Abstract
Objectives
To formally study the prevalence and histological classification of renal cell carcinoma (RCC) in a series of patients with PTEN Hamartoma Tumor syndrome (PHTS).
Methods
We evaluated prevalence of RCC within a prospectively-accrued series of 219 patients found to have pathogenic germline PTEN mutations. Clinical data including pathology reports were requested for all participants. Slides and tumor blocks were requested for central pathology re-review and immunohistochemistry (IHC) analysis.
Results
Nine patients were identified with RCC. Based on SEER data 0.28 RCC cases were expected for the group, giving an overall age-adjusted Standardized Incidence Ratio (SIR) of 31.7 (95% CI 15.4–58.1, p<0.001) with a higher sex-adjusted SIR for females (46.7 vs. 21.6 for males). Reported histology of each mutation positive patient’s RCC was variable. However, on central pathology re-review of 8 patients, six examined lesions were determined to be of papillary subhistology (pRCC), with the other two patients’ tumors consistent with the initial report of chromophobe RCC (chRCC). IHC demonstrated complete loss of PTEN protein in all PTEN mutation positive patients’ pRCCs and patchy positivity in one chRCC.
Conclusions
PHTS is a hereditary syndrome newly associated with pRCC, and PTEN IHC may be a helpful screening tool to identify pRCC patients with PHTS. Physicians caring for PHTS patients should note the >31-fold increased risk for RCC and have a low threshold for investigating possible RCC in patients with relevant complaints. Renal ultrasound is not sensitive for detecting pRCC and so PHTS patients should have alternate renal imaging (CT or MRI).
Keywords: PTEN germline mutations; Cowden syndrome; Bannayan-Riley-Ruvalcaba syndrome; Carcinoma, Kidney
INTRODUCTION
PTEN Hamartoma Tumor Syndrome (PHTS) is a molecularly-defined umbrella term used to describe individuals with Cowden Syndrome (CS, OMIM 158350), Bannayan-Riley-Ruvalcaba Syndrome (BRRS, OMIM 153480), and other conditions with germline mutations of the PTEN tumor suppressor gene, localized to 10q23.1,2 CS is an autosomal dominant condition which causes increased risk for benign and malignant neoplasias, most notably up to a 50% lifetime risk for female breast cancer and 10% lifetime risk for epithelial thyroid carcinoma.2,3 It is assumed that individuals with BRRS or another clinical diagnosis who have germline PTEN mutation, i.e. PHTS, carry similar risks, and management guidelines focus on reducing morbidity and mortality from the two known major component cancer types. Case reports exist which describe PHTS, CS and BRRS patients with an array of other cancer types, including renal cell carcinoma (RCC),4,5 and the operational diagnostic criteria for CS recognize genitourinary tumors and malformations as a minor feature.6 Previous studies have demonstrated loss of PTEN protein in a minority of sporadic RCC tissue and RCC-derived cell lines with one study correlating loss of heterozygosity at 10q23 with poor patient prognosis, but others finding no association between reduced PTEN expression and patient survival.7–13 It seems reasonable that patients with PHTS, who have one mutated PTEN gene in all bodily tissues, would be at increased risk for RCC. However, RCC prevalence and histology have yet to be studied systematically in a large PHTS patient series. Thus, we sought to determine the incidence of RCC in our PHTS patients and performed central pathology re-review to determine the histological types of RCC and PTEN expression analysis by immunohistochemistry (IHC) of these RCC.
MATERIALS AND METHODS
From October 15, 2005 to August 4, 2011, 3,333 eligible patients were enrolled into Cleveland Clinic IRB# 8458 (as approved by the Cleveland Clinic Human Subjects’ Protection Committee) if they minimally met the following: “relaxed” criteria for the diagnosis of CS (presence of 2 major/pathognomonic, 1 major/pathognomonic + 2 minor, or 3 minor characteristics of Cowden syndrome per the International Cowden Consortium diagnostic criteria6); presence of macrocephaly and developmental disability/autism; presence of penile freckling; or presence of a known germline PTEN alteration. Clinical data including pathology reports were requested for all participants.
