Abstract
Purpose
Insulin-like growth factors (IGFs) regulate a wide range of biological functions including cell proliferation, differentiation, and apoptosis through paracrine and autocrine mechanisms. Accordingly, the present study analyzed polymorphisms of IGF genes and their impact on the prognosis for patients with gastrointestinal stromal tumors (GISTs).
Methods
Two hundred-thirteen consecutive patients with GISTs who underwent curative surgery from 5 medical centers were enrolled in the present study. The genomic DNA was extracted from paraffin-embedded tumor tissue, and four IGF-1 (+2995C/A, +533C/T, IVS2-16540A/G, Ex4-177G/C) and one IGF-2 (IVS1+1280A/G) gene polymorphisms were determined using a Sequenom MassARRAY system.
Results
With a median follow-up of 18.4 months, the estimated 5-year relapse-free survival and overall survival rates were 69.9% and 86.7%, respectively. In a multivariate analysis including age, gender, primary site of disease, pathology, and risk stratification, no significant association was observed between the polymorphism of the IGF-1 and IGF-2 genes and survival.
Conclusion
None of the five IGF-1 and IGF-2 gene polymorphisms investigated in this study was found to be an independent prognostic marker for Korean patients with surgically resected GIST. However, further studies on a larger scale are warranted to clarify the role of IGF-1 and IGF-2 gene polymorphisms as a prognostic biomarker for GIST patients.
Keywords: Gastrointestinal stromal tumor, Insulin-like growth factor, Single nucleotide polymorphism, Prognosis
INTRODUCTION
The insulin-like growth factor (IGF) and its family play a key role in the growth and development of normal tissue and regulate a wide range of biological functions, such as cell proliferation, differentiation, and apoptosis, through paracrine and autocrine mechanisms [1]. In particular, IGF receptor-mediated initiation of signal transduction also activates important intracellular signal pathways in solid tumors, including the Ras/Raf/mitogen-activated protein kinase and phosphoinositide 3-kinase pathway [2]. Previous studies have shown that an increased expression of IGF or its family is associated with the grade of tumor growth and the prognosis for various solid tumors [3-6]. Recently, the targeting for IGF has emerged as an important solution for the resistance that arises after targeted treatment.
Gastrointestinal stromal tumors (GISTs) represent a heterogenous group of tumors that originate from the interstitial cells of Cajal in the nerve plexus of the muscularis in the gastrointestinal wall [7]. GISTs are characterized by KIT and platelet-derived growth factor alpha (PDGFRA) receptor abnormalities that represent the key oncogenic event, as well as the most important therapeutic target [8]. Recently, IGF or its family has emerged as a novel molecular signaling pathway other than KIT and PDGFRA on GISTs [9]. The expression of IGF-1 and IGF-2 family has also been shown to be correlated with a poor prognosis for GISTs [10]. In addition, epidemiologic studies have indicated that high plasma IGF-1 plays a role in energy balance, which has also been shown to influence risk for solid tumors including GIST [11]. Thus, given these results, IGF or its family would seem to play an important role in tumor growth and spread, thereby affecting the prognosis for GISTs.
Single nucleotide polymorphisms (SNPs) have already been widely implicated in cancer development, prognosis, and treatment response, yet similar evidence is lacking for IGF genes. Although IGF-1 tag SNPs have been associated with circulating IGF-1 levels, the functional polymorphisms that might be mediating these associations have not been identified [12]. Wong et al. [13] reported that a putative regulatory IGF-1 in the promoter region is associated with reduced colorectal cancer risk. In addition, IGF-1 haplotype and the IGF2 Ex4-233 C/T polymorphism was also found to be significantly associated with risk of pancreatic cancer [14].
To date, only a few studies have been published in regards with the relationship between the SNP of IGF or its family gene and clinical outcomes of GISTs. Therefore, the present study analyzed five IGF-1 and IGF-2 gene polymorphisms and their effect on the prognosis for GIST patients.
