Abstract
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are ubiquitously produced in cardiovascular systems. Under physiological conditions, ROS/RNS function as signaling molecules that are essential in maintaining cardiovascular function. Aberrant concentrations of ROS/RNS have been demonstrated in cardiovascular diseases due to increased production or decreased scavenging, which have been considered as common pathways for the initiation and progression of cardiovascular diseases such as atherosclerosis, hypertension, (re)stenosis, and congestive heart failure. NAD(P)H oxidases are primary sources of ROS and can be induced or activated by all known cardiovascular risk factors. Stresses, hormones, vasoactive agents, and cytokines via different signaling cascades control the expression and activity of these enzymes and of their regulatory subunits. But the molecular mechanisms by which NAD(P)H oxidase is regulated in cardiovascular systems remain poorly characterized. Investigations by us and others suggest that adenosine monophosphate-activated protein kinase (AMPK), as an energy sensor and modulator, is highly sensitive to ROS/RNS. We have also obtained convincing evidence that AMPK is a physiological suppressor of NAD(P)H oxidase in multiple cardiovascular cell systems. In this review, we summarize our current understanding of how AMPK functions as a physiological repressor of NAD(P)H oxidase.
Keywords: NAD(P)H oxidase, endothelial cells, vascular smooth muscle cells, blood cells, AMPK, cardiovascular diseases
Introduction
Overwhelming evidence indicates that reactive oxygen species (ROS) and reactive nitrogen species (RNS) are pathogenic species that cause tissue injury and/or impair tissue repair, mechanisms shared by many cardiovascular disease states including hypertension, atherosclerosis, stroke, (re)stenosis, and cardiac failure [1]. Emerging evidence suggests that ROS/RNS are essential signals in maintaining fundamental biological processes and physiological function [2]. ROS/RNS are established signaling molecules which regulate multiple cellular processes such as proliferation, differentiation, migration, and apoptosis in response to stimulation by growth factors, cytokines, and vasoactive agents [3].
Among the various cellular ROS-generating enzymes in the cardiovascular system, including xanthine oxidase, cyclo- and lipo-oxygenases, uncoupled endothelial nitric oxide synthase (eNOS) [4], mitochondrial respiratory enzymes [5], and peroxidases, reduced-form nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidases (Nox) appear to be the major contributors of ROS associated with cardiovascular diseases and health [6–11]. Cellular ROS can be scavenged by an additional set of enzymes, which includes superoxide dismutase (SOD), catalase, and glutathione peroxidase. The expression and activation of Nox isoforms and regulatory proteins can be regulated by different stimuli and signaling pathways.
Adenosine monophosphate-activated protein kinase (AMPK) is reported to be a critical energy sensor that regulates oxidative stress [12–14]. Recently, emerging data have indicated that Nox in the cardiovascular system can be regulated by AMPK [15]. In this review article, we will discuss our current understanding of cardiovascular Nox, particularly, their roles and regulation in physiological and pathological conditions. Furthermore, we will address AMPK regulation and function in the cardiovascular system as well as AMPK signaling pathways involved in cardiovascular Nox regulation. We will also emphasize the capacity of AMPK to lower the burden of cardiovascular disease (CVD).
NAD(P)H oxidases are major sources of ROS in cardiovascular systems
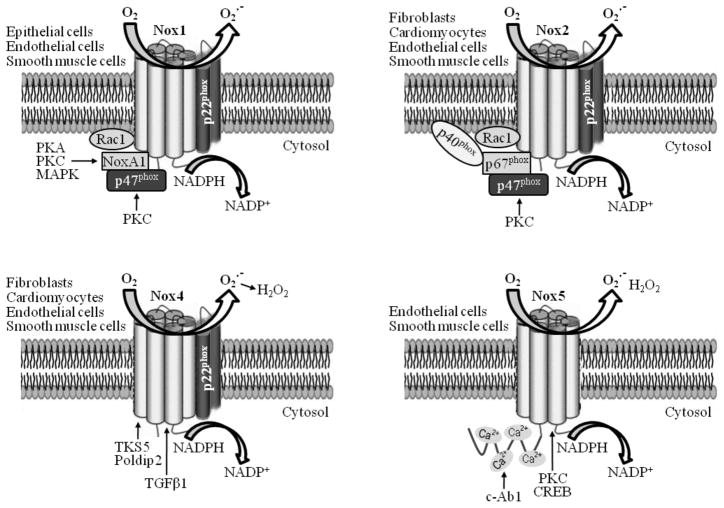
The Nox enzymes, also known as respiratory burst oxidases, are a family of multiple-subunit complex enzymes that generate O2•− via one-electron reduction of oxygen using NAD(P)H as an electron source (NAD(P)H +2O2 → NADP+ + H+ + 2O2•−) [16]. Originally designated as phagocytic oxidases (phox) involved in defense and innate immunity, the Nox family is now formally recognized to be widely expressed in different cell types. Importantly, Nox family members are pivotal and “professional” ROS generators in cardiovascular systems including vascular endothelial cells (ECs), vascular smooth muscle cells (VSMCs), adventitial fibroblasts, and cardiomyocytes [7, 9, 17, 18]. Seven Nox isoforms in mammalian cells have been identified: Nox1 to Nox5, and Dual oxidases (Duox1 and Duox2) [17, 19]. All Nox isoforms are transmembrane proteins with six transmembrane domains. Different Nox complexes with unique combinations of different subunits are found to function in various cardiovascular cells (Fig. 1). With regard to physiological responses, Nox-derived ROS have been linked to ECs migration and ischemia-induced angiogenesis [20]. Results from experimental animals [21] (Table 1) indicate that Nox isoforms have been implicated in cardiac fibrosis [22], preconditioning (PC) [23], cardiac remodeling post-myocardial infarction (MI) [24], angiotensin II (AngII)-mediated cardiac hypertrophy [25, 26], atherosclerosis [27–29], aortic aneurysm formation [30], and response to arterial injury [31]. Emerging evidences indicate that moderately elevated ROS have protective effects in multiple cells including in cardiomyocytes [32] and ECs [33, 34]. Thus, there are ample evidences for the role of Nox-derived ROS in modulating cardiovascular physiology and pathophysiology.
Fig. 1.
Distribution and regulation of NAD(P)H oxidases in cardiovascular systems. With the exception of Nox4, the Nox homologues are basally inactive. Regulation of the cardiovascular Nox complex is mediated by variations of Nox isoform structure as well as interactions with various partners. See text for details.
Table 1.
NAD(P)H oxidase knockout or transgenic mouse models associated with cardiovascular disease.
| KO or Tg mouse model | Phenotype or related cardiovascular outcome | Reference(s) |
|---|---|---|
| Nox1 null | Attenuated renal-sensitive signaling | [79] |
| Nox1 null | Lower blood pressure, reduction in aortic media hypertrophy | [80] |
| Nox1 null | Reduced neointima formation | [81] |
| Nox2 null | Attenuated cardiac remodeling and PC effect | [22–24] |
| Beneficial effects on neovascularization | [82, 83] | |
| Impaired ischemia-induced flow recovery | [84, 85] | |
| Decreased neointima formation | [31] | |
| Attenuated AngII-induced hypertrophy but not TAC | [25, 26, 86] | |
| Nox2 null/ApoE null | No significant effects on atherogenesis | [87] |
| Nox2 null/ApoE null | Reduced early atherosclerosis | [27] |
| Nox4 null | Exaggerated contractile dysfunction, hypertrophy, and cardiac dilatation | [32] |
| p47phox null | Impaired pressor responses to AngII | [88] |
| p47phox null/ApoE null | Reduced atherosclerosis and AngII-induced AAA | [28–30] |
| Cardiomyocyte-specific Rac1 null | Attenuated AngII-induced hypertrophy | [89] |
| Tg-SMC-Nox1 | Elevated AngII-induced hypertension and VSMC hypertrophy | [90, 91] |
| Tg-tie2-Nox2 | Elevated AngII-induced EC dysfunction and hypertension | [92] |
| Tg-VECad-Nox4 | Promote EC angiogenesis | [20] |
| Tg-tie2-Nox4 | Lower systemic blood pressure | [93] |
| Tg-αMHC-Nox4 | Protective myocardial angiogenesis to overload stress | [32] |
| Tg-SMC-p22phox | Elevated AngII-induced vascular hypertrophy | [94] |
| Tg-SMC-p22phox | Enhanced carotid arterial lesions | [95] |
Refer to the text for expanded forms of abbreviations.
NAD(P)H oxidases in the cardiovascular system
All ECs and VSMCs express the catalytic subunits Nox1 [35–39], Nox2 [38, 40–42], Nox4 [20, 39, 41, 43–48], and/or Nox5 [49, 50] (Table 2). Expression of both Nox2 and Nox4 has been described in adventitial fibroblasts [51, 52]. Both Nox2 [53, 54] and Nox4 [32, 55] are expressed in the heart. Additionally, Nox2 is also expressed in neutrophil [56], monocyte/macrophage [57, 58], and platelet [59]. Recently, it is reported that monocyte/macrophage express Nox4 [60]. Different Nox isoforms have distinct subcellular localization, regulation, and function (Table 2). Nox4 and Nox2 are abundantly expressed, while Nox1 is less expressed, in ECs [61, 62]. Nox1 and Nox4 are present in higher amounts than Nox2 in VSMCs [39]. Generally, the most abundant vascular Nox isoform is Nox4 [36, 45, 63, 64], based on the gene expression data. Unlike Nox1 and Nox2, Nox4 is constitutively active in cardiovasculature [65, 66], and generates predominantly H2O2 rather than O2•− [67, 68]. Several reports suggest that Nox4 is Rac1 independent [69–72], although some reports imply that Nox4 activation may be partially regulated by Rac1[73, 74]. Recently, two Nox4-binding proteins, polymerase (DNA-directed) δ-interacting protein 2 (Poldip2) in VSMCs [75], and tyrosine kinase substrate with five Src homology 3 domains (Tks5) [76], have been shown to be important (Fig. 1). Nox4 has been associated with CVDs such as hypertension, atherosclerosis, and cardiovascular and renal complications of diabetes, and is involved in the remodeling of pulmonary arteries during hypoxia-induced pulmonary hypertension [48, 77, 78], although the precise mechanisms are unclear. Recent studies suggest that Nox4 may also have protective effects. Specifically, Nox4-null mice develop exaggerated contractile dysfunction, hypertrophy, and cardiac dilatation in response to chronic overload, whereas the cardiomyocyte-targeted expression of Nox4 protects transgenic mice from these effects due to Nox4-enhanced myocardial angiogenesis [32]. In addition, elevated endothelial Nox4 promotes angiogenesis and flow recovery from limb ischemia in an eNOS-dependent manner [20], augments vasodilation, and decreases blood pressure [93]. These results suggest that Nox4 may be a potential therapeutic target for manipulating angiogenesis and tissue repair. Nox1 has been implicated in VSMCs migration, proliferation, and extracellular matrix production; these effects are mediated by cofilin [81]. Studies on transgenic mice suggest a possible role for Nox1 in acute, but not chronic, forms of AngII-dependent hypertension [79, 96–98] as well as in atherosclerosis, neointima formation of post-injury [81], endothelial dysfunction, and stroke [99]. In spite of the extensive experimental data implicating Nox1 in vascular disease (Table 1), there is a paucity of clinical data, although the expression of Nox1 and Nox activator 1 (NoxA1) is induced in human atherosclerotic vessels [100]. The physiological functions of Nox2 in the cardiovasculature remain unclear, but pathological increases in Nox2 contribute to oxidative injury and cardiovascular damage, including endothelial dysfunction [15, 42], vascular inflammation [42], and structural remodeling [54, 92].