PTEN mutation analysis was performed using genomic DNA isolated from research participants’ peripheral blood leukocytes or from banked germline DNA samples. Mutation analysis for all subjects included promoter sequencing and mutation scanning of all exons and flanking intronic regions by denaturing gel gradient electrophoresis (DGGE) or LightScanner technology; variants detected by DGGE or LightScanner were confirmed with single exon Sanger sequencing as per routine of the Eng lab as previously described.14 Samples from patients meeting ICC criteria, those with macrocephaly and developmental disability/autism, and those with a personal/family history of a known large PTEN rearrangement also underwent multiplex ligation probe assay (MLPA); MLPA-identified rearrangements were confirmed with quantitative PCR.14
Expected numbers of cancers were obtained using age-specific SEER incidence rates from 2004–2008 (http://seer.cancer.gov). OpenEpi software was used to calculate Standardized Incidence Rate (SIR). The 95% confidence interval and corresponding p-value were calculated using the mid-P exact test.
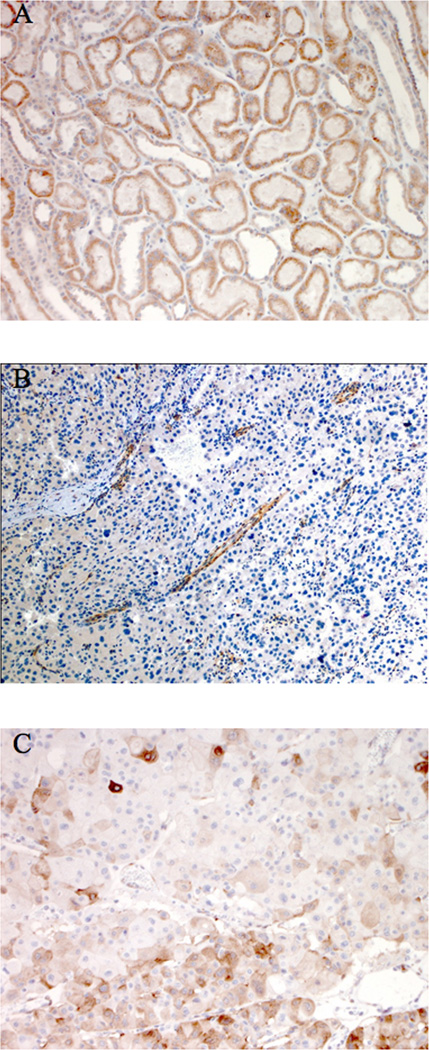
Hematoxylin and Eosin-stained (HE) sections and tumor blocks were requested for patients with an RCC diagnosis. Materials were available for 8 patients and were blindly reviewed by a dedicated uropathologist (MZ). PTEN IHC was performed on unstained sections using the 6H2.1 monoclonal antibody (Cascade Biosciences, dilution 1:50). Briefly, 4 µm sections were deparaffinized and antigen retrieval was performed for 30 minutes at 98°C in an EDTA solution (pH 8.0). Endogenous biotin was blocked with an avidin/biotin blocking step prior to primary antibody incubation. After overnight incubation of primary antibody at 4°C, sections were incubated with a biotinylated anti-mouse IgG secondary antibody. PTEN antigen detection was visualized by a 3,3’-diaminobenzadine chromogen step. Normal kidney tissue was used as an internal positive control (Figure 1a).
Figure 1.
PTEN IHC staining, demonstrating normal expression in normal kidney tissue (A), complete loss in a representative papillary RCC tumor with positive internal control staining of glomeruli and blood vessels (B), and patchy expression in the chromophobe carcinoma (C).
RESULTS
Of the 3,333 eligible research participants, 219 were found to have pathogenic germline PTEN mutations. To be conservative, patients carrying variants of unknown significance were ineligible for the current study. Nine of the 219 PTEN mutation positive patients were identified with a prevalent or incident history of RCC (Table 1). Based on SEER data, only 0.28 cases were expected for the total group. Thus, the age-adjusted SIR for RCC in this PTEN mutation positive group is 31.7 (95% CI 15.4–58.1, p<0.001). When stratified by gender, the SIR was higher for females (46.7, 95% CI 18.9–97.2) than for males (21.6, 95% CI 5.5–58.7). One patient had bilateral metachronous RCC; all other patients had unifocal tumors, and no patient had RCC-related metastases or mortality. Family history was absent for RCC in the relatives of our 9 patients with germline PTEN mutation and RCC.
Table 1.