METHODS
Study population
All the tissues investigated in this study were obtained from 213 consecutive Korean patients who underwent surgical resection between January 1998 and June 2008 at five medical centers. The GIST risk stratification was classified according to the National Institutes of Health (NIH) consensus classification system [7]. Retrospective information was also received concerning the patient characteristics and the date of diagnosis, relapse, and death. Written informed consent for gene expression analyses was received from the patients, and the study was approved by the Institutional Review Board at Kyungpook National University Hospital (KNUH).
Selection of target IGF-1/2 gene polymorphisms
Due to high number of SNPs in the human genome, the initial challenge was the efficient selection of the SNPs most likely to contribute phenotypic effects. Thus, a prioritizing strategy was created using public databases that provide diverse information on the potential phenotypic risks of SNPs. First candidate genes involved in IGF and related information were collected from web-based databases that included information on the biologic pathway and potential biologic effects of polymorphisms. Next, SNPs with frequencies lower than 0.1 were excluded based on the allele frequencies recorded for East Asian populations obtained from FASTSNP (function analysis and selection tool for SNP). The selected SNPs were then scored according to certain phenotypic risks and ordered according to the sum of risk scores based on the algorithm suggested in a previous report [15]. Finally, and four IGF-1 (+2995C/A, +533C/T, IVS2-16540A/G, Ex4-177G/C) and one IGF-2 (IVS1+1280A/G) gene polymorphisms were included in the current analysis.
Genotyping of IGF-1 and IGF-2 gene polymorphisms
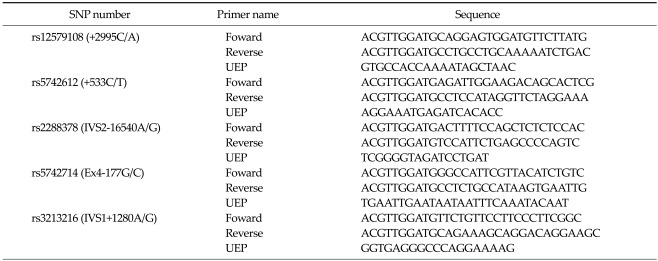
The genomic DNA was extracted from paraffin-embedded tissue, and four IGF-1 (+2995C/A, +533C/T, IVS2-16540A/G, Ex4-177G/C) and one IGF-2 (IVS1+1280A/G) gene polymorphisms were determined using a Sequenom MassARRAY system. The genotyping was undertaken using the Sequenom iPLEX platform, according to the manufacturer's instructions (www.sequenom.com; Sequenom Inc., San Diego, CA, USA). The detection of SNPs was carried out by analyzing the primer extension products generated from previously amplified genomic DNA using a Sequenom chip-based matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry platform. Multiplex SNP assays were designed using SpectroDESIGNER ver. 2.06 (Sequenom Inc.). The PCR amplification took place in a 5 µL mixture containing 10 ng genomic DNA, 100 nM of each amplification primer, 500 mM dNTP mix, 1.625 mM MgCl2, and 5.5 units of HotStarTaq DNA Polymerase (Qiagen, Hilden, Germany). The mixture was subjected to the following PCR conditions: a single denaturation cycle at 95℃ for 15 minutes, followed by 45 cycles at 94℃ for 20 seconds, 56℃ for 30 seconds, 72℃ for 60 seconds, and a final extension at 72℃ for 3 minutes. Any unincorporated nucleotides in the PCR product were deactivated using shrimp alkaline phosphatase. The allele discrimination reactions were conducted by adding allele-specific extension primers, DNA polymerase, and a cocktail mixture of deoxynucleotide triphosphates and di-deoxynucleotide triphosphates to each well. MassEXTEND clean resin (Sequenom Inc.) was also added to the mixture to remove any extraneous salts that could interfere with the MALDI-TOF analysis. The primer extension products were then cleaned and spotted onto a SpectroCHIP (Sequenom Inc.). The genotypes were determined by spotting an aliquot of each sample onto a 384 SpectroCHIP, which was subsequently read using the MALDI-TOF mass spectrometer. Triplicate samples and negative controls were included to check the genotyping quality. The primer sequences are summarized in the supplemental Table 1.
Table 1.
