Abstract
Objective
The purpose of this study was to prospectively compare pre-operative computed tomography (CT) perfusion parameters with tumor grade from colorectal adenocarcinoma (CRC) and to correlate pre-operative CT perfusion parameters with microvessel density (MVD) to evaluate angiogenesis in CRC.
Materials and Methods
Pre-operative perfusion CTs were performed with a 64-channel multidetector row CT in 27 patients (17 women and 10 men; age range 32-82 years) who were diagnosed with CRC involving the sigmoid and rectum between August 2006 and November 2007. All patients underwent surgery without pre-operative chemotherapy or radiation therapy. Dynamic perfusion CTs were performed for 65 seconds after intravenous injection of contrast medium (100 mL, 300 mg of iodine per mL, 5 mL/sec). Before surgery, blood flow (BF), blood volume, mean transit time (MTT), and permeability-surface area product were measured in the tumor. After surgery, one gastrointestinal pathologist evaluated tumor grade and performed immunohistochemical staining using CD 34 to determine MVD in each tumor. The Kruskal-Wallis test was used to compare CT perfusion parameters with tumor grade, and Pearson's correlation analysis was used to correlate CT perfusion parameters with MVD.
Results
In 27 patients with CRC, tumor grading was as follows: well differentiated (n = 8); moderately differentiated (n = 15); and poorly differentiated (n = 4). BF was higher in moderately differentiated CRC than well differentiated and poorly differentiated CRCs (p = 0.14). MTT was shorter in moderately differentiated than well differentiated and poorly differentiated CRCs (p = 0.039). The MVD was greater in poorly differentiated than well differentiated and moderately differentiated CRCs (p = 0.034). There was no significant correlation between other perfusion parameters and tumor grade. There was no significant correlation between CT perfusion parameters and MVD.
Conclusion
BF and MTT measurement by perfusion CT is effective in predicting moderately differentiated CRCs. However, perfusion CT is limited in distinguishing well differentiated and poorly differentiated CRCs. Pre-operative perfusion CT does not reflect the MVD of CRCs.
Keywords: Colon cancer, CT, Perfusion, Angiogenesis
INTRODUCTION
Colorectal adenocarcinoma (CRC) is the third most common cancer in the world, accounting for 8.9-9.4% of all cancers (1, 2). The worldwide prevalence of CRC is second only to that of breast cancer (2). The 5-year survival for poorly differentiated CRC (29%) is significantly less than well differentiated and moderately differentiated CRC (59%, p = 0.0002) (3). In addition, increased microvessel density (MVD) is correlated with poor outcome in CRC (4, 5). The histologic MVD technique is the current gold standard by which tumor angiogenesis is characterized (6). Several studies have shown a statistically significant correlation between MVD, invasion, and metastasis in various solid tumors such as breast, lung, uterine cervix, and prostate (6-12). Des Guetz et al. (13) reported that there is a statistically significant inverse relationship between angiogenesis, assessed by MVD or vascular endothelial growth factor expression, and survival in human invasive CRC.
Dynamic contrast-enhanced CT is also regarded as an appropriate non-invasive technique for assessment of angiogenesis, where positive correlations between CT parameters and immunohistologic measures, such as MVD, have been reported in studies of lung and kidney cancers (14). Goh et al. (14) reported that among perfusion parameters, the tumor permeability surface-area product (PS) and blood volume (BV) correlate positively with MVD and may reflect the degree of microvascularity within CRCs. In contrast, Li et al. (15) reported that there is no significant correlation between MVD and perfusion parameters in CRC. Indeed, debate continues regarding the correlation between MVD and CRC perfusion parameters, demonstrating the need for further investigation in this area of research.
Few studies have been conducted on the relationship between tumor grade and perfusion CT in CRC (1, 2). Thus, the purpose of the current study was to compare preoperative CT perfusion parameters with tumor grade of CRC and to evaluate the correlation between pre-operative CT perfusion parameters and MVD to evaluate angiogenesis in CRC.
MATERIALS AND METHODS
Patients
Prior to the beginning of this study, the Institutional Review Board approved of the experimental design and methods outlined here. Written informed consent was obtained from each patient or the patient's family before the pre-operative CT scan, including administration of radiation dose, was performed. Patients were excluded from the study if the patient had renal disease that precluded performing a contrast-enhanced CT (serum creatinine level, > 1.9 mg/dL). Between August 2006 and November 2007, 47 consecutive patients with CRC who underwent pre-operative CT scans were prospectively enrolled in the study.