Demographic and clinical data for PTEN-mutation positive patients with RCC
| Patien t ID |
Genotyp e |
Se x |
Age at RCC dx |
Laterali ty |
Tumor Size |
Presentation Leading to RCC Diagnosis |
Other CS Features |
|---|---|---|---|---|---|---|---|
| 105- 001 |
c.491del A |
M | 11 | Left | 4 cm | Fever, left flank pain, nausea; normal U/A, culture, and IVP |
L, T, ThyCA |
| 1731- 001 |
c.150del T |
M | 53 | Left | 1 cm | Incidentally identified during lung scan performed due to breathing difficulties |
AK, CP, GA, GP, L, M, MNG, OP, SF,T |
| 3147- 001 |
c.1003C> T (Arg335T er) |
F | 44 | Right | 10 cm | Right lower abdominal and flank pain |
M, ThyCA |
| 1495- 001 |
c.1003C> T (Arg335T er) |
F | 45 | Left | 6.5 cm | Microscopic hematuria, left flank pain |
GP, MNG, OP, UF |
| 1334- 001 |
c.210- 1G>A |
F | 53 | Left | 6.8 cm | Incidentally identified during PET scan performed due to breast cancer history |
DCIS, FBD, GA, GP, M, MNG |
| 857- 002 |
c.388C> T (Arg130T er) |
F | 64 | Right | 3.1 cm | Incidentally identified during MRI performed during endometrial cancer workup |
AK, CP, GM, GP, GT, M, MNG, OP, UtCA |
| 6092- 001 |
c. 164+1 G>T |
M | 49 | Right | 2 cm | Recurrent UTI, right flank pain |
GP, L, M, MNG |
| 4635- 001 |
c.70G>C (Asp24Hi s) |
F | 58 | Right | 1.3 cm | Incidentally identified during CT performed due to history of breast and uterine cancers |
BrCA, M, MNG, OP, SF, UtCA |
| 5266- 001 |
c.388C> T (Arg130T er) |
F | 32 | Bilatera I |
4 cm (R), 10 cm (L) |
Left flank pain | FBD, GP, MNG, UtCA |
AK = Acral keratoses; BrCA = Breast cancer; CP = Cutaneous papillomatosis; DCIS = Ductal carcinoma in situ; FBD = Fibrocystic breast disease; GA = Glycogenic acanthosis; GM = Genitourinary malformation; GP = GI Polyposis; GT = Genitourinary tumor; L= Lipoma; M = Macrocephaly; MNG = Multinodular goiter; OP = Oral papillomatosis; SF = Sclerotic fibromas; T = Trichilemmomas; ThyCA = Thyroid cancer; UF = Uterine fibroids; UtCA = Uterine cancer
Initial reporting of tumor histology was variable and complex, with papillary features identified in a majority of tumors. Re-review of nine tumors identified in eight patients for which materials were available showed that six patients had pRCC and the other two had chRCC (including one patient with bilateral chRCC, Table 2). Four cases were type I pRCC with tumor cells with scant cytoplasm and low grade nuclei. Two cases were type IIB pRCC with abundant eosinophilic cytoplasm and high grade (Fuhrman grade III or IV) nuclei. Precursor lesions consisting of papillary adenomas were identified in two cases of pRCC. The histologic appearance of these papillary and chromophobe RCCs is identical to sporadic cases. IHC analysis found complete loss of PTEN expression in all five pRCCs studied and patchy positivity in one chRCC (Figure 1b–c).
Table 2.
