Abstract
Apoptosis is a mechanism that regulates hepatic tissue homeostasis and contributes to both acute and chronic injury in liver disease. The apoptotic signaling cascade involves activation of the death-inducing signaling complex (DISC) and subsequent recruitment of proteins containing death-effector domains (DED) which regulate downstream effector molecules. Prominent among these are the Fas-associated death domain (FADD) and the cellular caspase 8 (FLICE)-like inhibitory protein (cFLIP) and alterations of these proteins can lead to severe disruption of physiological processes including acute liver failure or hepatocellular carcinoma. Their role in cell signaling events independent of the DISC remains undetermined. Oxidative stress can cause cell injury from direct effects on molecules or by activating intracellular signaling pathways including the mitogen activated protein kinases (MAPK). In this context, prolonged activation of the cJun N-terminal kinase (JNK)/AP-1/cJun signaling pathway promotes hepatocellular apoptosis, while activation of the extracellular signal regulated kinase (Erk) exerts protection. We investigated the role of FADD and cFLIP in acute oxidant stress induced by the superoxide generator menadione in hepatocytes. Menadione resulted in dose-dependent predominantly necrotic cell death. Hepatocytes expressing a truncated, dominant-negative FADD protein were partially protected, while cFLIP-deficient hepatocytes displayed increased cell death from menadione. In parallel, Erk phosphorylation was enhanced in hepatocytes expressing dnFADD and decreased in cFLIP-deficient hepatocytes. Hepatocyte injury was accompanied by increased release of proapoptotic factors and increased JNK/cJun activation. Thus, FADD and cFLIP contribute to the regulation of cell death from acute oxidant stress in hepatocytes involving MAPK signaling. This implies that DED-containing proteins are involved in the regulation of cellular survival beyond their role in cell death receptor-ligand mediated apoptosis.
Keywords: FADD, cFLIP, oxidant stress, MAPK, apoptosis, hepatocyte
Introduction
Apoptosis is a conserved and highly regulated mechanism of cell death which contributes to physiological tissue homeostasis and to tissue injury in acute and chronic disease. In the liver apoptosis is involved in the pathophysiology of toxic, viral and metabolic injuries [1]. Additionally, increased cell turnover from enhanced apoptosis was shown to promote the development of hepatocellular carcinoma [2,3]. Activation of the TNF-receptor superfamily through its cognate ligands triggers apoptosis involving the formation of an intracellular death inducing signaling complex (DISC) which elicits caspase activation. Hepatocytes require an additional amplification of the cell death signal through the release of proapoptotic factors from mitochondria and lysosomes [4]. The class of death effector domain (DED)-containing proteins constitutes and regulates the DISC through recruitment of apoptosis effector molecules involving protein-protein interactions without enzymatic activity [5]. Among these, the mediator of receptor-induced toxicity (MORT1), also called Fas-receptor associated death domain (FADD) and the cellular caspase 8 (FLICE)-like inhibitory protein (cFLIP) regulate the activation of caspase 8 [6,7]. FADD recruits procaspase 8 molecules to the DISC following activation of a TNF-receptor through its cognate ligand, while cFLIP antagonizes caspase 8 activation through binding to and blocking FADD or procaspase 8 [5]. Beyond their role in apoptosis DED-containing proteins have been implied in embryogenesis, tissue development, proliferation and regeneration [8–10].
Oxidative stress contributes to a wide spectrum of diseases and modulates acute and chronic cellular injury in the liver including alcoholic and non-alcoholic steatohepatitis [11–13]. The mechanisms of oxidant-induced cell death include direct injurious effects from biochemical reactions of oxidants with macromolecules as well as the activation of signal transduction pathways that regulate cellular survival [11]. Among these the class of mitogen activated protein kinases (MAPK) including c-Jun N-terminal kinase (JNK) and the extracellular-signal regulated kinase (Erk) are critically involved in hepatocyte survival. In response to oxidant stress increased activation of JNK and Erk occurs. While prolonged activation of JNK promotes cell death, Erk was shown to exhibit a protective effect by suppressing prolonged JNK activation and cell death.
The aim of the current study was to explore the role of the DED-containing proteins FADD and cFLIP in acute oxidant stress in hepatocytes using the superoxide generator menadione, which has previously been employed to study the involvement of superoxide in liver injury [14,15]. In vitro we observed increased expression and phosphorylation of proapoptotic FADD from menadione. To expand these findings to non-transformed cells, primary hepatocytes with targeted disruption of FADD and cFLIP derived form genetically modified mice were studied. Inhibition of FADD ameliorated injury from acute oxidant stress, while deletion of cFLIP caused increased sensitivity and cell death from apoptosis in hepatocytes. In parallel, Erk signaling was altered and we observed increased phosphorylation of Erk when FADD function was impaired, while decreased Erk phosphorylation occurred when cFLIP was deleted. Interestingly, cell death from menadione was only partially prevented by caspase inhibition. Thus we were able to show for the first time that DED-containing proteins are involved in the regulation of cell death from oxidant stress involving MAPKs and independent of cell death receptor activation in hepatocytes.
