Abstract
Behavioral activation that is associated with incentive-reward motivation increases in adolescence relative to childhood and adulthood. This quadratic developmental pattern is generally supported by behavioral and experimental neuroscience findings. We suggest that a focus on changes in dopamine neurotransmission is informative in understanding the mechanism for this adolescent increase in reward-related behavioral activation and subsequent decline into adulthood. We present evidence to indicate that incentive-reward motivation is modulated by mesoaccumbens dopamine and that it increases in adolescence before declining into adulthood due to normative developmental changes at the molecular level. Potential mechanisms of variation in functional mesoaccumbens dopamine transmission are discussed with a focus on the interplay between tonic and phasic modes of DA transmission in modulating both general incentive-motivational biases and the efficacy of reward learning during exposure to novel reward experiences. Interactions between individual difference factors and these age-related trends are discussed.
Keywords: risk-taking, reward, motivation, adolescence, dopamine
Human adolescence has long been recognized as a period of heightened exploration of novelty relative to levels observed in earlier childhood and later adulthood (Kelley, Schochet & Landry, 2004; Spear, 2000). This observation is intriguing, because while novel events and situations readily capture attention (Friedman, Cycowicz & Gaeta, 2001), one can respond in real-world contexts with unrestrained approach, caution or avoidance, depending on motivational bias. Approach to novelty reflects a positive motivational bias given that novelty presents a mixture of reward and threat (Hooks & Kalivas, 1995). Because of their willingness to explore unfamiliar situations and lack of experience with high-risk outcomes, adolescents may be vulnerable to various forms of risk-related harm.
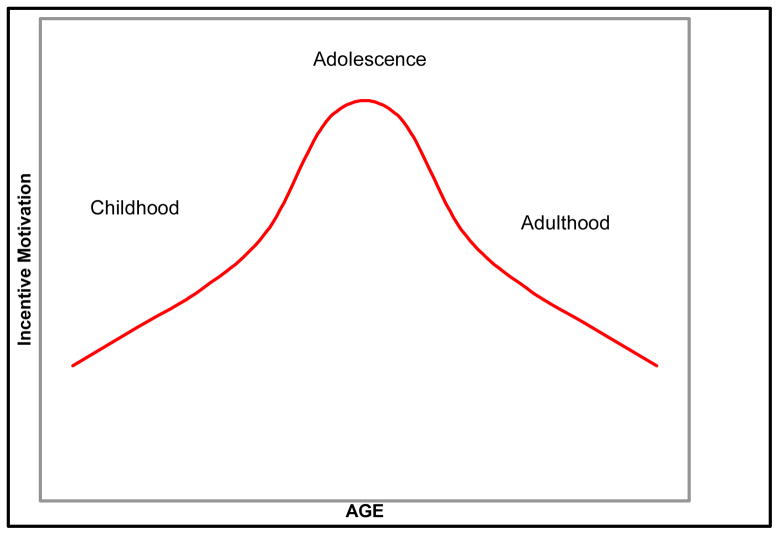
Individuals may engage in actions that have a high probability of adverse consequences because they are insensitive to those consequences, because they are more compelled by the prospect of gain, or because they do not have the requisite experience to develop any meaningful representation of probabilities of gain or loss and thus behave according to an inherent motivational bias. Within this paper, we address the nature of the adolescent’s motivational state and how that state biases response tendencies prior to the acquisition of consolidated experience. Our assertion is that adolescents experience an age-related increase in incentive motivation that is above and beyond what they experience in childhood and above and beyond levels experienced in adulthood (see quadratic pattern illustrated in Figure 1).
Figure 1.
Dopamine-driven acceleration of incentive motiviation from childhood to adolescence and subsequent decline from adolescence to adulthood.
What is Incentive Motivation?
Incentive motivation refers to the energizing of instrumental behavior by anticipation of reward acquisition, (Depue & Collins, 1999; Wise, 2004) a process fundamentally grounded in genetically-determined individual differences (Depue & Collins, 1999; Koob & LeMoal, 1997) but shaped through experience. Importantly, one cannot gain the requisite experience through inactivity. A biologically-grounded system is necessary to promote active exploration to ensure that rewards and reward-learning experiences are obtained.
Gray (1973) proposed that higher order dispositional traits are grounded in biologically-based neurobehavioral systems that vary quantitatively across individuals, i.e., they incorporate trait level variation. The system with incentive motivation at its core has been variously referred to as an expectancy, approach, behavioral facilitation or behavioral activation system (see Depue & Collins, 1999; Luciana, 2001; Wahlstrom, White, Collins & Luciana, 2010a for reviews). It is expressed through behavioral tendencies related to affiliation and agency and the higher order domain of extraversion (Depue & Collins, 1999). Affiliation directs individuals to approach others and to gain reward from interpersonal interactions, while agency promotes social dominance, mastery, efficacy, and achievement (Tellegen & Waller, 1997). While these characteristics can be measured in humans through questionnaire and laboratory paradigms, animal studies have operationalized motivation through measures of goal-directed motor activity, rates, speed and vigor of responding, and the extent to which an animal is willing to work for a given outcome (Beninger, 1983; Olds & Milner, 1954). Developmental psychologists have suggested that this system is represented by the temperament dimension of surgency (Rothbart, Ahadi, Hershey, & Fisher, 2001; Segalowitz, Santesso, Willoughby, Reker, Campbell et al., 2011). We have articulated its role in both adolescent and toddler behavior via dopamine activity (Luciana, 2001; Wahlstrom et al., 2010a,b). According to this framework, approach behaviors are primed by positive incentive stimuli (signals of reward), processed by neural circuits dedicated to reward processing, and then conditioned through instrumental learning using dopamine as a modulator.
A dedicated neural circuit is proposed to support incentive motivation, where the major nodes include (among other structures) the midbrain ventral tegmental area (VTA), its dopaminergic projections to medium spiny neurons of the nucleus accumbens, as well as the ventral pallidum, the amygdala, hippocampus, anterior cingulate cortex, and the medial orbitofrontal cortex (Depue & Collins, 1999; Kelly, Schochet & Landry, 2004). Dopamine (DA) is a primary transmitter that modulates the system’s activity through its facilitation of reward behavior (Damsa, Pfaus, Wenkstern, Phillips, & Fibiger, 1992; Frank, Manderscheid, Panicker, Williams & Kokoris, 1992; Gallistel, Schiegal & Yeomanns, 1981; Koob & Volkow, 2010; Panksepp, 1998). In humans, individual differences in emotional trait dispositions that reflect aspects of positive emotion within the construct of incentive motivation are impacted by genetic and neurophysiological variations in dopamine function (Depue, Luciana, Arbisi & Leon, 1994; Dreher, Kohn, Kolanchana, Weinberger & Berman, 2009; Hariri, 2009; King, Mefford, Wang, Murchison, Caligari, & Berger, 1986; Wacker, Chavanon, & Stemmler, 2006; Zald, Cowan, Ricccardi, Baldwin, Ansari, Shelby, et al., 2008).
Evidence in support of the developmental trajectory advanced in Figure 1 includes behavioral changes in motivational tendencies as well as neurobiological changes involving the structure and functioning of this circuitry.
Evidence for Increased Incentive Motivation in Adolescence
Epidemiological studies indicate that experimentation with substances including alcohol, nicotine, and illicit drugs begins in adolescence. National youth surveys indicate that 21.1% of youth in the United States have consumed alcohol (more than a few sips) and 7.5% have tried marijuana before age 13. By the end of high school, these percentages rise to 72.5% and 36.8%, respectively (Eaton, Kann, Kinchen, Shanklin, Ross et al., 2010). Sexual experimentation is also common. By age 15, up to 70% of adolescents, depending on culture, have engaged in sexual intercourse (Hawes, Wellings, & Stephenson, 2010), many without using adequate protection against pregnancy or disease (Eaton et al., 2010).
These statistics are supported by the manner in which adolescents describe their behavioral biases. For instance, using a measure of sensation-seeking that emphasized novelty-seeking, Steinberg and colleagues recently demonstrated that adolescents report high levels of sensation-seeking relative to children and adults. This tendency appeared to peak in early-to- mid adolescence (roughly between the ages of 12–15) and declined thereafter, as evidenced by a large-scale cross-sectional study of over 900 individuals who ranged in age from 10 to 30 years of age (Steinberg et al., 2008). In our lab, we have observed that mid-adolescents (ages 13–17) report increases in reward responsivity (from Carver and White’s (1994) BIS/BAS questionnaire) relative to levels reported earlier in the adolescent period (around ages 9–12) and relative to levels reported later, during young adulthood (ages 18–23) (Urosevic, Collins, Muetzel, Lim & Luciana, in press). The literature abounds regarding similar examples of self-reported increases in facets of novelty-seeking, sensation-seeking, and behavioral activation from childhood to adolescence as well as declines from adolescence to adulthood (see Steinberg, Albert, Cauffman, Banich, Graham, & Woolard, 2008 for discussion as well as Arnett, 1994; Kafry, 1982; Roth, Schumacher, & Brahler, 2005 for examples). At least one longitudinal twin study reported that genetic variation accounted for one third of the variance in behavioral activation system activity (Takashi, Yamagata, Kijima, Shigemasu, Ono, & Ando, 2007).
In addition, adolescents (relative to both children and adults) have high levels of social affiliation and spend more time with peers than with parents, validating the idea that social acceptance and affiliation are strong natural rewards for this age group (Csikszentmihalyi, Larson & Prescott, 1977; Sebastian, Viding, Williams & Blakemore, 2010; Steinberg, 2008). Affiliative tendencies are observed in pubescent animals as well (Spear, 2011). In contrast to other periods in the lifespan, peer affiliation in adolescence has been conceptualized as a central component of mutual promotion and facilitation of risk-taking behavior (Steinberg, 2008). Importantly, these behavioral domains (experimentation with substances and sexual behavior, peer affiliation in the context of heightened risk-taking) involve exploration of novelty in the pursuit of salient primary rewards, an observation that offers clues regarding potential neural substrates that might underlie approach to such stimuli.
Animal data coheres with the human literature given adolescent-limited increases in novelty preference, exploration, and risk-taking (Douglas et al. 2003; Spear, 2000; Stansfield & Kirstein, 2006), effects that are enhanced by individual dispositions as well as environmental stress (Philpot & Wecker, 2008; Toledo-Rodriguez & Sandi, 2011). In addition, adolescent rats show increased levels of incentive learning relative to adults (Burton, Noble & Fletcher, 2011).
Neurobiological Foundation
While neural structures that contribute to incentive behavior have been identified, it is critical to specify the biological mechanisms that might underlie its development given assertions that increases in sensation-seeking (a construct related, but not identical to, incentive motivation) has been suggested as a core substrate of adolescent risk taking behavior (Steinberg et al., 2008). Adolescent brain and behavioral development often fits a linear model with a plateau in early adulthood, at least as a first approximation. Comparatively fewer examples exist of behavioral domains characterized by accelerations to supra-adult levels followed by subsequent declines. Thus, the quadratic pattern observed via epidemiological, behavioral and animal data limits the number of biological substrates that might be invoked to account for it. Here we offer two potential explanations, both of which capitalize upon the idea that adolescence prepares individuals in an experience-expectant manner (Depue & Collins, 1999) for a range of social, emotional, and motivational experiences that come with emerging adulthood. Together, these experiences allow the individual to achieve reproductive success (in an evolutionary sense) but also permit the achievement of a personal sense of agency in pursuit of adult independence.
