Abstract
The safety of human exposure to an ever-increasing number and diversity of electromagnetic field (EMF) sources both at work and at home has become a public health issue. To date, many in vivo and in vitro studies have revealed that EMF exposure can alter cellular homeostasis, endocrine function, reproductive function, and fetal development in animal systems. Reproductive parameters reported to be altered by EMF exposure include male germ cell death, the estrous cycle, reproductive endocrine hormones, reproductive organ weights, sperm motility, early embryonic development, and pregnancy success. At the cellular level, an increase in free radicals and [Ca2+]i may mediate the effect of EMFs and lead to cell growth inhibition, protein misfolding, and DNA breaks. The effect of EMF exposure on reproductive function differs according to frequency and wave, strength (energy), and duration of exposure. In the present review, the effects of EMFs on reproductive function are summarized according to the types of EMF, wave type, strength, and duration of exposure at cellular and organism levels.
Keywords: Electromagnetic field, Reproduction
Introduction
Humans in modern society are exposed to an ever-increasing number of electromagnetic fields (EMFs) generated from the production and supply of electricity, television (TV) sets, personal computer (PC), radio communication, and mobile communication. Since the 1960s, when the biological hazard of EMF exposure was studied in the Soviet Union, the safety of humans exposed to EMFs both at home and during occupational activities has become an important issue in public health. The biological effect of EMFs is currently under debate and still a controversial issue. In the present review, the effects of EMFs on reproductive function are summarized according to the types of EMFs and duration of exposure at the cellular and organism levels.
Types of EMF and frequency of exposure
Humans in modern society are exposed to various kinds of EMFs. Extremely low frequency (ELF)-EMFs have 3 to 30 Hz frequencies and are generated from military communication. The EMFs to which humans are most frequently exposed are the 50 to 60 Hz super low frequency (SLF) EMFs generated from power cables for industrial and household electrical supplies and electronic goods. Very low frequency (VLF) EMFs with 3 to 30 kHz frequency are generated from PC monitors or TV sets. EMFs from TVs or PCs have a 6.25 µT intensity with a 20 kHz frequency [1]. The radio frequency (RF) EMFs generated from mobile phones, cordless phones, and broadcasting towers have frequencies of hundreds of MHz. All these EMFs are non-ionizing radiation, which do not have energy to release electrons from orbit. EMFs have a wave character in short frequency and act as a magnetic field in long frequency. The strength of the electric field and magnetic field is measured in units of kV/m and µT, respectively. Household electronic goods can produce a 4 µT EMF and EMF ranges from 0.01 to 1 µT inside and outside of house [2]. The strength of SLF-EMFs is dependent on the electrical current and distance from the conductor. Therefore, SLF-EMFs are the highest near the power cable and decrease rapidly by distance.
Cellular mechanism for EMF induced toxicity
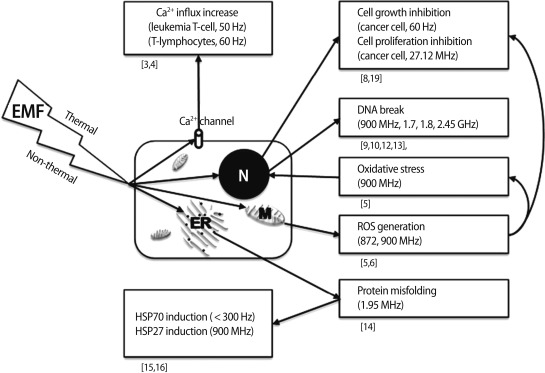
At the cellular level, an increase in free radicals and [Ca2+]i may mediate the effect of EMFs and lead to cell growth inhibition, protein misfolding, and DNA breaks. EMFs can disrupt Ca2+-dependent cell signaling. How does EL-EMF exposure affect signal transduction in cells? In human leukemia T-cell line Jurkat cells, a 50 Hz, 0.5 mT EMF was found to increase Ca2+ levels, blocking the effect of cholera toxin and the protein tyrosine kinase inhibitor genistein [3]. In thymic lymphocytes, Ca2+ influx increased during mitogen-activated signal transduction when exposed to a 60 Hz, 22 mT EMF [4], suggesting the modulating role of EMF on regulation of Ca2+ channels. RF EMFs of 900 and 872 MHz may enhance chemically induced reactive oxygen species (ROS) production, resulting in secondary DNA damage in human SH-SY5Y neuroblastoma cells [5,6]. Also in vivo experiments revealed the increased oxidative stress caused by a 900 MHz EMF, leading to endometrial histopathologic impairment in rats [7]. In prostate cancer cells, ROS induced by a 60 Hz sinusoidal EMF inhibited cell growth by apoptosis and arrested the cell cycle [8]. An RF EMF of 2,450 MHz exposure caused rearrangement of DNA segments and breakage of DNA in testes [9]. In another report, 1,800 MHz of EMF induced DNA breaks in human fibroblasts and rat granulosa cells in comet assay [10]. Similarly, an RF-EMF of 1,800 MHz induced DNA damage in Chinese hamster lung cells [11]. In addition, an RF EMF of 900 MHz and 1.7 GHz induced DNA breakage in cauda epididymal spermatozoa and embryonic stem cells in mice [12,13]. Some investigators have reported changes in protein folding by EMF. Changes in the structural fluctuation of tuna myoglobin protein was induced by EMF at the mobile phone frequency of 1.95 MHz, indicating RF EMFs as a potential risk for protein misfolding [14]. Heat-shock proteins (HSPs) were also increased by EMF exposure. In human endothelial cell line EA.hy926, HSP27 was activated by 900 MHz GSM non-thermal exposure [15]. HSP70 is induced by exposure to SLF (<300 Hz) EMFs [16]. Interestingly, HSP70 has non-thermal (EMF domain) and thermal (temperature domain) stress response promoter binding sites [17,18], suggesting that HSP70 is highly sensitive for EMF. In hepatocellular carcinoma, cell proliferation was inhibited with mitotic spindle disruption by a 27.12 MHz of RF EMF [19]. EMFs have been suggested as a cancer treatment tool with gamma irradiation. When a human breast cancer xenograft was treated with EMF and gamma irradiation at the same time, inhibitory effects on growth, angiogenesis, and metastasis were higher than in xenografts treated with gamma irradiation alone [20] (Figure 1).