Material and Methods
Cell lines, tissue culture, and viability assay
HepG2 and Huh7 hepatoblastoma cell lines were cultured in Dulbecco's modified Eagle medium: 10% FCS, 1% glutamine, 1% sodium pyruvate and 1% HEPES buffer (Gibco BRL, Life Technology, Carlsbad, CA) as previously described [16]. Where indicated, cells were pretreated with the MEK inhibitor U0126 (Cell Signaling, Beverly, MA) or the pancaspase inhibitor Val-Ala-Asp-fluoromethylketone (zVAD; Calbiochem, Darmstadt, Germany) dissolved in dimethyl sulfoxide (DMSO, Sigma, Steinheim, Germany). Cell viability was assessed by neutral red staining [17]. Briefly, cells were incubated for 2h at 37°C with medium containing neutral red (Sigma), washed and the absorbance was read using a spectrophotometer at 550 nm after extraction of the dye with 1.5 ml of N-propanol. The relative cell survival was calculated by dividing the optical density of a treatment group by the optical density of untreated control cells.
Animal models
Mice exhibiting liver-specific expression of a truncated and antagonistic dominant negative FADD protein (dnFADD) or mice with a hepatocyte-specific deletion of cFLIP using the cre-loxP system under control of the albumin promoter were employed [18,19]. All mice were bred and maintained in the animal facility of the University Medical Center of the Johannes Gutenberg University, Mainz, according to the criteria outlined by the “Guide for the care and Use of Laboratory Animals” and approved by the Committee for Experimental Animal Research. Mice were kept in a 12-h light/dark cycle under standard conditions with free access to water and food.
Isolation of primary hepatocytes by collagen perfusion
Murine hepatocytes were isolated by two-step collagenase perfusion. Briefly, mice were anesthetized, the portal vein was intubated and the liver perfused with a calcium-free buffer (0.14 M NaCl, 6.2 mM KCI, and 10 mM HEPES at pH 7.4) at 37°C. The perfusion was then switched to a collagenase solution (0.066 M NaCI, 6.7 mM KCI, 6.3 mM CaCl2, 0.1 M HEPES, and 75 collagen digestion units of collagenase isoform type I at pH 7.6). After 10 min of perfusion with collagenase liver cells were dispersed, filtered, resuspended (0.068 M NaCI, 5.4 mM KCI, 1.1 mM KH2PO4, 0.7 mM Na2SO4, 1.4 mM MgCl2, 1.6 mM CaCI2, 30 mM HEPES, 0.03 M TES, 0.03 M tricine at pH 7.6) and washed 3 times. Viability was assessed by trypan blue exclusion and reached 90% in all subsequent experiments. Collagenase and chemicals were purchased from Sigma.
Fluorescence microscopy
The numbers of apoptotic and necrotic cells were quantified by fluorescence microscopy as previously described [20]. In short, acridine orange (250 μg/ml) and ethidium bromide (250 μg/ml) were added to the medium of cultured cells, and the cells were visualized under fluorescent microscopy at a 200x magnification. Acridine orange intercalates into the DNA staining the nucleus green, while ethidium bromide is a membrane impermeable dye, and is only taken up by nonviable necrotic cells, staining them orange. Cells were considered apoptotic if they had a shrunken cytoplasm and nucleus and condensed chromatin or necrotic if the cytoplasm and nucleus were stained orange. A minimum of 200 cells/dish was examined, and the numbers of apoptotic and necrotic cells are expressed as a percentage of the total number of cells counted.
Western Blot
Western blotting was performed by denaturing 50 μg of protein at 100 °C for 5 min in Laemmli sample buffer containing 62.5 mM Tris, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromphenol blue, and 5% γ-mercaptoethanol. Samples were applied to 8% SDS-polyacrylamide gels and resolved at 100 V over 2 h. Proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell) in a Tris-glycine buffer containing 25 mM Tris, pH 8.3, 192 mM glycine, 0.01% SDS, and 15% methanol using a Bio-Rad Mini Trans Blot system according to the manufacturer’s specifications. Membranes were blocked in 5% nonfat dry milk, 20 mM Tris, pH 7.5, 500 mM sodium chloride, and 0.5% Tween 20 (TBS-T) for 1 h [21]. Primary antibodies included: actin (Sigma), phospho-JNK, cJun, phospho-cJun, phospho-Erk, Erk, Bid, cytochrome c oxidase (COX) IV, cytochrome c (all Cell Signaling Technology Inc., Danvers, MA), JNK, FADD, phospho-FADD, Mcl-1 (Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were exposed to goat anti-rabbit and anti-mouse secondary antibodies conjugated with horseradish peroxidase (Sigma).
Preparation of cytosolic and mitochondrial protein from liver tissue
Tissue pieces were rinsed in cold PBS and homogenized using a dounce homogenizer (Fisher Scientific, Schwerte) in buffer solution containing HEPES (20 mM, pH), KCl (10 mM), MgCl2 (1.5 mM), EDTA (1 mM), EGTA (1 mM), DTT (1 mM), PMSF (0.1 mM), and sucrose (250 mM). The homogenate was spun at 3000 rpm (750 g) and the supernatant was collected. After a repeated spin the supernatant was centrifuged at 11.000 rpm (10.000 g) for 15 minutes. The resulting pellet was dissolved in buffer solution and stored as mitochondrial fraction. The supernatant was saved as cytosolic fraction.
Statistical analysis
All numerical results are expressed as mean ±standard error and represent data from a minimum of three independent experiments. Statistical significance was determined by the Student’s t test. Calculations were made with Sigma Plot 2000 (SPSS Science, Chicago, IL).