Initial and Reviewed Histology for Examined RCCs from PHTS patients
| Patien t ID |
Initial histology | Central Review histology | ||||
|---|---|---|---|---|---|---|
| Fuhrm an Grade |
Histology Descriptio n |
Fuhrm an Grade |
Histology Description |
Pre- cursor lesions |
Normal tissue | |
| 105- 001 |
III–IV | Clear and granular cell |
Information unavailable | |||
| 857- 002 |
I | Mucinous tubular and spindle cell |
II | Type I Papillary, solid variant |
Papillary adenoma as |
Unremarkable |
| 1334- 001 |
IV | Chromoph obe |
N/A | Chromophob e, eosinophilic variant with raisinoid nuclei |
None | Unremarkable |
| 1495- 001 |
II | Predomina ntly papillary with focal clear cell features |
II | Type I Papillary with some clear cell changes next to necrosis |
Papillary adenom as |
Unremarkable |
| 1731- 001 |
III | Papillary | IV | Type IIB Papillary |
None | Glomerulosclero sis, diabetic changes |
| 3147- 001 |
III | Clear cell dominant with tubular, trabecular and papillary features |
II | Type I Papillary with secondary clear cell changes next to necrotic areas |
None | Histiocytes, extensive lymphoid follicles, hilar lymph node, perinephric renal capsule with lymphocytic reaction |
| 4635- 001 |
NOS | Papillary | II | Type IIB Papillary with solid areas |
None | Lymphoid infiltrates |
| 5266- 001 |
R: II L: III |
Chromophobe | N/A | R: Chromophob e, classical type L: Chromophob e, classical and eosinophilic type |
None | Unremarkable |
| 6092- 001 |
NOS | Papillary | II | Type I Papillary |
None | Unremarkable |
L = Left kidney tumor; R = Right kidney tumor
COMMENT
We observed an increased SIR for RCC in our PHTS patients, with an over-representation of papillary histology among these RCC. Given that papillary and chromophobe RCCs make up only 10–15% and 5% of all RCC cases, respectively,15,16 identifying these histologic types and not the more common clear cell histology in PHTS patients is notable. According to SEER data (www.seer.cancer.gov), the median age at diagnosis for cancer of the kidney and renal pelvis is 64 years of age, with 71.4% of cases diagnosed after age 55 and approximately a 2:1 male-to-female ratio. In our series, the median and mean ages at diagnosis were 49 and 45.4 years respectively, with a 2:1 female-to-male ratio. These data are consistent with the knowledge that patients with cancer as part of an inherited syndrome have an earlier age at diagnosis than sporadic cases.17
Previous studies of PTEN expression in sporadic RCC were mainly on clear cell RCC and a relatively small number of papillary and chromophobe RCCs. In the largest series, PTEN expression was negative in 2/44 (4.5%) papillary and 1/22 (4.5%) chromophobe RCCs, with the majority of these tumors (68% and 82% respectively) showing positive, but decreased, staining.9 A smaller study found PTEN expression in all studied RCCs, with reduced expression in 0/2 papillary and 4/8 chromophobe tumors. In contrast, the pRCC tumors from PHTS patients all showed completely negative PTEN expression, supporting a causative role for PTEN in the development of these tumors. Understanding the exact mechanism causing the observed loss of expression in these cases – whether a second mutation, loss of heterozygosity (i.e., deletion), promoter hypermethylation, or other – would make for an interesting follow-up study.
The patchy positivity observed in one chromophobe RCC studied does not allow us to draw a similar conclusion for PTEN’s role in the development of this tumor type. However, reduced Pten dose has been associated with risk of tumorigenesis in murine models18, implicating that this patient’s PTEN mutation may still have contributed to this cancer risk. More recently, we observed a hint of the importance of PTEN dosage by germline protein studies in our large PHTS series.14 Papillary adenomas are commonly identified in kidneys from sporadic pRCC cases19 and thus we were not surprised to identify them in two of our PHTS patients with papillary RCC.
All patients had other clinical features, which before the age of molecular diagnosis, would have granted them a clinical diagnosis of Cowden syndrome or sufficient suspicion for this condition to warrant a genetics evaluation. Eight of the nine patients in this series have germline PTEN mutations which are predicted to lead to protein truncation, the most common type of mutation observed in PHTS.14 Four patients possessed the mutations Arg130Ter and Arg335Ter which are well-studied “hot-spot” mutations. Arg130Ter is within PTEN’s phosphatase core motif; mutations in this area destroy PTEN’s ability to dephosphorylate protein and lipid substrates, thereby causing loss of its tumor suppression functions.20 This type of mutation has been shown to prevent PTEN from exiting the nucleus, limiting its ability to promote apoptosis through the AKT pathway.21 This evidence may show that RCC tends to occur in those patients with a PTEN mutation predicted to cause a more severe phenotypic outcome.
The National Comprehensive Cancer Network (NCCN) carries screening guidelines for PHTS patients, but they do not include RCC screening. We have recently recommended biannual RCC screening for PHTS patients beginning at age 40 because of the 34% lifetime risk calculated.22 The 31-fold SIR for RCC identified in this patient population is notable, and clinicians caring for patients with PHTS should have a low threshold for investigating possible RCC in PHTS patients with relevant symptoms such as hematuria. Renal ultrasound is not sensitive for detecting papillary and other solid histology RCC due to their hypovascular nature23, thus we recommend alternative imaging by CT or MRI.