Table 2.
Localization, regulation, and (patho)physiological functions of Nox isoforms in cardiovasculature
| Cell/tissue type | Nox isoform | Subcellular localization | Regulation | Function | Reference(s) |
|---|---|---|---|---|---|
| ECs | Nox2 | PM, Perinuclear, ER, Caveolae | AngII, ET-1 | Cell proliferation, angiogenesis | [41, 103] |
| Nox4 | Perinuclear, ER | AngII | Cell proliferation, angiogenesis, and senescence | [20, 41, 104, 105] | |
| Nox1 | Perinuclear, | AngII, shear stress | Angiogenesis | [35] | |
| Nox5 | Perinuclear | AngII, ET-1 | Angiogenesis | [49] | |
| VSMCs | Nox1 | Caveolae, PM, endosomes | AngII, EGF, PDI | Injury-induced neointima formation | [36, 39, 106, 107] [81, 108] |
| Nox4 | Focal adhesions, ER, mitochondria, nucleus | AngII, 7-KC, TGF-β1 | ER stress, apoptosis, proliferation, migration, differentiation | [39, 106, 109, 110] [64] |
|
| Nox2 | Perinuclear | AngII | SM contraction, neointima formation | [31, 111] | |
| Nox5 | PM | PDGF | Proliferation | [50] | |
| Cardiomyocyte | Nox2 | Sarcolemmal and ttubule membrane | Physiological stretch | Stretch-dependent ROS and Ca2+ release, new-onset AF | [53, 54] |
| Nox4 | Perinuclear | Pressure overload, hypoxia | Enhance myocardial angiogenesis | [32, 55] |
Refer to the text for the expanded forms of abbreviations.
Regulation of NAD(P)H oxidases
Different Nox complexes have respective partners (Fig.1). For example, cardiovascular Nox2 activity generally requires the association of a membrane-bound catalytic Nox subunit with a variety of regulatory subunits including Rac1 GTPase, cytosolic phox proteins including p40phox, p47phox, and p67phox, and proteins of the cytoskeleton including p22phox. This process is controlled through at least three distinct mechanisms: activation of Rac1 [101], the presence of lipids such as arachidonic acid and phosphatidic acid, as well as phosphorylation, oligomerization, and translocation of cytoplasmic subunits to the plasma membrane [102].
The translocation and oligomerization of the classical cytoplasmic domains (p47phox, p67phox, and p40phox) are initiated by the phosphorylation of p47phox by multiple kinases including protein kinase C (PKC) [112] and p21-activated kinase 1 (PAK1) [113]. P47phox acts as a scaffolding, transport, and molecular compass facilitating the arrival of p67phox at the correct cellular location. At the membrane, p67phox interacts with active Rac (GTP-loaded), which resides at the plasma membrane. Active Rac binds to proteins containing specific domains, such as the tandem tetratricopeptide repeat (TPR) motif in p67phox [114]. Therefore, not only is Rac necessary for Nox-dependent production of ROS, but the translocation of cytoplasmic subunits to the plasma membrane is also required before Rac can activate Nox. It is well known AngII exert its pathological functions via Nox activation in cardiovascular cells [115, 116]. Moreover, both high glucose level and palmitate enhance ROS production via PKC-mediated Nox activation in cultured vascular cells [117]. We recently found that Nox2 activation due to p67phox membrane translocation mediated by PKCζ contributes to elevated O2•− formation in ECs in response to thromboxane A2 receptor (TP) activation [118].
Besides the regulation of Nox activity by assembly, changes in the gene expression of Nox subtypes are essential for their function. Tumor necrosis factor α (TNFα) treatment increases the mRNA and protein levels of Nox subunits p47phox, p67phox, and gp91phox, as well as Nox activity in ECs (Wang S and Zou MH, unpublished data), MonoMac1 cells, and monocytes [119], suggesting the transcriptional upregulation of three essential Nox genes via the nuclear factor kappa B (NF-κB) pathway. In human aortic SMCs, NF-κB and activator protein-1 (AP-1) are important regulators of Nox activity and p22phox transcription, and a Janus tyrosine kinase/signal transducers and activators of transcription (JAK/STAT)-dependent mechanism is involved in the modulation of Nox1 and Nox4 expression and Nox-derived superoxide anion production [108, 120, 121]. These data indicate that cooperation among multiple transcription factors, co-activators, and co-repressors is essential for the precise control of Nox transcription and function.
AMPK in cardiovascular systems
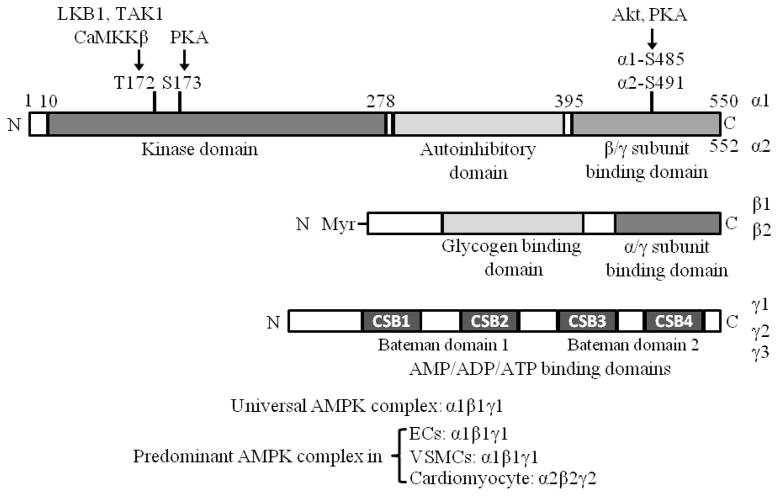
AMPK, a critical regulator of energy metabolic homeostasis at the cellular and whole organism levels [122], is a serine/threonine kinase which has been highly conserved during evolution. AMPK exists as a heterotrimeric complex consisting of a catalytic α subunit and regulatory β and γ units. There are two isoforms of α (α1 and α2) and β (β1 and β2) and three γ subunits (γ1, γ2, and γ3), which are differentially expressed in mammalian tissues (Fig. 2). AMPK is activated by allosteric regulation via an increased ratio of adenosine monophosphate (AMP) to adenosine triphosphate (ATP), and its activity is maintained by the inhibition of dephosphorylation through ADP binding [46, 123], and by the phosphorylation of the α subunit (T172) via upstream kinases including liver kinase B1 (LKB1) [124, 125], Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) [126, 127], and transforming growth factor-β-activated kinase 1(TAK1) [128, 129]. Protein phosphatase 2A (PP2A) [130, 131] and PP2C [132] are shown to dephosphorylate AMPK at T172. Moreover, both α1- and α2-containing AMPK complexes are inactivated by PP2C, and the α1 isoform is more resistant than α2 to inactivation by PP2A [133]. Recently, both protein kinase B (PKB) [134] and PKA [135] have been reported to phosphorylate AMPKα1/α2 at S485/491, which inhibits AMPK phosphorylation at T172. More recent evidence has shown that AMPKα1 phosphorylation at T172 can be impeded by AMPKα1 phosphorylation at S173 mediated by PKA in primary mouse adipocytes [136] (Fig.2).
Fig. 2.
Structure features of the catalytic α and regulatory β and γ subunits of AMPK. Residues phosphorylated by AMPKK (LKB1, CaMKKβ, TAK1), PKA, and Akt are shown within the α subunit. This figure is modified from Viollet et al [149]. Refer to the text for expanded forms of abbreviations.
Although AMPK is activated as a consequence of any cellular process, normal or anomalous, that either decreases ATP levels or increases AMP concentrations [137], AMPK is also activated by a variety of stimuli including cytokines (leptin, adiponectin, ghrelin, cannabinoids, IL-6) [138, 139], ciliary neutrotrophic factor (CNTF) [140], certain drugs including metformin [141], thiazolidinediones (TZD), which is PPARγ-independent [142], and may be AMP-dependent [143, 144], and some plant-derived compounds including berberine [145, 146], resveratrol [147], and curcumin [148]. Once AMPK is activated, it switches on catabolic pathways that can generate ATP (e.g., cellular uptake and utilization of glucose), while at the same time, terminates processes that consume ATP (e.g., cellular synthesis pathways). Thus, AMPK orchestrates the regulation of energy-generating and –consuming pathways. The rapid “switching” required to closely and quickly regulate and balance cellular energy resources is achieved by brisk phosphorylation of metabolic enzymes and of various transcription factors and co-activators that control gene expression [137].