Results
Menadione causes dose-dependent injury in hepatoma cell lines and primary hepatocyte
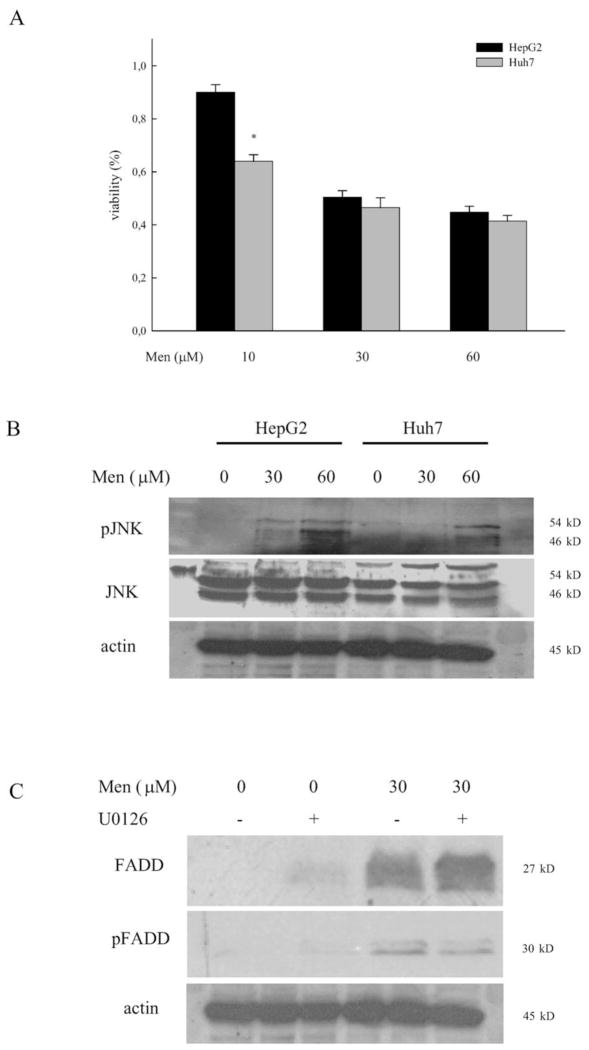
The superoxide generator menadione causes acute oxidative stress and cell death in non-transformed hepatocytes even at low concentrations [22]. We have previously shown that chronic oxidative stress induced by overexpression of cytochrome P450 2E1 partly desensitized cells from the toxic effects of menadione [20]. To further explore the mechanisms of oxidative injury in hepatocytes, transformed cell lines with a prolonged (Huh-7) or unaltered (HepG2) half-life of the tumor suppressor gene p53 were studied [23]. Cellular viability was assessed following 24h of treatment with increasing concentrations of menadione (fig. 1a). We observed dose-dependent loss of cellular viability in both HepG2 and Huh7 cells. Interestingly, toxicity in Huh7 cells occurred even at low concentrations indicating that the underlying mechanism maybe unrelated to the half-life of p53. At higher concentrations the loss of cellular viability was comparable between both cell lines. Hepatocyte injury from menadione involves prolonged activation of the MAPK JNK and the downstream transcription factor c-Jun [22]. In the two hepatoma cell lines menadione caused a dose-dependent increase in phosphorylation of the JNK p54 and p46 isoform, while levels of total JNK remained unchanged from treatment (fig 1b). Next we sought to determine if DED-containing proteins are involved in the regulation of injury from menadione. FADD is a critical regulator of cell death in hepatocytes and required for the activation of caspase 8 during cell death receptor mediated apoptosis. We have previously shown that inhibition of FADD protects from CD95/Fas/Apo-1 and TNF-induced liver injury [18]. In HepG2 cells increased levels of total and phosphorylated FADD protein were detected in response to menadione (fig 1c). This effect was independent of Erk activation and the MEK inhibitor U0126 did not affect the expression or phosphorylation of FADD. Thus, oxidant-induced injury from menadione is accompanied by activation of JNK, induction of FADD protein and occurred independently of p53 or Erk.
Figure 1. Cellular viability, JNK activation, and induction of FADD in hepatoma cell lines from menadione are independent of p53.
(a) HepG2 and Huh7 cells were treated with increasing concentrations of menadione and cellular viability was assessed by neutral red staining at 24h. Cellular viability is expressed in percent relative to untreated controls as mean ±standard error and * indicates a p-value <0.05. (b) Activation of JNK was assessed by immunoblotting using phospho-specific (pJNK) antibodies at 4h. Total JNK and actin served as a control for equal protein loading. (c) Levels of total and phosphorylated FADD (pFADD) protein were determined in HepG2 cells treated with menadione at 6h. Cells were pretreated with U0126 for 1h when indicated.