The following were exclusion criteria for the study: pre-operative radiation therapy or chemotherapy (n = 5), descending or ascending colon cancers with severe motion artifact (n = 3), poor identification of the tumor due to size (< 1 cm in diameter) at planning CT for perfusion scan (n = 3), no surgical intervention (n = 7), inability to place an intravenous cannula (n = 1), and mucinous carcinoma (n = 1). Thus, 27 consecutive patients (17 women and 10 men; mean age 59.3 years; range 32-82 years) who underwent pre-operative CT scans were prospectively enrolled in the study.
CT Technique
Pre-operative perfusion CT scans were performed with a 64 channel volume CT (Lightspeed VCT; GE Healthcare Technologies, Milwaukee, WI, USA) on all patients. To prevent peristaltic movement, 20 mg of the spasmolytic agent, hyoscine butylbromide (Buscopan®; Boehringer Ingelheim, Ingelheim am Rhein, Germany), was administered intravenously prior to data acquisition. Colonic dilatation with CO2 gas by automatic colon insufflators (protoCO2l; EZEM, Bicester, UK) was performed on all patients. Limited scout images were initially obtained from all patients in the lying position on the scanner table to identify colorectal tumor location by a supervising, experienced gastrointestinal radiologist before dynamic perfusion CT was performed. The scanning coordinates were used to plan the subsequent dynamic perfusion CT examination, which was focused on the mid-portion of the tumor. The following parameters for limited scout images were used: slice thickness, 5 mm; interval, 5 mm; scan type, helical mode; rotation speed, 1 sec; 80 Kvp, 200 mA, scan field of view, 33 cm; and matrix, 512 × 512 mm.
One hundred mL of iopramide (Ultravist®; Schering, Berlin, Germany) was injected intravenously at a rate of 5 mL/s via an automatic pump injector for dynamic perfusion CT scans. Dynamic perfusion CT scans were performed at the mid-portion of the tumor for 60 seconds, beginning 5 seconds after contrast injection using the following parameters: slice thickness, 5 mm; interval, 5 mm; scan type, cine mode; 8 contiguous sections of 40 mm diameter; rotation speed, 0.5 sec; 80 Kvp; 200 mA; scan field of view, 33 cm; and matrix, 512 × 512 mm. All patients underwent surgery without pre-operative chemotherapy or radiation therapy after perfusion CT was performed.
Image Analysis
All CT scanning data were sent to a workstation (Advantage Windows 4.2; GE Healthcare Technologies). Two gastrointestinal radiologists (YYJ and JWK, with abdominal imaging experience of 15 and 3 years, respectively) reviewed the eight contiguous section images and selected one section image as having the best visualized tumor, with consensus. One gastrointestinal radiologist analyzed the selected 5 mm tumor section image by using perfusion software based on distributed parameter analysis (Body Protocol, Perfusion 3.0; GE Healthcare Technologies). An arterial input was defined by using a mouse to place a circular region of interest (ROI), 25 mm2 in area, within the best visualized iliac or femoral artery on the selected image. The software automatically derived a smoothed arterial time-enhancement curve, and parametric maps were generated, with each pixel representing a parameter value.
Three circular or ovoid ROIs (mean area = 31.23 mm2; range = 13-67 mm2) were manually defined on the most enhanced area of the tumor. The ROIs of the tumor did not include central, poorly enhanced, low-attenuated areas, which were considered to be areas of necrosis or poorly vascularized areas within the tumor. Among three ROIs, the one with the highest attenuation was selected as the tumor ROI (mean area = 30.85 mm2; range = 13-57 mm2). Tumor blood flow (BF), BV, mean transit time (MTT), and PS measurements were obtained by manually defining a tumor ROI within the tumor boundary (Fig. 1). We took care to ensure that the tumor ROI remained within the tumor boundary, and excluded luminal gas when present, by viewing a cine loop of the perfusion CT images. The mean values for each of the four perfusion parameters (BF, BV, MTT, and PS) were recorded.