Structural Neuroplasticity
Studies of adolescent brain development focus on declines in gray matter volume throughout the cortex, which is taken as an index of synaptic pruning (Giedd, Clasen, Lenroot, Greenstein, Wallace, et al., 2006; Gogtay, Giedd, Lusk, Hayashi, Greenstein et al., 2004), on increases in white matter volume (Schmithorst & Yan, 2010), and on connectivity patterns between regions (Asato, Terwilliger, Wu & Luna, 2010). Perhaps structures that comprise the brain’s incentive-reward motivational system undergo a period of synaptic exuberance as development proceeds from childhood to adolescence. If so, this exuberance might be measurable in humans through changes in subcortical gray matter within medial structures such as the nucleus accumbens, dorsal striatum, amygdala, and hippocampus, and also within cortical regions to which they are directly connected (particularly anterior cingulate and ventromedial prefrontal cortex). During later adolescence, a subsequent wave of synaptic pruning and enhanced axonal connectivity (perhaps extending into young adulthood) would refine synaptic connections based on incentive-reward experiences uniquely encoded by each individual.
Few human structural MRI studies have specifically focused on adolescent changes in volumes of subcortical structures involved in mediation of incentive-reward motivation. Giedd et al. (2006) reported upon gray matter volumes within the caudate nucleus across adolescence with some evidence for a U-shaped sexually-dimorphic developmental trajectory. Volumes peaked prior to adolescence (around the age of 7) in girls and somewhat later (around the age of 10) in boys. In contrast, between the ages of 4 and 18, the amygdala increased in volume throughout adolescence only in boys, while hippocampal volumes increased in girls. Yurgelun-Todd et al. (2003) also reported increased amygdala volumes with increasing age in adolescents, while Bramen, Hranilovich, Dahl, Forbes, Chen et al. (2011) found a sexually dimorphic pattern where age and pubertal status were associated with increased amygdala volumes in boys while maturation in girls was associated with smaller volumes. Structures such as the medial orbitofrontal cortex and nucleus accumbens were not examined.
We recently observed increases in nucleus accumbens volumes from early to mid-adolescence and then a decline into adulthood (Urosevic et al., under review). Like our observation of quadratic changes in self-reported reward responsivity, this patterning was supported by a combination of cross-sectional and longitudinal data. Regions such as the caudate, amygdala and hippocampus did not show similar trends.
Teicher, Andersen & Hostetter (1995) examined rates of synaptic pruning (operationalized as changes in dopamine receptor density) in the nigrostriatal versus mesolimbic dopamine systems in rats from pre-puberty into adulthood. They found strong evidence that both D−1 and D−2 receptors are overproduced then pruned during early adolescence in the corpus striatam (nigrostriatal system) but little evidence for receptor pruning in the nucleus accumbens during the same period. There was moderate evidence for a transient decline in D−1 receptor density in the nucleus accumbens in late adolescence that rebounded by early adulthood.
Overall, while it is plausible that adolescence could be a period of synaptic exuberance followed by pruning in subcortical regions, it may be difficult to empirically document both phases of neurodevelopment, as regressive gray matter changes (e.g., gray matter volume reductions) appear to dominate adolescent structural development. Synaptogenesis is maximal in early childhood throughout the cortex with a gradual pruning of synapses during the late childhood and adolescent periods (Bourgeois, Goldman-Rakic, & Rakic, 1994; Gogtay et al., 2004). The pruning process, inferred from age-related changes in gray matter volumes and cortical thickness, appears to be regionally variant and unfolds in a posterior-to-anterior gradient (Gogtay et al., 2004). It has been generally accepted that subcortical structures stabilize in their maturation earlier than cortical regions, although this conclusion is based on limited evidence. Only recently have neuroimaging techniques allowed volumes of subcortical structures to be resolved with some reliability (Fischl et al. 2002).
In contrast, animal work indicates that subcortical structures of the reward system are malleable in the context of relevant experience. Full coverage of this topic is beyond the scope of this review, but two models will be briefly mentioned, both of which focus on the nucleus accumbens (NAcc). The first concerns basic studies of incentive-motivation in animal models, one of which is derived from a research program involving female Syrian hamsters. Meisel and Mullins (2006) demonstrated that the NAcc is part of a circuit that regulates incentive-motivated behavior, including sexual behavior, in this species. A single sexual encounter in a sexually naïve female activates NAcc neurons (Meisel, Camp & Robinson, 1993; Joppa, Meisel & Gardner, 1995; Kohlert & Meisel, 1999) and sexual behavior increases c-Fos expression in the NAcc core, but not the shell (Bradley & Meisel, 2001). The NAcc core has greater involvement in behavioral response activation based on reward-related information associated with conditioned reinforcers, as derived from information transmitted by limbic inputs, such as the basolateral amygdala (e.g., Ito, Robbins, & Everitt, 2004).
To study sexual behavior in the laboratory under maximally controlled conditions, the animals are ovariectomized and then their hormonal status is regulated by exogenous administrations of gonadal hormones. Estradiol administrations in adult females decreases synaptic density in medium spiny neurons of the NAcc core and alter the morphology of spines in that region. Dendritic spines are the primary anatomical sites of excitatory synapses, and this destabilization is thought to lead to a down regulation of accumbens excitability (Staffend, Loftus, & Meisel, 2011). The reduction is akin to what is observed during drug withdrawal (Kourrich, Rothwell, Klug & Thomas, 2007) and may lead to a similar state of motivational “craving”, which ultimately promotes sexual motivation. Therefore, the hormonal environment modulates the behavioral expression of incentive-motivation by altering synaptic structure within the NAcc (as well as the hippocampus, not discussed here). In the wild, this dynamic would ultimately serve to facilitate motivated behavior (in this case, receptivity to, and seeking of, sexual experience). Adolescence has not been a focus of this work, but given the hormonal changes that accompany puberty onset, as well as the observation that sexual behavior is frequently initiated during adolescence, it is possible that these accumbens synaptic alterations may play a role in motivating normative adolescent escalations in sexual and social behaviors.
A second model of structural plasticity is offered by studies of drug addiction, where it has been observed that drugs of abuse lead to changes in synaptic strength in several regions of the reward system (Luscher & Bellone, 2008). Structural plasticity in the NAcc has been hypothesized to account for drug sensitization effects in psychostimulant-exposed individuals given that medium spiny neuron density increases after repeated cocaine ingestions (Robinson & Kolb, 2004). Spine density changes also accompany cocaine exposure following periods of withdrawal and abstinence (Shen, Toda, Moussawi, Bouknight, Zahm, & Kalivas, 2009). These effects may be mediated by changes in glutamate levels (Kourrich et al., 2007) and can be reversed with injections of a glutamate agonist (Zhou & Kalivas, 2008; Zhou, 2010). While this particular example highlights a pathological process, it similarly illustrates the manner in which experience can alter synaptic structure, strength of responding, and neurochemistry in this region. Similar experience-driven changes in brain structure could occur selectively during adolescence in a manner that accounts for an increase then subsequent decline in incentive-driven behavior.
Neurochemical Dynamics
A second interrelated mechanism to explain this patterning is grounded more explicitly in neurochemical changes that occur between childhood and adulthood. Specifically, during adolescence a transient peak in neurochemical signaling may occur within circuitry that mediates incentive-reward motivation, thereby providing a push toward acquisition of experiences that are essential for developing independent, intrinsically motivated adult behavior in a sexually maturing individual. Here we provide support for this assertion, focusing on changes in limbic/striatal dopamine (DA) signaling from childhood into adulthood.
Overview of the Dopamine System
Dopamine (DA) has both neurotransmitter and neuromodulatory effects. While neurotransmitters influence postsynaptic cells via direct receptor stimulation, neuromodulators may simultaneously regulate numerous populations of neurons thereby impacting the functional status of other chemical systems, leading combinations of excitatory and inhibitory effects (Kazmarek & Levitan, 1987). Thus, while DA cell bodies are abundant in midbrain regions (ventral tegmental area, VTA; substantia nigra, SN), they project to adjacent midbrain and striatal sites (mesostriatal system), to structures historically defined as core to the limbic system (mesolimbic system), and to the cortex (mesocortical system) (Bjorklund & Dunnett, 2007). DA is often co-released with glutamate and/or GABA and impacts information processing through these systems (Koob & Volkow, 2010; Trudeau, 2004). The VTA-to-NAcc (mesoaccumbens) projection is particularly relevant to this review given that the latter structure represents a core node for behavioral activation associated with incentive-reward motivation; it has been estimated that 75% of this projection is dopaminergic (Swanson, 1982).
When depolarized, dopamine cells display two firing rates. One is represented by single-spike firing (in the 2–10 Hz frequency, termed “tonic”); the other is characterized by bursts of 2–6 action potentials (in the 15–30 Hz frequency; “phasic”) (Grace and Bunney, 1984 a, b; Wanat, Willuhn, Clark & Phillips, 2009). The tonic mode is lowin amplitude and steady, and underpins basal DA neuron firing patterns, resulting in extracellular DA concentrations ranging from 5 to 20 nM. DA diffuses several micrometers away from its synaptic release sites, which accounts for extracellular concentrations (Garris & Rebec, 2002; Rice & Cragg, 2008). In addition to presynaptic autoreceptor action, extracellular DA levels are regulated by the action of the dopamine transporter (via reuptake) and by catabolic enzymes. Tonic firing is controlled by membrane properties of the neuron and regulated by GABAergic inhibition (Floresco, West, Ash, Moore, & Grace 2003; Goto, Otani & Grace, 2007).
Phasic firing is rapid and transient, and results in DA concentrations of as much as 1 mM. It leads to high amplitude release of DA into neural synapses and, like extracellular DA from tonic release, is regulated by reuptake mechanisms as well as catabolic enzymes. The net result is that phasic DA acts locally within the synaptic cleft. Phasic activity is triggered by environmentally salient events (see Schulz, 2000, Willuhn, Wanat, Clark & Phillips, 2010 and Wanat et al. 2009 for reviews) and, within the striatal region, is dependent on glutaminergic excitatory input to DA neurons from sources such as the pontine tegmentum, ventral pallidum, and subthalamic nucleus.
The neural and behavioral correlates of each firing mechanism have been only minimally investigated, and it is still relatively unclear how the firing modes interact or the extent to which they are coupled. Tonic activity has been conceptualized as inhibitory to phasic signals (Goto et al. 2007), though it has also been suggested that phasic signals potentiate tonic levels (Niv, Joel & Dayan, 2006). Schultz (2000) demonstrated that phasic DA activity increases in the context of unexpected rewards and in response to reward during initial phases of instrumental reward learning, and that tonic DA levels transiently decline when anticipated rewards are not delivered or when animals encounter aversive stimuli.