Critical role of the Fas-associated death domain (FADD) in regulating hepatocyte-injury from acute oxidative stress
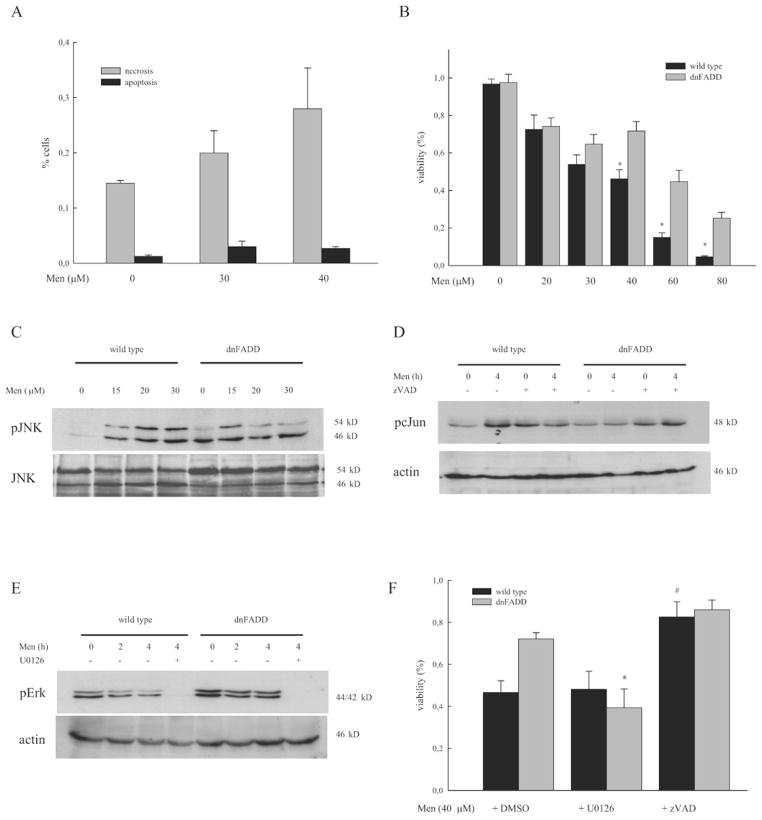
To extended these findings to non-transformed cells, primary hepatocytes were treated with increasing concentrations of menadione and apoptosis and necrosis were quantitated. We observed a dose-dependent increase of necrotic cell death from menadione in wild type hepatocytes while only low levels of apoptosis occurred (fig. 2a). To determine if FADD contributes mechanistically to menadione-induced cell death, hepatocytes derived from mice exhibiting a functional loss of FADD were studied. These mice express a truncated, dominant-negative FADD protein (dnFADD) in hepatocytes which acts as a competitive antagonist and protects from apoptosis induced by CD95/Apo-1/Fas and TNF [18,24]. Hepatocytes derived from dnFADD mice exhibited increased resistance towards acute oxidant injury even at high concentrations of menadione and cell death at 40 μM was reduced by 36% in dnFADD compared to the wild type (fig. 2b; viability dnFADD vs wt: 78.1% vs 50.3%, p<0.01). Resistance to menadione-induced cell death was previously shown to involve phosphorylation of ERK1/2 whereas sustained activation of JNK/c-Jun/AP-1 signaling promotes cell death [25]. Therefore we studied activation of JNK and Erk in dnFADD hepatocytes. As previously described, hepatocytes derived from wild type mice exhibited increased phosphorylation of JNK at 4h in response to menadione (fig. 2c). In hepatocytes derived from dnFADD mice, the degree of JNK activation was increased at baseline in untreated cells and did not increase further in response to menadione (fig. 2c). In parallel, menadione resulted in increased phosphorylation of the downstream transcription factor cJun in wild type but not in dnFADD hepatocytes (fig. 2d). Pretreatment with the pancaspase inhibitor zVAD ameliorated cJun phosphorylation in hepatocytes stressing the involvement of caspases during JNK/cJun activation despite the low degrees of apoptotic cell death. Strikingly the phosphorylation of the MAPK Erk was significantly higher in dnFADD hepatocytes compared to the wild type at baseline. Despite decreased phosphorylation following treatment, dnFADD hepatocytes exhibited increased levels of pErk at all times compared to the wild type (fig. 2e). Next we explored the mechanistic involvement of Erk in this context. Treatment with the MEK inhibitor U0126 abolished Erk activation in both genotypes (fig. 2e). In parallel, inhibition of Erk decreased cellular viability in dnFADD hepatocytes to a level comparable to the wild type, while the pancaspase inhibitor zVAD ameliorated cell death significantly in both genotypes (fig. 2f).
Figure 2. Inhibition of FADD ameliorates menadione-induced cell injury and JNK/c-Jun activation.
(a) Primary hepatocytes were treated with increasing concentrations of menadione and apoptosis and necrosis were assessed morphologically by staining with acridine orange/ethidium bromide as specified in material and methods. Results are expressed as mean ±standard error derived from 3 independent high power fields. (b) Cellular viability in hepatocytes derived from wild type and mice with liver-specific expression of a dominant-negative FADD protein (dnFADD) was assessed by neutral red staining following ex-vivo treatment with increasing concentrations of menadione for 24h and is expressed relative to untreated controls. * indicates a p-value <0.05 for wild type vs dnFADD. Activation of (c) JNK, (d) cJun, and (e) Erk were assessed by immunoblotting for phosphorylated and total protein, or actin as control following treatment with menadione. Pretreatment was done with DMSO, zVAD, or U0126 for 1h when indicated. (f) Cellular viability in response to menadione was reassessed following pretreatment with DMSO, U0126, or zVAD. * indicates a p-value <0.05 for dnFADD ±U0126 and # for wild type ±zVAD.