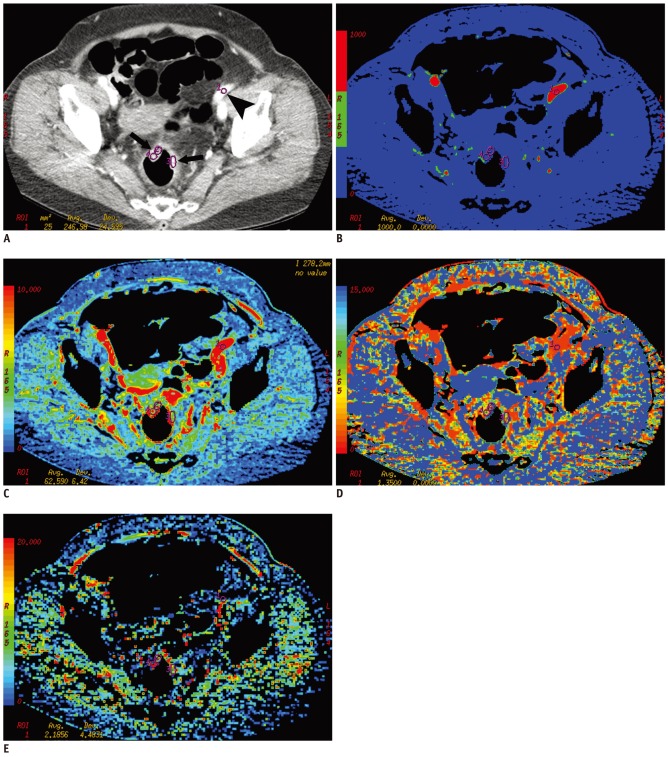
Fig. 1.
Perfusion CT of rectal cancer in 68 year-old woman.
A. Contrast-enhanced CT image shows tumor region of interests within enhancing rectal cancer (arrows) and arterial input (arrowhead) in left iliac artery. B-E. Corresponding functional color maps of rectal tumor (arrow) and iliac artery (arrowhead) for each perfusion parameter [(B) blood flow, (C) blood volume, (D) mean transit time, and (E) permeability surface-area product] are displayed according to color scale.
Tumor Grade, Immunohistochemical Staining and Microvessel Density
The surgical specimens were fixed with 10% formaldehyde and embedded in paraffin, then the tumor specimens were divided by one gastrointestinal pathologist (JHL, with 15 years of experience) into three histologic subgroups (well differentiated, moderately differentiated, and poorly differentiated CRC).
For evaluation of tumor angiogenesis, additional tissue blocks from each tumor were acquired for further immunohistochemical staining. The locations for additional tissue on tumor specimens were selected at the mid-portion of the tumor in the craniocaudal direction, then several 4 µm-thick sections (range, 3-7 µm) acquired by 5 mm intervals along the cranial and caudal direction from the selected tumoral mid-portion. Particular care was taken to ensure additional tissue did not include necrotic portions of the tumor, if possible.
A representative paraffin block of 10% formalin-fixed tumor tissue was cut to a thickness of 4 µm and immunohistochemical staining was carried out using a Microprobe Immuno/DNA stainer (Fisher Scientific, Pittsburgh, PA, USA). Immunohistochemical analysis of CD34 (titer 1:200, monoclonal; Zymed, San Francisco, CA, USA) was performed using the avidin-biotin complex method. Sections were deparaffinized, then incubated in the primary antibodies for 20 minutes at 45℃. Tissue sections for CD34 were incubated in pepsin solution (Research Genetics, Huntsville, AL, USA) for 4 minutes before application of primary antibody. Anti-mouse immunoglobulin G (Sigma Chemical, St. Louis, MO, USA) labeled with biotin was used, and a secondary antibody for the detection of primary antibodies was incubated for 7 minutes at 45℃. The streptavidin-horseradish peroxidase detection system (Research Genetics, Huntsville, AL, USA) was then applied to capillary channels, followed by 10 minutes of incubation at 45℃. After drainage, the tissue sections were ready for chromogen reaction with 3,3'-diamino-benzidine. The sections were counterstained with hematoxylin and mounted in Universal Mount (Research Genetics, Huntsville, AL, USA). Negative controls were treated similarly, with the exception of primary antibodies.
Hot spot analysis was used as criteria for microvessel counting, as established by Weidner (16). The rationale of counting microvessels in vascular 'hot spots,' or in areas giving the impression at low magnification of containing numerous microvessels, is that these areas originated from tumor cell clones with the highest angiogenic potential, and consequently, had the easiest access to the blood stream and an increased probability of producing metastasis capable of becoming angiogenic and growing tumors (17).