AMPK in the heart
AMPKα2 is the predominant AMPKα isoform expressed in the mouse and rat hearts [150], whereas both α1 and α2 subunits of AMPK equally contribute to total AMPK activity in the human heart [151]. The heart expresses both γ1 and γ2 isoforms but lacks the γ3 isoform. γ1 isoform complexes account for 70% of AMPK activity in the ischemic heart [150], but mutations in the γ2 subunit of AMPK causes familial hypertrophic cardiomyopathy [152]. AMPK can be activated in the heart by several factors, such as adiponectin [153], macrophage migration inhibitory factor (MIF) [154], follistatin-like 1 (Fstl1) [155], resveratrol, a cardioprotective polyphenol from red wine [156], and cardiac ischemia [157, 158]. Conversely, insulin inhibits AMPK activation [159, 160].
Animal experiments indicate that AMPK has distinct functions in the heart (Table 3). AMPK carries out important physiological roles such as regulating fatty acid and glucose metabolism in the heart [161, 162]. AMPK activation has been shown to reduce myocardial ischemic injury [163, 164]. AMPK activation may inhibit myocardial hypertrophy [156, 165, 166] and impede the transition from cardiac hypertrophy to heart failure [151]. In addition, the cardiac hypertrophy induced by isoproterenol or pressure-overload in AMPKα2−/− mice is significantly higher than in wild-type (WT) animals and is associated with p70S6K activation [167, 168]. AMPKα2 has been implicated in the positive regulation of myocardial estrogen-related receptor-α (ERRα) expression which is negatively associated with congestive heart failure [169]. Further, AMPK plays an important role in ischemia-induced autophagy in cardiomyocytes [170]. More recently, our research indicates that AMPKα2 inhibition impedes myocardium autophagy, accentuates cardiac dysfunction, and increases mortality in diabetic mice [171].
Table 3.
AMPK and transgenic or knockout mouse models related to cardiovascular disease
| Animal model | Tissue specificity | Phenotype or related cardiovascular outcome | Reference(s) |
|---|---|---|---|
| AMPKα1 KO | Whole body | Impaired eNOS activation in EC | [187] |
| Whole body | Impaired AICAR-enhanced vasorelaxation | [193] | |
| Whole body | Aggravated endothelial dysfunction and vascular inflammation in response to AngII | [42] | |
| Whole body | Impaired angiogenesis | [191] | |
| AMPKα2 KO | Whole body | More rapid onset of ischemia-induced contracture, Impaired glucose transport and glycogen metabolism in heart | [194, 195] [194, 195] |
| Whole body | Higher cardiac hypertrophy induced by isoproterenol or pressure-overload | [167] | |
| Whole body | Endothelial dysfunction, elevated atherosclerosis in LDLr−/− mice | [15] | |
| Whole body | Impaired SERCA activity, aberrant ER stress, Elevated atherosclerosis in ApoE−/− mice | [196] | |
| Whole body | Impaired angiogenesis | [191] | |
| Whole body | Hypertension | [197] | |
| Whole body | Elevated neointima formation after injury. | [198] | |
| TgAMPKγ2(CA-T400N) | Heart | Glycogen storage cardiomyopathy, greater infarct sizes and apoptosis after ischemia-reperfusion | [199] |
| TgAMPKγ2(CA-N488I) | Heart | Glycogen storage cardiomyopathy | [200, 201] |
| TgAMPKγ2(DN-R531G) | Heart | Glycogen accumulation and hypertrophy | [202] |
| TgAMPKγ2(DN-R302Q) | Heart | Glycogen accumulation cardiomyopathy | [203] |
| TgAMPKα2(DN-K45R) | Heart | Impaired glucose uptake and glycolysis, and elevated injury after ischemia-reperfusion | [163] |
| TgAMPKα2(DN-D157A) | Heart | Impaired glucose uptake, | [204] |
| Increase myocardial infarct size, | [205] | ||
| Impaired autophagy, aggravated cardiomyopathy and mortality in diabetic mice | [171] |
Refer to the text for expanded forms of abbreviations.
AMPK in endothelial cells
Both α subunits of AMPK are expressed in ECs [172, 173]. No compensatory upregulation of AMPKα2 or AMPKα1 is observed in either AMPKα1−/− or AMPKα2−/− mouse aortic ECs, respectively, although AMPKα1 is the predominant isoform of AMPKα in human and mouse ECs and aortae [42, 172, 174]. AMPKα2 however, has more important physiological functions such as anti-atherogenic effects [172] and pro-angiogenic effects in response to hypoxia [175, 176].
In ECs, stimuli such as peroxynitrite [177–179], the anti-diabetic drug metformin [178], and the lipid-lowing drug statin [180] have been reported to activate AMPK via LKB1 phosphorylation and activation. Furthermore, flow shear stress activates either AMPK signaling event for NO bioavailability [181] or extracellular signal-regulated kinase 5 (ERK5)-mediated PPARγ1 signaling pathway to facilitate antiinflammatory response in ECs [182]. However, AMPK can be inactivated in ECs by free fatty acids such as palmitate [131] as well as by high glucose [183]. Moreover, AMPK activity was lower in the aortic endothelium of obese rats compared to control rats [184].
AMPK plays an important role in maintaining vascular endothelial function [185]. First, AMPK signaling contributes to vasodilation of conduit and resistance vessels via the upregulation of eNOS phosphorylation at Ser1177 and Ser633 [186] and the subsequent association of phosphorylated eNOS with heat shock protein 90 (Hsp90) [187, 188]. In addition, AMPK activation by metformin or 5′-aminoimidazole-4-carboxamide ribonucleoside (AICAR) impairs high glucose/diabetes-induced 26S proteasome activity, which mediates the degradation of GTP cyclohydrolase 1 (GTPCH1), the rate-limiting enzyme in the de novo synthesis of tetrahydrobiopterin (BH4), an essential cofactor for eNOS. Thus, in vivo activation of AMPK attenuates diabetes-enhanced GTPCH1 degradation and restores the impaired endothelium-dependent relaxation in diabetic mice [183]. However, aortae from AMPKα2−/− mice, which exhibit elevated 26S proteasome activity, have reduced levels of GTPCH I and BH4, and consequent endothelial dysfunction [183]. Second, AMPK performs its role of counteracting oxidative stress in ECs via the upregulation of both manganese superoxide dismutase (MnSOD) and uncoupling protein 2 (UCP2) and the downregulation of Nox. Third, AMPK exerts a pivotal role in angiogenesis. Treatment of endothelial cell progenitor cells with AICAR increases their ability to form capillary-like tubes in vitro through an NO-dependent mechanism [189]. Inhibition of AMPK signaling suppresses both human umbilical-vein endothelial cell (HUVEC) migration and in vitro differentiation into tube-like structures in hypoxic, but not normoxic cultures. Dominant-negative AMPK also impairs in vivo angiogenesis in Matrigel plugs implanted subcutaneously in mice [175]. The AMPK-p38 MAPK signaling cascade elevates VEGF production in muscle and promotes angiogenesis in skeletal muscle in response to ischemic injury [190]. More recently, our research indicates that AMPK inhibition with either a pharmacological inhibitor (compound C) or by genetic means (transfection of AMPKα-specific siRNA or either AMPKα1 or AMPKα2 deletion) significantly lowers tube formation in HUVEC or mouse aortic ECs, associated with impaired UCP2 expression [191]. Finally, AMPK activation ameliorates hypoxia and glucose deprivation-induced ECs apoptosis by increasing the expression of anti-apoptotic proteins Bcl-2 and survivin [192].
AMPK in vascular smooth muscle cells
We and another group have found that both catalytic α subunits of AMPK are expressed in VSMCs. No compensatory upregulation of AMPKα2 or AMPKα1 is observed in AMPKα1−/− or AMPKα2−/− VSMCs, respectively, although AMPKα1 is the predominant isoform contributing to total AMPKα and its activation in VSMCs [198, 206]. AMPK in cultured VSMCs can be activated by the TP agonists IBOP and U46619, in a H2O2-dependent manner, whereas SQ29548, a selective TP antagonist, significantly attenuates TP-enhanced AMPK activation [207].
AMPK activation exerts its anti-proliferative role in VSMCs. Igata et al. [208] reported that the AMPK activator AICAR significantly inhibits proliferation of human aortic SMCs induced by both platelet-derived growth factor-BB (PDGF-BB) and fetal calf serum (FCS). Consistent with this finding, AICAR treatment inhibits the expression of p21 but not p27. AICAR increases p53 protein levels and p53-S15 phosphorylation in human aortic SMCs, with both of these effects being blocked by AMPK inhibition. These data suggest that AMPK suppresses VSMCs proliferation by blocking cell cycle progression through p53 up-regulation. Another study has shown that subcutaneous injection of AICAR for 2 weeks suppresses neointimal formation after transluminal mechanical injury of the rat femoral artery by blocking the phosphorylation of extracellular signal-regulated kinase 1/2 [209]. Recently, a potent anti-tumor agent, β-lapachone (3,4-dihydro-2,2- dimethyl-2H-naphtho[ 1,2-b]pyran-5,6-dione), was shown to inhibit FCS- or PDGF-induced proliferation of VSMCs and to reduce neointimal formation in balloon-injured rat carotid arteries through the LKB1-AMPK-p53-p21 pathway [210]. We have also found that AMPK activation inhibits VSMCs hypertrophy induced by thromboxane receptor activation [207]. Interestingly, our recent work revealed that AMPKα2 deletion exacerbates neointima formation through the upregulation of E3 ligase S-phase kinase-associated protein 2 (Skp2) mediated by the activation of p52 NF-κB-2, leading to p27 downregulation and a resultant accelerated cell cycle in VSMCs. AMPK activation by metformin blunts neointimal hyperplasia after carotid artery injury in WT mice [198]. In addition, AMPK activation in VSMCs has been implicated in attenuated smooth muscle contraction via direct phosphorylation of myosin light chain kinase [211] and consequent vasorelaxation [193]. Moreover, we reported that AMPKα2 deletion accentuates agonist-induced vascular smooth muscle contraction and high blood pressure in mice [197].