Loss of cFLIP sensitizes hepatocytes towards acute oxidative injury
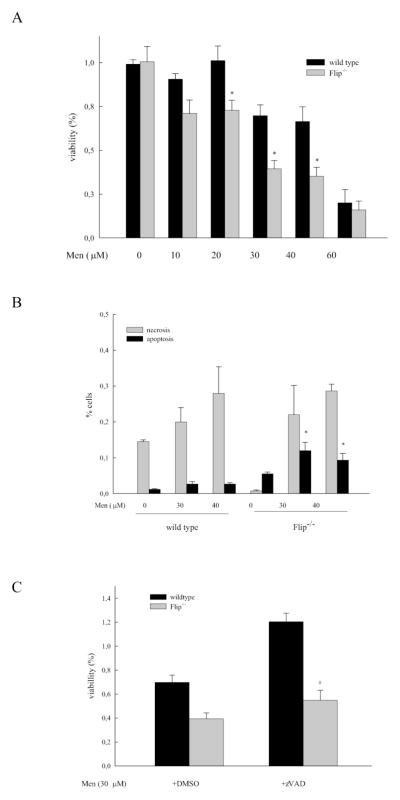
The caspase 8 homologue cFLIP is crucial for the regulation of tissue homeostasis in the liver. We have previously shown that hepatocytes lacking cFLIP exhibit increased liver injury and apoptosis in response to activation of the CD95/Fas/Apo-1 and TNF-receptor [19]. To explore the role of cFLIP in acute oxidant stress, primary hepatocytes derived from cFLIP knockout mice (flip−/−) were treated with increasing doses of menadione. In contrast to the degree of toxicity observed in wild type and dnFADD hepatocytes, the loss of cFLIP resulted in significant cell death which occurred even at low concentrations of menadione (fig. 3a). At higher concentrations the difference between wild type and flip−/− hepatocytes was lost (fig. 3a). While wild type hepatocytes exhibited primarily necrotic cell death, we observed increased apoptosis in hepatocytes derived from flip−/− mice (fig. 3b). To determine if increased vulnerability in flip−/− hepatocytes was caspase-dependent, the pancaspase inhibitor zVAD was employed. Pretreatment with zVAD significantly ameliorated cell death from 30 μM menadione in flip−/− hepatocytes (fig. 3c). These results point towards both caspase-dependent and -independent mechanisms of cell death from oxidant stress in hepatocytes.
Figure 3. Loss of cFLIP augments apoptotic, partly caspase-independent cell death from menadione.
(a; c) Hepatocytes derived from wild type or mice with liver-specific deletion of cFLIP (flip−/−) were isolated using collagen-perfusion and treated ex-vivo with menadione for 24h as indicated. Pretreatment with zVAD was for 1h when indicated. Cellular viability was assessed by neutral red staining. Results are expressed as mean viable cells ±standard error relative to untreated controls and * indicates a p-value <0.05 for wild type vs. flip−/−, # p-value <0.05 for flip−/− ±zVAD. (b) The degree of apoptosis and necrosis was assessed by costaining with acridine orange/ethidium bromide as specified in material and methods. Results are expressed as mean ±standard error and * indicates a p-value <0.01 for wild type vs flip−/−.
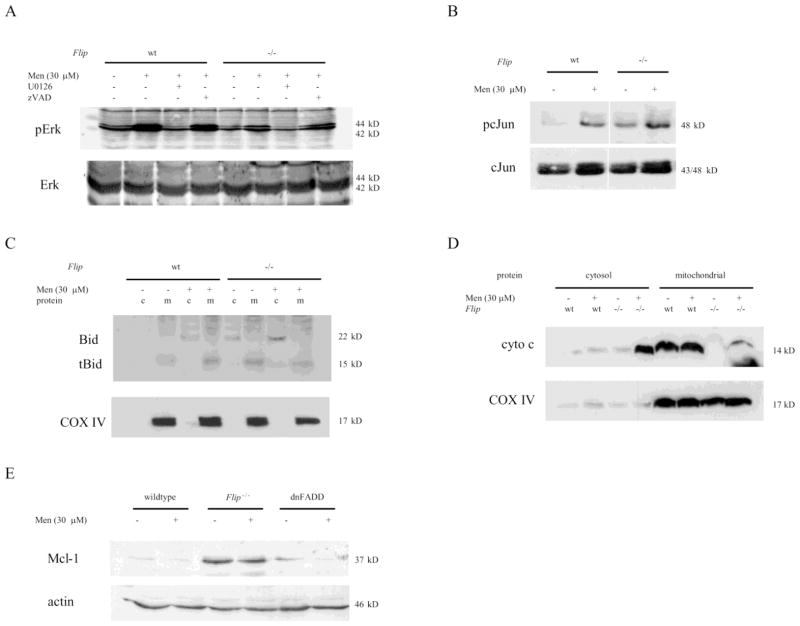
To examine the involvement of Erk phosphorylation in response to menadione in flip−/− hepatocytes, immunoblotting was performed (fig. 4a). Erk phosphorylation in cFLIP knockout mice was decreased both at baseline and following treatment with menadione. Pretreatment with the MEK inhibitor U0126, but not zVAD decreased phosphorylation from menadione effectively irrespective of the genotype (fig. 4a). In parallel, phosphorylation of the downstream target of JNK, cJun, was significantly increased in cFLIP-deficient hepatocytes (fig. 4b). Thus, in line with the observations in dnFADD hepatocytes, activation of cJun and decreased Erk activation are contributing to the increase in cellular injury from menadione in cFLIP-deficient cells.
Figure 4. Cell death from oxidative stress in cFLIP-deficient hepatocytes involves altered MAPKs signaling and proapoptotic mitochondrial factors.
