Abstract
Enterocutaneous (EC) fistula is an abnormal connection between the gastrointestinal (GI) tract and skin. The majority of EC fistulas result from surgery. About one third of fistulas close spontaneously with medical treatment and radiologic interventions. Surgical treatment should be reserved for use after sufficient time has passed from the previous laparotomy to allow lysis of the fibrous adhesion using full nutritional and medical treatment and until a complete understanding of the anatomy of the fistula has been achieved. The successful management of GI fistula requires a multi-disciplinary team approach including a gastroenterologist, interventional radiologist, enterostomal therapist, dietician, social worker and surgeons. With this coordinated approach, EC fistula can be controlled with acceptable morbidity and mortality.
Keywords: Intestinal fistula, Surgical procedures, Multidisciplinary approach
INTRODUCTION
In surgical textbooks, enterocutaneous (EC) fistulas are described as surgical tragedies, catastrophes or disasters. Most EC fistulas occur following abdominal surgeries and only 15-25% of spontaneous EC fistulas are the result of underlying diseases such as Crohn's disease, radiation enteritis or diverticular disease (1, 2). The incidence of traumatic EC fistula has been increasing due to the higher incidence of damage control surgery performed for major trauma (3).
EC fistulas are associated with significant morbidity and mortality. Patients with EC fistula are faced with the burden of overcoming septic complications resulting from early intra-abdominal infection, fluid electrolyte imbalance and malnutrition. The goals of EC fistula management are to restore gastrointestinal (GI) continuity and allow enteral nutrition with minimal morbidity and mortality. A step-by-step approach is recommended to achieve these goals.
Management of EC Fistulas
Recognition and Stabilization of EC Fistula
The initial stage of EC fistula management consists of its identification, followed by general supportive care with fluid and electrolyte replacement, control of sepsis, nutritional support and control of fistula drainage by pharmacologic means as well as through skin protection. Once a postsurgical or spontaneous EC fistula is identified, obtaining anatomic information is of the utmost importance to predict the site of intestinal opening and assess the need for surgery. Favorable external fistulas include esophageal, duodenal stump, pancreaticobiliary, and jejunal fistulas with small enteric defects (< 1 cm) and long tracts (> 2 cm). In contrast, gastric, lateral duodenal, ligament of Treitz, and ileal fistulas are less likely to close spontaneously (4). Additionally, non-healing EC fistulas are associated with a Foreign body, Radiation, Inflammation, Infection, Inflammatory bowel disease, Epithelization of the fistula tract, Neoplasms and Distal obstructions (or FRIEND). The presence of any FRIEND component in EC fistula is an indication for surgical intervention; however, surgery should be performed only after sufficient time has been afforded to restore overall patient condition and allow lysis of the intra-abdominal fibrous adhesions from previous operations.
Fistula output fluids rich in electrolytes, minerals and protein cause electrolyte imbalance and malnutrition.
Fluid replacement therapy is therefore the first step in the management of patients with EC fistulas. Crystalloid, colloid solutions and blood transfusions are generally required during early resuscitation.
After initial resuscitation, septic complications need to be controlled. Treatment is comprised of intra-abdominal infection control with antibiotics, computed tomography (CT)-guided drainage or sometimes open drainage for a "controlled fistula". A controlled fistula refers to an EC fistula without evidence of sepsis (high fever, rigors, and hypotension), or localized infection (cellulitis, pneumonia) (5). If the intestinal contents drain out through the matured tract, there is no longer intraperitoneal contamination or fluid accumulation to cause septic problems.
Sufficient parenteral or enteral nutritional support should be provided when the septic problems are under control. Parenteral nutrition has been shown to affect the spontaneous closure of EC fistulas (5-8). Recently, enteral feeding was found to have a protective effect on the mucosal barrier and immunologic function of the bowel, even in patients with high-output EC fistulas (9). Enteral feeding also improves hepatic protein synthesis. These advantages suggest that early enteral feeding with the combination of parenteral nutrition is a key component of nutritional support in patients with EC fistulas. A regular supplementation of trace minerals such as copper, zinc, and a vitamin complex is generally recommended.