Figure 1.
Summary of the effects of electromagnetic fields at the cellular level. EMF, electromagnetic field; N, nucleus; ER, endoplasmic reticulum; M, mitochondria.
Human diseases related to EMF exposure
Alterations in biomarkers following EMF exposure have been reported through in vitro and in vivo experiments using animal cells and animals, respectively. Most of what is known on the correlation between human health and EMF exposure has been drawn from epidemiological studies. The results on the hazards of EMF exposure are contradictory, leaving the conclusion unclear to date [21-23]. Currently, the biological hazard of EMF exposure is understood to be different according to frequency, type of wave, and strength (energy) of the EMF. Importantly, some people are very sensitive to certain types of EMFs. The effect of EMF exposure could be different at various toxicological endpoint levels according to the route and duration of EMF exposure and target human subjects. Therefore, the possible body or cellular functions susceptible for EMF exposure should be suggested according to the type of frequency, wave, and strength of EMF, and the safety guidelines for EMF exposure should be made according to this criteria.
Possible human diseases related with EMF exposure obtained from epidemiological studies include the life threatening diseases such as leukemia in children and adults [24,25], brain cancer in adults [26], Lou Gehrig's disease [27], depression [28], suicide [29], and Alzheimer's' disease [30]. Recently, EMFs were reported to cause DNA damage and neurological diseases at much lower levels than those proscribed by international safety guidelines. Most recently, the Bioinitiative Report (http://www.bioinitiative.org/) has noted that the current safety guidelines for EMF exposure are not sufficient and should be revised based on data from various toxicological tests [31].
Changes in the reproductive endocrine system by EMF exposure
There are many studies on the casual relationship between SLF-EMF exposure and pineal gland function [32]. Although still under debate, EMF exposure can affect the secreting activity of the pineal gland in several animal species as does the light. Indeed, static exposure to SLF-EMFs can affect cyclic secretions of melatonin in several species [33]. In Long-Evans rats exposed to a circularly polarized 50 Hz EMF for 6 weeks, the pineal and circulating melatonin levels decreased [34]. In cows, a 60 Hz EMF exposure for 4 weeks (16 hours per day) altered circulating melatonin and prolactin levels and the estrous cycle [35,36]. In an adult Djungarian hamster, a 60 Hz EMF exposure acutely affected the pineal and circulating melatonin levels [37]. SLF-EMF exposure directly affects the pineal gland, deteriorating the biological effect of melatonin [38]. Melatonin regulates the pulse of LHRH in the hypothalamus, influencing gonadotropin FSH and LH. Eventually, this can alter the production of gonadal sex steroids, resulting in changes in the reproductive cycle [39,40]. In cows, exposure to 60 Hz SLF-EMF at 30 µT for 24-27 days did not alter the progesterone levels but shortened the estrous stage [41]. RF-EMF exposure can affect ACTH, GH, TSH, FSH, and LH in the pituitary [42]. Most EMF-induced hormonal changes are mediated by the thermal effect of EMFs. In contrast, long-term exposure to RF-EMFs did not have a cumulative effect on the endocrine, serological, or immunological parameters [43].
Reproductive toxicity of EMF in females
Reproductive toxicity of EMF exposure has been studied in both sexes at various endpoints [44]. Undoubtedly, reproduction is under control of the nervous system and endocrine system. In female mice, neuroendocrinological alteration was believed to be a prime cause of loss of fertility with aging [45,46]. In female mice exposed to a 20 kHz saw tooth EMF generated from a TV set or PC for 6 weeks after weaning, the estrous cycle was extended [47]. In cows, similarly, a 60 Hz, 30 µT EMF 16 hours per day extended the estrous cycle [35]. Because extension of the estrous cycle can decrease total ovulation opportunities in females during their fertile period of life, decrease in fecundity can be expected. In mouse follicle cultures, exposure to a 33 Hz SLF-EMF at 5-day intervals resulted in defects in follicle growth. In contrast, the follicles exposed to a 50 Hz EMF continued growth. 33 Hz or 50 Hz SLF-EMF exposure for 3 days inhibited the antrum formation of follicles cultured in vitro [48]. Together, the estrous cycle regulated by ovarian steroids might be much more sensitive to EMF exposure than the fetal development and feto-maternal interaction. In contrast, in female rats a 10 kHz, 0.2 mT sine wave EMF does not affect the estrous cycle [1], suggesting that the effect of EMFs on the estrous cycle differs according to the frequency, energy, and animal species. In adult Wistar female rats exposed continuously to a 50 Hz SLF-EMF for 3 months, the weights of the uterus and ovaries, progesterone levels, and estrogen levels in relation to the varying periods of the estrous cycle were not significantly altered [49].