AMPK in blood cells
AMPK is also expressed and exerts critical effects in blood cells. It is well known that AMPKα1 is the predominant AMPKα isoform expressed in both human and mouse monocytes/macrophages; however, AMPKα2 is almost undetectable in macrophages [212, 213]. Either anti-inflammatory or proinflammatory stimuli rapidly activate or inactivate AMPK in macrophages, respectively. Activated AMPK supresses the production of proinflammatory cytokines and promotes macrophage polarization to an anti-inflammatory functional phenotype in a manner associated with NF-κB inhibition [212, 213], which results from SIRT1-mediated p65 deacetylation [213]. It has been reported that the stimulation of glycolysis by hypoxia in activated monocytes requires the phosphorylation and activation of inducible 6-phosphofructo-2-kinase (iPFK-2) by AMPK [214]. Interestingly, AMPK inactivation within monocytic MM6 cells has been implicated in the exercise-induced immunosuppression [215]. Further, it is reported that berberine represses proinflammatory responses through AMPK activation in macrophages [216]. AMPK activation also blunts proinflammatory cytokine production in neutrophils [217]. Recently, Bae et al. found that AMPK activation elevates the ability of both neutrophils and macrophages to ingest bacteria (Escherichia coli), and the capacity of macrophages to ingest apoptotic cells, due to cytoskeletal reorganization mediated by Rac1 activation. In vivo, AMPK activation leads to augmented phagocytosis of bacteria in the lungs [218]. These data imply that AMPKα1 may be a promising target for immunoregulation including the innate immune and adaptive immune response.
We and other groups have found that AMPKα1 is the predominant isoform of AMPKα expressed in murine erythrocytes [219-221]; however, AMPKα2 is nearly undetectable in erythrocytes [221]. The life span of erythrocytes from AMPKα1−/− mice is shorter than that in their WT littermates, possibly due to the increased osmotic fragility associated with elevated ROS levels and oxidized proteins [219]. AMPKα1−/− erythrocytes are significantly more susceptible to the eryptotic effect of energy depletion due to increased cytosolic Ca2+ [220]. Further, AMPKα1−/− erythrocytes have less deformability in response to shear stress, limiting their membrane flexibility [221]. Finally, AMPKα1−/−, but not AMPKα2−/−, mice are anemic despite excessive reticulocytosis, and exhibit severe splenomegaly [219, 220]. Interestingly, treatment of AMPKα1−/− mice with the antioxidant tempol results in reduced reticulocyte amounts and improved erythrocyte survival [219].
Both AMPKα1 and AMPKα2 isoforms are expressed in human and murine platelets [222]. Either thrombin or insulin activates AMPK in platelets [222, 223]. AMPK inhibition significantly impairs thrombin-induced platelet aggregation and clot retraction. Furthermore, compared with WT mice, AMPKα2−/−, but not AMPKα1−/−, mice have disrupted clot retraction, higher frequency of rebleeding, and unstable thrombi formation induced by FeCl3, which is attributed to the blunted Thr12 phosphorylation of Fyn, a Src-family kinase, and the consequently impaired Tyr747 phosphorylation of αIIbβ3 integrin [222]. These data suggest that AMPKα2, rather than AMPKα1, positively regulates platelet function.
Redox regulation of AMPK
Recent studies by our laboratory and others demonstrate that AMPK activity can be regulated by oxidative stress. Interestingly, AMPKα1 is more sensitive to oxidative stress [224], while AMPKα2 is much more sensitive to an increase in AMP concentration [133, 224]. Different oxidative stress mediates AMPK activity via a distinct mechanism, including either AMP/ATP ratio-dependent, upstream kinase-mediated, or oxidative modification manner. Our laboratory was the first to demonstrate that low concentrations of ONOO− (<10 μM) increase the activity of AMPK and its downstream enzymes such as eNOS and ACC in ECs [225, 226]. We have shown that ONOO−-dependent AMPK activation occurs after hypoxia-reoxygenation [227] and in metformin- [226], simvastatin- [180], or berberine- [146] stimulated ECs, and ONOO− is capable of activating AMPK independently of changes in AMP/ATP ratio [226, 227]. AMPK is also activated by hypoxia via mitochondrial ROS through an unknown mechanism [228] or the upstream kinase LKB1 [229]. Recently, Mungai et al. reported that hypoxia triggers AMPK activation even when there is an apparent lack of AMP elevation, through ROS-dependent calcium release-activated calcium (CRAC) channel activation, leading to an elevation of cytosolic Ca2+ that activates the AMPK upstream kinase CaMKKβ [230]. Moreover, we found that thromboxane receptor activates AMPK in VSMCs via LKB1 in an H2O2-dependent manner [207]. H2O2 transiently activates AMPK in a dose-dependent manner, which is associated with an increased AMP/ATP ratio [231] or via an unknown mechanism without altering the ratios of AMP/ATP [224]. Interestingly, H2O2 directly causes the oxidative modification of AMPKα including S-glutathionylation of C299/304 without depletion of cellular ATP, which contributes to AMPK activation [232]. On the other hand, AMPK becomes inactivated under disease conditions including diabetes and obesity [183], which is reportedly associated with elevated oxidative stress [233]. Collectively, several ROS/RNS acutely activate AMPK and cellular functions mediated by this molecule, including the maintenance of redox homeostasis, such that AMPK might function as an “early warning system” in response to oxidants to attenuate oxidative injury [234].
AMPK activation suppresses oxidative stress
Either AMPKα1 or AMPKα2 deletion/inhibition elevates oxidative stress in different cell types/tissues through distinct mechanisms [15, 42, 219]. For example, AMPKα1 silencing in HUVEC downregulates the expression of genes involved in antioxidant defense, including MnSOD, catalase, γ-glutamylcysteine synthase, and thioredoxin [174]. Furthermore, AMPKα1 deletion significantly downregulates Foxo3 and ROS scavenging enzymes including catalase, MnSOD, and glutathione peroxidase (GPx-1) in mice erythroblasts [219]. In contrast, Ruderman et al have reported [235] that AMPK activation diminishes oxidative stress in ECs. There is emerging evidence that AMPK signaling pathways can ameliorate the oxidative stress associated with cardiovascular disease by upregulating the expression of endogenous antioxidant genes. For example, AMPK activation by metformin or AICAR normalizes hyperglycemia-induced mitochondrial ROS production by induction of MnSOD and promotion of mitochondrial biogenesis, via the activation of the AMPK-peroxisome proliferator-activated response-gamma coactivator-1α (PGC-1α) pathway [236]. AMPK activation by AICAR or metformin attenuates either AngII- or lipopolysaccharide-induced oxidative endothelium injury, and c-Jun N-terminal kinase (JNK) activation, via mitochondrion stabilization and increased mitochondrial biogenesis by PGC-1α [237]. Additionally, our work demonstrates that AICAR upregulates the AMPK-dependent mitochondrial uncoupling protein 2 (UCP2) via p38, which is associated with a reduction in both O2•− and prostacyclin synthase nitration in diabetic WT mice, but not in their AMPKα2−/− counterparts [238]. Collectively, these results establish that chronic AMPK activation functions as a conductor of the redox orchestra.
Regulation of NAD(P)H oxidases by AMPK
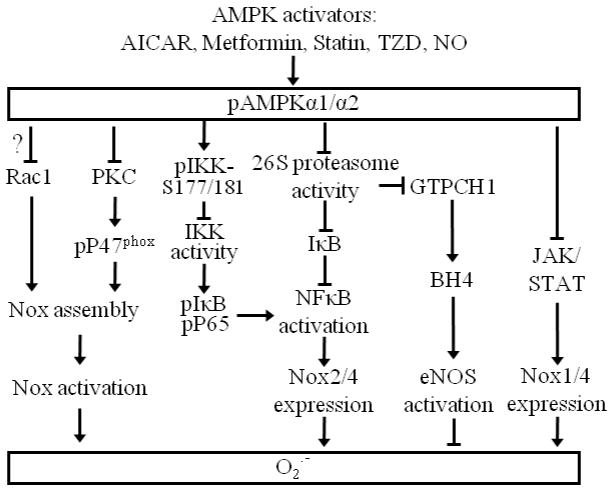
Nox enzymes are generally regulated via either Nox complex assembly or Nox subunits expression. Several lines of research indicate that AMPK activation may function as a Nox inhibitory mechanism. AMPK activators, such as AICAR or 5′-AMP, are reported to attenuate both phorbol 12-myristate 13-acetate (PMA)- and formyl methionyl leucyl phenylalanine (fMLP)-stimulated p47phox phosphorylation and translocation to the cell membrane in human neutrophils, which is consistent with reduced O2•− production [12]. AMPK activation by rosiglitazone may inhibit PKC [142], which phosphorylates p47phox at several serine sites [112], mediating p47 membrane translocation associated with Nox activation (Fig. 3). Thus, AMPK activation mitigates glucose-induced oxidative stress via Nox inhibition in ECs [142, 235]. These data suggest that AMPK may restrain Nox activation in the cardiovascular system [239] via assembly inhibition. Additionally, metformin, an AMPK activator, also suppresses Nox activity by an unknown mechanism in podocytes [13].
Fig. 3.
Schematic summary of available information on the regulation of cardiovascular Nox by AMPK. Refer to the text for expanded forms of abbreviations.
