Abstract
Objective
Tributyltin (TBT), an endocrine disrupting chemical, has been reported to decrease ovarian function by causing apoptosis in the ovary, but the mechanism is not fully understood. Therefore, we examined whether TBT increases the expression of adipogenesis-related genes in the ovary and the increased expression of these genes is associated with apoptosis induction.
Methods
Three-week-old Sprague-Dawley rats were orally administered TBT (1 or 10 mg/kg body weight) or sesame oil as a control for 7 days. The ovaries were obtained and weighed on day 8, and then they were fixed for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) or frozen for RNA extraction. Using the total RNA of the ovaries, adipogenesis- and apoptosis-related genes were analyzed by real-time polymerase chain reaction (PCR).
Results
The ovarian weight was significantly decreased in rats administered 10 mg/kg TBT compared to that in control rats. As determined by the TUNEL assay, the number of apoptotic follicles in ovary was significantly increased in rats administered 10 mg/kg TBT. The real-time PCR results showed that the expression of adipogenesis-related genes such as PPARγ, aP2, CD36, and PEPCK was increased after TBT administration. In addition, apoptosis-related genes such as TNFα and TNFR1 were expressed more in the TBT-administered rats compared with the control rats.
Conclusion
The present study demonstrates that TBT induces the expression of adipogenesis- and apoptosis-related genes in the ovary leading to apoptosis in the ovarian follicles. These results suggest that the increased expression of adipogenesis-related genes in the ovary by TBT exposure might induce apoptosis resulting in a loss of ovarian function.
Keywords: Adipogenesis, Apoptosis, Ovarian follicle, Ovary, Tributyltin, Sprague-Dawley rats
Introduction
The endocrine disruptors known as environmental hormones are the chemicals that interfere with the normal endocrine functions. They are discharged into the environment and remain to be introduced into organisms and act as hormones [1]. Nowadays, more than 100 species of endocrine disruptors including dioxin, polychlorinated biphenyl (PCB), bisphenol A, dichlorodiphenyltrichloroethane (DDT), and tributyltin (TBT) compounds have been reported. TBT, one of the organotin compounds is widely used in polyvinyl chloride (PVC) stabilizers, various plastic additives, industrial catalysts, insecticides, disinfectants, wood preservatives, and especially, as a biocide in antifouling coatings to prevent the attachment of organisms on underwater structures, ships, and other watercraft [2].
It has been reported that TBT is one of the main causes of marine pollution resulting in the abnormal development of reproductive organs in gastropods, mollusks, and fish [3]. In addition, TBT is known to be severely toxic to the liver and the nervous and immune systems when exposed in large quantities [4,5]. To date, it is known that TBT inhibits the reproductive functions by inactivating steroidogenic regulatory enzymes, causing the malfunctioning of mitochondria, and inducing cellular stress in ovarian cells. The exact mechanism, however, is largely unknown [6-8].
With the reports that the aromatase in human ovarian granulosa cells is inactivated by ligands for retinoid X receptor (RXR), a nuclear hormone receptor, and for peroxisome proliferator-activated receptor γ (PPARγ) as well as TBT, studies on the interactions of TBT and PPARγ are actively underway [9,10]. PPARγ, a nuclear receptor protein which forms a complex with RXR, acts as a transcription regulator of the target gene by binding on a specific DNA region. Specifically, PPARγ is involved in the gene transcription for adipocyte differentiation [11,12]. Recently, it has been reported that TBT induces obesity by promoting the differentiation of adipocytes in the body while stimulating the activity of RXR-PPARγ complex [13]. Furthermore, when a fetus or neonate is exposed to TBT, the differentiation of adipocytes is promoted by stimulating cellular PPARγ activity and obesity is induced with the accumulation of lipids in the cells [14]. The expression of PPARγ in the ovarian granulosa cells is involved in the regulation of cellular differentiation, glucose homeostasis, and inflammatory reaction [15]. On the other hand, it has been reported that the dysfunction of ovarian follicular growth and ovulation is associated with PPARγ mutation [16,17]. PPARγ also has a direct effect on ovarian function by regulating the expression of progesterone receptor in the ovaries [18].