Any single brown-staining endothelial cell or small clusters of brown-staining endothelial cells, with or without a lumen, clearly separate from adjacent microvessels, tumor cells, and other connective tissue elements, were considered as individual vessels. Vessels of a caliber larger than approximately eight red blood cells and vessels with a thick muscular wall were excluded from the final count.
Slides were examined at low-power magnification (× 40) to identify the areas with the highest density of microvessels. In each case, the most vascularized area was selected and the microvessels within a high-power magnification (× 200) field of this area were counted 3 times. The mean of the three highest counts per tumor was used for analysis (Fig. 2).
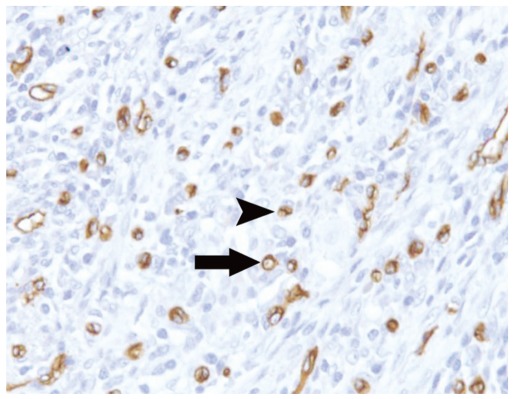
Fig. 2.
Increased CD34 immunostaining in moderately differentiated colorectal adenocarcinomas was observed.
Microvessels were defined as single brown-staining endothelial cells with lumen (arrow) and small clusters of brown-staining endothelial cells without lumen (arrowhead) (CD34 immunostaining at × 200 magnification).
Statistical Analysis
The correlation between the four perfusion parameters (BF, BV, MTT, and PS) and MVD, and the three histologic grades of CRC was examined using the Kruskal-Wallis test. The correlation between perfusion parameters and MVD was analyzed using Pearson's correlation analysis. All analyses were performed using the Statistical Package for the Social Sciences (SPSS, version 17; SPSS, Chicago, IL, USA). A value of p < 0.05 was considered significant.
RESULTS
All tumors were histologically proven to be non-mucinous CRCs after surgery. The mean interval between pre-operative CT scan and surgery was 23.5 days (range of 7-75 days). Of 27 CRCs, there were 8, 15, and 4 well differentiated, moderately differentiated, and poorly differentiated CRCs, respectively. The tumors were located in the rectum and sigmoid colon in 23 and 4 patients, respectively. There were 3 T2 tumors, 21 T3 tumors, and 3 T4 tumors.
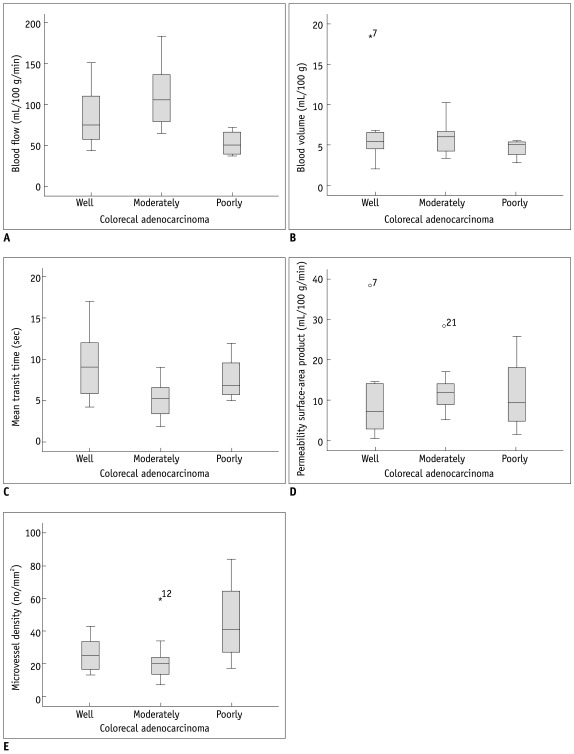
The correlation of perfusion parameters and tumor grade is summarized in Table 1. The mean BF was significantly higher in moderately differentiated than well differentiated and poorly differentiated CRCs (p = 0.014). The mean MTT was significantly shorter in moderately differentiated compared with well differentiated and poorly differentiated CRCs (p = 0.039). There was no significant correlation between the other perfusion parameters (BV and PS) and tumor grade (p > 0.05). The MVD was significantly greater in poorly differentiated than well differentiated and moderately differentiated CRCs (p = 0.034) (Table 1, Fig. 3).