(a) Activation of Erk and (b) c-Jun were assessed by immunoblotting for phosphorylated and total protein following treatment with menadione for 4h. Hepatocytes were pretreated with DMSO, U0126, or zVAD for 1h when indicated. (c; d) Immunoblotting for cytochrome c (cyto c), Bid and cytochrome c oxidase IV (COX IV) was performed following separation of cytosolic (c) from mitochondrial (m) protein and treatment with menadione. (e) Levels of Mcl-1 were determined by immunoblotting in wild type, dnFADD and flip−/− hepatocytes.
Receptor-mediated apoptosis in hepatocytes requires the release of proapoptotic mitochondrial proteins from the Bcl-2 family [26]. To determine if menadione-induced oxidant cell death resulted in activation of Bid and release of cytochrome c, cytosolic and mitochondrial proteins were examined separately by immunoblotting (fig. 4c and 4d). Menadione caused an increase in total Bid protein in the cytosolic fraction and increased truncated (tBid) in the mitochondrial fraction of wild type hepatocytes. In contrast, flip−/− hepatocytes exhibited higher levels of cytosolic Bid both untreated and in response to menadione (fig. 4c). In parallel, liberation of the proapoptotic cytochrome c from mitochondria into the cytosol was significantly enhanced in cFLIP-deficient hepatocytes following treatment with menadione (fig. 4d). Also, levels of cytochrome c were significantly lower in the mitochondrial fraction of cFLIP-deficient hepatocytes while levels of cytochrome c oxidase IV (COX IV) which served as a control for the separation of cytosolic and mitochondrial fractions and equal protein loading were comparable (fig. 4d). Mcl-1 is an anti-apoptotic factor of the Bcl-2 family that prevents activation of the mitochondrial loop of apoptosis [27]. To determine if Mcl-1 contributed to menadione-induced cell death in wild type, dnFADD and flip−/− hepatocytes cytosolic levels were examined. Although oxidant stress did not change Mcl-1 at 4h, we observed significantly higher levels of this anti-apoptotic protein in flip−/− hepatocytes (fig. 4e). Thus, despite a potentially compensatory up regulation of anti-apoptotic and alterations in the level of cytochrome c factors in flip−/− hepatocytes, the loss of cFLIP sensitized hepatocytes towards acute oxidant injury from menadione with increased cJun activation, increased activation, and release of pro-apoptotic mitochondrial proteins.
Discussion
The role of DED-containing proteins beyond the regulation of receptor-mediated apoptosis in hepatocytes is undefined. In the current study we evaluated the contribution of FADD and cFLIP in acute oxidant injury using two hepatoma cell lines and two murine models with hepatocyte-specific modification of DED-containing proteins. HepG2 and Huh7 cells displayed similar toxicity from menadione independent of their differences in the tumor-suppressor gene p53 and was accompanied by activation of JNK [22]. Interestingly we observed an induction of FADD protein in response to acute oxidant stress indicating that FADD potentially contributes to cellular injury independent of the classical receptor-mediated apoptosis signaling pathways. This type of ligand-independent death receptor activation has previously been implied for ethanol-mediated apoptosis involving DR5 and CD95/Fas signaling in hepatocytes [28,29]. The regulation of FADD is complex and only partly understood: both increased expression and phosphorylation have been implied in the regulation of cellular survival. Recent evidence has implicated FADD serine phosphorylation in its subcellular distribution and trafficking - one way by which FADD potentially exerts receptor-independent effects [30]. Additionally, activation of Erk which regulates toxicity from menadione, has been shown to contribute to FADD-dependent apoptosis in transformed non-hepatic tissue [22,31]. In the current study we did not observe an effect of Erk inhibition on menadione-induced FADD expression or phosphorylation.
The loss of FADD function in hepatocytes expressing an antagonistic, dominant negative protein protected from menadione-induced cell death and decreased the activation of JNK. Previous studies have shown that the dynamics of JNK activation determines the amount of cell death. Czaja et al. demonstrated that prolonged activation of JNK in the context of Erk inhibition dramatically increased oxidant-induced injury in hepatocytes while transient activation of JNK did not promote cell death [22]. In the current study inhibition of FADD resulted in increased Erk phosphorylation at baseline and ameliorated JNK phosphorylation in response to menadione treatment. The inhibition of Erk using an MEK inhibitor completely abolished the protective effect of dnFADD in menadione-induced cell death. These findings imply that activation of Erk underlies the protection of hepatocytes with impaired FADD function from menadione potentially through its ability to modulate JNK activation. Interestingly, only a small amount of cell death was due to apoptosis in wild type and dnFADD hepatocytes. Nonetheless, the pancaspase inhibitor zVAD partly protected from menadione, indicating that despite low levels of caspase activation during the initial priming phase of injury, cellular protection can be achieved through caspase inhibition. Thus, these results identify FADD as a factor that promotes oxidative injury due its ability to modulate Erk and JNK signaling in hepatocytes. In patients with hepatocellular carcinoma the resistance of cancer cells towards apoptosis and the activation of Erk signaling has been linked with a poor prognosis. With respect to our findings this could partly result from a decreased sensitivity of cancer cells towards oxidative stress [32,33].
To further explore the role of DED-containing proteins hepatocytes lacking the caspase 8 homologue cFLIP were studied. We have previously shown that cFLIP-deficient hepatocytes are more sensitive to apoptosis induced by activation of the CD95/Apo1/Fas- or TNF signaling pathway [19]. Acute oxidant stress resulted in a significant increase of cell death and apoptosis in cFLIP-deficient hepatocytes. Interestingly, this type of cell death was only partly dependent on caspase activation but resulted in increased activation of proapoptotic Bid and augmented release of cytochrome c from mitochondria. Thus, oxidant stress from menadione involves the release of proapoptotic factors independent of caspase and death receptor activation. These findings are in line with recent observations, that loss of FADD and caspase 8 protect cells from breakdown of the mitochondrial membrane potential, the release of cytochrome c and the activation of caspases [34].