When ovariectomized female Sprague-Dawley rats were exposed to a 1,439 MHz time division multiple access (TDMA) EMF for 4 hours per day for 3 days, there were no differences in uterine wet mass or serum estradiol level, suggesting no estrogenic activity related to high frequency EMFs used in cellular phones [50]. When female rats were exposed to a 900 MHz EMF for 30 min/day for 30 days, endometrial apoptosis and oxidative stress were increased [51]. Also, as an in vivo experiment, increased oxidative stress by a 900 MHz EMF led to the endometrial histopathologic impairment in rats [7].
Reproductive toxicity of EMFs in males
A code division multiple access (CDMA) EMF of 848.5 MHz has no significant effect on the body, testicular, or epididymal weights, or the sperm count or apoptosis of germ cells in adult Sprague-Dawley rats [52]. Similarly, in adult male Sprague-Dawley rats exposed 30 minutes per day, 5 days a week for 4 weeks to a 900 MHz EMF, the testes weight, the testicular biopsy score count, and the percentage of interstitial tissue out of the entire testicular tissue were not significantly changed. However, the diameter of the seminiferous tubules, the mean height of the germinal epithelium, and serum total testosterone levels were significantly decreased in the EMF group. Plasma LH and FSH levels had not significantly changed following EMF exposure [53]. In prepubertal male rats (age of 5 weeks) exposed to a 1.95-GHz wide-band CDMA signal for 5 hours per day, 7 days a week for 5 weeks, neither the body weight gain or weights of the testis, epididymis, seminal vesicles and prostate, nor the number of sperm in the testis and epididymis had changed. Of note, the testicular sperm count was significantly increased without abnormalities of sperm motility or morphology, suggesting a positive effect of W-CDMA on spermatogenesis [54]. However, at a higher frequency, specifically 2.45 GHz, an EMF induced a decrease in the Leydig cell number and increase of apoptosis-positive cells in the seminiferous tubules of Wistar rats [55]. Exposure to an RF EMF of 2,450 MHz resulted in rearrangement of DNA segments and breakage of DNA in the testis [9]. An RF EMF of 900 MHz and 1.7 GHz induced DNA breakage in mouse cauda epididymal spermatozoa and embryonic stem cells [12,13]. Taken together, the RF-EMF generated by a mobile phone might be harmful to male reproductive function.
In male mice, a 60 Hz EMF did not decrease the body or testes weights but significantly increased germ cell death and abnormality in the seminiferous tubules. A 60 Hz EMF at 0.5 mT increased the TUNEL-positive spermatogonia, indicating the potentiation of DNA fragmentation, but in flow cytometry, the cell survival was not significantly altered [56]. Similarly, 60 Hz EMFs of 14 and 200 µT also induced the apoptosis of spermatogenic cells in mice [57]. In human sperm, the acrosome reaction was not altered by a 900 MHz RF-EMF from mobile phone at 2.0 W/kg strength for 1 hour though the sperm morphology was changed together with a decrease in sperm-hemizona binding, indicating that the RF-EMF exposure can decrease sperm fertility [58]. In contrast, acute exposure to a sinusoidal SLF-EMF (50 Hz, 1 mT) did not affect boar sperm viability and morphology during the first hour of incubation. SLF-EMF treated spermatozoa showed resting intracellular Ca2+ levels significantly lower than those recorded for controls. As a consequence, MF-SLF exposed sperm displayed a reduced motility, a modest reactivity when co-incubated with solubilized zonae pellucidae, and a reduction in oocyte penetrating ability. After 2 or 4 hours of incubation, signs of morphological damage appeared on the plasma membrane and at the acrosome, and decreased the fertilization rate. A 1 mT EMF decreased sperm function in vivo [59,60]. In rabbits, a 50 Hz SLF-EMF was found to be able to alter sperm motility and decrease their viability [61]. Together, SLF-EMFs negatively influence spermatozoa first by impairing cell Ca2+ homeostasis then by affecting sperm morphology and function. In contrast, the positive effect of SLF-EMFs on sperm was also reported. In human sperm, a 50 Hz, 5 mT square wave SLF-EMF increased sperm motility within 3 hours, and this effect was sustained for 21 hours. In contrast, a 50 Hz, 5 mT sine wave EMF and 50 Hz, 2.5 mT square wave SLF-EMF did not affect sperm motility. Therefore, the positive effect of a SLF-EMF on sperm motility was dependent on the type and strength of the EMF [62].
The toxicity of EMF exposure to the next generation has also been reported. Male rat offspring exposed to 60 Hz, 1 mT SLF-EMF from gestation day 13 through postnatal day 21 showed a decrease in the number, diameter, area, and volume of seminiferous tubules, the height of seminiferous epithelium, and the number of Leydig cells, but connective tissues, vasculature, plasma testosterone levels, Sertoli cells, the length of seminiferous tubules, and gonadosomatic index remained unchanged. This suggests that gestational and lactational exposure to SLF-EMFs has adverse effects on the testis development [63]. In contrast, when pregnant rats were exposed to a 60 Hz, 500 µT SLF-EMF for 21 hours per day from gestation day 6 to postnatal day 21, spermatogenesis and the fertility of male offspring were not significantly different from the control [64].