The Sequenom MassARRAY primer sequence
SNP, single nucleotide polymorphisms; UEP, unique-event polymorphism.
Statistical analysis
The genotypes for each SNP were analyzed as a three-group categorical variable (reference model) and grouped according to the dominant and recessive model. The survival estimates were calculated using the Kaplan-Meier method. The differences in overall survival (OS) or relapse-free survival (RFS) according to the gene polymorphisms were compared using log-rank tests. For the multivariate analysis, Cox's proportional hazard regression model was used for the survival analyses. The analyses were adjusted for age, gender, primary site of disease, pathology, and risk stratification. The hazard ratio and 95% confidence interval were also estimated. A cut-off P-value of 0.05 was adopted for all the statistical analyses. All the analyses were performed using the SPSS ver. 14 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics and survival analysis
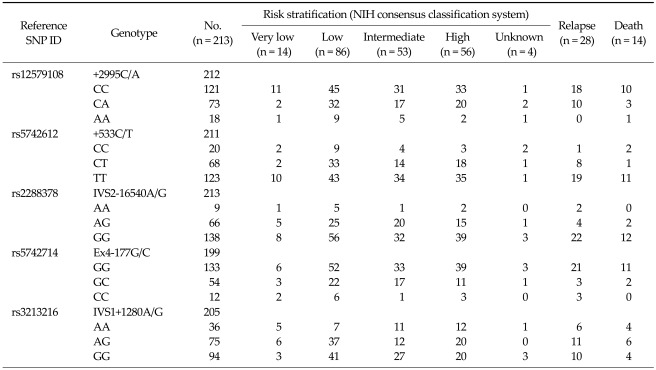
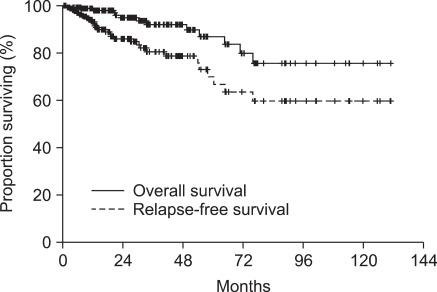
The patient characteristics are shown in Table 2. The median age was 62.0 years (range, 25 to 87 years), and the ratio of males to females was 89 to 124. The stomach was the most common primary site (n = 136, 63.8%). The most frequently observed pathology was a spindle cell type (n = 172, 80.8%). One hundred seventy-eight patients (83.6%) underwent complete resection. The median primary tumor size was 4.3 cm and the median mitotic count was 4/50 HPFs. According to the NIH consensus classification system, the risk groups were as follows: very low (n = 14, 6.6%), low (n = 86, 40.4%), intermediate (n = 53, 24.9%), and high (n = 56, 26.3%). The genotype distribution according to the risk and clinical outcomes are also shown in Table 3. With a median follow-up duration of 18.4 months, 28 relapses (13.1%) were documented and 14 patients died. The estimated 5-year RFS and OS rates were 69.9% and 86.7%, respectively (Fig. 1). Plus, the survival differed according the NIH consensus classification system (OS, P = 0.046; RFS, P < 0.001) (Fig. 2).
Table 2.
Patient characteristics (n = 213)
Values are presented as median (range) or number.
NIH, National Institutes of Health.
Table 3.
Genotype distribution according to the risk and clinical outcomes
SNP, single nucleotide polymorphisms; NIH, National Institutes of Health.
Fig. 1.
Survival curves for all patients.
Fig. 2.
Survival curves of overall survival (A) and relapse-free survival (B) according to National Institutes of Health consensus classification system.