To further substantiate the role of MAPKs in menadione induced cell death, we evaluated Erk and cJun activation. In line with our previous findings in wild type and FADD-deficient hepatocytes, we observed lower levels and decreased activation of Erk and increased phosphorylation of c-Jun from menadione in cFLIP-deficient hepatocytes, indicating that Erk activation represents a protective signal, which is partly responsible for the suppression of injurious JNK/c-Jun/AP1 activation. Mcl-1, an anti-apoptotic member of the Bcl-2 family, was unaffected by treatment with menadione indicating that in the studied model selectively alters proteins that are involved in the mitochondrial cell death pathway. Interestingly, we observed increased levels of Mcl-1 protein in cFLIP-deficient hepatocytes, which could represent a counter regulatory effect in these cells which are prone to increased activation of the cell death pathway.
In conclusion we have identified the DED-containing proteins FADD and cFLIP as regulators of cell injury from acute, oxidant stress in hepatocytes which occurred partly independent of death receptor ligand- and caspase-activation. Resistance of hepatocytes lacking FADD is mediated by increased Erk activation and decreased phosphorylation of JNK and c-Jun. In analogy, loss of cFLIP resulted in decreased Erk phosphorylation, increased cJun activation, and increased oxidant-induced cell death. Thus, our study implies a novel function of DED-containing proteins which are capable of regulating cellular survival independent of death-receptor and DISC activation. These findings are especially relevant to patients with hepatocellular carcinoma, which is associated with resistance towards cell death signaling and exhibit alterations of tyrosine kinase signaling pathways.
Highlights.
The role of DED-containing proteins in oxidant-induced cell injury is undefined
Cell death from oxidative stress in hepatocytes is regulated by FADD and cFLIP
Increased JNK activation and decreased Erk activation promote oxidant cell death
DED-proteins regulate oxidant cell death independent of receptor-ligand interaction
Acknowledgments
We thank Irina Wagner, Anne Pratz, Sandra Weyer, and Brigitte Bartsch for their excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) grant SCHA 1015/3-1 to JMS and SCHU 1239/3-1 to MS and intramural funds of the University Medical Center Mainz.
Abbreviations
- cFLIP
cellular FLICE-inhibitory protein
- FADD
Fas-associated death domain
- DED
death effector domain
- DISC
death-inducing signaling complex
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen activated protein kinase
- Erk
extracellular signal regulated kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guicciardi ME, Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis. 2010;30:402–410. doi: 10.1055/s-0030-1267540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Weber A, Boger R, Vick B, Urbanik T, Haybaeck J, Zoller S, Teufel A, Krammer PH, Opferman JT, Galle PR, Schuchmann M, Heikenwalder M, Schulze-Bergkamen H. Hepatocyte-specific deletion of the antiapoptotic protein myeloid cell leukemia-1 triggers proliferation and hepatocarcinogenesis in mice. Hepatology. 2010;51:1226–1236. doi: 10.1002/hep.23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schattenberg JM, Schuchmann M, Galle PR. Cell death and hepatocarcinogenesis: Dysregulation of apoptosis signaling pathways. J Gastroenterol Hepatol. 2011;26(Suppl 1):213–219. doi: 10.1111/j.1440-1746.2010.06582.x. [DOI] [PubMed] [Google Scholar]
- 5.Valmiki MG, Ramos JW. Death effector domain-containing proteins. Cell Mol Life Sci. 2009;66:814–830. doi: 10.1007/s00018-008-8489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 7.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 8.Imtiyaz HZ, Zhou X, Zhang H, Chen D, Hu T, Zhang J. The death domain of FADD is essential for embryogenesis, lymphocyte development, and proliferation. J Biol Chem. 2009;284:9917–9926. doi: 10.1074/jbc.M900249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SM, Schickel R, Peter ME. Nonapoptotic functions of FADD-binding death receptors and their signaling molecules. Curr Opin Cell Biol. 2005;17:610–616. doi: 10.1016/j.ceb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Schuchmann M, Ruckert F, Garcia-Lazaro JF, Karg A, Burg J, Knorr N, Siebler J, Varfolomeev EE, Wallach D, Schreiber W, Lohse AW, Galle PR. MORT1/FADD is involved in liver regeneration. World J Gastroenterol. 2005;11:7248–7253. doi: 10.3748/wjg.v11.i46.7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czaja MJ. Cell signaling in oxidative stress-induced liver injury. Semin Liver Dis. 2007;27:378–389. doi: 10.1055/s-2007-991514. [DOI] [PubMed] [Google Scholar]
