Abstract
The granular cell tumor (GCT) is an uncommon, benign lesion with a preference for subcutaneous sites. In the head and neck, the tongue is the most common site, followed by the larynx. We experienced a case of a 27-year-old woman with lingual squamous cell carcinoma (SCC) surrounded by GCT. The pathological findings established that the lesion was SCC covered by GCT in the midline of the tongue. The size of the mass was very small, however, so we excised it in a diamond shape. There is an interesting association between GCTs and other malignant neoplasms. However, no causal relationship between GCT and these other carcinomas has been established. Here we report on an SCC coexisting with GCT at the same site as a median tongue lesion and review the literature.
Keywords: Tongue; Granular cell tumor; Squamous cell carcinoma; Excision, pathology
INTRODUCTION
Granular cell tumor (GCT) is a soft-tissue neoplasm of unclear origin. It is a relatively uncommon lesion, most often affecting the tongue, skin, and breast, although it may occur at any site.1 The tongue is the most common site of occurrence, accounting for nearly 30% to 60% of cases.1 GCT was first described by Abrikossoff in 1926 as granular cell myoblastoma, assuming a myogenic origin.2 However, other investigators have since proposed histiocytic, fibroblastic, myoepithelial, and neuronal origins.1 Recently, GCT has been referred to as the benign neural cell tumor.
There is an interesting association between GCTs and other malignant neoplasms. Saito et al.3 reported that esophageal GCT colocalized with squamous cell carcinoma (SCC), and Al-Ahmadie et al.4 reported that GCT of the breast colocalized with an in situ and infiltrating ductal carcinoma. Caltabiano et al.5 reported a unique, simultaneous occurrence of an SCC and a GCT of the tongue at the same site. Strong et al.6 reported that the incidence of GCT in association with malignancies is 9%.
The association of GCT and carcinoma in these cases raises the suspicion of an interrelationship that seems more than casual. However, no causal relationship between GCT and these other carcinomas has been established.
We experienced a case of lingual SCC surrounded by GCT. Here, we discuss this patient and provide a review of the relevant literature.
CASE REPORT
A 27-year-old woman was admitted to our hospital for investigation and treatment of a tongue cancer that was observed on a routine medical check-up. The patient was a nonsmoker and had a history of alcohol abuse, but physical examination revealed the tongue lesion as a shallow protrusion. The size of the mass was 1.0×0.5 cm, and it had an antero-posterior longitudinal round shape in the midportion of the tongue. The previous punch biopsy scar was shown in the midline of the tongue. A punch biopsy from the lesion showed SCC colocalized with GCT. The computed tomography (CT) suggested a tumor localized to the superficial layer of the tongue. Radiographic examination and CT revealed no evidence of nodal or visceral metastases.
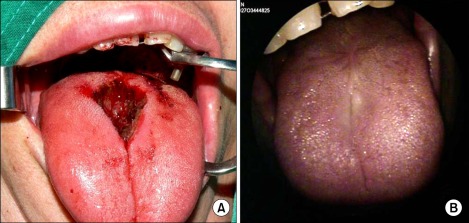
We planned a surgical excision. Because the mass was located in the midline of the tongue, it was small and localized to the superficial layer, and the patient was a young woman, we excised the mass in a diamond shape (Fig. 1A). The excision procedure was continued until the resection margin was clear. The defect was then closed by simple sutures.
FIG. 1.
(A) Intraoperative view: The mass was located in the midline of the tongue. Because the mass was so small and was localized to the superficial layer and the patient was a young woman, we excised the mass in a diamond shape. (B) Postoperative view 12 months later: The defect site was clearly closed.
PATHOLOGIC FINDINGS
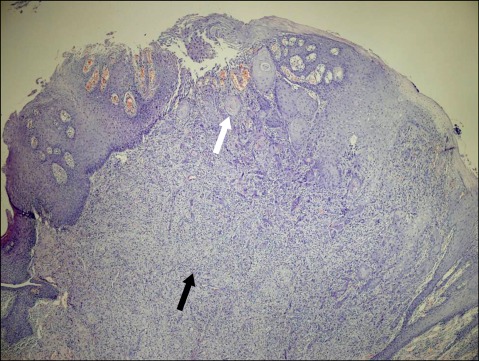
The surgical specimen consisted of a 1.5×1.0 cm section of the tongue. Microscopic examination of the specimen showed an SCC (3×1 mm) arising from the basal portion of the mucosa and infiltrating downward into the stroma (Fig. 2). The tumor cells of SCC show moderate to severe nuclear pleomorphism. The basement membrane is not seen and the border between the carcinoma and subepithelial connective tissue is indistinct owing to irregular infiltration of tumor cells into the connective tissue. The above features of nuclear pleomorphism and infiltration into the stroma are the characteristics of malignant tumors, which are not seen in pseudoepitheliomatous hyperplasia (PEH). The SCC was covered by GCT, and most of the specimen was GCT.