IHC of mismatch repair proteins is a cost-effective screening tool to identify patients with Lynch syndrome, an inherited condition causing increased risks for colorectal, uterine, and other cancer types. A study by the Centers for Disease Control and Prevention estimated cost-effectiveness ratios of $45,000–$75,000 per year-life saved when mutation analysis is performed on colorectal cases with abnormal IHC results, implicating a risk for germline mutation in one of the four mismatch repair genes. Cost-effectiveness is increased further when family members of a mutation positive individual are able to discover their mutation status and pursue earlier and more frequent colonoscopy screening to remove adenomatous polyps and identify tumors at the earliest, most treatable stage. 24 Given the findings in this study, a similar approach may be feasible for pRCC tumors in PHTS.
Until such a tumor screening protocol is established, patients with papillary or chromophobe RCC may be referred for clinical cancer genetics consultation, which includes genetic counseling, for a comprehensive personal and family history analysis assessing for features consistent with PHTS or other inherited neoplasia syndromes (Table 3). Several of these syndromes have considerable overlap in features, and thorough assessment by a genetics professional can help pinpoint which condition is most likely so that testing can proceed in a step-wise and cost-saving manner. Should an inherited syndrome be identified, the patient as well as their family members can benefit greatly from this knowledge. The conditions listed in Table 3 are inherited in an autosomal dominant manner, meaning that if a patient has the condition, their first-degree relatives (parents, siblings, and children) are assumed to be at 50% risk to also be affected. If a gene mutation can be identified, predictive testing can be offered to at-risk relatives to help them learn their genetic status, and if mutation positive, seek preventive screening and surgical measures to reduce their cancer risks. Genetic counseling is a vital part of this process. The American Society of Clinical Oncology recommends pre- and post-test counseling when genetic testing is considered to ensure the patient is aware of the benefits and limitations of testing and provides their informed consent.25 Genetic counselors are health professionals with specialized graduate degrees, most commonly Master’s level, skilled at analyzing a personal and family history to assess risk for an inherited syndrome, educating patients about this risk, facilitating appropriate testing, and helping the patient understand what results mean for themselves as well as their family members (www.nsgc.org).
Table 3.
Genetic Differential Diagnoses of Papillary and Chromophobe RCC
| Syndrome | Gene | OMIM # | Renal features | Other features |
|---|---|---|---|---|
| Familial papillary renal cell cancer |
MET | 605074 | Multiple bilateral type 1 papillary RCCs |
None |
| Hereditary leiomyomatosis and renal cell cancer |
FH | 605839 | Type 2 papillary RCC |
Cutaneous and uterine leiomyomas; leiomyosarcoma |
| Birt-Hogg-Dubé syndrome |
FLCN | 135150 | Multiple chromophobe- oncocytoma hybrid, clear cell, papillary RCCs |
Facial fibrofolliculomas, lung cysts, spontaneous pneumothoraces |
| Familial Paraganglioma Syndrome |
SDHB, SDHD |
115310, 168000 |
Chromophobe, clear cell, papillary RCC; oncocytoma |
Benign and malignant paragangliomas, papillary thyroid carcinoma |
|
PTEN Hamartoma Tumor syndrome |
PTEN | 601728 |
Unifocal papillary RCC, chromophobe RCC |
Macrocephaly; GI polyposis; malignant and benign breast, non- medullary thyroid, and uterine tumors; autism spectrum/developmental delays; benign mucocutaneous lesions (trichilemmomas, skin and oral papillomas, acral keratoses, lipomas, freckling of the glans penis) |
In many inherited cancer syndromes, patients are at increased risk for second primary tumors and increased surveillance and prophylactic surgical options are offered. For the patient him/herself, knowing which specific gene is mutated is important to understand which non-RCC malignancies he/she is at risk for and to be able to tailor surveillance or prophylactic measures in a gene-specific manner. For example, female breast and epithelial thyroid cancers are the two most common cancer risks in CS, with similar risks presumed in PHTS. The NCCN (www.nccn.org) recommends screening start at age 18 with a baseline thyroid ultrasound, and that women begin having semiannual clinical breast exams