Genotype frequency effect on survival
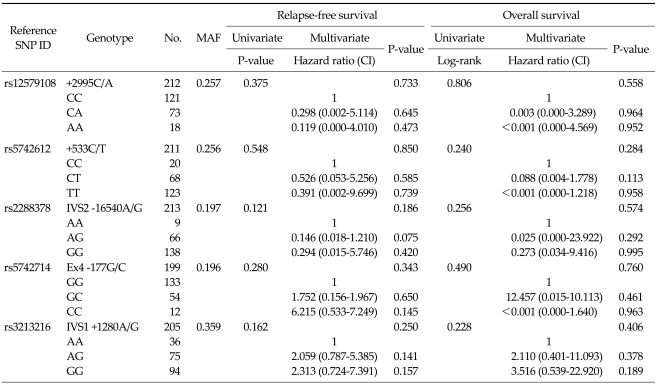
The five IGF-1 and IGF-2 gene polymorphisms were successfully amplified in all cases. The frequencies of each genotype are shown in Table 4 and conformed to the Hardy-Weinberg equilibrium (P < 0.05). The linkage of the Ex4-177G/C polymorphisms with the IVS2-16540A/G, +533C/T, or +2995C/A was relatively strong (correlation coefficient, D' = 0.88; correlation coefficient, D' = 0.78; or correlation coefficient, D' = 0.85). In the univariate and multivariate analyses including age, gender, primary site of disease, pathology, and risk stratification, none of the five IGF-1 and IGF-2 gene polymorphisms had a significant effect on survival (Table 4). In a haplotype analysis of the 4 polymorphisms in the IGF-1 gene, none of the haplotypes was found to be associated with the GIST prognosis. Among the clinicopathologic variables, the risk stratification was a significant prognostic factor in the Cox model for RFS (P = 0.002).
Table 4.
Univariate and multivariate survival analysis according to five IGF-1 and IGF-2 gene polymorphisms
P-values correspond to univariate using log-lank test and multivariate Cox model adjusted for age, gender, primary site of disease, pathology, and risk stratification.
IGF, insulin-like growth factors; SNP, single nucleotide polymorphisms; MAF, minor allele frequency; CI, confidence interval.
DISCUSSION
The prognostic impact of five SNPs of the IGF and IGF-2 gene was investigated in patients with a surgically resected GIST. However, no significant association was observed between the five polymorphisms and survival in these patients. Given the homogenous ethnic background of Korean patients, any potential confounding effect due to ethnicity was likely to be small in the present study.
Since IGF or its family plays a critical role in tumor-related growth and metastasis, the association of IGF gene polymorphisms with the risk or prognosis of several solid tumors, such as liver, prostate, pancreas, and breast cancer has already been demonstrated [12,14,16-19]. Recently, the current authors also reported that IGF gene polymorphisms are no significant association with survival in patients with colorectal cancer [20]. Although no data has been published on the relationship between the SNPs of the IGF genes and the clinical outcomes of GISTs, in-depth analysis of polymorphisms can provide useful information in terms of prognosis of GISTs. In fact, some studies on SNPs have been started to evaluate the pathogenesis or prognosis in patients with GISTs. For example, Vashist et al. [21] reported that haeme oxygenase-1 promotor GTn polymorphism was a potential prognostic marker.
In the previous studies, polymorphic variants of the IGF-1 gene and elevated serum levels of IGF-1 protein have been associated with an increased risk of common cancers, while information on IGF-2 polymorphisms and their correlation with cancer risk or prognosis is scarce, and the results that have been published are similarly inconsistent [17,22,23]. For example, significant associations between the SNPs in the IGF-1 promoter region (IGF-1-2995G/A) and the risk of cancer were found in 298 Chinese patients with colorectal cancer and 1,142 controls, suggesting that IGF-1 plays a role in colonic carcinogenesis and genetically inherited variation in IGF-1 expression influences risk of colorectal cancer [13]. Tsuchiya et al. [19] also reported that IGF-1 (cytosine-adenine) repeat polymorphism in the promoter region was associated with prognosis in 111 prostate cancer patients with bone metastasis at the diagnosis. However, these polymorphisms were not found to have any prognostic significance in the survival of the patients with GIST in the current study. There are two possible explanations for this result. First, the sample size of 213 patients was relatively small to draw a statistically significant power in the polymorphism study. Second, somatic alteration in the process of tumorigenesis may have developed, as the DNA was extracted from paraffin-embedded tumor tissue, although no data has been reported on differences between germline and somatic SNPs of the IGF gene. Interestingly, some investigators have reported that IGF-1 receptor was strongly expressed in pediatric or wild type GIST by SNP analysis [24,25]. These studies implicate IGF-1 receptor as a potential therapeutic target in these patients with GIST.