FIG. 2.
A squamous cell carcinoma was seen arising from the basal portion of the mucosa and infiltrating downward into the stroma (white arrow). Beneath the squamous cell carcinoma was a granular cell tumor (black arrow) (H&E ×40).
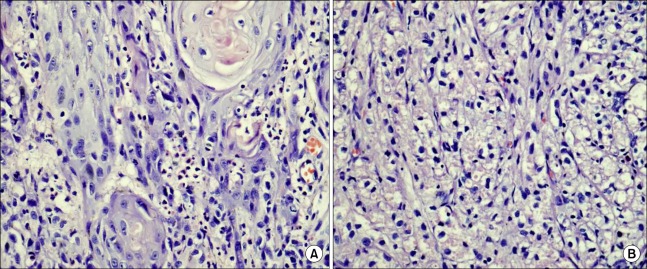
The tumor was composed of nests of oval or polygonal cells with small, round, and hyperchromatic nuclei in the center of the cells with eosinophilic granular cytoplasm. The cytoplasmic granules were positive for PAS staining with diastase resistance. Immunohistochemical analysis was done. The SCC showed positive staining for cytokeratin and Ki-67 and p53 at the invasive front but was negative for muscle markers and actin, the neural marker S-100, and the mesenchymal markers vimentin and desmin. The GCT was strongly positive for vimentin, S-100, and neuron-specific enolase, but was negative for cytokeratin and the muscle markers.
Twelve months have now passed since the surgery, and the patient has been under follow-up observation at our outpatient clinic without any recurrence (Fig. 1B). In addition, she has experienced no discomfort with tongue movement.
DISCUSSION
Abrikossoff first described the entity of GCT of the tongue in 1926 as granular cell myoblastoma, claiming a myogenic origin.7 These tumors usually occur as small, poorly defined, solitary or multiple, painless, nonulcerated, and nonencapsulated nodules. Complete excision is curative in most cases. Although the histogenesis of GCT is still disputed, owing to its close relationship with peripheral nerves and the presence of some residual axons in some GCTs, it is thought to originate from Schwann cells. GCT is characterized by infiltrating sheets and cords of polygonal bland cells with abundant eosinophilic granular cytoplasm, which stains positively for S100 protein, vimentin, and neuron-specific enolase. The granular appearance is due to the presence of numerous lysosomes. The presence of similar granular cells in degenerating nerves, as well as the positivity for S-100 protein and neuron-specific enolase, further support the neural origin of these tumors.7 These granules are then autophagocytosed (autodigested by lysosomes), resulting in the characteristic ultrastructural appearance of the granules seen in the cytoplasm of GCTs.
GCTs can develop almost anywhere in the body, including the skin, subcutaneous tissue, larynx, trachea, bladder, uterus, vulva, and central nervous system, and are most commonly found in the tongue, which accounts for nearly 30% to 60% of all GCTs.1
There is an interesting association between GCTs and other malignant neoplasms. The coexistence of GCT and a true malignant neoplasm in the same organ has been described as an association of GCT with SCC in the esophagus,3,8 on the tongue,5,7 and in the larynx,9 as well as GCT with adenocarcinoma in the bronchi,8 the stomach,10 and the mammary glands.11 Two of these cancers arose from the epithelium overlying the GCT.8 Extensive review of the literature revealed only two cases of synchronous benign GCT of the tongue coexisting with SCC at different sites.7 To our knowledge, the case we describe here is the first in which SCC coexisted with GCT at the same site as a median tongue lesion. Some investigators speculate that the pathogenesis of the overlying type (as in our case) may involve chronic irritation of the mucosa, leading to carcinoma development.7
GCTs have been reported to induce PEH of the associated mucosal epithelium that closely mimics the invasion patterns of SCC. GCT could induce growth factors (such as transforming growth factor-α) leading to PEH and, in rare circumstances, to SCC. Caltabiano et al.5 reported that an immunohistochemical analysis with p63 was useful to distinguish PEH from invasive SCC.