Considering the above studies, it can be postulated that TBT stimulates the expression of the PPARγ gene in the ovaries while inducing the differentiation of adipocytes resulting in the increase of apoptosis by suppressing steroidogenesis. Therefore, we investigated the relationship between the apoptosis increase and the expression of apoptosis- and adipogenesis-related genes in rat ovaries after TBT administration to prove this hypothesis.
Methods
1. Experimental animals and TBT administration
Three-week-old female Sprague-Dawley rats were purchased from Samtako (Ohsan, Korea) and divided into three groups that included nine animals per group in the Animal Care Room of Seoul Women's University. Tributyltin acetate (TBT; Sigma, St. Louis, MO, USA) dissolved in sesame oil was orally administered at a concentration of 1 to 10 mg/kg body weight for seven days. The controls received sesame oil (Sigma) only for seven days [19]. This study was undertaken after the approval of the Institutional Review Board and all the experimental procedures were undertaken according to the terms of use and care for experimental animals.
2. Ovarian and body weight measurements
At eight days, the rats were sacrificed and the weights of the body and ovaries were measured. The gonad somatic indexes were calculated by dividing the ovarian weight by the body weight and multiplying by 100.
3. TUNEL staining
The apoptosis of the ovarian cells induced by TBT were identified by the terminal deoxynucleotidyl transferase-mediated dUTP-digoxigenin nick end-labeling (TUNEL) method. Ovarian paraffin sections were placed in xylene for 3 minutes and in 100%, 95%, and 80% of ethanol for 3 minutes, and then washed twice with phosphate buffered saline (PBS). The TUNEL reaction mixture (In Situ Cell Death Detection Kit, Fluorescein; Roche, Basel, Switzerland) was dropped onto the ovarian sections and incubated for 1 hour at 37℃ under the parafilm cover. The sections were counter-stained with 4',6-diamidino-2-phenylindole (DAPI) after washing with PBS and mounted with fluorescence mounting solution. TUNEL positive cells were analyzed under a fluorescence microscope.
4. Analyses of gene expression using real-time polymerase chain reaction (PCR)
The ovaries were homogenized with a tissue homogenizer in 300 µL of Tri reagent (RNA isoplus; Takara Bio Inc., Shiga, Japan). The homogenized tissues were left to stand for 5 minutes at room temperature. After centrifugation (14,000 rpm, 4℃, 20 minutes), the supernatants were moved into new test tubes. Then, 60 µL of chloroform (Sigma) was added and left to stand for 10 minutes at room temperature, and centrifuged. The supernatant was collected in a tube and after the addition of 150 µL of isopropanol, the tube was left to stand for 10 minutes at room temperature and centrifuged. After removing the supernatant, 1 mL of ethanol (Sigma) was added in a pellet, mixed, and centrifuged. The ethanol remaining in the tube was removed and replaced with DEPC (Takara Bio Inc., Shiga, Japan) water. Complementary DNA (cDNA) was synthesized with 3 µg of total RNA. The expressions of specific genes using cDNAs synthesized from the template DNAs were analyzed by a Light Cycler 480 (Roche) with each specific primer and SYBR green I (Roche). Using 18S DNA as an internal control, the relative expression levels were compared and calculated. The gene specific primer sequences were as follows:
18S Forward(F) 5'-GTCTSTGATGCCCTTAGATG-3'; Reverse(R) 5'-AGCTTATGACCCGCACTTAC; PPARγ F 5'-ACAAGACTACCCTTTACTGAAATTACCAT-3'; R 5'-TGCGAGTGGTCTTCCATCAC-3'; aP2 F 5'-GCGTGGAATTCGATGAAATCA-3'; R 5'-CCCGCCATCTAGGGTTATGA-3'; CD36 F 5'-CCTGCAAATGTCAGAGGAAA-3'; R 5'-GCGACATGATTAATGGCACA-3'; PEPCK F 5'-AACTGTTGGCTGGCTCTC-3'; R 5'-GAACCTGGCGTTGAATGC-3'; TNFα F 5'-AGGGTCTGGGCCATAGAACT-3'; R 5'-CCACCACGCTCTTCTGTCTAC-3'; Resistin F 5'-CTTCCCTCTGGAGGAGACTG-3'; R 5'-TGAAGCCATCGACAAGAAGA-3'; TNFR1 F 5'-ATGGATGTATCCCCATCAGC-3'; R 5'-ATTGGGGGAGTGAGAGGC-3'; FasL F 5'-TTAAATGGGCCACACTCCTC-3'; R 5'-ACTCCGTGAGTTCACCAACC-3'.
