PubMed] [Google Scholar]
- 2.Treiman GS, Treiman RL, Ichikawa L, van Allan R. Should percutaneous transluminal angioplasty be recommended for treatment of infrageniculate popliteal artery or tibioperoneal trunk stenosis? J Vasc Surg. 1995;22:457–463. doi: 10.1016/s0741-5214(95)70015-3. [DOI] [PubMed] [Google Scholar]
- 3.Parsons RE, Suggs WD, Lee JJ, Sanchez LA, Lyon RT, Veith FJ. Percutaneous transluminal angioplasty for the treatment of limb threatening ischemia: do the results justify an attempt before bypass grafting? J Vasc Surg. 1998;28:1066–1071. doi: 10.1016/s0741-5214(98)70033-3. [DOI] [PubMed] [Google Scholar]
- 4.Clair DG, Dayal R, Faries PL, et al. Tibial angioplasty as an alternative strategy in patients with limb-threatening ischemia. Ann Vasc Surg. 2005;19:63–68. doi: 10.1007/s10016-004-0136-0. [DOI] [PubMed] [Google Scholar]
- 5.Söder HK, Manninen HI, Jaakkola P, et al. Prospective trial of infrapopliteal artery balloon angioplasty for critical limb ischemia: angiographic and clinical results. J Vasc Interv Radiol. 2000;11:1021–1031. doi: 10.1016/s1051-0443(07)61332-3. [DOI] [PubMed] [Google Scholar]
- 6.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31(1 Pt 2):S1–S296. [PubMed] [Google Scholar]
- 7.Schwarten DE. Clinical and anatomical considerations for nonoperative therapy in tibial disease and the results of angioplasty. Circulation. 1991;83(2 Suppl):I86–I90. [PubMed] [Google Scholar]
- 8.Hanna GP, Fujise K, Kjellgren O, et al. Infrapopliteal transcatheter interventions for limb salvage in diabetic patients: importance of aggressive interventional approach and role of transcutaneous oximetry. J Am Coll Cardiol. 1997;30:664–669. doi: 10.1016/s0735-1097(97)00216-7. [DOI] [PubMed] [Google Scholar]
- 9.Siablis D, Kraniotis P, Karnabatidis D, Kagadis GC, Katsanos K, Tsolakis J. Sirolimus-eluting versus bare stents for bailout after suboptimal infrapopliteal angioplasty for critical limb ischemia: 6-month angiographic results from a nonrandomized prospective single-center study. J Endovasc Ther. 2005;12:685–695. doi: 10.1583/05-1620MR.1. [DOI] [PubMed] [Google Scholar]
- 10.Tsetis D, Belli AM. The role of infrapopliteal angioplasty. Br J Radiol. 2004;77:1007–1015. doi: 10.1259/bjr/97382129. [DOI] [PubMed] [Google Scholar]
- 11.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 12.Olin JW. Thromboangiitis obliterans (Buerger's disease) In: Rutherford RB, editor. Rutherford's Vascular Surgery. 6th ed. Philadelphia: Elsevier Saunders; 2005. pp. 404–419. [Google Scholar]
- 13.Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 14.Feiring AJ, Wesolowski AA, Lade S. Primary stent-supported angioplasty for treatment of below-knee critical limb ischemia and severe claudication: early and one-year outcomes. J Am Coll Cardiol. 2004;44:2307–2314. doi: 10.1016/j.jacc.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 15.Krankenberg H, Sorge I, Zeller T, Tübler T. Percutaneous transluminal angioplasty of infrapopliteal arteries in patients with intermittent claudication: acute and one-year results. Catheter Cardiovasc Interv. 2005;64:12–17. doi: 10.1002/ccd.20237. [DOI] [PubMed] [Google Scholar]
- 16.Romiti M, Albers M, Brochado-Neto FC, Durazzo AE, Pereira CA, De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47:975–981. doi: 10.1016/j.jvs.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Rastogi S, Stavropoulos SW. Infrapopliteal angioplasty. Tech Vasc Interv Radiol. 2004;7:33–39. doi: 10.1053/j.tvir.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 19.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 20.Balmer H, Mahler F, Do DD, Triller J, Baumgartner I. Balloon angioplasty in chronic critical limb ischemia: factors affecting clinical and angiographic outcome. J Endovasc Ther. 2002;9:403–410. doi: 10.1177/152660280200900403. [DOI] [PubMed] [Google Scholar]
- 21.Bailey CM, Saha S, Magee TR, Galland RB. A 1 year prospective study of management and outcome of patients presenting with critical lower limb ischaemia. Eur J Vasc Endovasc Surg. 2003;25:131–134. doi: 10.1053/ejvs.2002.1817. [DOI] [PubMed] [Google Scholar]
- 22.Kudo T, Chandra FA, Ahn SS. The effectiveness of percutaneous transluminal angioplasty for the treatment of critical limb ischemia: a 10-year experience. J Vasc Surg. 2005;41:423–435. doi: 10.1016/j.jvs.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 23.Löfberg AM, Lörelius LE, Karacagil S, Westman B, Almgren B, Berqgvist D. The use of below-knee percutaneous transluminal angioplasty in arterial occlusive disease causing chronic critical limb ischemia. Cardiovasc Intervent Radiol. 1996;19:317–322. doi: 10.1007/BF02570182. [DOI] [PubMed] [Google Scholar]