DA acts on D1-like and D2-like receptors (Beaulieu & Gainetdinov, 2011) found presynaptically on the spines of projection neurons (Caille, Durmartin & Bloch, 1996; Hersch, Ciliax, Gutekunst, Rees, Heilman, Yung, et al., 1995) and postsynaptically on the heads of dendritic spines contacted by glutamatergic terminals (Levey, Hersch, Rye, Sunahara, Niznik, Kitt, et al., 1983). The tonic and phasic modes of firing may interact differentially with each receptor subtypes. In subcortical projections, phasic DA release activates D1 receptors to facilitate inputs from the VTA to limbic and striatal structures (Goto et al. 2007). Tonic release has bidirectional effects on VTA-prefrontal inputs through the action of D2 receptors. Increases attenuate the activity of PFC afferents while decreases facilitate them, perhaps enabling switches to new response strategies when current responses fail to yield anticipated rewards (Goto & Grace, 2005). Importantly, there is an optimal level of activity at which DA energizes behavior. An inverted-U-shaped performance function (Yerkes & Dodson, 1908) has been proposed to characterize individual differences in DA mediation of reward-based learning (Cohen, Krohn-Grimberghe, Elger, & Weber 2007).
The critical question in the context of this review concerns which physiological parameters of DA neurotransmission are most tightly coupled to individual differences in the behavioral activation associated with incentive-reward motivation. Here we focus on tonic versus phasic modes of action and their effects on behavioral and neurobiological activation. At this point it is important to distinguish between two basic sources of individual differences: genetic variation and learning experiences. In the case of incentive motivation, although accounts tend to focus on the energizing of behavior during a specific incentive-reward learning experience (e.g., Schulz, 2000), individuals vary in their capacities for incentive-related behavioral activation at the outset, prior to any specific learning experience (Depue & Collins, 1999). While phasic DA activity has garnered most of the attention in the literature because of its role in reward learning, more enduring cross-situational individual differences in the magnitude and frequency of incentive-driven behavioral activation appear to be modulated by tonic DA levels (Ostlund, Wassum, Murphy, Balleine, & Maidment, 2011; Niv, Daw, Joel & Dayan, 2007; Willuhn et al. 2010).
The importance of tonic DA levels for motivation can be seen in research on mechanisms of drug reward. For instance, non-contingent systemic administrations of drugs of abuse (nicotine, alcohol, cannabinoids, opiates, amphetamine and cocaine) consistently increase extracellular tonic levels of DA in the NAcc, while drugs with low abuse potential do not (Wanat et al., 2009). Drugs such as amphetamine and cocaine impact extracellular concentrations via clearance mechanisms, notably reuptake inhibition. Alcohol exerts similar effects but through a more complex cascade of signaling events. When tonic DA levels fall below a certain threshold, animals will titrate their levels of drug self-stimulation to re-establish a given level and to achieve optimal levels of intoxication (Willuhn et al. 2010). Thus, tonic DA levels contribute to the drugs’ reinforcing properties but across a time scale of minutes to hours as opposed to the millisecond precision within which phasic signals operate (Wanat et al. 2009; Willuhn et al. 2010). Moreover, decreases in striatal DA transporter activity (achieved through knockout preparations) increase tonic firing and enhance incentive motivation in the context of well-learned behaviors (Cagniard, Balsam, Brunner & Zhuang, 2006a; Cagniard, Beeler, Britt, McGeehee, Marinelli et al., 2006b). Increases in incentive motivation are associated with higher levels of tonic DA activity in the striatum and with increasing response vigor, as demonstrated through behavioral and computational models (Niv, 2007; Weiner & Joel, 2002).
Phasic DA responses are more contextually bound in regard to drug reward. Phasic DA responses occur following drug administrations (reward delivery), notably alcohol, cannabinoids and nicotine, as well as following instrumental responses during the course of cue-drug learning. Conditioned drug cues that are followed by drug administration consistently activate phasic DA, while phasic bursts do not reliably occur in the context of non-contingent pairings. However, spontaneous phasic responses due to pharmacological properties of drugs are also evident and may alter tonic levels of DA, thereby complicating efforts to distinguish tonic versus phasic effects.
Willuhn et al. (2010) suggest that different time scales of dopamine transmission may have different functions. Distinct aspects of phasic signaling may relate to both reinforcement learning and approach behavior, while tonic signaling enables motivational and motor systems but not reinforcement learning. Overall, then, receptor stimulation by tonic DA activity may index the individual’s ongoing incentive-motivational disposition (Willuhn et al. 2010; Niv, 2007) whereas phasic signals primarily influence encoding of stimulus and response reward associations by modulating synaptic plasticity in response to naturally occurring rewards, novel events, and reward learning experiences.
These models may be integrated through a proposed developmental cascade through which elevated tonic DA levels that promote incentive motivation and response vigor serve to direct action in contexts where initial cues for reward learning experiences are ambiguous or simply absent. This context may characterize early adolescence. By definition, higher levels of incentive-motivation (accompanying higher levels of tonic DA) energize exploratory and approach behaviors that bring the individual into contact with reinforcing experiences of reward acquisition, each of which has specific stimulus and response associations. These experiences would then drive both incentive-reward learning and phasic DA responding, and sufficiently potent learning experiences may lead to further alterations in tonic DA levels as bouts of phasic DA responses accumulate (Niv, 2007). With increasing age, prefrontal circuitry and its connections with dorsal and ventral striatal regions become more directionally organized (Asato et al., 2010). This refinement of PFC-striatal connectivity may permit phasic DA signals to achieve optimal signal-to-noise ratios in the relay to cortical circuits. The medial orbitofrontal region, for instance, is among the last cortical brain regions to reach full synaptic maturity (Gogtay et al. 2004) but is in receipt of outcome-related signals from the striatum during reward learning (Tobler, O’Doherty, Dolan & Schulz, 2007). These signals permit an accurate calculation of expected value to be achieved during learning, allowing an organism to make probabilistic judgments about potential outcomes during decision-making (Schulz, 2000; Tobler et al. 2007). Thus, tonic DA signals code individual differences in incentive motivation, propelling individuals at sufficient levels to engage rewarding experiences. These experiences serve as learning contexts through which phasic signals will be generated. As phasic DA signaling becomes more reliable with advancing age (Robinson, Zitzman, Smith, & Spear, 2011) and as the prefrontal cortex achieves its full maturational potential, the organism can maximally benefit from this informational stream in making decisions in potentially risky contexts.
This model is admittedly difficult to operationalize and relies on assessing distinct aspects of DA neurotransmission not only between childhood and adolescence and between adolescence and adulthood but within adolescence as experience consolidates. If tonic levels of dopamine underlie incentive-reward motivation, and if such levels increase through adolescence, there should be evidence of increased DA release, decreased DA transporter activity, increased extracellular DA concentrations and/or decreased autoreceptor regulation, all of which are predicted to have effects on tonic concentrations of DA (Willuhn et al., 2010; Goto et al., 2007). Similarly, as adolescence progresses, we might expect changes in phasic DA responses that have been enabled by this tonic shift. Accordingly, shifts in age-related patterning of tonic versus phasic activity might differentially characterize early adolescence, when individuals initiate a significant expansion in the scope and intensity of reward experiences, as opposed to later adolescence as such experience become more consolidated.
Does the Dopamine System Change During Adolescence?
Animal data supports the notion that various aspects of DA transmission change in the course of the mammalian lifespan from childhood to adolescence (see Table 1). Human studies indicate a functional decline in activity between middle adulthood and old age (Bäckman, Lindenberger, Li & Nyberg, 2010; Dreher, Meyer-Lindenberg, Kohn & Berman, 2008).
TABLE 1.
Summary of major changes in dopaminergic signaling in primates and rodents. Highlighted boxes indicate evidence suggesting heightened dopamine activity compared to adulthood as discussed in the text.
| Cortical | Subcortical | References | |
|---|---|---|---|
| Tissue Concentrations | Peaks during adolescence | NA | Brown & Goldman, 1977; Goldman-Rakic & Brown, 1982 |
| Dopaminergic Innervation | Peaks in PFC layer III during adolescence | NA | Rosenberg & Lewis, 1994; 1995; Lambe et al., 2000 |
| D1-Type Density | Peaks in childhood. Elevated in adolescents compared to adults | Peaks in childhood. Elevated in adolescents compared to adults | Lidow et al., 1991; Lidow & Rakic, 1992, Montague et al., 1999; Seeman et al., 1987 |
| D2-Like Density | Peaks in childhood. Elevated in adolescents compared to adults | Peaks in childhood. Elevated in adolescents compared to adults | Lidow et al., 1991; Lidow & Rakic, 1992, Seeman et al., 1987 |
| Tissue Concentrations | Monotonic increases between childhood and adulthood, but adolescent peaks in dopamine synthesis | Monotonic increases between childhood and adulthood, but adolescent peaks in synaptic availability | Andersen, Dumont, & Teicher, 1997; Giorgi et al., 1987; Nomura, Naitoh, & Seqawa, 1976, Stamford, 1989; Ungethüm et al., 1996 |
| Dopaminergic Innervation | Monotonic increase between childhood and adulthood. | NA | Berger et al., 1985; Kalsbeek et al., 1988 |
| D1-Type Density | Monotonic increases between childhood and adulthood. | Peaks during periadolescence | Andersen et al., 1997; Gelbard et al., 1989; Giorgi et al., 1987; Rao et al., 1991; Tarazi et al., 1999; Tarazi & Baldessarini, 2000; Teicher et al., 1995 |
| D2-Like Density | Monotonic increases between childhood and adulthood. | Peaks during periadolescence | Andersen et al., 1997; Gelbard et al., 1989; Rao et al., 1991; Tarazi et al., 1998b; Teicher et al., 1995 |
Table reprinted from Wahlstrom, White & Luciana (2010b), with permission.
Primate and rodent research generally indicates the most extensive changes in DA transmission during the prenatal period into early childhood, as full synaptic capacity develops. There appear to be more subtle but measurable changes in adolescence and declines in DA functional activity thereafter. We have summarized this work elsewhere (Wahlstrom, Collins, White & Luciana, 2010a; Wahlstrom, White & Collins, 2010b), and other recent reviews have discussed it as well (Ernst, Romeo & Andersen, 2009; Spear, 2011). We will emphasize points most salient to the impact of tonic versus phasic signaling.
Table 1, reprinted from Wahlstrom et al. (2010b), summarizes evidence of adolescent-specific changes in dopamine function. The Table presents data separately from primate versus rodent studies, because distinctions between species have been observed, particularly in comparing subcortical versus cortical patterns of DA activity along several parameters. Both primates and rodents exhibit alterations in substrates of DA signaling during adolescence. Subcortical changes are most relevant to this review.