5. Statistical analyses
Nine rats per experimental group were used; the weights and the expression levels of all genes were represented as mean±SE. Statistical significance was analyzed using the Student's t-test and one-way analysis of variance with Tukey test. By comparing the experimental values to the controls, it was considered significant when p was less than 0.05.
Results
1. Changes of body and ovarian weights after TBT administration
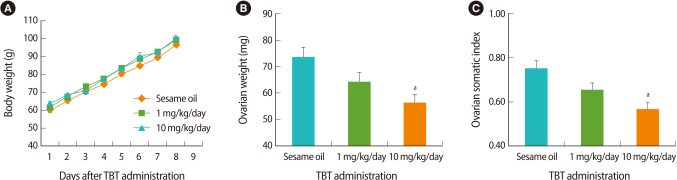
The body weight was measured before and after the treatment of TBT for 7 days. It was slightly increased in the TBT-treated experimental group, but there was no significance when compared to the sesame oil-treated control (Figure 1A). At 8 days of TBT treatment, both the ovarian weight and the gonad somatic index were decreased in a dose-dependent manner in the TBT-treated group compared to the control (Figure 1B, C).
Figure 1.
(A) The body weight of rats after tributyltin (TBT) administration. Three-week-old female rats were orally administered sesame oil, or 1 mg/kg or 10 mg/kg of TBT, daily for 7 days (n=9, each group). (B) The ovary weight of rats after TBT administration. The ovary weight was significantly decreased in 10 mg/kg TBT-treated rats compared to the control rats treated with sesame oil. (C) The gonad somatic index of the rats treated with 10 mg/kg TBT was also decreased compared to that of the control rats treated with sesame oil. The data is represented as mean±SE. aIndicates a significant difference between the sesame oil- and TBT-treated rats (p<0.05).
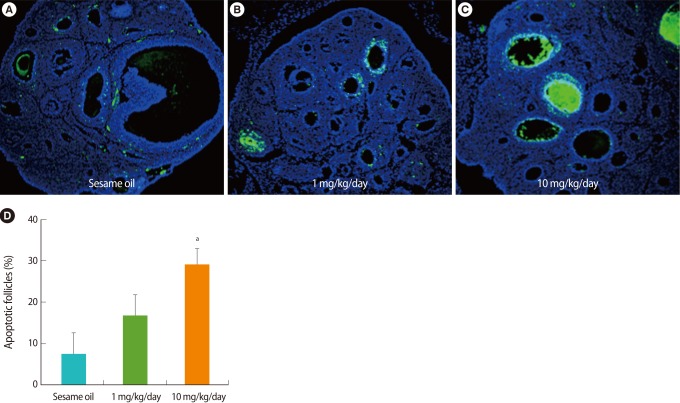
2. Increase in ovarian apoptosis after TBT administration
To identify the apoptotic ovarian follicles after TBT treatment, the ovarian sections were stained by TUNEL and observed under a fluorescence microscope. In the ovaries treated with TBT, not in the sesame oil control, the number of TUNEL-positive follicles was increased in a dose-dependent manner relative to TBT (Figure 2).
Figure 2.