at 25 and annual mammography and breast MRI at age 30–35, with these screenings beginning even earlier should family history dictate. Risk-reducing prophylactic mastectomy is also offered as an option. These aggressive approaches to medical management are offered to prevent cancer diagnoses in their entirety and to detect any cancers at the earliest, most treatable stages. If a syndromic diagnosis is missed, the patient would not be aware of these increased neoplasia risks and would be directed to adhere to the American Cancer Society’s general population screening guidelines, which do not include screening for thyroid cancer at all and include only mammography for women beginning at age 40 with high-risk screening offered to only those women with a 20–25% risk for breast cancer based on personal and family history factors.26,27
Should genetic testing be negative, genetics providers can also offer appropriate opportunities for research and be a source of continued contact for a patient should new genes be discovered or new family history information come to light which alter the patient’s risk assessment. New genetics research uncovers additional genes involved in cancer predisposition on a regular basis. Our group has previously identified variants in the SDHB and SDHD genes and germline hypermethylation of KILLIN as being involved in PTEN mutation negative Cowden and Cowden-like syndromes. This preliminary work and other reports have shown that variation in SDHB/D and germline KILLIN hypermethylation are associated with increased risks of RCC over those with germline PTEN mutations.28–30
CONCLUSIONS
Patients with PHTS are at increased risk of developing RCC with an adjusted SIR>31. Papillary RCC was the most common histology identified when tumor materials underwent central re-review by a dedicated GU pathologist. Until larger studies are done to determine the sensitivity and specificity of reflexing to germline PTEN gene testing for pRCC patients with negative PTEN IHC, patients with papillary and chromophobe RCC may be referred to cancer genetics clinics for personal and family history assessment to determine if testing for PHTS or another hereditary syndrome is indicated. Given the decreased sensitivity of ultrasound for detecting pRCC, CT or MRI should be utilized when investigating symptoms concerning for RCC in PHTS patients.
ACKNOWLEDGEMENTS
We are grateful to our patients and their families who have taken part in our research over the last many years. We wish to acknowledge all the genetic counselors, physicians, and other healthcare providers who have referred patients to our study and Dawn Caraballo for assistance with obtaining patient consent and release of materials. This study was funded in part by R01CA118980 and P01CA124570 from the National Cancer Institute, the William Randolph Hearst Foundations and Healthnet Foundation (to CE). CE is the Sondra J. and Stephen R. Hardis Endowed Chair in Cancer Genomic Medicine at the Cleveland Clinic and is an ACS Clinical Research Professor, generously funded, in part, by the F.M. Kirby Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Marsh DJ, Kum JB, Lunetta KL, et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet. 1999;8:1461–1472. doi: 10.1093/hmg/8.8.1461. [DOI] [PubMed] [Google Scholar]
- 2.Zbuk KM, Eng C. Cancer phenomics: RET and PTEN as illustrative models. Nat Rev Cancer. 2007;7:35–45. doi: 10.1038/nrc2037. [DOI] [PubMed] [Google Scholar]
- 3.Starink TM, van der Veen JP, Arwert F, et al. The Cowden syndrome: a clinical and genetic study in 21 patients. Clin Genet. 1986;29:222–233. doi: 10.1111/j.1399-0004.1986.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 4.Lynch ED, Ostermeyer EA, Lee MK, et al. Inherited mutations in PTEN that are associated with breast cancer, cowden disease, and juvenile polyposis. Am J Hum Genet. 1997;61:1254–1260. doi: 10.1086/301639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capitan Canadas LM, Salinas Sanchez JL, Martinez Castillo SL, et al. Multiple oral fibropapillomatosis as an initial manifestation of Cowden Syndrome. Case report. Med Oral Patol Oral Cir Bucal. 2006;11:E319–E324. [PubMed] [Google Scholar]
- 6.Pilarski R, Eng C. Will the real Cowden syndrome please stand up (again)? Expanding mutational and clinical spectra of the PTEN hamartoma tumour syndrome. J Med Genet. 2004;41:323–326. doi: 10.1136/jmg.2004.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner W, Farber G, Herget T, et al. Loss of tumor suppressor protein PTEN during renal carcinogenesis. Int J Cancer. 2002;99:53–57. doi: 10.1002/ijc.10303. [DOI] [PubMed] [Google Scholar]