Developmental toxicity of EMFs
The developmental toxicity of EMF exposure has been studied at various endpoints [44]. The effect of EMF exposure on implantation and fetal development was reported. When female mice were superovulated and mated following exposure to a 50-Hz EMF at 0.5 mT 4 hours per day, 6 days a week for 2 weeks, the number of blastocysts was significantly decreased together with an increase in DNA fragmentation. This suggests that EMF exposure in the preimplantation stage could have detrimental effects on embryo development [65]. In swine, cleavage of fertilized eggs in the oviduct was delayed under 50 Hz, 0.75 mT EMF exposure 4 hours before ovulation, suggesting that SLF-EMFs negatively affect early embryo development [60]. When pregnant mice were exposed to a 50 Hz, 20 mT sine wave EMF during gestation day 0 to 17, embryonic survival, sex ratio, and embryonic malformation were not significantly changed, but the height and body weight of offspring were significantly increased [66]. When males and females were exposed to a 50 Hz, sine wave EMF at 5.0 mT for 9 weeks and 2 weeks before copulation, respectively, the fertility of both gametes and fetal development were not affected [67]. In mice, when the dams were exposed to a 20 kHz saw tooth EMF at 6.5 µT for 8 hours per day during pregnancy, fetal abnormality was not increased [68]. The potential hazard of EMF exposure to the dam and fetus was reviewed in [1]. There was a positive relationship between occupational monitor labor during pregnancy and the natural abortion rate [69-71]. Epidemiological studies on birth defects and abortions in pregnant women working in offices revealed that the EMF generated from a computer monitor can negatively affect human reproduction [69,72]. However, none of these kinds of reports have been verified by experiments [73]. Importantly, the reproductive hazard of EMF exposure in non-pregnant young women has not been studied well. Together, the effect of SLF-EMF exposure on embryonic development is still controversial, but some negative effects of EMFs have been reported in some animal models. Thus, it would be better to avoid or minimize casual exposure to EMFs during pregnancy.
Conclusions and perspectives
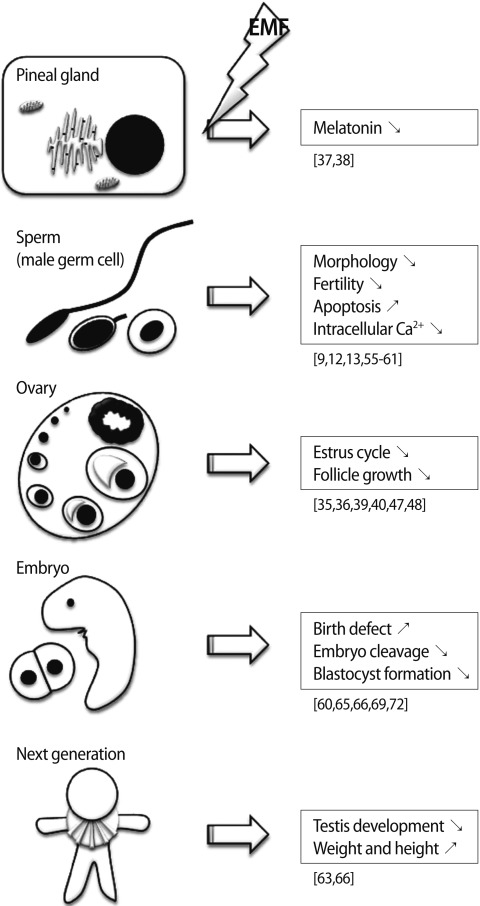
Through in vitro and in vivo studies, EMF exposure was found to alter the reproductive endocrine hormones, gonadal function, embryonic development, pregnancy, and fetal development (Table 1, Figure 2). These effects were different according to the frequency, duration of exposure, and strength of EMFs. Humans in modern society cannot avoid various kinds of EMFs during household and occupational activities, but should be aware of the biological hazard of EMFs. The effort to avoid EMF exposure and techniques to protect or relieve EMF radiation are required to preserve our reproductive potential.
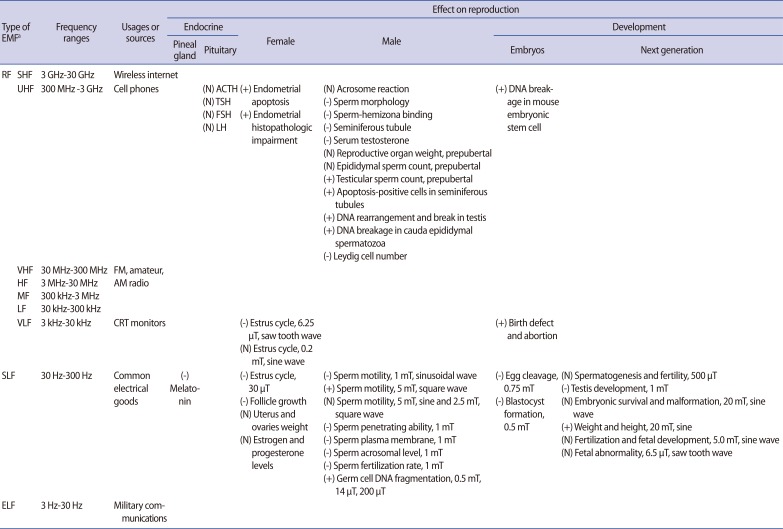
Table 1.