Detection of apoptotic cells in the ovary after tributyltin (TBT) administration (A-C). Paraffin sections of week-old rat ovaries were stained by using a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay kit and counter-stained with 4',6-diamidino-2-phenylindole after TBT exposure for 7 days. Apoptotic cells stained by TUNEL show as green. ×200. (D) The percent of apoptotic follicles counted visibly increased in the ovary after TBT exposure.
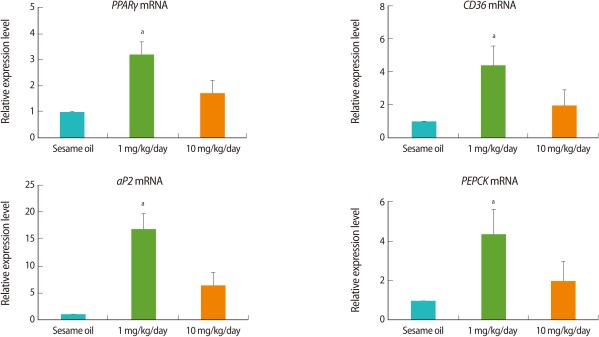
3. Increase in ovarian adipogenesis-related gene expression after TBT administration
The expression of adipogenesis-related genes in the ovaries was analyzed by real-time PCR. In the TBT-treated group, the expressions of mRNAs of adipogenesis-related genes including PPARγ, aP2, CD36, and PEPCK were increased. The expressions were increased in all TBT-treated groups when compared to the control. However, it was noted that the expressions in the 1 mg/kg TBT treated group were higher than those in the 10 mg/kg TBT group (Figure 3).
Figure 3.
The expression levels of adipogenesis-related genes in the rat ovary after tributyltin (TBT) administration. The female rats (n=9, each group) were orally administrated with 1 mg/kg, 10 mg/kg TBT, or sesame oil alone for 7 days. Following dissection, cDNA was prepared from ovaries for real-time polymerase chain reaction analysis. Expression levels were normalized to 18S. Data is represented as mean±SE. aIndicates a significant difference between sesame oil- and TBT-treated rats (p<0.05).
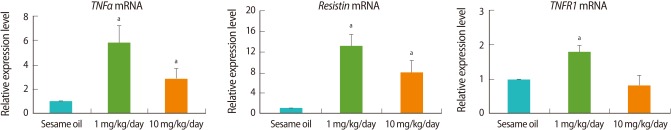
4. Increase of ovarian adipokine gene expression after TBT administration
The expression of the representative adipokine genes, TNFα and resistin was analyzed by real-time PCR. TNFα expression was increased in TBT-treated group similar to the pattern of adipocyte induction-related genes expression, whereas resistin was increased in a dose-dependent manner (Figure 4). In addition, the expression of apoptosis-related gene, TNFR1 was increased in a dose-dependent manner in the TBT-treated group relative to the control (Figure 4).
Figure 4.
Real-time polymerase chain reaction (PCR) analysis of adipokine- and apoptosis-related genes. The female rats (n=9, each group) were orally administrated with 1 mg/kg, 10 mg/kg tributyltin (TBT), or sesame oil alone for 7 days. Following dissection, cDNA was prepared from ovaries for real-time PCR analysis. Expression levels were normalized to 18S. Data is represented as mean±SE. aIndicates a significant difference between sesame oil- and TBT-treated rats (p<0.05).
Discussion
It is well known that PPARγ plays an important role in the development of adipocytes by inducing the differentiation of adipocytes and fatty acid metabolism [20,21]. On the other hand, it has been reported that TBT, an endocrine disrupter, stimulates PPARγ action to promote the differentiation of adipocytes [14,22]. However, whether the differentiation of fat cells from the ovarian cells could be induced by TBT or if there is any relationship between adipocyte differentiation and the apoptosis of ovarian cells is largely unknown. Therefore, this study analyzed the changes of adipogenesis-related gene expression after TBT administration, and also investigated the effects of adipocyte differentiation on apoptosis in rat ovaries.