- 12.Marra F, Gastaldelli A, Svegliati BG, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14:72–81. doi: 10.1016/j.molmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- 14.Hong JY, Lebofsky M, Farhood A, Jaeschke H. Oxidant stress-induced liver injury in vivo: role of apoptosis, oncotic necrosis, and c-Jun NH2-terminal kinase activation. Am J Physiol Gastrointest Liver Physiol. 2009;296:G572–G581. doi: 10.1152/ajpgi.90435.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Singh R, Xiang Y, Czaja MJ. Macroautophagy and chaperone-mediated autophagy are required for hepatocyte resistance to oxidant stress. Hepatology. 2010;52:266–277. doi: 10.1002/hep.23645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuchmann M, Schulze-Bergkamen H, Fleischer B, Schattenberg JM, Siebler J, Weinmann A, Teufel A, Worns M, Fischer T, Strand S, Lohse AW, Galle PR. Histone deacetylase inhibition by valproic acid down-regulates c-FLIP/CASH and sensitizes hepatoma cells towards. Oncol Rep. 2006;15:227–230. doi: 10.3892/or.15.1.227. [DOI] [PubMed] [Google Scholar]
- 17.Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3:1125–1131. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- 18.Schuchmann M, Varfolomeev EE, Hermann F, Rueckert F, Strand D, Koehler H, Strand S, Lohse AW, Wallach D, Galle PR. Dominant negative MORT1/FADD rescues mice from CD95 and TNF-induced liver failure. Hepatology. 2003;37:129–135. doi: 10.1053/jhep.2003.50011. [DOI] [PubMed] [Google Scholar]
- 19.Schattenberg JM, Zimmermann T, Worns M, Sprinzl MF, Kreft A, Kohl T, Nagel M, Siebler J, Schulzebergkamen H, He YW, Galle PR, Schuchmann M. Ablation of c-FLIP in hepatocytes enhances death-receptor mediated apoptosis and toxic liver injury in vivo. J Hepatol. 2011 doi: 10.1016/j.jhep.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Schattenberg JM, Wang Y, Rigoli RM, Koop DR, Czaja MJ. CYP2E1 overexpression alters hepatocyte death from menadione and fatty acids by activation of ERK1/2 signaling. Hepatology. 2004;39:444–455. doi: 10.1002/hep.20067. [DOI] [PubMed] [Google Scholar]
- 21.Schattenberg JM, Wang Y, Singh R, Rigoli RM, Czaja MJ. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J Biol Chem. 2005;280:9887–9894. doi: 10.1074/jbc.M410310200. [DOI] [PubMed] [Google Scholar]
- 22.Czaja MJ, Liu H, Wang Y. Oxidant-induced hepatocyte injury from menadione is regulated by ERK and AP-1 signaling. Hepatology. 2003;37:1405–1413. doi: 10.1053/jhep.2003.50233. [DOI] [PubMed] [Google Scholar]
- 23.Bressac B, Galvin KM, Liang TJ, Isselbacher KJ, Wands JR, Ozturk M. Abnormal structure and expression of p53 gene in human hepatocellular carcinoma. Proceedings of the National Academy of Sciences. 1990;87:1973–1977. doi: 10.1073/pnas.87.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni HM, Chen X, Ding WX, Schuchmann M, Yin XM. Differential roles of JNK in ConA/GalN and ConA-induced liver injury in mice. Am J Pathol. 2008;173:962–972. doi: 10.2353/ajpath.2008.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh R, Czaja MJ. Regulation of hepatocyte apoptosis by oxidative stress. J Gastroenterol Hepatol. 2007;22(Suppl 1):S45–S48. doi: 10.1111/j.1440-1746.2006.04646.x. [DOI] [PubMed] [Google Scholar]
- 26.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 27.Vick B, Weber A, Urbanik T, Maass T, Teufel A, Krammer PH, Opferman JT, Schuchmann M, Galle PR, Schulze-Bergkamen H. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology. 2009;49:627–636. doi: 10.1002/hep.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang P, Zheng Y, Shi J, Zhang Y, Liu S, Liu Y, Zheng D. Targeting a novel N-terminal epitope of death receptor 5 triggers tumor cell death. J Biol Chem. 2010;285:8953–8966. doi: 10.1074/jbc.M109.070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McVicker BL, Tuma DJ, Kubik JL, Tuma PL, Casey CA. Ethanol-induced apoptosis in polarized hepatic cells possibly through regulation of the Fas pathway. Alcohol Clin Exp Res. 2006;30:1906–1915. doi: 10.1111/j.1530-0277.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- 30.Tourneur L, Chiocchia G. FADD: a regulator of life and death. Trends Immunol. 2010;31:260–269. doi: 10.1016/j.it.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Tewari R, Sharma V, Koul N, Sen E. Involvement of miltefosine-mediated ERK activation in glioma cell apoptosis through Fas regulation. J Neurochem. 2008;107:616–627. doi: 10.1111/j.1471-4159.2008.05625.x. [DOI] [PubMed] [Google Scholar]
- 32.Newell P, Toffanin S, Villanueva A, Chiang DY, Minguez B, Cabellos L, Savic R, Hoshida Y, Lim KH, Melgar-Lesmes P, Yea S, Peix J, Deniz K, Fiel MI, Thung S, Alsinet C, Tovar V, Mazzaferro V, Bruix J, Roayaie S, Schwartz M, Friedman SL, Llovet JM. Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo. J Hepatol. 2009;51:725–733. doi: 10.1016/j.jhep.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schattenberg JM, Galle PR. Show me your signaling--and I'll tell you who you are. J Hepatol. 2009;51:638–639. doi: 10.1016/j.jhep.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 34.von Haefen C, Wendt J, Semini G, Sifringer M, Belka C, Radetzki S, Reutter W, Daniel PT, Danker K. Synthetic glycosidated phospholipids induce apoptosis through activation of FADD, caspase-8 and the mitochondrial death pathway. Apoptosis. 2011;16:636–651. doi: 10.1007/s10495-011-0592-2. [DOI] [PubMed] [Google Scholar]