Effects of EMF on mammalian endocrine system and reproduction
EMF, electromagnetic field; +, increase; -, decrease; N, no change; RF, radio frequency; SHF, super high frequency; UHF, ultra high frequency; VHF, very high frequency; HF, high frequency; MF, medium frequency; LF, low frequency; VLF, very low frequency; SLF, super low frequency; ELF, extremely low frequency; CRT, cathode ray tube.
aRadio spectrums were divided into nine group according to Tanenbaum (2002), Beasley and Miller (2008).
Figure 2.
Summary of the effects of electromagnetic fields (EMFs) on reproduction. ↗, increase; ↘, decrease or inhibition.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Dawson BV, Robertson IG, Wilson WR, Zwi LJ, Boys JT, Green AW. Evaluation of potential health effects of 10 kHz magnetic fields: a rodent reproductive study. Bioelectromagnetics. 1998;19:162–171. doi: 10.1002/(sici)1521-186x(1998)19:3<162::aid-bem4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Hydro-Québec. Electricity in the air. Montreal: Hydro-Québec; 1989. [Google Scholar]
- 3.Lindström E, Mild KH, Lundgren E. Analysis of the T cell activation signaling pathway during ELF magnetic field exposure, p56lck and [Ca2+]i-measurements. Bioelectrochem Bioenerget. 1998;46:129–137. [Google Scholar]
- 4.Liburdy RP. Calcium signaling in lymphocytes and ELF fields. Evidence for an electric field metric and a site of interaction involving the calcium ion channel. FEBS Lett. 1992;301:53–59. doi: 10.1016/0014-5793(92)80209-y. [DOI] [PubMed] [Google Scholar]
- 5.Zeni O, Di Pietro R, d'Ambrosio G, Massa R, Capri M, Naarala J, et al. Formation of reactive oxygen species in L929 cells after exposure to 900 MHz RF radiation with and without co-exposure to 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone. Radiat Res. 2007;167:306–311. doi: 10.1667/RR0595.1. [DOI] [PubMed] [Google Scholar]
- 6.Luukkonen J, Hakulinen P, Maki-Paakkanen J, Juutilainen J, Naarala J. Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872 MHz radiofrequency radiation. Mutat Res. 2009;662:54–58. doi: 10.1016/j.mrfmmm.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Guney M, Ozguner F, Oral B, Karahan N, Mungan T. 900 MHz radiofrequency-induced histopathologic changes and oxidative stress in rat endometrium: protection by vitamins E and C. Toxicol Ind Health. 2007;23:411–420. doi: 10.1177/0748233707080906. [DOI] [PubMed] [Google Scholar]
- 8.Koh EK, Ryu BK, Jeong DY, Bang IS, Nam MH, Chae KS. A 60-Hz sinusoidal magnetic field induces apoptosis of prostate cancer cells through reactive oxygen species. Int J Radiat Biol. 2008;84:945–955. doi: 10.1080/09553000802460206. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar S, Ali S, Behari J. Effect of low power microwave on the mouse genome: a direct DNA analysis. Mutat Res. 1994;320:141–147. doi: 10.1016/0165-1218(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 10.Diem E, Schwarz C, Adlkofer F, Jahn O, Rüdiger H. Non-thermal DNA breakage by mobile-phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutat Res. 2005;583:178–183. doi: 10.1016/j.mrgentox.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Zhang DY, Xu ZP, Chiang H, Lu DQ, Zeng QL. Effects of GSM 1800 MHz radiofrequency electromagnetic fields on DNA damage in Chinese hamster lung cells. Zhonghua Yu Fang Yi Xue Za Zhi. 2006;40:149–152. [PubMed] [Google Scholar]
- 12.Aitken RJ, Bennetts LE, Sawyer D, Wiklendt AM, King BV. Impact of radio frequency electromagnetic radiation on DNA integrity in the male germline. Int J Androl. 2005;28:171–179. doi: 10.1111/j.1365-2605.2005.00531.x. [DOI] [PubMed] [Google Scholar]
- 13.Nikolova T, Czyz J, Rolletschek A, Blyszczuk P, Fuchs J, Jovtchev G, et al. Electromagnetic fields affect transcript levels of apoptosis-related genes in embryonic stem cell-derived neural progenitor cells. FASEB J. 2005;19:1686–1688. doi: 10.1096/fj.04-3549fje. [DOI] [PubMed] [Google Scholar]
- 14.Mancinelli F, Caraglia M, Abbruzzese A, d'Ambrosio G, Massa R, Bismuto E. Non-thermal effects of electromagnetic fields at mobile phone frequency on the refolding of an intracellular protein: myoglobin. J Cell Biochem. 2004;93:188–196. doi: 10.1002/jcb.20164. [DOI] [PubMed] [Google Scholar]
- 15.Leszczynski D, Joenväärä S, Reivinen J, Kuokka R. Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells: molecular mechanism for cancer- and blood-brain barrier-related effects. Differentiation. 2002;70:120–129. doi: 10.1046/j.1432-0436.2002.700207.x. [DOI] [PubMed] [Google Scholar]
- 16.Goodman R, Blank M. Insights into electromagnetic interaction mechanisms. J Cell Physiol. 2002;192:16–22. doi: 10.1002/jcp.10098. [DOI] [PubMed] [Google Scholar]
- 17.Lin H, Blank M, Goodman R. A magnetic field-responsive domain in the human HSP70 promoter. J Cell Biochem. 1999;75:170–176. doi: 10.1002/(sici)1097-4644(19991001)75:1<170::aid-jcb17>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Lin H, Blank M, Rossol-Haseroth K, Goodman R. Regulating genes with electromagnetic response elements. J Cell Biochem. 2001;81:143–148. doi: 10.1002/1097-4644(20010401)81:1<143::aid-jcb1030>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman JW, Pennison MJ, Brezovich I, Yi N, Yang CT, Ramaker R, et al. Cancer cell proliferation is inhibited by specific modulation frequencies. Br J Cancer. 2012;106:307–313. doi: 10.1038/bjc.2011.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron IL, Sun LZ, Short N, Hardman WE, Williams CD. Therapeutic Electromagnetic Field (TEMF) and gamma irradiation on human breast cancer xenograft growth, angiogenesis and metastasis. Cancer Cell Int. 2005;5:23. doi: 10.1186/1475-2867-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brainard GC, Kavet R, Kheifets LI. The relationship between electromagnetic field and light exposures to melatonin and breast cancer risk: a review of the relevant literature. J Pineal Res. 1999;26:65–100. doi: 10.1111/j.1600-079x.1999.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 22.Preece AW, Hand JW, Clarke RN, Stewart A. Power frequency electromagnetic fields and health. Where's the evidence? Phys Med Biol. 2000;45:R139–R154. doi: 10.1088/0031-9155/45/9/201. [DOI] [PubMed] [Google Scholar]
- 23.BioInitiative report: a rationale for a biologically-based public exposure standard for electromagnetic fields (ELF and RF) [Internet] BioInitiative. 2010. [cited 2012 Feb 26]. Available from: http://www.bioinitiative.org.