A fistula output greater than 500 mL per day is classified as a high-output fistula and less than 200 mL is classified as a low-output fistula. Fistula output is a significant single prognostic factor for determining the possibility of spontaneous closure and mortality (9-11). Control of output is therefore also very important in achieving spontaneous closure. Traditionally, NPO and nasogastric suction have been used to decrease output, but there is little evidence to support the effectiveness of these strategies. H2-receptor antagonists and proton pump inhibitors are recommended to control fistula output. Somatostatin and its analog, octreotide, inhibit endocrine and exocrine secretion in the GI tract. Recent randomized trials did not consistently show a positive effect of octreotide on fistula closure or a reduction in fistula output (12-15). High doses of antimotility drugs such as loperamide (up to 36 mg daily) and codeine phosphate (up to 240 mg daily) are also used to decrease fistula output (16).
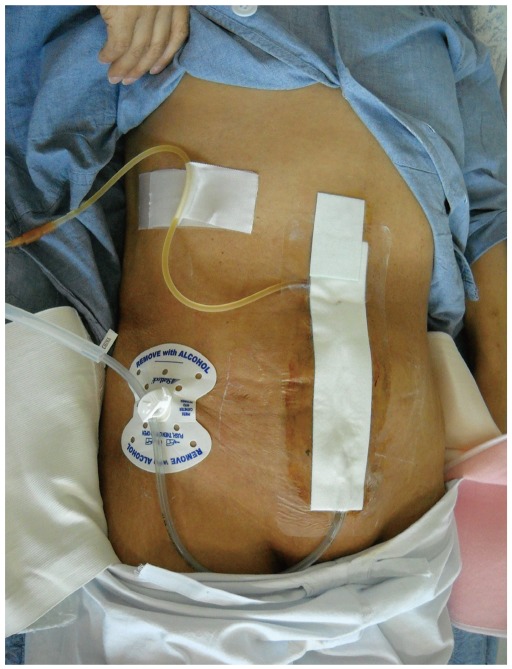
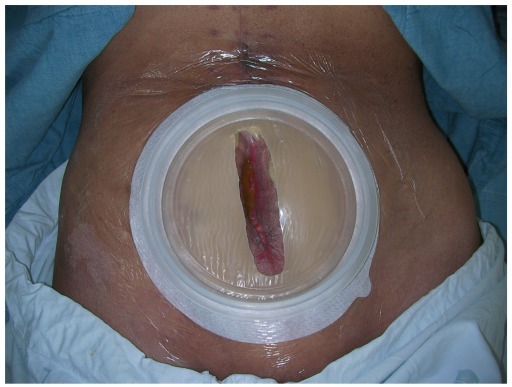
Because the small bowel contents that drain from an EC fistula are rich in digestive enzymes and electrolytes, skin protection is a crucial step in wound management. It also aids in the secure closure of the abdomen when surgery is mandatory to control the fistula. Skin should be protected from maceration and breakdown when stoma appliances and protective films are used. Several studies of vacuum-assisted closure (VAC) reported successful closure of EC fistulas (2, 5, 17), but the main advantages of VAC is skin protection and ease of dressing (Figs. 1, 2). Fibrin glue has also been introduced to close EC fistulas, but the success of the study was not definitive due to the small sample size (18).
Fig. 1.
Application of vacuum-assisted closure systems in enterocutaneous fistulas.
Fig. 2.
Application of skin protection appliance after stabilization of enterocutaneous fistula.
Investigation to Defining the Anatomy of EC Fistula
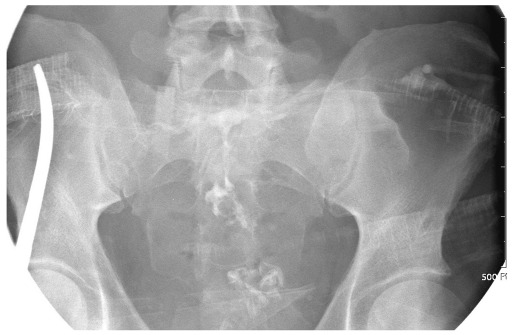
After seven to ten days of general supportive care to stabilize patients and fistula output, an investigation to determine the anatomic location of the EC fistula should be considered. The gold-standard for examination of fistula anatomy involves a fistulogram using water-soluble contrast material (Fig. 3). The information gained via a fistulogram includes: 1) the source of the fistula; 2) the nature of the fistula such as length, course and relationship to the bowel; 3) the absence or presence of bowel continuity (end vs. side fistula); 4) the absence or presence of distal obstruction; 5) the nature of the bowel adjacent to the fistula (inflammation and stricture); and 6) the absence or presence of an abscess cavity in communication with the fistula (5). Other studies such as abdominal CT scans and MRI of the abdomen also provide useful information in selected cases (16).