Fig. 3.
Relationship between CT perfusion parameters (A-D) and microvessel densities (E) of well differentiated, moderately differentiated, and poorly differentiated colorectal adenocarcinoma (CRC). There were significant differences in blood flow (A), mean transit time (C), and microvessel densities (E) between well differentiated, moderately differentiated, and poorly differentiated CRC (p < 0.05).
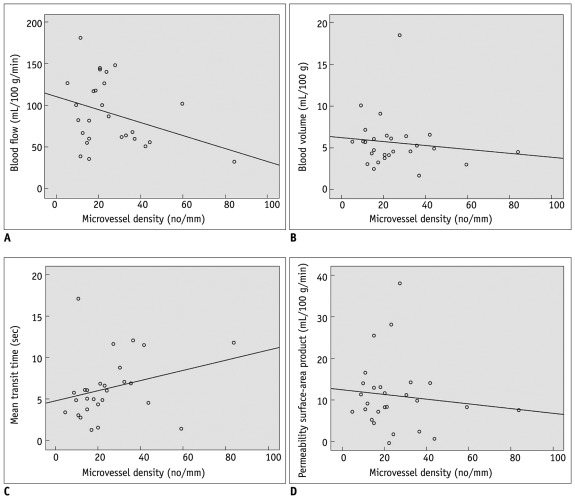
The Pearson's correlation coefficients (r) and p values between the four perfusion parameters (BF, BV, MTT, and PS) and MVD in CRCs are summarized in Table 2. There was no significant correlation between the four perfusion parameters and MVD in CRCs (Fig. 4).
Fig. 4.
Correlation plots of microvessel density (MVD) and CT vascular parameters. (A) blood flow, (B) blood volume, (C) mean transit time, and (D) permeability surface-area product. No perfusion parameters correlated significantly with MVD.
DISCUSSION
Our study showed that the mean BF was significantly lower in poorly differentiated than well differentiated and moderately differentiated CRCs. There was no significant difference between tumor grades with respect to the other perfusion parameters. Among the four perfusion parameters (BF, BV, MTT and PS), it is well known that BF reflects the flow rate through the vasculature in the tissue region and is related to tumor vascularity and tumor grade. In general, as the tumor grade is higher, the BF value further increases. Dugdale et al. (18) demonstrated a correlation between higher tumor BF and higher grade of lymphoma based on histopathologic examination. There are some conflicting results between previous reports and our study. These observations stand in contrast to the conventional belief that high-grade tumors have increased vascularity. Contrary to current dogma, Sahani et al. (19) reported that the mean BF values were higher in well differentiated HCC than in moderately differentiated or poorly differentiated HCCs. They suggested that a relatively larger tumor diameter (mean of 9 cm) and the presence of tumor necrosis in the high-grade tumor group could account for their results. There were several problems in comparing directly HCC and CRC because HCC and CRC are different in tumor nature and behavior. We found that the vascularities of CRC were increased in moderately differentiated CRCs compared to well differentiated CRCs, and decreased in poorly differentiated CRCs compared to moderately differentiated and well differentiated CRCs. There are two hypotheses to account for our results. First, the growth rate of CRCs was too rapid to develop angiogenesis of mature vessels in poorly differentiated CRCs. Second, poorly differentiated CRCs have larger regions in which endothelial cells have high vascular permeability, therefore the interstitial pressure will increase and can compress the capillary sprouts and small capillaries more than in well differentiated and moderately differentiated CRCs.