Our recent work might reveal an alternative mechanism for AMPK-mediated Nox inhibition [15]. In that study, we found that AMPK negatively regulates NF-κB activation by inhibiting the 26S proteasome-dependent IκBα degradation pathway and the consequent expression of Nox4, Nox2 and its partners p47phox and p67phox. The finding that either AICAR-induced AMPK activation or constitutively active AMPK increases IκBα protein levels in TNFα-treated ECs, without affecting IκBα mRNA levels, suggests that AMPK regulates IκBα only at the protein level. The ability of pharmacological inhibition of NF-κB to block the endothelial Nox gene as well as protein expression induced by either TNFα or AMPKα deletion suggests that both stimuli regulate Nox gene transcription via NF-κB. In addition, the ability of NF-κB inhibition to decrease components p67phox and p47phox protein levels in the absence of either stimulus suggests that the basal expression of these subunits is also regulated by the NF-κB pathway. These results are consistent with several recent reports indicating that AMPK activation by either metformin, AICAR, or NO inhibits NF-κB activation by decreasing IKK-dependent IκBα phosphorylation in ECs [240, 241]. More recently, Foretz et al. report that Nox2, neither Nox1 nor p22phox, is upregulated by unknown means in the aortae of AMPKα1−/− mice chronically infused with AngII at low subpressor doses; this is associated with elevated Nox activity [42]. In addition, AMPK inhibition by high glucose upregulates Nox4 via an unreported mechanism, increases Nox activity, and causes podocyte apoptosis through p53 upregulation [242]. These results indicate that AMPK may exert its anti-oxidant role by inhibiting the expression of Nox subunits. Although these studies are highly suggestive of potential crosstalk between AMPK and Nox, how AMPK suppresses Nox remains to be established. Additionally, it is reported that JAK/STAT inhibitors significantly diminish the interferon γ (IFNγ)-mediated upregulation of Nox1 and Nox4 expression, Nox activity, and resultant O2•− production in human VSMCs [108]. Interestingly, AMPKα2−/− VSMCs show elevated STAT1/3 activation (Song P and Zou MH, unpublished data). Whether AMPK activation blunts Nox expression via STAT warrants further investigation.
Taken together, AMPK activation suppresses Nox activity may via either blocking Nox assembly by inhibiting p47phox phosphorylation and translocation to cell membrane or inactivation of transcription factors including NF-κB and STAT for Nox or the expression of its partners (Fig.3). In contrast to a direct phosphorylation of IKK at Ser-177/181 by AMPKα2 in vitro [241], currently there is no evidence suggesting that AMPK can phosphorylate p47phox, IκBα, NFκB components, or STATs. Thus, how AMPK regulates Nox in monocytes/macrophages and platelet remains to be established.
AMPK and NAD(P)H oxidases in cardiovascular diseases
Numerous cellular and animal experiments (Table 3) report cardiovascular-protective effects of AMPK [234, 243–246]. Many therapeutic agents used for the treatment of diabetes and atherosclerosis, including metformin [141, 226], thiazolidinediones [142], and statins [180, 247] may exert their cardiovascular protective effects by the activation of AMPK. AMPK activation has a number of potentially beneficial anti-atherosclerotic effects including reducing the adhesion of inflammatory cells to the blood vessel endothelium, reducing lipid accumulation and the proliferation of inflammatory cells caused by oxidised lipids, stimulation of gene expression responsible for cellular antioxidant defenses [248], and stimulation of enzymes responsible for NO formation [181, 183, 249].
Recently, we showed that AMPKα2 deletion upregulates Nox2/4 and its partners p67phox and p47phox via NF-κB activation. Increased Nox activity results in elevated O2•− production in ECs, which leads to endothelial dysfunction contributing to exacerbated atherosclerosis in low-density lipoprotein receptor knockout (LDLr−/−) mice given a high-fat diet [15]. AMPKα1 deletion also upregulates Nox2, associated with elevated Nox activity in response to AngII. The increased Nox activity contributes to augmented O2•− production and the resultant endothelial dysfunction [42]. In addition, we found that oxidized and glycated LDL (HOG-LDL) enhances the p47phox membrane translocation associated with Nox activation [196]. Augmented Nox activity causes ROS elevation, which oxidizes the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA), and subsequently increases cytosolic Ca2+, which is associated with endoplasmic reticulum (ER) stress in ECs. The aberrant ER stress results in impaired endothelium-dependent vasorelaxation in isolated aortae from ApoE−/−/AMPKα2−/− mice fed a high-fat diet, which contributes to severe atherosclerosis. However, AMPK activation by AICAR blunts p47phox membrane translocation and ER stress. These data indicate that AMPK activation suppresses HOG-LDL–induced ER stress by inhibiting Nox–derived ROS. More recently, it is reported that early atrial fibrillation (AF) causes to the upregulation of Nox2 expression and activity. Ex vivo atorvastatin inhibits atrial Rac1 and Nox2 activity by unknown method in patients with postoperative AF [54]. Whether the function of statin is mediated by AMPK warrants further investigation. Overall, AMPK activation attempts to suppress oxidative injury by suppressing Nox-derived ROS and associated ER and mitochondria dysfunction. This feedback mechanism might be essential for maintaining cardiovascular homeostasis, therefore AMPK exerts its critical role in preventing cardiovascular disease including heart disease [151], atherogenesis [15, 196, 250], neointima formation [198, 209], and hypertension [197, 251].
Conclusions and perspectives
Several reported cellular and animal experiments indicate that either the expression of Nox and its partners or the assembly and activation of Nox complex are regulated by AMPK via different mechanisms (Fig.3). AMPK activators such as metformin may exert their cardiovascular protective function through Nox inhibition by AMPK activation. It is not clear whether other clinical AMPK activators including TZD and statin elicit their cardiovascular protective function via Nox inhibition mediated by AMPK. Treatment of Nox isoform knock out animals or Nox/AMPK double-knock out animals with these drugs will be beneficial for answering the question.
The AMPKα1 and α2 isoforms have ~90% homology in their N-terminal catalytic domains and ~60% homology in their C-terminal domains [252], suggesting that they likely have distinct upstream and downstream signaling pathways [133], although both AMPKα isoforms have common downstream pathways. It is therefore important to develop activators which specifically target different AMPKα isoforms. Different AMPK isoforms are predominantly expressed in different cardiovascular cell types and tissues. Nox subcellular localization and its components may be differentially regulated by distinct AMPKα isoforms via its respective molecular mechanisms. Tissue-specific conditional knockout of α isoforms in mouse/rat would afford the ability to assign downstream signaling to individual α subunits of AMPK for Nox regulation. It is also critical to re-evaluate the distinct functions of the different catalytic AMPKα subunits in both normal and pathological settings.
Highlights.
AMPK is activated by oxidants;
AMPK suppresses NAD(P)H oxidase;
Dysfunctional AMPK results in oxidative stress.
Acknowledgments
The authors sincerely apologize to our colleagues whose original contributions were not cited owing to page limitations. The authors also thank all current and former members of Dr Zou’s laboratory for the work described in this review. Dr. Ming-Hui Zou’s laboratory is supported by funding from the following agencies: National Institutes of Health RO1 (HL110488, HL105157, HL089920, HL080499, HL079584, and HL074399), the American Diabetes Association, the American Heart Association (11SDG5560036), and the Warren Chair in Diabetes Research of the University of Oklahoma Health Sciences Center. Dr. Zou is a recipient of the National Established Investigator Award of the American Heart Association.
Abbreviations
- 7-KC
7-ketocholesterol
- AAA
abdominal aortic aneurysm
- AICAR
5′-aminoimidazole-4-carboxamide ribonucleoside
- AMPK
adenosine monophosphate-activated protein kinase
- AngII
angiotensin II
- ApoE
apolipoprotein E
- BH4
5,6,7,8-tetrahydrobiopterin
- CAD
coronary artery disease
- CaMKKβ
Ca2+/calmodulin-dependent protein kinase kinase β
- CRAC
calcium release-activated calcium
- CVD
cardiovascular disease
- ECs
endothelial cells
- EGF
epidermal growth factor
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ERRα
estrogen-related receptor-α
- ET-1
Endothelin 1
- GPx-1
glutathione peroxidase
- GTPCH1
GTP-cyclohydrolase 1
- H2O2
hydrogen peroxide
- HF
heart failure
- HUVEC
human umbilical vein endothelial cells
- LKB1
liver kinase B1
- MI
myocardial infarction
- MPO
myeloperoxidase
- NAD(P)H
nicotinamide adenine dinucleotide phosphate reduced form
- NF-κB
nuclear factor kappa B
- NO
nitric oxide
- Nox
NAD(P)H oxidase
- NoxA1
Nox activator1
- NoxO1
Nox organizer 1
- O2•−
superoxide anion
- ONOO−
peroxynitrite
- PC
preconditioning
- PDGF
platelet-derived growth factor
- PDI
protein disulfide isomerase
- PGC-1α
peroxisome proliferator-activated response-gamma coactivator-1α
- PKA
protein kinase A
- PKC
protein kinase C
- PM
plasma membrane
- RAS
renin-angiotensin system
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SERCA
sarcoplasmic/endoplasmic reticulum Ca2+ ATPase
- Skp2
S-phase kinase-associated protein 2
- SOD
superoxide dismutase
- TAC
transverse aortic constriction
- TAK1
transforming growth factor-β-activated kinase 1
- TGF-β1
transforming growth factor β1
- TNFα
Tumor necrosis factor α
- TP
thromboxane A2 receptor
- TPR
tetratricopeptide repeat
- TZD
thiazolidinediones
- SMC
smooth muscle cell
- VSMCs
vascular smooth muscle cells
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sugamura K, Keaney JF., Jr Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 3.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 4.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wosniak J, Jr, Santos CX, Kowaltowski AJ, Laurindo FR. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal. 2009;11:1265–1278. doi: 10.1089/ars.2009.2392. [DOI] [PubMed] [Google Scholar]
- 6.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandes RP, Weissmann N, Schroder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal. 2009;11:841–860. doi: 10.1089/ars.2008.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Touyz RM, Briones AM, Sedeek M, Burger D, Montezano AC. NOX isoforms and reactive oxygen species in vascular health. Mol Interv. 2011;11:27–35. doi: 10.1124/mi.11.1.5. [DOI] [PubMed] [Google Scholar]