Fig. 3.
Fistulogram with water soluble contrast material provided detailed information regarding anatomy of enterocutaneous fistula.
Decision Making for Surgery
The duration of conservative management should be based on the anatomic studies of the fistula tract. In the absence of the adverse prognostic factors (FRIEND) described previously, the reported success rate of fistula closure varies from 30% to 74% in patients within a time frame of 4 to 12 weeks.
Determining the optimal time for surgical intervention has not been well defined in the literature. However, surgery should be delayed until the intra-abdominal and systemic conditions of the patient are conducive to major surgery. Intraperitoneal adhesion can be performed from as early as postoperative day 4 and adhesive procedures are most frequently performed at postoperative day 35. Evenson and Fischer (5) proposed waiting at least four months from the date of the previous operation. Datta et al. (16) delayed the operation for a median of nine months from the initial surgery or the occurrence of a fistula. The timing of definitive surgery should be individualized according to patient characteristics.
Surgery for EC Fistula
The aims of surgery for EC fistulas are: 1) refunctionalization of the entire bowel; 2) resection of the fistula with end-to-end anastomosis of the bowel; and 3) secure abdominal wall closure. Once surgery is planned, care should be taken not to injure the adjacent bowels while opening the abdomen. The fistula tract is detected by staining with methylene blue or guided probes. After identifying the fistula opening in the bowel, resection of the diseased bowel and end-to-end anastomosis is the preferred method compared to over-sewing the fistula opening of the bowel, as recurrent fistula is more likely after over-sewing (36%) than resection (16%) (19). To facilitate early feeding and decompression of the proximal bowel, selected cases require gastrostomy, diverting ileostomy or jejunostomy.
Abdominal closure is challenging when fistula closure is attempted. Skin defects and the increased probability of wound contamination make secure skin closure difficult. Wound complications can result in new or recurring EC fistulas. To promote normal healing of the abdominal wound after surgery, optimal parenteral or enteral nutrition with a supplement comprised of trace minerals and vitamins is essential.
CONCLUSION
Treatment of EC fistula remains a surgical challenge despite the recent improvement of supportive patient care. Once EC fistula occurs, adequate stabilization of the patient, a thorough investigation of the fistula anatomy, and non-operative management should intially be attempted. If surgery is required, careful planning, meticulous dissection, resection of the bowel, reanastomosis and reconstruction of the abdominal wall are critical. A multi-disciplinary team approach with a gastroenterologist, interventional radiologists, dieticians, enterostomal therapy nurses and surgeons will maximize successful EC fistula closure.
References
- 1.Berry SM, Fischer JE. Classification and pathophysiology of enterocutaneous fistulas. Surg Clin North Am. 1996;76:1009–1018. doi: 10.1016/s0039-6109(05)70495-3. [DOI] [PubMed] [Google Scholar]
- 2.Draus JM, Jr, Huss SA, Harty NJ, Cheadle WG, Larson GM. Enterocutaneous fistula: are treatments improving? Surgery. 2006;140:570–576. doi: 10.1016/j.surg.2006.07.003. discussion 576-578. [DOI] [PubMed] [Google Scholar]
- 3.Fischer PE, Fabian TC, Magnotti LJ, Schroeppel TJ, Bee TK, Maish GO, 3rd, et al. A ten-year review of enterocutaneous fistulas after laparotomy for trauma. J Trauma. 2009;67:924–928. doi: 10.1097/TA.0b013e3181ad5463. [DOI] [PubMed] [Google Scholar]