One study reported on changes in midbrain cell firing through adolescence in the rat (McCutcheon & Marinelli, 2009). Slow spike firing, in the range of 3–5 Hz, increased from days 24–27 (late childhood), peaked around day 48 (peri-adolescence) and then showed a steady decline through day 70 (young adulthood). Others, using microdialysis, have reported a quadratic patterning of basal DA concentrations with late adolescent peaks relative to early adolescence or adulthood (Badanich, Adler & Kirstein, 2006; Philpot, Wecker & Kirstein, 2009). Concordantly, there is a decline in synthesis-modulating DA autoreceptor function in the striatum, nucleus accumbens, and—most notably—the prefrontal cortex between childhood and adolescence (Andersen, Dumont & Teicher, 1997), leading to increases in synaptic availability. In adolescent primates, subcortical tissue concentrations of DA increase relative to both childhood and adulthood (Goldman-Rakic & Brown, 1982; Irwin, DeLanney, McNeil, Chan,, Forno, & Murphy, 1994). D1 and D2 densities peak in subcortical structures such as the striatum and NAcc in late childhood to early adolescence, as do the densities of D2-like D3 and D4 receptors (Andersen, Thompson, Krenzel & Teicher, 2002; Tarazi, Tomasini, & Baldessarini, 1998b). Both D1 and D2 densities are heightened during adolescence relative to adulthood, a pattern that characterizes both the cortex and subcortical regions (Lidow & Rakic, 1992; Seeman, Bzowej, Guan, Bergeron, Becker, & Reynolds, 1987). The decline in receptor density from adolescence to adulthood is in the range of 35–50%, depending on region, and is accompanied by dramatic changes in DA basal activity (Andersen et al. 2002).
We have not previously reviewed changes in transporter density, but these could impact adolescents’ tonic DA levels as well as pharmacological responses to DA drugs (Wanat et al., 2009). If transporter density declines with consequent increases in DA tone, then adolescents might be particularly sensitive to pharmacological manipulations that impact reuptake inhibition. Indeed, adolescent animals, relative to adults, are more sensitive to the rewarding properties of psychostimulant drugs including cocaine (Brenhouse and Andersen, 2008), nicotine (Torres et al. 2008) and alcohol (Pautassi et al. 2008). Spear has demonstrated through an elegant series of studies (see Doremus-Fitzwater, Varlinska & Spear, 2010; Spear, 2011) that adolescent rats are particularly sensitive to the rewarding effects of alcohol, and concordant with the human work of Chein et al. (2010), to alcohol’s facilitation of social behavior (Varlinska & Spear, 2002). However, at least one study has demonstrated that cocaine-induced blockade of the DA transporter does not differentially impact extracellular DA levels in adolescents versus adults (Frantz, O’Dell & Parsons, 2007).
Findings regarding postnatal changes in DA transporters have been inconsistent. This is especially true in the SN and VTA, where different lines of evidence have indicated no consistent developmental changes (Moll et al., 2000), steady increases between birth and adulthood (Galineau et al., 2004), and peaks in transporter density at postnatal day 21 in the rat, which is prepubertal (Coulter, Happe, & Murrin, 1996). Importantly, different binding agents were used in each study and the age groups studied are not directly comparable. For example, neither the Moll et al. (2000) nor the Galineau et al. (2004) studies examined transporter levels at the age at which density peaked according to the evidence of Coulter, Happe, and Murrin (1996). Findings in subcortical regions are more consistent, with converging lines of evidence indicating that transporter density increases into periadolescence and plateaus thereafter in the striatum, NAcc, thalamus, and bed nucleus of the stria terminalis (Coulter, Happe, & Murrin, 1996; Galineau et al., 2004; Tarazi, Tomasini, & Baldessarini, 1998a; but see Moll et al., 2000 for contrary findings).
As comprehensively summarized by Spear (2011), although the animal evidence is overwhelming for a remodeling of the DA system during adolescence, these various changes do not map onto developmental changes in motivated behavior in a straightforward manner. For example, adolescent rats appear to show differential responsivity to the motor-activating vs. rewarding properties of stimulant drugs, possibly depending on the specific mechanisms of drug action, e.g., DA release vs. inhibition of the DA transporter (Walker et al. 2010). Accordingly, although DA projection systems to both dorsal and ventral striatum (NAcc) are functionally immature in adolescent rats, the functional consequences differ in terms of DA release, reuptake, and behavior, at least in the case of stimulant drugs. In general, components of DA projection systems are highly interactive and there is the potential for researchers to reach different conclusions based on the use of different methodological techniques (see also Ernst et al. 2009; Willuhn et al. 2009 for further discussion of this point), as well as different experimental designs.
This complexity is illustrated well by Robinson et al. (2011), who studied phasic DA fluctuations in early adolescent rats and reported a baseline rate of fluctuation similar to adult rats but an unreliable coupling of phasic DA responses to novel stimuli, along with a non-habituating phasic DA responsivity to repeated brief social interactions with other rats (unlike adult rats). As shown by this example, adolescents may differ from adults regarding the information carried by fluctuations in DA transmission, even when overall transmission levels are equivalent. Therefore, it is challenging to pinpoint which developmental changes in DA transmission have the most potent and direct influence over adolescent reward behavior, as well as which changes are antecedents rather than consequences of reward experiences (Spear, 2011).
This complexity is reflected, too, by human functional neuroimaging studies using tasks that deliver rewards and punishments (summarized in Table 2), which generally have not been specifically designed to assess DA-related processes but contribute nonetheless to this discussion. Paradigms used across studies vary considerably in the extent to which they assess incentive-related learning, brain responses to anticipation versus delivery of rewards, whether incentives are designed to promote improvement on an otherwise non-rewarding task, and whether outcomes can be predicted in the course of task performance. Analytic strategies similarly vary from whole-brain analysis to a priori region-of-interest approaches with variation in the stringency of applied statistical thresholds. Given this heterogeneity in design and analysis, the studies vary considerably in the extent to which they identify age-related differences in activation.
Table 2.
fMRI Studies of Reward Processing in Healthy Adolescents
| Reference | Age Groups | Task Paradigm | Findings |
|---|---|---|---|
| May 2004 | 18 children and adols, ages 8–18 | Guessing game with no predictable contingencies; guesses were rewarded, not rewarded, or had neutral outcomes in a predetermined sequence | Many regions activated by reward feedback including the NAcc and orbital frontal region; no effects of age on patterns of activation |
| Bjork et al 2004 | 12 adols (ages 12–17); 12 adults (ages 22–28) | Monetary Incentive Delay Task: Subjects saw cues signaling opportunities to win or avoid losing rewards of different magnitudes; Task was to respond quickly to a subsequent target to receive the reward; feedback was provided on each trial | No group diffs in performance; anticipation of gain activated NAcc in both groups; right insula, dorsal midbrain, thalamus and ACC also activated; gain vs. nongain outcomes activated the NAcc, PFC, and putamen; posthoc group comparison revealed an adolescent decrement in gain anticipation activation in the right NAcc |
| Ernst 2005 | 18 adols (ages 9–17); 16 adults (ages 20–40) | Wheel of Fortune: Subjects selected one slice of the wheel based on color (red or blue); the wheel was spun; the subject won the dollar amount paired with the selected slice if the slice stopped under the pointer. If not, the subject won nothing. | Win versus no-win contrast showed greater NAcc activation in adols vs. adults; negative feedback activated the amygdala in adults moreso than adols |
| Eshel et al. 2007 | 18 adols, 16 adults; same as Ernst et al. 2005 | Wheel of Fortune task; focus was on risky selections | Increased risky selections in moderate risk range negatively correlated w/age; risky choice selection was associated with greater OFC/VLPFC/ACC activation in adults vs. adols; this activation correlated w/risky selections |
| Galvan et al 2006 | 16 children (aged 7–11), 13 adols (ages 13–17), 12 adults (ages 23–29) | Delayed response two-choice task during event-related fMRI: passive pairings of cues and rewards; task was to select the side of cue presentation; responses not explicitly rewarded | Focus on nucleus accumbens and OFC responses to large rewards; adols showed greater NAcc activity relative to both children and adults; adols showed less OFC activation than children and were equivalent to adults |
| Galvan et al 2007 | 10 children, 7 adols, 9 adults (subset of the above sample) | Delayed response two-choice task during event-related fMR (same as above) plus self-reported measures of risk-taking and risk perception | Self-reported likelihood of engaging in risk-taking was associated with magnitude of NAcc activation in response to large rewards (r = .61); those who anticipated positive consequences from risk-taking activated the NAcc more |
| van Leijenhorst et al., 2010 | N= 53; 15 adults (ages 18–23); 18 adol (ages 14–15); 17 pre-adol (ages 10–12) | Slot Machine Task: three possible outcomes (XYZ (50%), XXY (25%), XXX (25%); wins only for XXX; examined how adols respond to uncertain rewards | when anticipating uncertain rewards, all age groups showed increased activation in the striatum; a cluster in the anterior insula showed a linear decrease in activation with age; when processing outcomes, middle adols were more responsive to received rewards as indicated by increased activation in the ventral striatum; young adults responded most to the omission of rewards as indicated by increased activation in the OFC |
| Bjork, Smith, Chen, & Hommer, 2010 | 24 adols (ages 12–17) and 24 adults (ages 22–42) | Compared adol response to reward cues vs. reward delivery using Monetary Incentive Delay Task | Both adols and adults recruit the NAcc and medial OFC during task performance; Adols showed reduced NAcc recruitment by reward-predictive cues compared to adults in a linear contrast with non-incentive cues, and in a volume-of-interest analysis of signal change in the NAcc. Adols showed little difference in striatal and frontocortical responsiveness to reward deliveries compared to adults; suprathreshold activation of ACC by loss outcomes vs. avoided losses was present in adults but not adols |
| Cohen et al 2010 | 18 children, aged 8–12, 16 adols aged 14–19, and 11 adults aged 25–30 | Probabilistic learning task Participants classified abstract stimuli into one of two categories; feedback provided on each trial; correct responses included a monetary reward. |
Adols had faster reaction times than the other groups to stimuli w/large rewards; Neural responses to stimulus presentation vs. feedback were modeled using linear and quadratic functions; Neural prediction error signals in the striatum peaked in adolescence, whereas neural decision value signals varied depending on how value was modeled but decreased with age. |
| Geier et al. 2010 | 18 adols (ages 13–17) and 16 adults (ages 18–30) | Rewarded antisaccade task; antisaccade trials cued by possibility of reward or not on that trial; correct inhibitions were rewarded; no trial-by-trial feedback provided | Both groups performed better under incentive conditions; Adols vs. adults showed attenuated VS responses during the incentive cue, but heightened VS and sPFC responses during reward anticipation |
| Somerville et al 2010 | Children (ages 6–12); adols (ages 13–17); adults (ages 18–29) | Affective go no-go paradigm with happy versus calm faces | Groups did not differ in hit rate; false alarms were greatest for adols vs. the other two groups for happy targets; adols activated the VS moreso that adults and children in response to happy faces; activation of frontal regions (IFG) showed a linear pattern across development |
| Van Leijenhorst et al 2010 | 4 age groups: 8–10 yo; 12–14yo; 16–17yo; 19–26yo; total n= 58 | Goal was to examine whether cognitive control patterns develop linearly and whether reward responsiveness is elevated in adol using a two-choice decision-making task; participants repeatedly chose between a low risk gamble and a high-risk gamble; the size of the reward associated with the high-risk gamble varied | All age groups made more high-risk decisions as rewards increased; there was a decrease in risk-taking with age in the most ambiguous condition; older participants were more risk averse; Across ages, risky choices were associated with activation in the medial PFC and the ventral striatum; cautious choices were associated with DLPFC activation; there was an adolescent-specific peak in activation in a VMPFC/subcallosal region during the decision phase, and in the VS/caudate during the outcome phase. |
| Chein et al 2011 | 14 adols (ages 14–18); young adults (ages 19–22 years); adults (ages 24–29) | Investigated peer influence on risk-taking; Stoplight Task: simulates driving intersections w/traffic lights; At each intersection subjects decide whether or not to brake as the vehicle approaches a changing traffic signal (cycling from green to yellow to red); timing of traffic signals and probability of crash both unpredictable; incentives offered for completing the drive in a fast time period | Adolescents took significantly more risks when observed by peers than when alone; behavior was predicted by sensation-seeking tendencies and not by impulsivity; significant age by social context interactions were found selectively in the VS and OFC; presence of peers activated these sites in adols but not other groups; Among adols, greater activity in the VS and OFC was associated with risky decision-making (go versus stop trials); Adults engaged lateral PFC sites more robustly than did adolescents when making decisions; this age difference did not interact with social context |
Abbreviations in the tables are as follows: Adols = adolescents; NAcc= nucleus accumbens; OFC= orbitofrontal cortex; PFC = prefrontal cortex; VS = ventral striatum; DLPFC = dorsolateral prefrontal cortex; VMPFC= ventromedial prefrontal cortex; VLPFC = ventrolateral prefrontal cortex; IFG = inferior frontal gyrus; ACC = anterior cingulated cortex; sPFC= superior prefrontal cortex
For instance, age differences between children and adolescents were not found in blood oxygen level dependent (BOLD) responses to feedback when a guessing game was utilized (May, Delgado, Dahl, Stenger, Ryan, Fiez and Carter, 2004), but in a comparison of adolescents and adults that utilized a “Wheel of Fortune” gambling task (Ernst, Nelson, Jazbec, McClure, Monk and Leibenluft (2005), a win versus no-win contrast revealed greater NAcc activation in adolescents. In contrast, negative feedback (non-wins relative to wins) elicited greater amygdala activation in adults than adolescents, suggesting less sensitivity to punishment for adolescents, a pattern also observed by van Leijenhorst, Zanolie, van Meel, Westenberg, Rombouts, and Crone, (2010) in the context of orbitofrontal activation and by Bjork, Smith, Chen and Hommer (2010) in the ACC. In addition, van Leijenhorst, Gunther et al. (2010) found adults to be behaviorally more risk-averse.