- 10.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 11.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alba G, El Bekay R, Alvarez-Maqueda M, Chacon P, Vega A, Monteseirin J, Santa Maria C, Pintado E, Bedoya FJ, Bartrons R, Sobrino F. Stimulators of AMP-activated protein kinase inhibit the respiratory burst in human neutrophils. FEBS Lett. 2004;573:219–225. doi: 10.1016/j.febslet.2004.07.077. [DOI] [PubMed] [Google Scholar]
- 13.Piwkowska A, Rogacka D, Jankowski M, Dominiczak MH, Stepinski JK, Angielski S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem Biophys Res Commun. 2010;393:268–273. doi: 10.1016/j.bbrc.2010.01.119. [DOI] [PubMed] [Google Scholar]
- 14.Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl) 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chanock SJ, el Benna J, Smith RM, Babior BM. The respiratory burst oxidase. J Biol Chem. 1994;269:24519–24522. [PubMed] [Google Scholar]
- 17.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 18.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- 19.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF., Jr NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sirker A, Zhang M, Shah AM. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic research in cardiology. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20:1546–1548. doi: 10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- 23.Bell RM, Cave AC, Johar S, Hearse DJ, Shah AM, Shattock MJ. Pivotal role of NOX-2-containing NADPH oxidase in early ischemic preconditioning. FASEB J. 2005;19:2037–2039. doi: 10.1096/fj.04-2774fje. [DOI] [PubMed] [Google Scholar]
- 24.Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–325. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- 25.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 26.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 27.Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 28.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vendrov AE, Hakim ZS, Madamanchi NR, Rojas M, Madamanchi C, Runge MS. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arterioscler Thromb Vasc Biol. 2007;27:2714–2721. doi: 10.1161/ATVBAHA.107.152629. [DOI] [PubMed] [Google Scholar]
- 30.Thomas M, Gavrila D, McCormick ML, Miller FJ, Jr, Daugherty A, Cassis LA, Dellsperger KC, Weintraub NL. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation. 2006;114:404–413. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Keaney JF, Jr, Schulz E, Levison B, Shan L, Sakuma M, Zhang X, Shi C, Hazen SL, Simon DI. Decreased neointimal formation in Nox2-deficient mice reveals a direct role for NADPH oxidase in the response to arterial injury. Proc Natl Acad Sci U S A. 2004;101:13014–13019. doi: 10.1073/pnas.0405389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peshavariya H, Dusting GJ, Jiang F, Halmos LR, Sobey CG, Drummond GR, Selemidis S. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn-Schmiedeberg’s archives of pharmacology. 2009;380:193–204. doi: 10.1007/s00210-009-0413-0. [DOI] [PubMed] [Google Scholar]
- 34.Basuroy S, Tcheranova D, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase-derived reactive oxygen species, via endogenous carbon monoxide, promote survival of brain endothelial cells during TNF-alpha-induced apoptosis. American journal of physiology. Cell physiology. 2011;300:C256–265. doi: 10.1152/ajpcell.00272.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L, Arbiser J, Ruegg C, Krause KH, Imhof BA. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PLoS One. 2011;6:e14665. doi: 10.1371/journal.pone.0014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 37.Muzaffar S, Shukla N, Bond M, Newby AC, Angelini GD, Sparatore A, Del Soldato P, Jeremy JY. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J Vasc Res. 2008;45:521–528. doi: 10.1159/000129686. [DOI] [PubMed] [Google Scholar]
- 38.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 39.Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, Schmidt HH. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radic Biol Med. 2001;31:1456–1464. doi: 10.1016/s0891-5849(01)00727-4. [DOI] [PubMed] [Google Scholar]
- 40.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, Neff C, Shah AM, Wingler K, Schmidt HH. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension. 2010;56:490–497. doi: 10.1161/HYPERTENSIONAHA.109.149187. [DOI] [PubMed] [Google Scholar]
- 41.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 42.Schuhmacher S, Foretz M, Knorr M, Jansen T, Hortmann M, Wenzel P, Oelze M, Kleschyov AL, Daiber A, Keaney JF, Jr, Wegener G, Lackner K, Munzel T, Viollet B, Schulz E. alpha1AMP-activated protein kinase preserves endothelial function during chronic angiotensin II treatment by limiting Nox2 upregulation. Arterioscler Thromb Vasc Biol. 2011;31:560–566. doi: 10.1161/ATVBAHA.110.219543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 45.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 46.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montezano AC, Burger D, Ceravolo GS, Yusuf H, Montero M, Touyz RM. Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci (Lond) 2011;120:131–141. doi: 10.1042/CS20100384. [DOI] [PubMed] [Google Scholar]
- 48.Schroder K. Isoform specific functions of Nox protein-derived reactive oxygen species in the vasculature. Curr Opin Pharmacol. 2010;10:122–126. doi: 10.1016/j.coph.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, Lambeth D, Quinn MT, Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res. 2010;106:1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med. 2008;45:329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rey FE, Li XC, Carretero OA, Garvin JL, Pagano PJ. Perivascular superoxide anion contributes to impairment of endothelium-dependent relaxation: role of gp91(phox) Circulation. 2002;106:2497–2502. doi: 10.1161/01.cir.0000038108.71560.70. [DOI] [PubMed] [Google Scholar]
- 52.Chamseddine AH, Miller FJ., Jr Gp91phox contributes to NADPH oxidase activity in aortic fibroblasts but not smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H2284–2289. doi: 10.1152/ajpheart.00459.2003. [DOI] [PubMed] [Google Scholar]
- 53.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 54.Reilly SN, Jayaram R, Nahar K, Antoniades C, Verheule S, Channon KM, Alp NJ, Schotten U, Casadei B. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. 2011;124:1107–1117. doi: 10.1161/CIRCULATIONAHA.111.029223. [DOI] [PubMed] [Google Scholar]
- 55.Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. Journal of leukocyte biology. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 57.Casbon AJ, Allen LA, Dunn KW, Dinauer MC. Macrophage NADPH oxidase flavocytochrome B localizes to the plasma membrane and Rab11-positive recycling endosomes. J Immunol. 2009;182:2325–2339. doi: 10.4049/jimmunol.0803476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chlopicki S, Olszanecki R, Janiszewski M, Laurindo FR, Panz T, Miedzobrodzki J. Functional role of NADPH oxidase in activation of platelets. Antioxid Redox Signal. 2004;6:691–698. doi: 10.1089/1523086041361640. [DOI] [PubMed] [Google Scholar]
- 60.Lee CF, Qiao M, Schroder K, Zhao Q, Asmis R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ Res. 2010;106:1489–1497. doi: 10.1161/CIRCRESAHA.109.215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bengtsson SH, Gulluyan LM, Dusting GJ, Drummond GR. Novel isoforms of NADPH oxidase in vascular physiology and pathophysiology. Clin Exp Pharmacol Physiol. 2003;30:849–854. doi: 10.1046/j.1440-1681.2003.03929.x. [DOI] [PubMed] [Google Scholar]
- 62.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 63.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 64.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 66.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 67.Gordillo G, Fang H, Park H, Roy S. Nox-4-dependent nuclear H2O2 drives DNA oxidation resulting in 8-OHdG as urinary biomarker and hemangioendothelioma formation. Antioxid Redox Signal. 2010;12:933–943. doi: 10.1089/ars.2009.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Price MO, McPhail LC, Lambeth JD, Han CH, Knaus UG, Dinauer MC. Creation of a genetic system for analysis of the phagocyte respiratory burst: high-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood. 2002;99:2653–2661. doi: 10.1182/blood.v99.8.2653. [DOI] [PubMed] [Google Scholar]
- 70.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 71.Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. American journal of physiology. Cell physiology. 2009;296:C422–432. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meng D, Lv DD, Fang J. Insulin-like growth factor-I induces reactive oxygen species production and cell migration through Nox4 and Rac1 in vascular smooth muscle cells. Cardiovasc Res. 2008;80:299–308. doi: 10.1093/cvr/cvn173. [DOI] [PubMed] [Google Scholar]
- 73.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol. 2003;285:F219–229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 74.Zhuang J, Jiang T, Lu D, Luo Y, Zheng C, Feng J, Yang D, Chen C, Yan X. NADPH oxidase 4 mediates reactive oxygen species induction of CD146 dimerization in VEGF signal transduction. Free Radic Biol Med. 2010;49:227–236. doi: 10.1016/j.freeradbiomed.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 75.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2:ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol. 2009;296:L489–499. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 79.Yogi A, Mercure C, Touyz J, Callera GE, Montezano AC, Aranha AB, Tostes RC, Reudelhuber T, Touyz RM. Renal redox-sensitive signaling, but not blood pressure, is attenuated by Nox1 knockout in angiotensin II-dependent chronic hypertension. Hypertension. 2008;51:500–506. doi: 10.1161/HYPERTENSIONAHA.107.103192. [DOI] [PubMed] [Google Scholar]
- 80.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 81.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haddad P, Dussault S, Groleau J, Turgeon J, Michaud SE, Menard C, Perez G, Maingrette F, Rivard A. Nox2-containing NADPH oxidase deficiency confers protection from hindlimb ischemia in conditions of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1522–1528. doi: 10.1161/ATVBAHA.109.191437. [DOI] [PubMed] [Google Scholar]
- 83.Haddad P, Dussault S, Groleau J, Turgeon J, Maingrette F, Rivard A. Nox2-derived reactive oxygen species contribute to hypercholesterolemia-induced inhibition of neovascularization: effects on endothelial progenitor cells and mature endothelial cells. Atherosclerosis. 2011;217:340–349. doi: 10.1016/j.atherosclerosis.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 84.Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, Ushio-Fukai M. Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res. 2008;103:212–220. doi: 10.1161/CIRCRESAHA.108.176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 86.Maytin M, Siwik DA, Ito M, Xiao L, Sawyer DB, Liao R, Colucci WS. Pressure overload-induced myocardial hypertrophy in mice does not require gp91phox. Circulation. 2004;109:1168–1171. doi: 10.1161/01.CIR.0000117229.60628.2F. [DOI] [PubMed] [Google Scholar]
- 87.Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2000;20:1529–1535. doi: 10.1161/01.atv.20.6.1529. [DOI] [PubMed] [Google Scholar]