First, to assess the overall toxicity of TBT, changes in bodyweight were investigated after TBT treatment. The bodyweight for 7 days of administration was slightly increased in the TBT-treated group; however, there was no statistical significance when compared to the sesame oil-treated control group. On the other hand, the ovarian weight showed a tendency to decrease after TBT administration, and gonad somatic indices in the experimental group were significantly lower than in the control. It may be that the reduction of the ovarian weight results from the inhibition of follicular growth which is induced by the decrease of the ovarian steroid hormones and various growth factors by exogenous TBT [23-25].
To evaluate the cause of the inhibition of follicular development according to TBT treatment, the degree of the ovarian adipocyte differentiation was analyzed. In order to assess the ovarian adipocyte differentiation degree after TBT treatment, the expression of the adipogenesis-related genes including PPARγ, aP2, CD36, and PEPCK was analyzed by real-time PCR. It has been reported that adipocyte fatty acid binding protein (aP2) plays an important role in the adipocyte differentiation processes by being involved in lipid metabolism and intracellular fatty acid transport [26]. The expression of aP2 in fat cells is increased rapidly by treatment with TBT and this aP2 expression is regulated by PPARγ [27,28]. CD36, a scavenger receptor expressed on adipocytes, removes the oxidized LDL and functions to transport the fatty acid into the adipocytes [29]. It is known that CD36 expression, like aP2, is regulated by PPARγ during the differentiation of fat cells [30]. Phosphoenol-pyruvate carboxykinase, an enzyme that is involved in the gluconeogenesis pathway, is abundant in adipocytes as well as the kidney and liver [31].
In this study, adipogenesis-related genes in the TBT-treated group were increased when compared to sesame oil-treated controls. It may be concluded that TBT has a direct effect on the ovary to promote the expression of adipogenesis-related genes such as PPARγ by which the ovarian cells are changed into adipocytes resulting in the ovarian dysfunction. On the other hand, these genes showed the maximum expression in group treated with 1 mg/kg TBT and slightly decreased in the 10 mg/kg TBT group. It is thought that the main cause of the decrease in gene expression with the high dose of TBT treatment was the cytotoxicity of TBT that resulted in the death of the ovarian cells, and hence the gene expression did not happen.
Meanwhile, it is well known that the fat cells secrete adipokines including leptin, adiponectin, TNFα, IL-1β, and resistin influencing the metabolism of the surrounding cells and tissues [32]. It was shown, in this study, that the expression of TNFα and resistin genes was higher in the TBT-treated group than the control. It is thought that the increase of adipogenesis-related genes expression in the ovaries by TBT causes the differentiation of adipocytes by which adipokine synthesis is increased in the ovaries. Therefore, it can be postulated that resistin and TNFα increased in the ovaries induces apoptosis and dysfunction in steroidogenesis of the ovarian cells including the granulosa cells, which results in abnormality in follicular development [33].
It is well known that ovarian apoptosis is increased by TBT. The present study also demonstrated that the number of apoptotic follicles detected by TUNEL was increased in a dose-dependent manner after TBT administration. The mechanism of ovarian apoptosis induced by TBT has not yet been clearly demonstrated. To verify this, the expression of TNFR1, an apoptosis-related gene, was investigated in the ovarian tissues after TBT treatment.
It is well known that TNFR1 in the various tissues binds to TNFα to activate cellular TNF receptor-associated death domain and caspases promoting apoptosis [34-36]. In this study, the expression of TNFR1 was significantly increased in rats treated with TBT. These results indicate that the high concentration of TBT promotes directly ovarian apoptosis, which leads to ovarian dysfunction.
In conclusion, the present study demonstrates that TBT exposure can promote adipokine gene expression with an increase in adipogenesis-related gene expression in the rat ovary. It also suggests that the adipokines stimulate apoptosis-related gene expression and hence induce apoptosis in the ovary. These results are postulated to show that the chronic exposure to TBT causes the accumulation of endocrine disruptors in the body, resulting in ovarian dysfunction.