Our study showed that the mean MTT was significantly shorter in moderately differentiated than in well differentiated and poorly differentiated CRCs. It is well established that the MTT is defined as the average time required to travel from the artery to the vein; the MTT is considered to be a marker of perfusion pressure. Sahani et al. (19) reported that well differentiated HCCs had a shorter MTT and higher BF than moderately differentiated and poorly differentiated HCCs. Our study showed that moderately differentiated CRCs had a shorter MTT and higher mean BF than well differentiated and poorly differentiated CRCs, thus the results in the current study are similar to of the results of Sahani et al. (19)
MVD assessment is the most commonly used technique to quantify intratumoral angiogenesis in cancer (13). Our study also showed a correlation between tumor grades and MVD. Tumor vessels, irrespective of tumor size, demonstrate a zonal distribution in which vascularity decreases from the tumor edge to the center (20, 21). As the tumor grows, the central portions become relatively hypovascular and eventually necrotic. MVD is a surrogate marker of tumoral angiogenesis and has been proposed to identify patients at high risk of recurrence, since high values of MVD have been correlated with poor outcome in CRC (4, 5). This association is greatest when measurements of MVD are restricted to the peripheral angiogenesis zone (20, 22). However, other studies suggest that the relationship between MVD and CRC is controversial (6, 8, 15). Chen et al. (23) reported that the gross enhancement morphology of HCC lesions is also correlated with tumor MVD, and reflects the distributive features of tumor microvessels within HCC lesions. Jinzaki et al. (24) reported that the enhancement pattern on CT was different among the subtypes of RCCs, and correlated with either MVD or with the existence of intratumoral necrosis or hemorrhage. Therefore, we hypothesized that the enhanced area of tumors without necrosis in defining tumor ROI reflects MVD for angiogenesis more than including the entire tumor with a central, poorly vascularized area in CRC. Our study used an enhanced focal area as a tumor ROI "so called hot spot analysis" rather than the entire tumoral area. Hot spot analysis is a widely performed technique that has been shown to yield results that are reflective of both tumor neovascularization and prognosis (13, 17). In poorly differentiated CRCs, MVD was significantly increased compared to well differentiated and moderately differentiated CRCs. However, there was no significant correlation between perfusion parameters and MVD. The results are similar to the findings reported by Li et al. (15). The study defined the maximally enhanced large area of the tumor as the tumor ROI and there was no clinical correlation between MVD and perfusion parameters. In contrast, Goh et al. (14) reported that the tumor PS and BV correlate positively with MVD and may reflect the microvascularity of CRCs. They defined the entire area of the tumor on selected CT images as the tumor ROI and used two counting methods of systematic field and hot spot analysis (17, 25). However, there is a paucity of CRC data using systemic field analysis with the former approach. This difference in the methods of defining tumor ROI and microvessel counting between our study and the study of Goh et al. (14) may be one of the causes accounting for the different results, and as such there is still a need for standardization of microvessel counting. Because tumor angiogenesis can be assessed with different microvessel counting methods, the issue of which method is the most effective remains controversial (26).
There were several limitations to our study. First, the study sample size was small and there were few cases of poorly differentiated CRCs because the prospective consecutive selection of patients was done prior to surgery. Accordingly, a study with a larger number of patients is needed. Second, there is no precise correlation between tumor ROI in perfusion CT and pathologic specimen after surgery. Third, radiation hazards still remain a limitation in perfusion CT. Nevertheless, as CT machines and software have been developed and additional research is performed, the radiation dose of perfusion CT to patients may decrease.
In conclusion, BF and MTT may be effective and clinically useful in the prediction of moderately differentiated CRCs, although the BV and PS are not effective in prediction of tumor grade in CRCs. Pre-operative perfusion CT does not reflect MVD of CRCs.
References