- 88.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci U S A. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 91.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 92.Murdoch CE, Alom-Ruiz SP, Wang M, Zhang M, Walker S, Yu B, Brewer A, Shah AM. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic research in cardiology. 2011;106:527–538. doi: 10.1007/s00395-011-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 94.Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;288:H37–42. doi: 10.1152/ajpheart.00638.2004. [DOI] [PubMed] [Google Scholar]
- 95.Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation. 2004;109:520–525. doi: 10.1161/01.CIR.0000109698.70638.2B. [DOI] [PubMed] [Google Scholar]
- 96.Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens. 2004;22:535–542. doi: 10.1097/00004872-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 97.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 98.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2010;299:H673–679. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rivera J, Sobey CG, Walduck AK, Drummond GR. Nox isoforms in vascular pathophysiology: insights from transgenic and knockout mouse models. Redox Rep. 2010;15:50–63. doi: 10.1179/174329210X12650506623401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Niu XL, Madamanchi NR, Vendrov AE, Tchivilev I, Rojas M, Madamanchi C, Brandes RP, Krause KH, Humphries J, Smith A, Burnand KG, Runge MS. Nox activator 1: a potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation. 2010;121:549–559. doi: 10.1161/CIRCULATIONAHA.109.908319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 102.Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 103.Yang B, Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H954–962. doi: 10.1152/ajpheart.00758.2006. [DOI] [PubMed] [Google Scholar]
- 104.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10:1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 105.Lener B, Koziel R, Pircher H, Hutter E, Greussing R, Herndler-Brandstetter D, Hermann M, Unterluggauer H, Jansen-Durr P. The NADPH oxidase Nox4 restricts the replicative lifespan of human endothelial cells. Biochem J. 2009;423:363–374. doi: 10.1042/BJ20090666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 107.Stanic B, Katsuyama M, Miller FJ., Jr An oxidized extracellular oxidation-reduction state increases Nox1 expression and proliferation in vascular smooth muscle cells via epidermal growth factor receptor activation. Arterioscler Thromb Vasc Biol. 2010;30:2234–2241. doi: 10.1161/ATVBAHA.110.207639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Manea A, Tanase LI, Raicu M, Simionescu M. Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2010;30:105–112. doi: 10.1161/ATVBAHA.109.193896. [DOI] [PubMed] [Google Scholar]
- 109.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 110.Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O’Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gupte SA, Kaminski PM, George S, Kouznestova L, Olson SC, Mathew R, Hintze TH, Wolin MS. Peroxide generation by p47phox-Src activation of Nox2 has a key role in protein kinase C-induced arterial smooth muscle contraction. Am J Physiol Heart Circ Physiol. 2009;296:H1048–1057. doi: 10.1152/ajpheart.00491.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Faust LR, el Benna J, Babior BM, Chanock SJ. The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site-directed mutagenesis. J Clin Invest. 1995;96:1499–1505. doi: 10.1172/JCI118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Knaus UG, Morris S, Dong HJ, Chernoff J, Bokoch GM. Regulation of human leukocyte p21-activated kinases through G protein--coupled receptors. Science. 1995;269:221–223. doi: 10.1126/science.7618083. [DOI] [PubMed] [Google Scholar]
- 114.Diekmann D, Abo A, Johnston C, Segal AW, Hall A. Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science. 1994;265:531–533. doi: 10.1126/science.8036496. [DOI] [PubMed] [Google Scholar]
- 115.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 116.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 118.Zhang M, Song P, Xu J, Zou MH. Activation of NAD(P)H oxidases by thromboxane A2 receptor uncouples endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2011;31:125–132. doi: 10.1161/ATVBAHA.110.207712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gauss KA, Nelson-Overton LK, Siemsen DW, Gao Y, DeLeo FR, Quinn MT. Role of NF-kappaB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-alpha. Journal of leukocyte biology. 2007;82:729–741. doi: 10.1189/jlb.1206735. [DOI] [PubMed] [Google Scholar]
- 120.Manea A, Manea SA, Gafencu AV, Raicu M. Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Archives of physiology and biochemistry. 2007;113:163–172. doi: 10.1080/13813450701531235. [DOI] [PubMed] [Google Scholar]
- 121.Manea A, Manea SA, Gafencu AV, Raicu M, Simionescu M. AP-1-dependent transcriptional regulation of NADPH oxidase in human aortic smooth muscle cells: role of p22phox subunit. Arterioscler Thromb Vasc Biol. 2008;28:878–885. doi: 10.1161/ATVBAHA.108.163592. [DOI] [PubMed] [Google Scholar]
- 122.Lage R, Dieguez C, Vidal-Puig A, Lopez M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008;14:539–549. doi: 10.1016/j.molmed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 123.Chen L, Jiao ZH, Zheng LS, Zhang YY, Xie ST, Wang ZX, Wu JW. Structural insight into the autoinhibition mechanism of AMP-activated protein kinase. Nature. 2009;459:1146–1149. doi: 10.1038/nature08075. [DOI] [PubMed] [Google Scholar]
- 124.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 126.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 127.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 128.Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 129.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci U S A. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 131.Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- 132.Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, Lopaschuk GD. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochimica et biophysica acta. 1996;1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 133.Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J. 1998;334(Pt 1):177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, Rider MH. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 135.Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem. 2006;281:36662–36672. doi: 10.1074/jbc.M606676200. [DOI] [PubMed] [Google Scholar]
- 136.Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, Vaahtomeri K, Auchli Y, Rechsteiner H, Brunisholz RA, Viollet B, Makela TP, Wallimann T, Neumann D, Krek W. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 2010;29:469–481. doi: 10.1038/emboj.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 138.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 139.Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Watt MJ, Dzamko N, Thomas WG, Rose-John S, Ernst M, Carling D, Kemp BE, Febbraio MA, Steinberg GR. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med. 2006;12:541–548. doi: 10.1038/nm1383. [DOI] [PubMed] [Google Scholar]
- 141.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A, Avogaro A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27:2627–2633. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- 143.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 144.LeBrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, Tomas E. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291:E175–181. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- 145.Kim WS, Lee YS, Cha SH, Jeong HW, Choe SS, Lee MR, Oh GT, Park HS, Lee KU, Lane MD, Kim JB. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am J Physiol Endocrinol Metab. 2009;296:E812–819. doi: 10.1152/ajpendo.90710.2008. [DOI] [PubMed] [Google Scholar]
- 146.Han Y, Wang Q, Song P, Zhu Y, Zou MH. Redox regulation of the AMP-activated protein kinase. PLoS One. 2010;5:e15420. doi: 10.1371/journal.pone.0015420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim T, Davis J, Zhang AJ, He X, Mathews ST. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem Biophys Res Commun. 2009;388:377–382. doi: 10.1016/j.bbrc.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 149.Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45:276–295. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li J, Coven DL, Miller EJ, Hu X, Young ME, Carling D, Sinusas AJ, Young LH. Activation of AMPK alpha- and gamma-isoform complexes in the intact ischemic rat heart. Am J Physiol Heart Circ Physiol. 2006;291:H1927–1934. doi: 10.1152/ajpheart.00251.2006. [DOI] [PubMed] [Google Scholar]
- 151.Beauloye C, Bertrand L, Horman S, Hue L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc Res. 2011;90:224–233. doi: 10.1093/cvr/cvr034. [DOI] [PubMed] [Google Scholar]
- 152.Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, Salmon A, Ostman-Smith I, Watkins H. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10:1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 153.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 155.Shimano M, Ouchi N, Nakamura K, van Wijk B, Ohashi K, Asaumi Y, Higuchi A, Pimentel DR, Sam F, Murohara T, van den Hoff MJ, Walsh K. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1108559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem. 2008;283:24194–24201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baron SJ, Li J, Russell RR, 3rd, Neumann D, Miller EJ, Tuerk R, Wallimann T, Hurley RL, Witters LA, Young LH. Dual mechanisms regulating AMPK kinase action in the ischemic heart. Circ Res. 2005;96:337–345. doi: 10.1161/01.RES.0000155723.53868.d2. [DOI] [PubMed] [Google Scholar]
- 158.Nishino Y, Miura T, Miki T, Sakamoto J, Nakamura Y, Ikeda Y, Kobayashi H, Shimamoto K. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res. 2004;61:610–619. doi: 10.1016/j.cardiores.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 159.Gamble J, Lopaschuk GD. Insulin inhibition of 5′ adenosine monophosphate-activated protein kinase in the heart results in activation of acetyl coenzyme A carboxylase and inhibition of fatty acid oxidation. Metabolism. 1997;46:1270–1274. doi: 10.1016/s0026-0495(97)90229-8. [DOI] [PubMed] [Google Scholar]
- 160.Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL, Hue L. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett. 2001;505:348–352. doi: 10.1016/s0014-5793(01)02788-0. [DOI] [PubMed] [Google Scholar]
- 161.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 162.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 163.Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Young LH. AMP-activated protein kinase conducts the ischemic stress response orchestra. Circulation. 2008;117:832–840. doi: 10.1161/CIRCULATIONAHA.107.713115. [DOI] [PubMed] [Google Scholar]
- 165.Li HL, Yin R, Chen D, Liu D, Wang D, Yang Q, Dong YG. Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. Journal of cellular biochemistry. 2007;100:1086–1099. doi: 10.1002/jcb.21197. [DOI] [PubMed] [Google Scholar]