- 24.London SJ, Thomas DC, Bowman JD, Sobel E, Cheng TC, Peters JM. Exposure to residential electric and magnetic fields and risk of childhood leukemia. Am J Epidemiol. 1991;134:923–937. doi: 10.1093/oxfordjournals.aje.a116176. [DOI] [PubMed] [Google Scholar]
- 25.Bastuji-Garin S, Richardson S, Zittoun R. Acute leukaemia in workers exposed to electromagnetic fields. Eur J Cancer. 1990;26:1119–1120. doi: 10.1016/0277-5379(90)90266-v. [DOI] [PubMed] [Google Scholar]
- 26.Harrington JM, McBride DI, Sorahan T, Paddle GM, van Tongeren M. Occupational exposure to magnetic fields in relation to mortality from brain cancer among electricity generation and transmission workers. Occup Environ Med. 1997;54:7–13. doi: 10.1136/oem.54.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansen C, Olsen JH. Mortality from amyotrophic lateral sclerosis, other chronic disorders, and electric shocks among utility workers. Am J Epidemiol. 1998;148:362–368. doi: 10.1093/oxfordjournals.aje.a009654. [DOI] [PubMed] [Google Scholar]
- 28.Verkasalo PK, Kaprio J, Varjonen J, Romanov K, Heikkilä K, Koskenvuo M. Magnetic fields of transmission lines and depression. Am J Epidemiol. 1997;146:1037–1045. doi: 10.1093/oxfordjournals.aje.a009232. [DOI] [PubMed] [Google Scholar]
- 29.Reichmanis M, Perry FS, Marino AA, Becker RO. Relation between suicide and the electromagnetic field of overhead power lines. Physiol Chem Phys. 1979;11:395–403. [PubMed] [Google Scholar]
- 30.Sobel E, Dunn M, Davanipour Z, Qian Z, Chui HC. Elevated risk of Alzheimer's disease among workers with likely electromagnetic field exposure. Neurology. 1996;47:1477–1481. doi: 10.1212/wnl.47.6.1477. [DOI] [PubMed] [Google Scholar]
- 31.Johansson O. Disturbance of the immune system by electromagnetic fields-A potentially underlying cause for cellular damage and tissue repair reduction which could lead to disease and impairment. Pathophysiology. 2009;16:157–177. doi: 10.1016/j.pathophys.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Stevens RG, Davis S, Thomas DB, Anderson LE, Wilson BW. Electric power, pineal function, and the risk of breast cancer. FASEB J. 1992;6:853–860. doi: 10.1096/fasebj.6.3.1740235. [DOI] [PubMed] [Google Scholar]
- 33.Reiter RJ. Static and extremely low frequency electromagnetic field exposure: reported effects on the circadian production of melatonin. J Cell Biochem. 1993;51:394–403. doi: 10.1002/jcb.2400510403. [DOI] [PubMed] [Google Scholar]
- 34.Kato M, Honma K, Shigemitsu T, Shiga Y. Circularly polarized 50-Hz magnetic field exposure reduces pineal gland and blood melatonin concentrations of Long-Evans rats. Neurosci Lett. 1994;166:59–62. doi: 10.1016/0304-3940(94)90840-0. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez M, Petitclerc D, Burchard JF, Nguyen DH, Block E, Downey BR. Responses of the estrous cycle in dairy cows exposed to electric and magnetic fields (60 Hz) during 8-h photoperiods. Anim Reprod Sci. 2003;77:11–20. doi: 10.1016/s0378-4320(02)00273-7. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez M, Petitclerc D, Burchard JF, Nguyen DH, Block E. Blood melatonin and prolactin concentrations in dairy cows exposed to 60 Hz electric and magnetic fields during 8 h photoperiods. Bioelectromagnetics. 2004;25:508–515. doi: 10.1002/bem.20024. [DOI] [PubMed] [Google Scholar]
- 37.Yellon SM. Acute 60 Hz magnetic field exposure effects on the melatonin rhythm in the pineal gland and circulation of the adult Djungarian hamster. J Pineal Res. 1994;16:136–144. doi: 10.1111/j.1600-079x.1994.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 38.Stevens RG, Davis S. The melatonin hypothesis: electric power and breast cancer. Environ Health Perspect. 1996;104(Suppl 1):135–140. doi: 10.1289/ehp.96104s1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lincoln GA, Maeda KI. Reproductive effects of placing micro-implants of melatonin in the mediobasal hypothalamus and preoptic area in rams. J Endocrinol. 1992;132:201–215. doi: 10.1677/joe.0.1320201. [DOI] [PubMed] [Google Scholar]