Bjork, Knutson, Fong, Caggiano, Bennett, & Homer (2004) compared 12–17 year-olds to young adults using the Monetary Incentive Delay Task. On each trial, participants viewed cues that signaled the opportunity to either win or lose money. A subsequent target appeared, and the task was to respond as quickly as possible to the target with a button press. If the response was sufficiently quick, then the participant either won or avoided losing money. Feedback was provided. This task would seem to be an excellent candidate for the recruitment of incentive motivation. Yet, there were no group differences in performance. Two aspects of reward processing were assessed in terms of brain activation, the anticipation of gain and responses to reward delivery. Gain anticipation activated the NAcc in adolescents and adults, along with other neural structures associated with reward processing (see Table 2). Posthoc group comparisons revealed that adolescents showed a decrement in gain anticipation(decreased right NAcc activation), suggesting motivational deficiency in this age group. A more recent examination of reward anticipation using the same paradigm (Bjork et al., 2010) indicated reduced recruitment of the NAcc in adolescents when responses to incentive and non-incentive cues were compared.
Others find stronger evidence for distinctive adolescent patterns of activation in reward-linked neural structures (Galvan, Hare, Parra, Penn, Voss, Glover, & Casey, 2006; Somerville, Hare and Casey, 2010; van Leijenhorst et al., 2010). Galvan et al. (2006) focused on NAcc and OFC responses to large rewards in a task that required no explicit reward-related learning. Adolescents showed greater NAcc activity relative to both children and adults, while they showed less OFC activation than children. The magnitude of NAcc activation in response to large rewards was associated with the self-reported likelihood of engaging in risk-taking (Galván, Hare, Voss, Glover, & Casey 2007), and those who anticipated positive consequences from risk-taking activated the NAcc more strongly, a finding that supports the impact of individual difference factors on observed age-related activations. Eshel, Nelson, Blair, Pine, and Ernst (2007) similarly focused on the extent to which adolescents made risky selections and the neural activations associated with those selections. Increased risky selections in a moderate risk range negatively correlated with age. In addition, these selections were associated with less activation of the OFC, ventrolateral PFC, and ACC in adolescents versus adults.
Somerville, Hare and Casey (2010) utilized a distinct measure of affective bias (an affective target detection task) and compared children, adolescents, and adults in their hit rates, false alarm rates, and patterns of brain activation. Adolescents were more likely to generate false alarms, misinterpreting calm faces as happy, implying a positive bias. A region-of-interest analysis indicated that adolescents had greater activation of the ventral striatum than adults or children in response to happy faces. Activation of prefrontal cortical areas showed a more linear pattern across development.
Van Leijenhorst et al. (2010) examined how adolescents, as compared to children and adults, responded to reward anticipation vs. uncertainty using a gambling task. All age groups showed increased activation in the striatum during reward anticipation. However, during the processing of reward outcomes, adolescents showed a unique pattern of increased activation in the ventral striatum, a pattern that was not observed by Bjork et al. (2004, 2010) but is more akin to Ernst et al.’s (2005) findings. In a second study, van Leijenhorst, Gunther Moor, Op de Macks, Rombouts, Westenberg, and Crone (2010) assessed whether cognitive control patterns develop linearly between childhood and adulthood and whether reward responsiveness is elevated in adolescents relative to other groups. They used a two-choice decision-making task requiring participants to repeatedly choose between a low risk gamble and a high-risk gamble. The size of the reward associated with the high-risk gamble varied. All age groups made more high-risk decisions as rewards increased. However, there was a decrease in risk-taking with age. Across ages, risky choices were associated with activation in the medial PFC and the ventral striatum. Cautious choices were associated with DLPFC activation. In terms of unique patterns in adolescents, there was an adolescent-specific peak in activation in a VMPFC/subcallosal region during the decision phase of the task and in the ventral striatum/caudate during the outcome phase.
Chein, Albert, O”Brien, Uckert, and Steinberg, (2011) potentially lend some interpretive clarity to these disparate findings by showing significant age-by-social context interactions in the context of a driving simulation. The presence of peers activated the ventral striatum and orbitofrontal cortex in adolescents but not other groups. Among adolescents, greater activity in both regions was associated with risky decision-making (running yellow lights at stoplight intersections). Concordant with Ernst et al. (2005), adults engaged lateral PFC sites more robustly than did adolescents, perhaps suggesting more sensitivity to punishment conditions. This activation did not interact with social context. Thus, under conditions that elicit particularly strong incentive-motivation in adolescents, i.e., peer interaction, adolescents may exhibit stronger neural responses to both reward anticipation (also concordant with Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010) and reward delivery and may show heightened ventral striatal responses.
Taking this literature as a whole, six studies (Chein et al., 2011; Galvan et al., 2006, 2007; Somerville et al., 2010; van Leijenhorst et al., 2010) compared children or early adolescents to mid-adolescents and adults, allowing quadratic trends to be assessed. Nearly every paradigm was associated with some form of increased activity in dopamine-innervated structures of the reward system in adolescents as compared to younger versus older participants, although the conditions that elicit reliable region-specific elevations in activation for adolescents remain to be clarified. The strongest contrary evidence comes from Bjork et al. (2004; 2010), who used a measure explicitly designed to elicit incentive motivation and failed to find significant age differences. In contrast, Somerville et al’s (2010) finding of a bias for perception of positive facial affect in adolescents versus children and adults coheres with the notion that incentive motivation is primed most strongly in a social context during this period, and as shown by Chein et al. (2011) contributes to elevated risk taking in a peer context. While this body of work may be suggestive of a quadratic trajectory in the development of neural signals associated with reward processing from a broad perspective, at this early point in the research the findings are conflicting in regard to elevated NAcc responsivity and associated incentive-reward activation as the specific neurobehavioral substrate for the quadratic pattern. In addition, because complex decision-making strategies are frequently incorporated into task designs, some experimental paradigms are more adept at assessing developmental trends in cognitive and behavioral control as opposed to incentive-reward motivation.
While any finding of increased adolescent NAcc (or other reward structure) activation in the context of a risk-reward paradigm is interesting in terms of the neurobiology of risk-reward decision making in general, direct connections to levels of DA transmission are lacking. Experimentally, the most direct connection to DA phasic responses requires that the task involve trial-and-error learning of incentive-reward response contingencies, thereby utilizing the magnitude of the reward prediction error to guide learning and thus improve behavioral performance (e.g., Cohen, Asarnow, Sabb, Bildner, Bookheimer et al. 2010; Pessiglione, Seymour, Flandin, Dolan & Frith, 2006). Alternatively, a task with tradeoffs between level of response effort and magnitude of reward may provide an assessment of individual differences in tonic DA levels (see Niv, 2007; Pinkston & Lamb, 2011).
At present, findings from the varied (albeit interesting) risk-reward tasks in this literature tend to obscure important conceptual distinctions. For instance, if punishment contingencies play a large role, as opposed to limiting a task to reward-delivery and reward-omission contingencies, another emotional-motivational system that regulates behavioral inhibition will be activated in simultaneous competition with the system that regulates incentive-reward motivation, as suggested by Ernst, Pine and Hardin (2006). Conversely, if a task induces NAcc activation in response to simply viewing outcome-feedback stimuli that do notguide learningor elicit modifications of future responses, the interpretive linkto DA phasic responses and incentive-motivation is relatively weak.
Finally, most neuroimaging studies of human adolescent development have focused on cortical regions (where structural and functional changes have been clear-cut) and have not been designed to maximize assessment of diencephalic and midbrain structures. Recently D’Ardenne and colleagues have reported results using methods to tailor fMRI to assessment of brainstem responses, including within areas rich in DA cells such as the ventral tegmental area (D’Ardenne,, McClure, Nystrom, & Cohen, 2007). However, fMRI remains targeted at phasic responses, as tonic activation levels typically are removed as sources of nuisance variance in standard statistical processing of BOLD signals. Positron emission tomography (PET) scanning is more suited to assessment of individual differences in tonic DA levels (e.g., Cools, Frank, Gibbs, Miyakawa, Jagust, & D’Esposito, 2009), which introduces the risk of radiation exposure. In addition, because sample sizes tend to be small in fMRI studies, the role of individual differences that cut across age groups cannot be explicitly assessed. This is particularly important given the variability in individual BOLD activation responses observed within age groups, which can greatly exceed the magnitude of the age group average effects (Bjork et al., 2010).