- 1.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993;54:594–606. doi: 10.1002/ijc.2910540413. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Burton S, Norman AR, Brown G, Abulafi AM, Swift RI. Predictive poor prognostic factors in colonic carcinoma. Surg Oncol. 2006;15:71–78. doi: 10.1016/j.suronc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Takebayashi Y, Aklyama S, Yamada K, Akiba S, Aikou T. Angiogenesis as an unfavorable prognostic factor in human colorectal carcinoma. Cancer. 1996;78:226–231. doi: 10.1002/(SICI)1097-0142(19960715)78:2<226::AID-CNCR6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Gardy R, Seon BK, Duff SE, Abdalla S, Renehan A, et al. Both high intratumoral microvessel density determined using CD105 antibody and elevated plasma levels of CD105 in colorectal cancer patients correlate with poor prognosis. Br J Cancer. 2003;88:1424–1431. doi: 10.1038/sj.bjc.6600874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawighorst H, Knapstein PG, Knopp MV, Weikel W, Brix G, Zuna I, et al. Uterine cervical carcinoma: comparison of standard and pharmacokinetic analysis of time-intensity curves for assessment of tumor angiogenesis and patient survival. Cancer Res. 1998;58:3598–3602. [PubMed] [Google Scholar]
- 7.Macchiarini P, Fontanini G, Hardin MJ, Squartini F, Angeletti CA. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet. 1992;340:145–146. doi: 10.1016/0140-6736(92)93217-b. [DOI] [PubMed] [Google Scholar]
- 8.Bossi P, Viale G, Lee AK, Alfano R, Coggi G, Bosari S. Angiogenesis in colorectal tumors: microvessel quantitation in adenomas and carcinomas with clinicopathological correlations. Cancer Res. 1995;55:5049–5053. [PubMed] [Google Scholar]
- 9.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 10.Gasparini G, Weidner N, Maluta S, Pozza F, Boracchi P, Mezzetti M, et al. Intratumoral microvessel density and p53 protein: correlation with metastasis in head-and-neck squamous-cell carcinoma. Int J Cancer. 1993;55:739–744. doi: 10.1002/ijc.2910550507. [DOI] [PubMed] [Google Scholar]
- 11.Wakui S, Furusato M, Itoh T, Sasaki H, Akiyama A, Kinoshita I, et al. Tumour angiogenesis in prostatic carcinoma with and without bone marrow metastasis: a morphometric study. J Pathol. 1992;168:257–262. doi: 10.1002/path.1711680303. [DOI] [PubMed] [Google Scholar]
- 12.Olivarez D, Ulbright T, DeRiese W, Foster R, Reister T, Einhorn L, et al. Neovascularization in clinical stage A testicular germ cell tumor: prediction of metastatic disease. Cancer Res. 1994;54:2800–2802. [PubMed] [Google Scholar]
- 13.Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823–1832. doi: 10.1038/sj.bjc.6603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goh V, Halligan S, Daley F, Wellsted DM, Guenther T, Bartram CI. Colorectal tumor vascularity: quantitative assessment with multidetector CT--do tumor perfusion measurements reflect angiogenesis? Radiology. 2008;249:510–517. doi: 10.1148/radiol.2492071365. [DOI] [PubMed] [Google Scholar]
- 15.Li ZP, Meng QF, Sun CH, Xu DS, Fan M, Yang XF, et al. Tumor angiogenesis and dynamic CT in colorectal carcinoma: radiologic-pathologic correlation. World J Gastroenterol. 2005;11:1287–1291. doi: 10.3748/wjg.v11.i9.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- 17.Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38:1564–1579. doi: 10.1016/s0959-8049(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 18.Dugdale PE, Miles KA, Bunce I, Kelley BB, Leggett DA. CT measurement of perfusion and permeability within lymphoma masses and its ability to assess grade, activity, and chemotherapeutic response. J Comput Assist Tomogr. 1999;23:540–547. doi: 10.1097/00004728-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue--initial experience. Radiology. 2007;243:736–743. doi: 10.1148/radiol.2433052020. [DOI] [PubMed] [Google Scholar]
- 20.Konerding MA, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer. 2001;84:1354–1362. doi: 10.1054/bjoc.2001.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goh V, Halligan S, Gharpuray A, Wellsted D, Sundin J, Bartram CI. Quantitative assessment of colorectal cancer tumor vascular parameters by using perfusion CT: influence of tumor region of interest. Radiology. 2008;247:726–732. doi: 10.1148/radiol.2473070414. [DOI] [PubMed] [Google Scholar]
- 22.Miles KA. Tumour angiogenesis and its relation to contrast enhancement on computed tomography: a review. Eur J Radiol. 1999;30:198–205. doi: 10.1016/s0720-048x(99)00012-1. [DOI] [PubMed] [Google Scholar]
- 23.Chen WX, Min PQ, Song B, Xiao BL, Liu Y, Ge YH. Single-level dynamic spiral CT of hepatocellular carcinoma: correlation between imaging features and density of tumor microvessels. World J Gastroenterol. 2004;10:67–72. doi: 10.3748/wjg.v10.i1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jinzaki M, Tanimoto A, Mukai M, Ikeda E, Kobayashi S, Yuasa Y, et al. Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumor angiogenesis. J Comput Assist Tomogr. 2000;24:835–842. doi: 10.1097/00004728-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Beresford MJ, Harris AL, Ah-See M, Daley F, Padhani AR, Makris A. The relationship of the neo-angiogenic marker, endoglin, with response to neoadjuvant chemotherapy in breast cancer. Br J Cancer. 2006;95:1683–1688. doi: 10.1038/sj.bjc.6603491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Offersen BV, Borre M, Overgaard J. Quantification of angiogenesis as a prognostic marker in human carcinomas: a critical evaluation of histopathological methods for estimation of vascular density. Eur J Cancer. 2003;39:881–890. doi: 10.1016/s0959-8049(02)00663-9. [DOI] [PubMed] [Google Scholar]