The present study also evaluated a SNP of IGF-2 gene, yet it was not found to have a significant influence on the prognosis of GIST. In a previous study by Suzuki et al. [14] that compared the frequency of 6 SNPs of IGF-1 and IGF-2 in a large-scale case control study to determine whether genetic variations of IGF modify pancreatic cancer risk, the IGF-2 3'-UTR Ex4 -233T/T genotype was significantly associated with a reduced risk of pancreatic cancer. In contrast, Lai et al. [22] reported that polymorphism of IGF-2 gene is not likely to contribute to the pathogenesis of prostate cancer or be involved in tumor progression, although the expression of IGF-2 and androgen receptors in prostate suggested that IGF-2 plays a role in regulating androgen receptor expression in prostate cancer cells. Thus, given these results, a better understanding of the distinct polymorphisms in IGF genes and protein expression regulation in different cancers will be a critical step toward the clinical utilization of this new subclass of genetic variations in management of GIST.
In conclusion, none of the five IGF-1 and IGF-2 gene polymorphisms investigated in this study was found to be an independent prognostic marker for Korean patients with surgically resected GISTs. However, further studies on a larger scale are warranted to clarify the role of IGF and its family gene polymorphisms as a prognostic biomarker for GIST patients.
ACKNOWLEDGEMENTS
The bio-specimens for this study were provided by the National Biobank of Korea-KNUH, which is supported by the Ministry of Health, Welfare and Family Affairs. All materials derived from the National Biobank of Korea-KNUH were obtained (with informed consent) under Institutional Review Board (IRB)-approved protocols.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Furstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3:298–302. doi: 10.1016/s1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 2.Werner H, Le Roith D. New concepts in regulation and function of the insulin-like growth factors: implications for understanding normal growth and neoplasia. Cell Mol Life Sci. 2000;57:932–942. doi: 10.1007/PL00000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(11 Suppl):3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 4.Kim WY, Jin Q, Oh SH, Kim ES, Yang YJ, Lee DH, et al. Elevated epithelial insulin-like growth factor expression is a risk factor for lung cancer development. Cancer Res. 2009;69:7439–7448. doi: 10.1158/0008-5472.CAN-08-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan PD, Goss PE. The emerging role of the insulin-like growth factor pathway as a therapeutic target in cancer. Oncologist. 2008;13:16–24. doi: 10.1634/theoncologist.2007-0199. [DOI] [PubMed] [Google Scholar]
- 6.Van der Ven LT, Roholl PJ, Gloudemans T, Van Buul-Offers SC, Welters MJ, Bladergroen BA, et al. Expression of insulin-like growth factors (IGFs), their receptors and IGF binding protein-3 in normal, benign and malignant smooth muscle tissues. Br J Cancer. 1997;75:1631–1640. doi: 10.1038/bjc.1997.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 8.Reichardt P, Reichardt A, Pink D. Molecular targeted therapy of gastrointestinal stromal tumors. Curr Cancer Drug Targets. 2011;11:688–697. doi: 10.2174/156800911796191042. [DOI] [PubMed] [Google Scholar]
- 9.Pantaleo MA, Astolfi A, Nannini M, Biasco G. The emerging role of insulin-like growth factor 1 receptor (IGF1r) in gastrointestinal stromal tumors (GISTs) J Transl Med. 2010;8:117. doi: 10.1186/1479-5876-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braconi C, Bracci R, Bearzi I, Bianchi F, Sabato S, Mandolesi A, et al. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293–1298. doi: 10.1093/annonc/mdn040. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E. Nutrition, insulin, insulin-like growth factors and cancer. Horm Metab Res. 2003;35:694–704. doi: 10.1055/s-2004-814147. [DOI] [PubMed] [Google Scholar]