Individual Differences in Dopaminergic Tone Predict the Outcomes of Adolescent-Specific Over-Activation
The third important point within this review concerns the notion that individual differences in mesoaccumbens dopamine transmission predict the specific outcomes of normative adolescent elevations in incentive-motivation and associated behavioral activation, as well as the eventual adult phenotypes.
Despite the general tenor of literature on adolescent risk-taking, many healthy typically developing teens do not apparently demonstrate unusually strong motivational drives and impulsive response tendencies. Any model of adolescent development will have greater explanatory power if it can account for both group-based profiles as well as individual differences in behavior, and incorporate interactions between the two sources of variation. To this point, we have asserted that incentive-reward motivation and behavioral activation are relatively greater in adolescence as compared to other points in the lifespan because of increases in mesoaccumbens dopamine tonic activity during this time. Nevertheless, we recognize that individual differences undoubtedly set a range around which this adolescent-specific reaction can occur, and that individuals enter the adolescent period with pre-existing variation in levels of incentive-reward motivation and behavioral activation due to genetic and environmental influences operating throughout development (Depue & Collins, 1999; Frank & Hutchison, 2009). For individuals with low levels of basal mesoaccumbens DA activity, increases during the adolescent period will increase their absolute levels of incentive-reward motivation and behavioral activation but still place them at relatively lower levels than their peers, resulting in less reward seeking and more limited exploration of the novel experiences that typically become available during adolescence. For individuals who occupy the middle of the distribution of basal mesoaccumbens DA activity, adolescent increases may bring them to a maximally adaptive peak of incentive-reward motivation and behavioral activation, with increases in reward seeking and exploration of novelty that do not entail frequent exposure to highly dangerous risks. For those entering adolescence with high levels of basal activity, further increases may predispose them toward behavioral engagements with excessively risky situations, repeated drug and alcohol use, inability to maintain focus in an achievement context, and complaints of chronic boredom. This can be seen as a form of response perseveration or stereotypy (in accord with the Yerkes-Dodson formulation) in which the individual repeats the prepotent response despite the presence of environmental cues that signal a need for caution.
This three-fold typology was illustrated in adults by Cools et al. (2009), who used baseline PET assessment and pharmacological manipulation to establish a link between basal striatal DA activity and reward-based learning. Specifically, it was demonstrated that DA synthesis capacity impacts learning rates as well as the behavioral effects of D2 receptor stimulation. Individuals with relatively high baseline DA synthesis (a component of basal DA activity) as indexed by uptake of a PET dopamine synthesis tracer showed relatively better reversal learning from unexpected rewards relative to punishments. These individuals were also more impaired by the administration of a D−2 receptor agonist, supporting the hypothesis that induction of significantly increased activity in an already high functioning DA system results in an “overdose” behavioral reaction, analogous to the dysfunctional motor response stereotypy observed under conditions of excessive mesoaccumbens DA transmission in animal studies. Conversely, individuals with relatively low levels of basal DA synthesis showed relatively poorer reversal learning from unexpected rewards relative to punishments, but improved their performance significantly following administration of the D−2 receptor agonist. Individuals with intermediate levels of basal DA synthesis maintained intermediate levels of task performance both at baseline and during the drug challenge condition. Together these findings suggest that there may be a linkage between tonic DA levels that influence overall levels of incentive-motivation and behavioral activation, on the one hand, and the efficacy of phasic mesolimbic DA signals that guide incentive-reward learning in specific environmental contexts, on the other. Accordingly, adolescent-limited elevations in mesoaccumbens DA transmission would be predicted to differentially affect individuals, depending on their baseline pre-adolescent levels, as they engage the novel opportunities for potential reward acquisition (and exposure to punishment) that arise during adolescence.
It must be emphasized that these illustrative scenarios are constructed in a type of “dimensional isolation” for clarity of presentation. The reality of adolescent behavioral change obviously is much more complex, beginning with interactions between the magnitude and frequency of incentive-reward activation of behavior and the strength of constraining influences exerted by behavioral inhibition and cognitive control systems. Moreover, adolescent reward experiences can induce plasticity in the mesoaccumbens DA system that determines forward-going developmental trajectories in ways that defy direct linear prediction from pre-adolescent behavioral characteristics (see Wahlstrom, Collins, White & Luciana (2010a) for a similar example of genotypically-variant patterns of working memory development from childhood to adulthood).
Concluding Comments
Few researchers have investigated the potential uniqueness of the adolescent period in terms of the mechanisms that underlie the time-limited heightening of incentive-reward motivation and behavioral activation during adolescence, bracketed by lower levels in both earlier childhood and later adulthood. As of now, there have not been structural MRI reports of NAcc expansion then shrinkage that correspond in magnitude to the more dramatic changes in cortical gray matter that occur throughout adolescence (Gogtay et al, 2004). Accordingly, we suggest a neurochemical account focusing on mesoaccumbens DA transmission that provides a potential mechanistic explanation for this observed quadratic trajectory in incentive-reward behavior. We emphasize that at a molecular level various aspects of DA neurotransmission are under both genetic and environmental influence and thus capable of forming a foundation for individual differences, as well as normative trends, in levels of incentive-reward motivation and associated behavioral activation. An important next step in the study of adolescent brain development is to generate empirical data that can be more directly interpreted in terms of DA-modulated variation in levels of ventral striatal activation in adolescents vs. children and adults, as well as direct assessment of individual differences that may have as much behavioral impact as the normative adolescent trends. Acquiring and integrating this evidence is crucial, because the behavioral activation associated with incentive-reward motivation contributes to the executive load that must be managed by the more linearly developing cognitive-behavioral control system (Luciana & Collins, under review). Thus, adolescence may be a period of nonlinear challenge to the ongoing development of emotional self-regulation.
Limitations
This discussion has been restricted to the dynamics of the incentive-reward motivational system and its subcortical substrate, particularly with respect to functional variation in the VTA-NAcc DA projections. To simplify the presentation, we have not discussed interactions with the cognitive control and behavioral inhibition systems, nor mechanisms for adaptive self-regulation of emotional behavior in the context of cortical/subcortical interactions. In addition, it is important to point out that other groups have suggested a role for DA in adolescent risk-taking behavior (Ernst et al., 2009), mediated through its facilitation of sensation-seeking (Steinberg et al., 2008, 2010) as well as through the balance of activation between cortical vs. subcortical structures (Casey, Jones & Hare, 2008). However, other groups also have expressed reservations concerning the centrality of a dopaminergic mechanism. For example, Casey and Jones (2010, p. 5) recently stated that “It remains unclear how changes in the dopamine system may relate to motivated behavior, because controversy remains as to whether reward sensitivity is modulated by dopamine systems.” While Steinberg et al. (2008) suggest that increases in limbic dopamine activity, in concert with puberty-driven hormonal changes, might drive adolescents’ increases in sensation-seeking, they note that this connection has not been established. Our view is that a comprehensive evaluation of both animal and human research literature clearly indicates a central role for dopamine, in concert with glutaminergic and gabaergic systems, in modulating levels of incentive-reward motivation. Moreover, we hypothesize that individuals’ levels of functional dopamine activity in ventral striatal regions are predictive of their behavioral biases in the presence of incentive-reward cues. We suggest that functional activity in the mesoaccumbens dopamine system is heightened in the adolescent period but with the caveat that this association has relied nearly exclusively on animal research. The human functional neuroimaging literature is contradictory on this point (see Table 2, as well as Spear (2011) for discussion), and within the animal research literature the primary mechanisms within the mesoaccumbens dopamine system that may induce these adolescent effects are unclear. Given our specific hypotheses regarding tonic mesoaccumbens DA transmission levels, we suggest that changes in autoreceptor and transporter function merit particular scrutiny given their roles in regulating extracellular DA.
We suspect that many investigators in the field of adolescent brain development, while questioning our specific hypotheses, would agree in principle with this model. Several labs have reported quadratic trajectories for aspects of reward-based responding and/or sensation-seeking (Somerville et al., 2010; Steinberg et al., 2008; van Leijenjorst et al., 2010a,b). What makes our perspective unique is that we view this accumulation of evidence as a foundation for moving beyond speculation to direct human pharmacological and neurobehavioral testing of a DA-based model.
Such testing in adolescent samples would not be without challenges, since systemic pharmacological agents can carry risks and side effects, and also can modify both tonic and phasic signaling (Willuhn et al. 2010). However, it may be possible to combine pharmacological probes with behavioral tasks that are more specific in assessing tonic DA levels versus phasic DA responses. For instance, phasic signaling is inferred through prediction error as quantified in probabilistic learning paradigms, while tonic DA levels may be more readily associated with response vigor during effortful behavior, as well as individual differences in responding to tradeoffs between level of response effort and magnitude of reward. Moreover, rather than lumping adolescents into one large group, it may be more productive to assess neural and behavioral distinctions as a function of level of exposure to novel reward experiences, e.g., by separating early, mid- and late adolescents into discrete groups or by quantifying the amount of exposure using self-report instruments.
Future Directions
Beyond direct assessment of the dopamine system, which would add considerable interpretive clarity to the neuroimaging and behavioral findings already reported, other future directions include refining the construct of incentive-reward motivation and behavioral activation as it applies to children and adolescents, perhaps by creating questionnaire measures that provide more developmentally appropriate representations the construct. Laboratory and neuroimaging probes, too, could be better informed by the animal pharmacology literature (as with Cohen et al., 2010), allowing the various components of incentive motivation and behavioral activation to be assessed more cleanly. Good candidates, in our view, would include assessments of response vigor (Niv, 2007), manipulations of the expenditure of effort to achieve immediate goals (Treadway, Buckholtz, Lambert, Schwartzman, & Zald, 2009), and discrimination of reward valuation as it varies in adolescents as compared to children and adults (for example, see findings related to delay discounting tasks reported by Olson, Collins, Hooper, Muetzel, Lim & Luciana, (2009) and Steinberg, Graham, O’Brien, Woolard, Cauffman & Banich, (2009)). Several recent studies have incorporated electrophysiological recordings to examine aspects of putative phasic DA responding (Hammerer, Li, Müller & Lindenberger, 2010; Eppinger & Kray, 2010), an assessment strategy that can be applied with relative ease to both adolescent and adult samples. For instance, Hammerer et al. (2010) examined the feedback-related negativity component of the ERP during probabilistic learning and found that adolescents and young adults were superior to children or elderly adults in their differential classification of task-related outcomes, that they needed fewer trials to learn from choice outcomes, and that they learned more from gains than losses. A research program of this type serves to refine a DA-based model with a more substantial consideration of the roles that tonic versus phasic modes of DA transmission have in modulating incentive motivation and guiding reward-related learning. Longitudinal work would allow age by individual difference factors to be more cleanly dissociated. Finally, as a whole, to the extent that pharmacological probes can be utilized, this work would elicit targets for future intervention studies given that neurochemistry is malleable.