- 4.Galie KL, Whitlow CB. Postoperative enterocutaneous fistula: when to reoperate and how to succeed. Clin Colon Rectal Surg. 2006;19:237–246. doi: 10.1055/s-2006-956446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evenson AR, Fischer JE. Current management of enterocutaneous fistula. J Gastrointest Surg. 2006;10:455–464. doi: 10.1016/j.gassur.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 6.MacFadyen BV, Jr, Dudrick SJ, Ruberg RL. Management of gastrointestinal fistulas with parenteral hyperalimentation. Surgery. 1973;74:100–105. [PubMed] [Google Scholar]
- 7.Rose D, Yarborough MF, Canizaro PC, Lowry SF. One hundred and fourteen fistulas of the gastrointestinal tract treated with total parenteral nutrition. Surg Gynecol Obstet. 1986;163:345–350. [PubMed] [Google Scholar]
- 8.Soeters PB, Ebeid AM, Fischer JE. Review of 404 patients with gastrointestinal fistulas. Impact of parenteral nutrition. Ann Surg. 1979;190:189–202. doi: 10.1097/00000658-197908000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lévy E, Frileux P, Cugnenc PH, Honiger J, Ollivier JM, Parc R. High-output external fistulae of the small bowel: management with continuous enteral nutrition. Br J Surg. 1989;76:676–679. doi: 10.1002/bjs.1800760708. [DOI] [PubMed] [Google Scholar]
- 10.Campos AC, Andrade DF, Campos GM, Matias JE, Coelho JC. A multivariate model to determine prognostic factors in gastrointestinal fistulas. J Am Coll Surg. 1999;188:483–490. doi: 10.1016/s1072-7515(99)00038-1. [DOI] [PubMed] [Google Scholar]
- 11.Edmunds LH, Jr, Williams GM, Welch CE. External fistulas arising from the gastro-intestinal tract. Ann Surg. 1960;152:445–471. doi: 10.1097/00000658-196009000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez C, McFadden DW, Reber HA. Complicated enterocutaneous fistulas: failure of octreotide to improve healing. World J Surg. 2000;24:533–537. doi: 10.1007/s002689910086. discussion 538. [DOI] [PubMed] [Google Scholar]
- 13.Hernández-Aranda JC, Gallo-Chico B, Flores-Ramírez LA, Avalos-Huante R, Magos-Vázquez FJ, Ramírez-Barba EJ. [Treatment of enterocutaneous fistula with or without octreotide and parenteral nutrition] Nutr Hosp. 1996;11:226–229. [PubMed] [Google Scholar]
- 14.Nubiola-Calonge P, Badía JM, Sancho J, Gil MJ, Segura M, Sitges-Serra A. Blind evaluation of the effect of octreotide (SMS 201-995), a somatostatin analogue, on small-bowel fistula output. Lancet. 1987;2:672–674. doi: 10.1016/s0140-6736(87)92452-4. [DOI] [PubMed] [Google Scholar]
- 15.Sancho JJ, di Costanzo J, Nubiola P, Larrad A, Beguiristain A, Roqueta F, et al. Randomized double-blind placebo-controlled trial of early octreotide in patients with postoperative enterocutaneous fistula. Br J Surg. 1995;82:638–641. doi: 10.1002/bjs.1800820521. [DOI] [PubMed] [Google Scholar]
- 16.Datta V, Engledow A, Chan S, Forbes A, Cohen CR, Windsor A. The management of enterocutaneous fistula in a regional unit in the United kingdom: a prospective study. Dis Colon Rectum. 2010;53:192–199. doi: 10.1007/DCR.0b013e3181b4c34a. [DOI] [PubMed] [Google Scholar]
- 17.McNaughton V, Brown J, Hoeflok J, Martins L, McNaughton V, Nielsen EM, et al. Canadian Association for Enterostomal Therapy ECF Best Practice Recommendations Panel. Summary of best practice recommendations for management of enterocutaneous fistulae from the Canadian Association for Enterostomal Therapy ECF Best Practice Recommendations Panel. J Wound Ostomy Continence Nurs. 2010;37:173–184. doi: 10.1097/WON.0b013e3181cf850b. [DOI] [PubMed] [Google Scholar]
- 18.Avalos-González J, Portilla-deBuen E, Leal-Cortés CA, Orozco-Mosqueda A, Estrada-Aguilar Mdel C, Velázquez-Ramírez GA, et al. Reduction of the closure time of postoperative enterocutaneous fistulas with fibrin sealant. World J Gastroenterol. 2010;16:2793–2800. doi: 10.3748/wjg.v16.i22.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch AC, Delaney CP, Senagore AJ, Connor JT, Remzi FH, Fazio VW. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg. 2004;240:825–831. doi: 10.1097/01.sla.0000143895.17811.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]