Our patient had a history of alcohol abuse, similar to other reported cases of both esophageal GCT alone and in a case associated with cancer.2,5,8 The association between alcohol use and GCT seems high and may indicate a relationship based on the evidence of ethanol's influence on Schwann cell proliferation and myelin formation in vitro. Moreover, alcohol is also implicated in the pathogenesis of SCC. However, there is still an unexplained association between GCT and other malignant cancers.5,8 The proximity of the two tumors raises the suspicion of an interrelationship that seems more than casual. However, no causal relationship between GCT and these other carcinomas has been established.
The optimal treatment for GCTs remains controversial. However, the current treatment options are as follows: a conservative approach with regular follow-up for tumors <10 mm in diameter without evidence of malignant change8 and surgical excision for tumors >20 mm in diameter, benign GCTs causing symptoms, or cases in which malignancy is suspected.5,8 If no malignant changes are detected in the removed specimen, additional treatment or follow-up is not considered necessary.5,8
We decided to treat our patient with midline partial glossectomy for the SCC that occupied a very small portion of the tongue with GCT. The causal relationship and appropriate management between GCT and the other carcinomas will require more research and experience.
FIG. 3.
(A) The squamous cell carcinoma is composed of tumor cells having pleomorphic nuclei and irregular infiltrates into the subepithelial connective tissue (H&E ×400). (B) The granular cell tumor is composed of round tumor cells having distinct cell borders and containing eosinophilic granules in the cytoplasm (H&E ×400).
References
- 1.Ordóñez NG, Mackay B. Granular cell tumor: a review of the pathology and histogenesis. Ultrastruct Pathol. 1999;23:207–222. doi: 10.1080/019131299281545. [DOI] [PubMed] [Google Scholar]
- 2.Abrikosov AA. Über Myome ausgehend von der quergestreiften willkürlichen Muskulatur. Virchows Arch. 1926;260:215–233. [Google Scholar]
- 3.Saito K, Kato H, Fukai Y, Kimura H, Miyazaki T, Kashiwabara K, et al. Esophageal granular cell tumor covered by intramucosal squamous cell carcinoma: report of a case. Surg Today. 2008;38:651–655. doi: 10.1007/s00595-007-3694-2. [DOI] [PubMed] [Google Scholar]
- 4.Al-Ahmadie H, Hasselgren PO, Yassin R, Mutema G. Colocalized granular cell tumor and infiltrating ductal carcinoma of the breast. Arch Pathol Lab Med. 2002;126:731–733. doi: 10.5858/2002-126-0731-CGCTAI. [DOI] [PubMed] [Google Scholar]
- 5.Caltabiano R, Cappellani A, Di Vita M, Lanzafame S. The unique simultaneous occurrence of a squamous cell carcinoma and a granular cell tumor of the tongue at the same site: a histological and immunohistochemical study. J Craniofac Surg. 2008;19:1691–1694. doi: 10.1097/SCS.0b013e31818973ad. [DOI] [PubMed] [Google Scholar]
- 6.Strong EW, McDivitt RW, Brasfield RD. Granular cell myoblastoma. Cancer. 1970;25:415–422. doi: 10.1002/1097-0142(197002)25:2<415::aid-cncr2820250221>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Simsir A, Osborne BM, Greenebaum E. Malignant granular cell tumor: a case report and review of the recent literature. Hum Pathol. 1996;27:853–858. doi: 10.1016/s0046-8177(96)90462-1. [DOI] [PubMed] [Google Scholar]
- 8.Vinco A, Vettoretto N, Cervi E, Villanacci V, Baronchelli C, Giulini SM, et al. Association of multiple granular cell tumors and squamous carcinoma of the esophagus: case report and review of the literature. Dis Esophagus. 2001;14:262–264. doi: 10.1046/j.1442-2050.2001.00198.x. [DOI] [PubMed] [Google Scholar]
- 9.Koźmińska J, Wilczyński K. A rare case of coexistence of plano-epithelial carcinoma and Abrikosow's tumor of the larynx. Otolaryngol Pol. 1995;49:244–245. [PubMed] [Google Scholar]
- 10.Xuan ZX, Yao T, Ueyama T, Tsuneyoshi M. Coincident occurrence of granular cell tumor of the stomach with an early gastric carcinoma. Fukuoka Igaku Zasshi. 1992;83:21–26. [PubMed] [Google Scholar]
- 11.Tai G, D'Costa H, Lee D, Watkins RM, Jones P. Case report: coincident granular cell tumour of the breast with invasive ductal carcinoma. Br J Radiol. 1995;68:1034–1036. doi: 10.1259/0007-1285-68-813-1034. [DOI] [PubMed] [Google Scholar]