- 40.Malpaux B, Daveau A, Maurice F, Gayrard V, Thiery JC. Short-day effects of melatonin on luteinizing hormone secretion in the ewe: evidence for central sites of action in the mediobasal hypothalamus. Biol Reprod. 1993;48:752–760. doi: 10.1095/biolreprod48.4.752. [DOI] [PubMed] [Google Scholar]
- 41.Burchard JF, Nguyen DH, Block E. Progesterone concentrations during estrous cycle of dairy cows exposed to electric and magnetic fields. Bioelectromagnetics. 1998;19:438–443. [PubMed] [Google Scholar]
- 42.Bortkiewicz A. A study on the biological effects of exposure mobile-phone frequency EMF. Med Pr. 2001;52:101–106. [PubMed] [Google Scholar]
- 43.Black DR, Heynick LN. Radiofrequency (RF) effects on blood cells, cardiac, endocrine, and immunological functions. Bioelectromagnetics. 2003;(Suppl 6):S187–S195. doi: 10.1002/bem.10166. [DOI] [PubMed] [Google Scholar]
- 44.Pourlis AF. Reproductive and developmental effects of EMF in vertebrate animal models. Pathophysiology. 2009;16:179–189. doi: 10.1016/j.pathophys.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging. 1995;16:837–843. doi: 10.1016/0197-4580(95)00072-m. [DOI] [PubMed] [Google Scholar]
- 46.Tappa B, Amao H, Ogasa A, Takahashi KW. Changes in the estrous cycle and number of ovulated and fertilized ova in aging female IVCS mice. Jikken Dobutsu. 1989;38:115–119. doi: 10.1538/expanim1978.38.2_115. [DOI] [PubMed] [Google Scholar]
- 47.Jung KA, Ahn HS, Lee YS, Gye MC. Effect of a 20 kHz sawtooth magnetic field exposure on the estrous cycle in mice. J Microbiol Biotechnol. 2007;17:398–402. [PubMed] [Google Scholar]
- 48.Cecconi S, Gualtieri G, Di Bartolomeo A, Troiani G, Cifone MG, Canipari R. Evaluation of the effects of extremely low frequency electromagnetic fields on mammalian follicle development. Hum Reprod. 2000;15:2319–2325. doi: 10.1093/humrep/15.11.2319. [DOI] [PubMed] [Google Scholar]
- 49.Aydin M, Cevik A, Kandemir FM, Yuksel M, Apaydin AM. Evaluation of hormonal change, biochemical parameters, and histopathological status of uterus in rats exposed to 50-Hz electromagnetic field. Toxicol Ind Health. 2009;25:153–158. doi: 10.1177/0748233709102717. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita H, Hata K, Yamaguchi H, Tsurita G, Wake K, Watanabe S, et al. Short-term exposure to a 1439-MHz TDMA signal exerts no estrogenic effect in rats. Bioelectromagnetics. 2010;31:573–575. doi: 10.1002/bem.20593. [DOI] [PubMed] [Google Scholar]
- 51.Oral B, Guney M, Ozguner F, Karahan N, Mungan T, Comlekci S, et al. Endometrial apoptosis induced by a 900-MHz mobile phone: preventive effects of vitamins E and C. Adv Ther. 2006;23:957–973. doi: 10.1007/BF02850217. [DOI] [PubMed] [Google Scholar]
- 52.Lee HJ, Pack JK, Kim TH, Kim N, Choi SY, Lee JS, et al. The lack of histological changes of CDMA cellular phone-based radio frequency on rat testis. Bioelectromagnetics. 2010;31:528–534. doi: 10.1002/bem.20589. [DOI] [PubMed] [Google Scholar]
- 53.Ozguner M, Koyu A, Cesur G, Ural M, Ozguner F, Gokcimen A, et al. Biological and morphological effects on the reproductive organ of rats after exposure to electromagnetic field. Saudi Med J. 2005;26:405–410. [PubMed] [Google Scholar]
- 54.Imai N, Kawabe M, Hikage T, Nojima T, Takahashi S, Shirai T. Effects on rat testis of 1.95-GHz W-CDMA for IMT-2000 cellular phones. Syst Biol Reprod Med. 2011;57:204–209. doi: 10.3109/19396368.2010.544839. [DOI] [PubMed] [Google Scholar]
- 55.Saygin M, Caliskan S, Karahan N, Koyu A, Gumral N, Uguz A. Testicular apoptosis and histopathological changes induced by a 2.45 GHz electromagnetic field. Toxicol Ind Health. 2011;27:455–463. doi: 10.1177/0748233710389851. [DOI] [PubMed] [Google Scholar]
- 56.Lee JS, Ahn SS, Jung KC, Kim YW, Lee SK. Effects of 60 Hz electromagnetic field exposure on testicular germ cell apoptosis in mice. Asian J Androl. 2004;6:29–34. [PubMed] [Google Scholar]
- 57.Kim YW, Kim HS, Lee JS, Kim YJ, Lee SK, Seo JN, et al. Effects of 60 Hz 14 microT magnetic field on the apoptosis of testicular germ cell in mice. Bioelectromagnetics. 2009;30:66–72. doi: 10.1002/bem.20448. [DOI] [PubMed] [Google Scholar]