Footnotes
This research was supported by a special research grant from Seoul Women's University (2011).
No potential conflict of interest relevant to this article was reported.
References
- 1.Lee CW, Choi KH, Jeong SW, Kim HL, Seo YR. An overview and future perspective on endocrine disruptors. J Korean Endocr Soc. 2009;24:7–14. [Google Scholar]
- 2.Appel KE. Organotin compounds: toxicokinetic aspects. Drug Metab Rev. 2004;36:763–786. doi: 10.1081/dmr-200033490. [DOI] [PubMed] [Google Scholar]
- 3.Blaber SJ. The occurrence of a penis-like outgrowth behind the right tentacle in spent females of Nucella lapillus. J Molluscan Stud. 1970;39:231–233. [Google Scholar]
- 4.Ogata R, Omura M, Shimasaki Y, Kubo K, Oshima Y, Aou S, et al. Two-generation reproductive toxicity study of tributyltin chloride in female rats. J Toxicol Environ Health A. 2001;63:127–144. doi: 10.1080/15287390151126469. [DOI] [PubMed] [Google Scholar]
- 5.Boyer IJ. Toxicity of dibutyltin, tributyltin and other organotin compounds to humans and to experimental animals. Toxicology. 1989;55:253–298. doi: 10.1016/0300-483x(89)90018-8. [DOI] [PubMed] [Google Scholar]
- 6.Heidrich DD, Steckelbroeck S, Klingmuller D. Inhibition of human cytochrome P450 aromatase activity by butyltins. Steroids. 2001;66:763–769. doi: 10.1016/s0039-128x(01)00108-8. [DOI] [PubMed] [Google Scholar]
- 7.Powers MF, Beavis AD. Triorganotins inhibit the mitochondrial inner membrane anion channel. J Biol Chem. 1991;266:17250–17256. [PubMed] [Google Scholar]
- 8.Philbert MA, Billingsley ML, Reuhl KR. Mechanisms of injury in the central nervous system. Toxicol Pathol. 2000;28:43–53. doi: 10.1177/019262330002800107. [DOI] [PubMed] [Google Scholar]
- 9.Mu YM, Yanase T, Nishi Y, Takayanagi R, Goto K, Nawata H. Combined treatment with specific ligands for PPARgamma:RXR nuclear receptor system markedly inhibits the expression of cytochrome P450arom in human granulosa cancer cells. Mol Cell Endocrinol. 2001;181:239–248. doi: 10.1016/s0303-7207(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 10.Saitoh M, Yanase T, Morinaga H, Tanabe M, Mu YM, Nishi Y, et al. Tributyltin or triphenyltin inhibits aromatase activity in the human granulosa-like tumor cell line KGN. Biochem Biophys Res Commun. 2001;289:198–204. doi: 10.1006/bbrc.2001.5952. [DOI] [PubMed] [Google Scholar]
- 11.Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58:726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 12.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 13.le Maire A, Grimaldi M, Roecklin D, Dagnino S, Vivat-Hannah V, Balaguer P, et al. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 2009;10:367–373. doi: 10.1038/embor.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol. 2010;24:526–539. doi: 10.1210/me.2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komar CM. Peroxisome proliferator-activated receptors (PPARs) and ovarian function: implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod Biol Endocrinol. 2005;3:41. doi: 10.1186/1477-7827-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Sato M, Li Q, Lydon JP, Demayo FJ, Bagchi IC, et al. Peroxisome proliferator-activated receptor gamma is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol. 2008;28:1770–1782. doi: 10.1128/MCB.01556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minge CE, Bennett BD, Norman RJ, Robker RL. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reverses the adverse effects of diet-induced obesity on oocyte quality. Endocrinology. 2008;149:2646–2656. doi: 10.1210/en.2007-1570. [DOI] [PubMed] [Google Scholar]