To conclude, behavioral self-regulation is achieved, at least in part, by one’s ability to control motivational impulses (Blair & Diamond, 2008). With respect to the study of human adolescence, incentive-reward motivational systems show a period of elevated activity between childhood and adolescence and a decline from adolescence into young adulthood. The mechanisms that underlie this acceleration and subsequent decline are unknown. Here we have suggested that adolescent changes in tonic neuromodulatory activity dopamine within mesostriatal projections may represent a central mechanism that should be further investigated to understand both this nonlinear developmental trend and the individual difference factors that interact with it.
Acknowledgments
The preparation of this paper was supported by grant DA017843 awarded by the National Institute on Drug Abuse to M. Luciana and which provided support to P. Collins and by grant MH017069, which supported J. Porter’s work on this project.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/dev
References
- Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+)-7-OH-DPAT. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1997;356:173–181. doi: 10.1007/PL00005038. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–691. doi: 10.1016/S0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Arnett J. Sensation seeking: A new conceptualization and a new scale. Personality and Individual Differences. 1994;16:289–296. doi: 10.1016/0191-8869(94)90165-1. [DOI] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: A DTI study. Cerebral Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Lindenberger U, Li S, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neuroscience and Biobehavioral Reviews. 2010;34:670–677. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacology Review. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Research Reviews. 1983;6:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- Berger B, Verney C, Febvret A, Vigny A, Helle K. Postnatal ontogenesis of the dopaminergic innervation in the rat anterior cingulate cortex (area 24). Immunocytochemical and catecholamine fluorescence histochemical analysis. Developmental Brain Research. 1985;21:31–47. doi: 10.1016/0165-3806(85)90021-5. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar B, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends in Neuroscience. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-X. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Homer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. The Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using FMRI. PLoS One. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends in Neuroscience. 2007;30(5):194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Blair C, Diamond A. Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Development and Psychopathology. 2008;20:899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois J, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bradley KC, Meisel RL. Sexual behavior induction of c-Fos in the nucleus accumbens and amphetamine-stimulated locomotor activity are sensitized by previous sexual experience in female Syrian hamsters. Journal of Neuroscience. 2001;21:2123–2130. doi: 10.1523/JNEUROSCI.21-06-02123.2001. Retrieved from http://www.jneurosci.org/content/21/6/2123.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cerebral Cortex. 2011;21(3):636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Goldman PS. Catecholamines in neocortex of rhesus monkeys: regional distribution and ontogenetic development. Brain Research. 1977;124:576–580. doi: 10.1016/0006-8993(77)90960-X. [DOI] [PubMed] [Google Scholar]
- Burton CL, Noble K, Fletcher PJ. Enhanced incentive motivation for sucrose-paired cues in adolescent rats: Possible roles for dopamine and opioid systems. Neuropsychopharmacology. 2011;36:1631–1643. doi: 10.1038/npp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated Dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006a;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006b;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Caille I, Dumartin B, Bloch B. Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Research. 1996;730:17–31. doi: 10.1016/0006-8993(96)00424-6. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67(2):319–333. doi: 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Somerville LH. Braking and accelerating of the adolescent brain. Journal of Research on Adolescence. 2011;21(1):21–33. doi: 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS) 2011 Available from URL: www.cdc.gov/ncipc/wisqars.
- Chein J, Albert D, O”Brien L, Uckert K, Steinberg L. Peers increase adolescent risk-taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14(2):F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nature Neuroscience, Volume. 2010;13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Krohn-Grimberghe A, Elger CE, Weber B. Dopamine gene predicts the brain response to dopaminergic drug. European Journal of Neuroscience. 2007;26:3652–3660. doi: 10.1111/j.1460-9568.2007.05947.x. [DOI] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. The Journal of Neuroscience. 2009;29(5):1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter CL, Happe HK, Murrin LC. Postnatal development of the dopamine transporter: a quantitative autoradiographic study. Developmental Brain Research. 1996;92:172–181. doi: 10.1016/0165-3806(96)00004-1. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, Prescott S. The ecology of adolescent activity and experience. Journal of Youth and Adolescence. 1977;6:281–294. doi: 10.1007/BF02138940. [DOI] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106:181–191. doi: 10.1037/0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–517. doi: 10.1017/S0140525X99002046. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF, Luciana M. A model of neurobiology–environment interaction in developmental psychopathology. In: Lenzenweger MF, editor. Frontiers of developmental psychopathology. New York: Oxford University Press; 1996. pp. 44–77. [Google Scholar]
- Depue R, Luciana M, Arbisi P, Collins P, Leon A. Dopamine and the structure of personality: Relation of agonist-induced dopamine D2 activity to positive emotionality. Journal of Personality and Social Psychology. 1994;67:485–498. doi: 10.1037/0022-3514.67.3.485. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol Behav. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinska EI, Spear LP. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain & Cognition. 2010;72(1):114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana P, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proceedings of the National Academy of Sciences. 2009;106(2):617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Meyer-Lindenberg AM, Kohn P, Bermann KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proceedings of the National Academy of Sciences. 2008;105(39):15106–15111. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Ross J, et al. Youth Risk Behavior Surveillance—United States, 2009. Morbidity and Mortality Weekly Report. 2010;59:SS-5. [PubMed] [Google Scholar]
- Eppinger B, Kray J. To choose or to avoid: age differences in learning from positive and negative feedback. Journal of Cognitive Neuroscience. 2010;23(1):41–52. doi: 10.1162/jocn.2009.21364. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec SP, McClure EB, Monk CS, Leibenluft, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choices selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. doi:0.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nature Neuroscience. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Frank RA, Manderscheid PZ, Panicker S, Williams HP, Kokoris D. Cocaine euphoria, dysphoria, and tolerance assessed using drug-induced changes in brain-stimulation reward. Pharmacol Biochem Behav. 1992;42:771–779. doi: 10.1016/00913057(92)90028-E. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Hutchison KE. Genetic contributions to avoidance-based decisions: Striatal D2 receptor polymorphisms. Neuroscience. 2009;164:131–140. doi: 10.1016/j.neuroscience.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz KJ, O’Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescenct and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: An event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience and Biobehavioral Review. 2001;25:355–373. doi: 10.1016/S0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Galineau L, Kodas E, Guilloteau D, Vilar M, Chalon S. Ontogeny of the dopamine and serotonin transporters in the rat brain: an autoradiographic study. Neuroscience Letters. 2004;363:266–271. doi: 10.1016/j.neulet.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Shizgal P, Heomans JS. A portrait of the substrate for self-stimulation. Psychol Rev. 1981;88:338–273. doi: 10.1037/0033-295X.88.3.228. [DOI] [PubMed] [Google Scholar]
- Galván A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in Adolescents. The Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A, Hare TA, Voss H, Glover G, Casey BJ. Risk-taking and adolescent brain: who Is at risk? Developmental Science. 2007;10:F8–F14. doi: 10.1111/j.14677687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Gan JO, Walton ME, Phillips PE. Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nature Neuroscience. 2010;13:1–27. doi: 10.1038/nn.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardener M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41:625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Garris PA, Rebec GV. Modeling fast dopamine neurotransmission in the nucleus accumbens during behavior. Behavioural Brain Research. 2002;137:47–63. doi: 10.1016/S0166-4328(02)00284-X. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20(7):1613–29. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard HA, Teicher MH, Faedda G, Baldessarini RJ. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. Brain Research. 1989;49:123–130. doi: 10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, et al. Puberty-related influences on brain development. Molecular and Cellular Endocrinology. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Giorgi O, De Montis G, Porceddu ML, Mele S, Calderini G, Toffano G, Biggio G. Developmental and age-related changes in D1-dopamine receptors and dopamine content in the rat striatum. Developmental Brain Research. 1987;35:283–290. doi: 10.1016/0165-3806(87)90053-8. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Developmental Brain Research. 1982;4:339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nature Neuroscience. 2005;8(6):805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Goto Y, Otani S, Grace AA. The Yin and Yang of dopamine release: a new perspective. Neuropharmacology. 2007;53(5):583–587. doi: 10.1016/j.neuropharm.2007.07.007. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17709119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984a;4 (11):2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. Retrieved from http://www.jneurosci.org/content/4/11/2877.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984b;4(11):2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. Retrieved from http://www.jneurosci.org/content/4/11/2866.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behavior. Trends in Neuroscience. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gray JA. Causal theories of personality and how to test them. In: Royce JR, editor. Multivariate analysis and psychological theory. Academic Press; 1973. [Google Scholar]
- Hammerer D, Li SC, Müller V, Lindenberger U. Life span differences in dlectrophysiological correlates of monitoring gains and losses during probabilistic reinforcement learning. Journal of Cognitive Neuroscience. 2010;23(3):579–592. doi: 10.1162/jocn.2010.21475. [DOI] [PubMed] [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annual Reviews of Neuroscience. 2009;32:255–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes ZC, Wellings K, Stephenson J. First heterosexual intercourse in the United Kingdom: A review of the literature. Journal of Sex Research. 2010;47:137–152. doi: 10.1080/00224490903509399. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. Journal of Neurochemistry. 2003;87:574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, … &, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. Journal of Neuroscience. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. Retrieved from http://www.jneurosci.org/content/15/7/5222.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Kalivas PW. The role of mesoaccumbenspallidal circuitry in novelty-induced behavioral activation. Neuroscience. 1995;64:587–597. doi: 10.1016/0306-4522(94)00409-X. [DOI] [PubMed] [Google Scholar]
- Irwin I, DeLanney LE, McNeil T, Chan P, Forno LS, Murphy GM, Jr, et al. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegeneration. 1994;3:251–265. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7531106. [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nature Neuroscience. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Joppa MA, Meisel RL, Garber MA. Fos expression in female hamster brain following sexual and aggressive behaviors. Neuroscience. 1995;68(3):783–792. doi: 10.1016/0306-4522(95)00179-M. [DOI] [PubMed] [Google Scholar]
- Kafry D. Sensation seeking of young children. Personality and Individual Differences. 1982;3:161–166. doi: 10.1016/0191-8869(82)90030-7. [DOI] [Google Scholar]
- Kazmarek LK, Levitan I. Neuromodulation: the biochemical control of neuronal excitability. Oxford: Oxford University Press; 1987. [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk-taking and novelty-seeking in adolescence. Annals of the NY Academy of Sciences. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- King RJ, Mefford IN, Wang C, Murchison A, Caligari EJ, Berger PA. CSF dopamine levels correlate with extraversion in depressed patients. Psychiatry Research. 1986;19(4):305–310. doi: 10.1016/0165-1781(86)90123-X. [DOI] [PubMed] [Google Scholar]