- 58.Fabra I, Roig JV, Sancho C, Mir-Labrador J, Sempere J, García-Ferrer L. Cocaine-induced ischemic colitis in a high-risk patient treated conservatively. Gastroenterol Hepatol. 2011;34:20–23. doi: 10.1016/j.gastrohep.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Bernabò N, Tettamanti E, Pistilli MG, Nardinocchi D, Berardinelli P, Mattioli M, et al. Effects of 50 Hz extremely low frequency magnetic field on the morphology and function of boar spermatozoa capacitated in vitro. Theriogenology. 2007;67:801–815. doi: 10.1016/j.theriogenology.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 60.Bernabò N, Tettamanti E, Russo V, Martelli A, Turriani M, Mattoli M, et al. Extremely low frequency electromagnetic field exposure affects fertilization outcome in swine animal model. Theriogenology. 2010;73:1293–1305. doi: 10.1016/j.theriogenology.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 61.Roychoudhury S, Jedlicka J, Parkanyi V, Rafay J, Ondruska L, Massanyi P, et al. Influence of a 50 hz extra low frequency electromagnetic field on spermatozoa motility and fertilization rates in rabbits. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2009;44:1041–1047. doi: 10.1080/10934520902997029. [DOI] [PubMed] [Google Scholar]
- 62.Iorio R, Scrimaglio R, Rantucci E, Delle Monache S, Di Gaetano A, Finetti N, et al. A preliminary study of oscillating electromagnetic field effects on human spermatozoon motility. Bioelectromagnetics. 2007;28:72–75. doi: 10.1002/bem.20278. [DOI] [PubMed] [Google Scholar]
- 63.Tenorio BM, Jimenez GC, Morais RN, Torres SM, Albuquerque Nogueira R, Silva Junior VA. Testicular development evaluation in rats exposed to 60 Hz and 1 mT electromagnetic field. J Appl Toxicol. 2011;31:223–230. doi: 10.1002/jat.1584. [DOI] [PubMed] [Google Scholar]
- 64.Chung MK, Lee SJ, Kim YB, Park SC, Shin DH, Kim SH, et al. Evaluation of spermatogenesis and fertility in F1 male rats after in utero and neonatal exposure to extremely low frequency electromagnetic fields. Asian J Androl. 2005;7:189–194. doi: 10.1111/j.1745-7262.2005.00007.x. [DOI] [PubMed] [Google Scholar]
- 65.Borhani N, Rajaei F, Salehi Z, Javadi A. Analysis of DNA fragmentation in mouse embryos exposed to an extremely low-frequency electromagnetic field. Electromagn Biol Med. 2011;30:246–252. doi: 10.3109/15368378.2011.589556. [DOI] [PubMed] [Google Scholar]
- 66.Kowalczuk CI, Robbins L, Thomas JM, Butland BK, Saunders RD. Effects of prenatal exposure to 50 Hz magnetic fields on development in mice: I. Implantation rate and fetal development. Bioelectromagnetics. 1994;15:349–361. doi: 10.1002/bem.2250150409. [DOI] [PubMed] [Google Scholar]
- 67.Ohnishi Y, Mizuno F, Sato T, Yasui M, Kikuchi T, Ogawa M. Effects of power frequency alternating magnetic fields on reproduction and pre-natal development of mice. J Toxicol Sci. 2002;27:131–138. doi: 10.2131/jts.27.131. [DOI] [PubMed] [Google Scholar]
- 68.Kim SH, Song JE, Kim SR, Oh H, Gimm YM, Yoo DS, et al. Teratological studies of prenatal exposure of mice to a 20 kHz sawtooth magnetic field. Bioelectromagnetics. 2004;25:114–117. doi: 10.1002/bem.10164. [DOI] [PubMed] [Google Scholar]
- 69.Goldhaber MK, Polen MR, Hiatt RA. The risk of miscarriage and birth defects among women who use visual display terminals during pregnancy. Am J Ind Med. 1988;13:695–706. doi: 10.1002/ajim.4700130608. [DOI] [PubMed] [Google Scholar]
- 70.McDonald AD, McDonald JC, Armstrong B, Cherry N, Nolin AD, Robert D. Work with visual display units in pregnancy. Br J Ind Med. 1988;45:509–515. doi: 10.1136/oem.45.8.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schnorr TM, Grajewski BA, Hornung RW, Thun MJ, Egeland GM, Murray WE, et al. Video display terminals and the risk of spontaneous abortion. N Engl J Med. 1991;324:727–733. doi: 10.1056/NEJM199103143241104. [DOI] [PubMed] [Google Scholar]
- 72.Bergqvist UO. Video display terminals and health. A technical and medical appraisal of the state of the art. Scand J Work Environ Health. 1984;10(Suppl 2):1–87. [PubMed] [Google Scholar]
- 73.Bryant HE, Love EJ. Video display terminal use and spontaneous abortion risk. Int J Epidemiol. 1989;18:132–138. doi: 10.1093/ije/18.1.132. [DOI] [PubMed] [Google Scholar]