- 18.Komar CM, Curry TE., Jr Inverse relationship between the expression of messenger ribonucleic acid for peroxisome proliferator-activated receptor gamma and P450 side chain cleavage in the rat ovary. Biol Reprod. 2003;69:549–555. doi: 10.1095/biolreprod.102.012831. [DOI] [PubMed] [Google Scholar]
- 19.Yamasaki K, Sawaki M, Takatsuki M. Immature rat uterotrophic assay of bisphenol A. Environ Health Perspect. 2000;108:1147–1150. doi: 10.1289/ehp.001081147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, et al. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2005;102:6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi T. Organotin-induced toxicity and nuclear receptor signaling. Biomed Res Trace Elem. 2006;17:373–381. [Google Scholar]
- 23.Cangialosi MV, Puccia E, Mazzola A, Mansueto V, Arukwe A. Screening of ovarian steroidogenic pathway in Ciona intestinalis and its modulation after tributyltin exposure. Toxicol Appl Pharmacol. 2010;245:124–133. doi: 10.1016/j.taap.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi T. Endocrine disruption induced by organotin compounds; organotins function as a powerful agonist for nuclear receptors rather than an aromatase inhibitor. J Toxicol Sci. 2008;33:269–276. doi: 10.2131/jts.33.269. [DOI] [PubMed] [Google Scholar]
- 25.McVey MJ, Cooke GM. Inhibition of rat testis microsomal 3beta-hydroxysteroid dehydrogenase activity by tributyltin. J Steroid Biochem Mol Biol. 2003;86:99–105. doi: 10.1016/s0960-0760(03)00256-5. [DOI] [PubMed] [Google Scholar]
- 26.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 27.Carfi' M, Croera C, Ferrario D, Campi V, Bowe G, Pieters R, et al. TBTC induces adipocyte differentiation in human bone marrow long term culture. Toxicology. 2008;249:11–18. doi: 10.1016/j.tox.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Shao D, Lazar MA. Peroxisome proliferator activated receptor gamma, CCAAT/enhancer-binding protein alpha, and cell cycle status regulate the commitment to adipocyte differentiation. J Biol Chem. 1997;272:21473–21478. doi: 10.1074/jbc.272.34.21473. [DOI] [PubMed] [Google Scholar]
- 29.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahimi A, Sfeir Z, Magharaie H, Amri EZ, Grimaldi P, Abumrad NA. Expression of the CD36 homolog (FAT) in fibroblast cells: effects on fatty acid transport. Proc Natl Acad Sci U S A. 1996;93:2646–2651. doi: 10.1073/pnas.93.7.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakravarty K, Cassuto H, Reshef L, Hanson RW. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit Rev Biochem Mol Biol. 2005;40:129–154. doi: 10.1080/10409230590935479. [DOI] [PubMed] [Google Scholar]
- 32.Kaipia A, Chun SY, Eisenhauer K, Hsueh AJ. Tumor necrosis factor-alpha and its second messenger, ceramide, stimulate apoptosis in cultured ovarian follicles. Endocrinology. 1996;137:4864–4870. doi: 10.1210/endo.137.11.8895358. [DOI] [PubMed] [Google Scholar]
- 33.Sharov AA, Falco G, Piao Y, Poosala S, Becker KG, Zonderman AB, et al. Effects of aging and calorie restriction on the global gene expression profiles of mouse testis and ovary. BMC Biol. 2008;6:24. doi: 10.1186/1741-7007-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 35.Schneider P, Tschopp J. Apoptosis induced by death receptors. Pharm Acta Helv. 2000;74:281–286. doi: 10.1016/s0031-6865(99)00038-2. [DOI] [PubMed] [Google Scholar]
- 36.Korzekwa A, Murakami S, Wocławek-Potocka I, Bah MM, Okuda K, Skarzynski DJ. The influence of tumor necrosis factor alpha (TNF) on the secretory function of bovine corpus luteum: TNF and its receptors expression during the estrous cycle. Reprod Biol. 2008;8:245–262. doi: 10.1016/s1642-431x(12)60015-1. [DOI] [PubMed] [Google Scholar]