- Kohlert JG, Meisel RL. Sexual experience sensitizes mating-related nucleus accumbens dopamine responses of female Syrian hamsters. Behavioural Brain Research. 1999;99:45–52. doi: 10.1016/S0166-4328(98)00068-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug Abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow N. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. Journal of Neuroscience. 2007;27(30):7921–8. doi: 10.1523/JNEUROSCI.1859-07.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkeys. The Journal of Neuroscience. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. Retrieved from http://www.jneurosci.org/content/20/23/8780.full.pdf+html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, et al. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P. Synchronized overproduction of neurotransmitter receptors in diverse regions of the primate cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10218–10221. doi: 10.1073/pnas.88.22.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Rakic P. Scheduling of monoaminergic neurotransmitter receptor expression in the primate neocortex during postnatal development. Cerebral Cortex. 1992;2:401–416. doi: 10.1093/cercor/2.5.401. [DOI] [PubMed] [Google Scholar]
- Luciana M. Dopamine-opiate modulation of reward-seeking behavior: Implications for the functional assessment of prefrontal development. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge: MIT; 2001. pp. 647–661. [Google Scholar]
- Lüscher C, Bellone C. Cocaine-evoked synaptic plasticity: a key to addiction? Nature Neuroscience. 2008;11(7):737–8. doi: 10.1038/nn0708-737. [DOI] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Mullins AJ. Sexual experience in female rodents: cellular mechanisms and functional consequences. Brain Research. 2006;1126:66–75. doi: 10.1016/j.brainres.2006.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Camp DM, Robinson TE. A microdialysis study of ventral striatal dopamine during sexual behavior in female Syrian hamsters. Behavioral Brain Research. 1993;55:151–157. doi: 10.1016/0166-4328(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Rüther E, Huether G. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Developmental Brain Research. 2000;119:251–257. doi: 10.1016/S0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Montague DM, Lawler CP, Mailman RB, Gilmore JH. Developmental regulation of the dopamine D1 receptor in human caudate and putamen. Neuropsychopharmacology. 1999;21:641–649. doi: 10.1016/S0893-133X(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: Opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007:507–520. doi: 10.1007/s00213006-0502-4. [DOI] [PubMed] [Google Scholar]
- Niv Y, Joel D, Dayan P. A normative perspective on motivation. Trends in Cognitive Science. 2006:375–381. doi: 10.1016/j.tics.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Naitoh F, Segawa T. Regional changes in monoamine content and uptake of the rat brain during postnatal development. Brain Research. 1976;101:305–315. doi: 10.1016/0006-8993(76)90271-7. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. JComp Physiolo Psycholo. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in adolescents: a diffusion tensor imaging study. Journal of Cognitive Neuroscience. 2009;21(7):1401–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Wassum KM, Murphy NP, Balleine BW, Maidment NT. Extracellular dopamine levels in striatal subregions track shifts in motivation and response cost during instrumental conditioning. J Neurosci. 2011;31(1):200–207. doi: 10.1523/JNEUROSCI.4759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotion. New York: Oxford University Press; 1998. [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent, but not adult, rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008;32(11):1–12. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot RM, Wecker L. Dependence of adolescent novelty-seeking behavior on response phenotype and effects of apparatus scaling. Behavioral Neuroscience. 2008;122:861–875. doi: 10.1037/0735-7044.122.4.861. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Wecker L, Kirstein CL. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int J Dev Neurosci. 2009;27:805–815. doi: 10.1016/j.ijdevneu.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Pinkston JW, Lamb RJ. Delay discounting in C57BL/6J and DBA/2J mice: Adolescent-limited and life-persistent patterns of impulsivity. Behavioral Neuroscience. 2011;125(2):194–201. doi: 10.1037/a0022919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PA, Molinoff PB, Joyce JN. Ontogeny of dopamine D1 and D2 receptor subtypes in rat basal ganglia: a quantitative autoradiographic study. Developmental Brain Research. 1991;60:161–177. doi: 10.1016/0165-3806(91)90045-K. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine Transmission in the nigrostriatal pathway. Brain Research Reviews. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Zitzman DL, Smith KJ, Spear LP. Fast dopamine release events in the nucleus accumbens of early adolescent rats. Neuroscience. 2011;176:296–307. doi: 10.1016/j.neuroscience.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structure plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: A tyrosine hydroxylase immunohistochemical study. Biological Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: A tyrosine hydroxylase immunohistochemical analysis. The Journal of Comparative Neurology. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Roth M, Schumacher J, Brahler E. Sensation seeking in the community: Sex, age and sociodemographic comparisons on a representative German population sample. Personality and Individual Differences. 2005;39:1261–1271. doi: 10.1016/j.paid.2005.05.003. [DOI] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at 3–7 years: The Children’s Behavior Questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Yuan WY. White matter development during adolescence as shown by diffusion MRI. Brain & Cognition. 2010;72(1):16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Elmenhorst D, Lang M, Winz OH, Bauer A. Mesolimbic functional magnetic resonance imaging activation during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10(3):272–283. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams K, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain & Cognition. 2010;72(1):134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Willoughby T, Reker DL, Campbell K, Chalmers H, Rose-Krasnor L. Adolescent peer interaction and trait surgency weaken medial prefrontal cortex responses to failure. Social, Cognitive and Affective Neuroscience. 2011 doi: 10.1093/scan/nsq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in Cocaine-withdrawn rats. Journal of Neuroscience. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare TA, Casey BJ. Frontostriatal maturation predicts behavioral regulation failures to appetitive cues in adolescence. J Cogn Neuroscience. 2010;23(9):2103–2114. doi: 10.1162/jocn.2010.21572. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20809855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Reward, aversions and affect in adolescence: Emerging controversies across laboratory, animal and human data. Developmental Cognitive Neuroscience. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Structure and Function. 2011;215(3–4):187–194. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA. Development and aging of the rat nigrostriatal system dopamine system studied with fast cyclic voltammetry. Journal of Neurochemistry. 1989;52:1582–1589. doi: 10.1111/j.1471-4159.1989.tb09212.x. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Developmental Psychobiology. 2006;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Stephenson MT, Hoyle RH, Palmgreen P, Slater MD. Brief measures of sensation seeking for screening and large-scale surveys. Drug and Alcohol Dependence. 2003;72:279–286. doi: 10.1016/j.drugalcdep.2003.08.00. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52:216–224. doi: 10.1002/dev.20445. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20213754. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Development. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Yamagata S, Kijima N, Shigemasu K, Ono Y, Ando J. Continuity and change in behavioral inhibition and activation systems: A longitudinal behavioral genetic study. Personality and Individual Differences. 2007;43:1616–1625. doi: 10.1016/j.paid.2007.04.030. [DOI] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D1, D2, and D4 receptors in rat forebrain. International Journal of Developmental Neuroscience. 2000;18:29–37. doi: 10.1016/S0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neuroscience Letters. 1998a;254:21–24. doi: 10.1016/S0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparisons with D2-like receptors. Developmental Brain Research. 1998b;110:227–233. doi: 10.1016/S0165-3806(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Developmental Neuroscience. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-Q. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Barber NI, Gelbard HA, Gallitano AL, Campbell A, Marsh E, Baldessarini RJ. Developmental differences in acute nigrostriatal and mesocorticolimbic system response to haloperidol. Neuropsychopharmacology. 1993;9:147–156. doi: 10.1038/npp.1993.53. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12136507. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Waller NG. Exploring personality through test construction: Development of the multidimensional personality questionnaire. In: Briggs S, Cheek J, editors. Personality measures: Development and evaluation. Vol. 1. JAI Press; 1997. [Google Scholar]
- Tobler PN, O’Doherty JP, Dolan RJ, Schulz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. Journal of Neurophysiology. 2007;97:1621–1632. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress during adolescence increases novelty seeking and risk-taking behavior in male and female rats. Frontiers in Behavioral Neuroscience. 2011;5:17. doi: 10.3389/fnbeh.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Lambert WE, Schwartzman AN, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009;4(8):e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE. Glutamate co-transmission as an emerging concept in monoamine neuron function. Journal of Psychiatry and Neuroscience. 2004;29(4):296–310. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC446224/pdf/20040700s00006p296.pdf. [PMC free article] [PubMed] [Google Scholar]
- Urošević S, Collins P, Muetzel RM, Lim KO, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Developmental Psychology. doi: 10.1037/a0027502. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Gunther Moor B, Op de Macks ZA, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision making: Neurocognitive development of reward and control regions. NeuroImage. 2010;51:345– 355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26(10):1502–1511. doi: 10.1111/j.1530-0277.2002.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Wacker J, Chavanon M, Stemmler G. Investigating the dopaminergic basis of extraversion in humans: A multilevel approach. Journal of Personality and Social Psychology. 2006;91(1):171–187. doi: 10.1037/0022-3514.91.1.171. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Collins PF, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: Behavioral implications and issues in assessment. Brain & Cognition. 2010a;72:146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Hooper CJ, Vrshek-Schallhorn S, Oetting WS, Brott MJ, Luciana M. Association of the catechol-O-methyltransferase (COMT) gene to prefrontally-mediated cognitions in adolescents. Biological Psychiatry. 2007;61:626–632. doi: 10.1016/j.biopsych.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and Biobehavioral Reviews. 2010b;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Morris SE, Arrant AE, Nagel JM, Parylak S, et al. Dopamine uptake inhibitors but not dopamine releasers induce greater increases in motor behavior and extracellular dopamine in adolescent rats than in adult male rats. The Journal of Pharmacology and Experimental Therapeutics. 2010;335:124–132. doi: 10.1124/jpet.110.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PE. Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev. 2009;2(2):195–213. doi: 10.2174/1874473710902020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner I, Joel D. Dopamine in schizophrenia: dysfunctional information processing in basal ganglia-thalamocortical split circuits. In: DiChiara G, editor. Handbook of Experimental Pharmacology Vol 154/II, Dopamine in the CNS II. Berlin: Springer-Verlag; 2002. pp. 417–472. [Google Scholar]
- Willuhn I, Wanat MJ, Clark MJ, Phillips PEM. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. In: Self DW, Staley JK, editors. Behavioral Neuroscience of Drug Addiction, Current Topics in Behavioral Neurosciences. 2010. pp. 29–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning, and motivation. Nature Reviews Neuroscience. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology. 1908;18:459–482. doi: 10.1002/cne.920180503. [DOI] [Google Scholar]
- Yurgelun-Todd DA, Killgore WD, Cintron CB. Cognitive correlates of medial temporal lobe development across adolescence: a magnetic resonance imaging study. Percept Mot Skills. 2003;96(1):3–17. doi: 10.2466/pms.2003.96.1.3. [DOI] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Kessler RM. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. The Journal of Neuroscience. 2008;28(53):14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W. Normalizing drug-induced neuronal plasticity in nucleus accumbens weakens enduring drug-seeking behavior. Neuropsychopharmacology. 2010;35:352–353. doi: 10.1038/npp.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]