- 8.Guo SP, Zhai YQ, Wang WL, et al. Expression and significance of a new tumor suppression gene PTEN in primary renal cell carcinoma. Ai Zheng. 2002;21:582–587. [PubMed] [Google Scholar]
- 9.Hager M, Haufe H, Kemmerling R, et al. PTEN expression in renal cell carcinoma and oncocytoma and prognosis. Pathology. 2007;39:482–485. doi: 10.1080/00313020701570012. [DOI] [PubMed] [Google Scholar]
- 10.Hara S, Oya M, Mizuno R, et al. Akt activation in renal cell carcinoma: contribution of a decreased PTEN expression and the induction of apoptosis by an Akt inhibitor. Ann Oncol. 2005;16:928–933. doi: 10.1093/annonc/mdi182. [DOI] [PubMed] [Google Scholar]
- 11.Kondo K, Yao M, Kobayashi K, et al. PTEN/MMAC1/TEP1 mutations in human primary renal-cell carcinomas and renal carcinoma cell lines. Int J Cancer. 2001;91:219–224. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1034>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Shin Lee J, Seok Kim H, Bok Kim Y, et al. Expression of PTEN in renal cell carcinoma and its relation to tumor behavior and growth. J Surg Oncol. 2003;84:166–172. doi: 10.1002/jso.10302. [DOI] [PubMed] [Google Scholar]
- 13.Klatte T, Pantuck AJ, Said JW, et al. Cytogenetic and molecular tumor profiling for type 1 type 2 papillary renal cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:1162–1169. doi: 10.1158/1078-0432.CCR-08-1229. [DOI] [PubMed] [Google Scholar]
- 14.Tan MH, Mester J, Peterson C, et al. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am J Hum Genet. 2011;88:42–56. doi: 10.1016/j.ajhg.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Leibovich BC, Lohse CM, Crispen PL, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. The Journal of urology. 2010;183:1309–1315. doi: 10.1016/j.juro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Lynch HT, Fusaro RM, Lynch J. Hereditary cancer in adults. Cancer detection and prevention. 1995;19:219–233. [PubMed] [Google Scholar]
- 18.Shen-Li H, Koujak S, Szablocs M, et al. Reduction of Pten dose leads to neoplastic development in multiple organs of Pten (shRNA) mice. Cancer biology & therapy. 2010;10:1194–1200. doi: 10.4161/cbt.10.11.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang KL, Weinrach DM, Luan C, et al. Renal papillary adenoma--a putative precursor of papillary renal cell carcinoma. Human pathology. 2007;38:239–246. doi: 10.1016/j.humpath.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Myers MP, Stolarov JP, Eng C, et al. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci U S A. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung JH, Eng C. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res. 2005;65:8096–8100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 22.Tan MH, Ngeow J, Rybicki L, et al. Lifetime Cancer Risks in Individuals with Germline PTEN Mutations. Clinical Cancer Research. 2011 doi: 10.1158/1078-0432.CCR-11-2283. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choyke PL, Walther MM, Glenn GM, et al. Imaging features of hereditary papillary renal cancers. J Comput Assist Tomogr. 1997;21:737–741. doi: 10.1097/00004728-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Mvundura M, Grosse SD, Hampel H, et al. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genetics in medicine : official journal of the American College of Medical Genetics. 2010;12:93–104. doi: 10.1097/GIM.0b013e3181cd666c. [DOI] [PubMed] [Google Scholar]
- 25.Statement of the American Society of Clinical Oncology: genetic testing for cancer susceptibility, Adopted on February 20, 1996. J Clin Oncol. 1996;14:1730–1736. doi: 10.1200/JCO.1996.14.5.1730. discussion 1737–40. [DOI] [PubMed] [Google Scholar]
- 26.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 2006;56:11–25. doi: 10.3322/canjclin.56.1.11. quiz 49–50. [DOI] [PubMed] [Google Scholar]
- 27.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 28.Ni Y, Zbuk KM, Sadler T, et al. Germline mutations and variants in the succinate dehydrogenase genes in Cowden and Cowden-like syndromes. Am J Hum Genet. 2008;83:261–268. doi: 10.1016/j.ajhg.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA. 2010;304:2724–2731. doi: 10.1001/jama.2010.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricketts CJ, Forman JR, Rattenberry E, et al. Tumor risks and genotype-phenotype-proteotype analysis in 358 patients with germline mutations in SDHB and SDHD. Human mutation. 2010;31:41–51. doi: 10.1002/humu.21136. [DOI] [PubMed] [Google Scholar]