- 12.Al-Zahrani A, Sandhu MS, Luben RN, Thompson D, Baynes C, Pooley KA, et al. IGF1 and IGFBP3 tagging polymorphisms are associated with circulating levels of IGF1, IGFBP3 and risk of breast cancer. Hum Mol Genet. 2006;15:1–10. doi: 10.1093/hmg/ddi398. [DOI] [PubMed] [Google Scholar]
- 13.Wong HL, Koh WP, Probst-Hensch NM, Van den Berg D, Yu MC, Ingles SA. Insulin-like growth factor-1 promoter polymorphisms and colorectal cancer: a functional genomics approach. Gut. 2008;57:1090–1096. doi: 10.1136/gut.2007.140855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki H, Li Y, Dong X, Hassan MM, Abbruzzese JL, Li D. Effect of insulin-like growth factor gene polymorphisms alone or in interaction with diabetes on the risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3467–3473. doi: 10.1158/1055-9965.EPI-08-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan HY, Chiou JJ, Tseng WH, Liu CH, Liu CK, Lin YJ, et al. FASTSNP: an always up-to-date and extendable service for SNP function analysis and prioritization. Nucleic Acids Res. 2006;34(Web Server issue):W635–W641. doi: 10.1093/nar/gkl236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canzian F, McKay JD, Cleveland RJ, Dossus L, Biessy C, Rinaldi S, et al. Polymorphisms of genes coding for insulin-like growth factor 1 and its major binding proteins, circulating levels of IGF-I and IGFBP-3 and breast cancer risk: results from the EPIC study. Br J Cancer. 2006;94:299–307. doi: 10.1038/sj.bjc.6602936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YJ, Yoon JH, Kim CY, Kim LH, Park BL, Shin HD, et al. IGF2 polymorphisms are associated with hepatitis B virus clearance and hepatocellular carcinoma. Biochem Biophys Res Commun. 2006;346:38–44. doi: 10.1016/j.bbrc.2006.05.080. [DOI] [PubMed] [Google Scholar]
- 18.Patel AV, Cheng I, Canzian F, Le Marchand L, Thun MJ, Berg CD, et al. IGF-1, IGFBP-1, and IGFBP-3 polymorphisms predict circulating IGF levels but not breast cancer risk: findings from the Breast and Prostate Cancer Cohort Consortium (BPC3) PLoS One. 2008;3:e2578. doi: 10.1371/journal.pone.0002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuchiya N, Wang L, Suzuki H, Segawa T, Fukuda H, Narita S, et al. Impact of IGF-I and CYP19 gene polymorphisms on the survival of patients with metastatic prostate cancer. J Clin Oncol. 2006;24:1982–1989. doi: 10.1200/JCO.2005.02.9439. [DOI] [PubMed] [Google Scholar]
- 20.Cho YY, Kim JG, Chae YS, Sohn SK, Kang BW, Moon JH, et al. No association of insulin-like growth factor gene polymorphisms with survival in patients with colorectal cancer. Cancer Res Treat. 2011;43:189–194. doi: 10.4143/crt.2011.43.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vashist YK, Uzunoglu G, Cataldegirmen G, Kalinin V, Schurr P, Koenig AM, et al. Haeme oxygenase-1 promoter polymorphism is an independent prognostic marker of gastrointestinal stromal tumour. Histopathology. 2009;54:303–308. doi: 10.1111/j.1365-2559.2009.03221.x. [DOI] [PubMed] [Google Scholar]
- 22.Lai MT, Chen RH, Tsai FJ, Wan L, Chen WC. Glutathione S-transferase M1 gene but not insulin-like growth factor-2 gene or epidermal growth factor gene is associated with prostate cancer. Urol Oncol. 2005;23:225–229. doi: 10.1016/j.urolonc.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 24.Janeway KA, Zhu MJ, Barretina J, Perez-Atayde A, Demetri GD, Fletcher JA. Strong expression of IGF1R in pediatric gastrointestinal stromal tumors without IGF1R genomic amplification. Int J Cancer. 2010;127:2718–2722. doi: 10.1002/ijc.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarn C, Rink L, Merkel E, Flieder D, Pathak H, Koumbi D, et al. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci U S A. 2008;105:8387–8392. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]