- 166.Tian R, Musi N, D’Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 167.Zarrinpashneh E, Beauloye C, Ginion A, Pouleur AC, Havaux X, Hue L, Viollet B, Vanoverschelde JL, Bertrand L. AMPKalpha2 counteracts the development of cardiac hypertrophy induced by isoproterenol. Biochem Biophys Res Commun. 2008;376:677–681. doi: 10.1016/j.bbrc.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 168.Zhang P, Hu X, Xu X, Fassett J, Zhu G, Viollet B, Xu W, Wiczer B, Bernlohr DA, Bache RJ, Chen Y. AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension. 2008;52:918–924. doi: 10.1161/HYPERTENSIONAHA.108.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hu X, Xu X, Lu Z, Zhang P, Fassett J, Zhang Y, Xin Y, Hall JL, Viollet B, Bache RJ, Huang Y, Chen Y. AMP Activated Protein Kinase-{alpha}2 Regulates Expression of Estrogen-Related Receptor-{alpha}, a Metabolic Transcription Factor Related to Heart Failure Development. Hypertension. 2011;58:696–703. doi: 10.1161/HYPERTENSIONAHA.111.174128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 171.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, Choi HC, Zou MH. Reduction of AMP-activated protein kinase {alpha}2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 2010;121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 174.Colombo SL, Moncada S. AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J. 2009;421:163–169. doi: 10.1042/BJ20090613. [DOI] [PubMed] [Google Scholar]
- 175.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 176.Neurath KM, Keough MP, Mikkelsen T, Claffey KP. AMP-dependent protein kinase alpha 2 isoform promotes hypoxia-induced VEGF expression in human glioblastoma. Glia. 2006;53:733–743. doi: 10.1002/glia.20326. [DOI] [PubMed] [Google Scholar]
- 177.Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, Neumann D, Schlattner U, Zou MH. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–6375. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 178.Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117:952–962. doi: 10.1161/CIRCULATIONAHA.107.744490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xie Z, Dong Y, Zhang J, Scholz R, Neumann D, Zou MH. Identification of the serine 307 of LKB1 as a novel phosphorylation site essential for its nucleocytoplasmic transport and endothelial cell angiogenesis. Mol Cell Biol. 2009;29:3582–3596. doi: 10.1128/MCB.01417-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Choi HC, Song P, Xie Z, Wu Y, Xu J, Zhang M, Dong Y, Wang S, Lau K, Zou MH. Reactive nitrogen species is required for the activation of the AMP-activated protein kinase by statin in vivo. J Biol Chem. 2008;283:20186–20197. doi: 10.1074/jbc.M803020200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 181.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JY. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Akaike M, Che W, Marmarosh NL, Ohta S, Osawa M, Ding B, Berk BC, Yan C, Abe J. The hinge-helix 1 region of peroxisome proliferator-activated receptor gamma1 (PPARgamma1) mediates interaction with extracellular signal-regulated kinase 5 and PPARgamma1 transcriptional activation: involvement in flow-induced PPARgamma activation in endothelial cells. Mol Cell Biol. 2004;24:8691–8704. doi: 10.1128/MCB.24.19.8691-8704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang S, Xu J, Song P, Viollet B, Zou MH. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 2009;58:1893–1901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo I, Oh GT, Park IS, Youn JH, Park SW, Lee KU, Park JY. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:2488–2494. doi: 10.1161/01.ATV.0000190667.33224.4c. [DOI] [PubMed] [Google Scholar]
- 185.Zou MH, Wu Y. AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function. Clin Exp Pharmacol Physiol. 2008;35:535–545. doi: 10.1111/j.1440-1681.2007.04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 187.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 188.Wang Y, Huang Y, Lam KS, Li Y, Wong WT, Ye H, Lau CW, Vanhoutte PM, Xu A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc Res. 2009;82:484–492. doi: 10.1093/cvr/cvp078. [DOI] [PubMed] [Google Scholar]
- 189.Li X, Han Y, Pang W, Li C, Xie X, Shyy JY, Zhu Y. AMP-activated protein kinase promotes the differentiation of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1789–1795. doi: 10.1161/ATVBAHA.108.172452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 191.Xu MJ, Song P, Shirwany N, Liang B, Xing J, Viollet B, Wang X, Zhu Y, Zou MH. Impaired Expression of Uncoupling Protein 2 Causes Defective Postischemic Angiogenesis in Mice Deficient in AMP-Activated Protein Kinase {alpha} Subunits. Arterioscler Thromb Vasc Biol. 2011;31:1757–1765. doi: 10.1161/ATVBAHA.111.227991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu C, Liang B, Wang Q, Wu J, Zou MH. Activation of AMP-activated protein kinase alpha1 alleviates endothelial cell apoptosis by increasing the expression of anti-apoptotic proteins Bcl-2 and survivin. J Biol Chem. 2010;285:15346–15355. doi: 10.1074/jbc.M110.102491. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 193.Goirand F, Solar M, Athea Y, Viollet B, Mateo P, Fortin D, Leclerc J, Hoerter J, Ventura-Clapier R, Garnier A. Activation of AMP kinase alpha1 subunit induces aortic vasorelaxation in mice. J Physiol. 2007;581:1163–1171. doi: 10.1113/jphysiol.2007.132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zarrinpashneh E, Carjaval K, Beauloye C, Ginion A, Mateo P, Pouleur AC, Horman S, Vaulont S, Hoerter J, Viollet B, Hue L, Vanoverschelde JL, Bertrand L. Role of the alpha2-isoform of AMP-activated protein kinase in the metabolic response of the heart to no-flow ischemia. Am J Physiol Heart Circ Physiol. 2006;291:H2875–2883. doi: 10.1152/ajpheart.01032.2005. [DOI] [PubMed] [Google Scholar]
- 195.Carvajal K, Zarrinpashneh E, Szarszoi O, Joubert F, Athea Y, Mateo P, Gillet B, Vaulont S, Viollet B, Bigard X, Bertrand L, Ventura-Clapier R, Hoerter JA. Dual cardiac contractile effects of the alpha2-AMPK deletion in low-flow ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2007;292:H3136–3147. doi: 10.1152/ajpheart.00683.2006. [DOI] [PubMed] [Google Scholar]
- 196.Dong Y, Zhang M, Wang S, Liang B, Zhao Z, Liu C, Wu M, Choi HC, Lyons TJ, Zou MH. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes. 2010;59:1386–1396. doi: 10.2337/db09-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang S, Liang B, Viollet B, Zou MH. Inhibition of the AMP-activated protein kinase-alpha2 accentuates agonist-induced vascular smooth muscle contraction and high blood pressure in mice. Hypertension. 2011;57:1010–1017. doi: 10.1161/HYPERTENSIONAHA.110.168906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Song P, Wang S, He C, Liang B, Viollet B, Zou MH. AMPKalpha2 deletion exacerbates neointima formation by upregulating Skp2 in vascular smooth muscle cells. Circ Res. 2011;109:1230–1239. doi: 10.1161/CIRCRESAHA.111.250423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 199.Banerjee SK, Ramani R, Saba S, Rager J, Tian R, Mathier MA, Ahmad F. A PRKAG2 mutation causes biphasic changes in myocardial AMPK activity and does not protect against ischemia. Biochem Biophys Res Commun. 2007;360:381–387. doi: 10.1016/j.bbrc.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 200.Arad M, Moskowitz IP, Patel VV, Ahmad F, Perez-Atayde AR, Sawyer DB, Walter M, Li GH, Burgon PG, Maguire CT, Stapleton D, Schmitt JP, Guo XX, Pizard A, Kupershmidt S, Roden DM, Berul CI, Seidman CE, Seidman JG. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation. 2003;107:2850–2856. doi: 10.1161/01.CIR.0000075270.13497.2B. [DOI] [PubMed] [Google Scholar]
- 201.Luptak I, Shen M, He H, Hirshman MF, Musi N, Goodyear LJ, Yan J, Wakimoto H, Morita H, Arad M, Seidman CE, Seidman JG, Ingwall JS, Balschi JA, Tian R. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest. 2007;117:1432–1439. doi: 10.1172/JCI30658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Davies JK, Wells DJ, Liu K, Whitrow HR, Daniel TD, Grignani R, Lygate CA, Schneider JE, Noel G, Watkins H, Carling D. Characterization of the role of gamma2 R531G mutation in AMP-activated protein kinase in cardiac hypertrophy and Wolff-Parkinson-White syndrome. Am J Physiol Heart Circ Physiol. 2006;290:H1942–1951. doi: 10.1152/ajpheart.01020.2005. [DOI] [PubMed] [Google Scholar]
- 203.Sidhu JS, Rajawat YS, Rami TG, Gollob MH, Wang Z, Yuan R, Marian AJ, DeMayo FJ, Weilbacher D, Taffet GE, Davies JK, Carling D, Khoury DS, Roberts R. Transgenic mouse model of ventricular preexcitation and atrioventricular reentrant tachycardia induced by an AMP-activated protein kinase loss-of-function mutation responsible for Wolff-Parkinson-White syndrome. Circulation. 2005;111:21–29. doi: 10.1161/01.CIR.0000151291.32974.D5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 205.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 206.Rubin LJ, Magliola L, Feng X, Jones AW, Hale CC. Metabolic activation of AMP kinase in vascular smooth muscle. J Appl Physiol. 2005;98:296–306. doi: 10.1152/japplphysiol.00075.2004. [DOI] [PubMed] [Google Scholar]
- 207.Zhang M, Dong Y, Xu J, Xie Z, Wu Y, Song P, Guzman M, Wu J, Zou MH. Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide. Circ Res. 2008;102:328–337. doi: 10.1161/CIRCRESAHA.107.163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, Toyonaga T, Asano T, Nishikawa T, Araki E. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res. 2005;97:837–844. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- 209.Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110:444–451. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- 210.Kim SY, Jeoung NH, Oh CJ, Choi YK, Lee HJ, Kim HJ, Kim JY, Hwang JH, Tadi S, Yim YH, Lee KU, Park KG, Huh S, Min KN, Jeong KH, Park MG, Kwak TH, Kweon GR, Inukai K, Shong M, Lee IK. Activation of NAD(P)H:quinone oxidoreductase 1 prevents arterial restenosis by suppressing vascular smooth muscle cell proliferation. Circ Res. 2009;104:842–850. doi: 10.1161/CIRCRESAHA.108.189837. [DOI] [PubMed] [Google Scholar]
- 211.Horman S, Morel N, Vertommen D, Hussain N, Neumann D, Beauloye C, El Najjar N, Forcet C, Viollet B, Walsh MP, Hue L, Rider MH. AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J Biol Chem. 2008;283:18505–18512. doi: 10.1074/jbc.M802053200. [DOI] [PubMed] [Google Scholar]
- 212.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Marsin AS, Bouzin C, Bertrand L, Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem. 2002;277:30778–30783. doi: 10.1074/jbc.M205213200. [DOI] [PubMed] [Google Scholar]
- 215.Moir H, Hughes MG, Potter S, Sims C, Butcher LR, Davies NA, Verheggen K, Jones KP, Thomas AW, Webb R. Exercise-induced immunosuppression: roles of reactive oxygen species and 5′-AMP-activated protein kinase dephosphorylation within immune cells. J Appl Physiol. 2010;108:1284–1292. doi: 10.1152/japplphysiol.00737.2009. [DOI] [PubMed] [Google Scholar]
- 216.Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 217.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L497–504. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bae HB, Zmijewski JW, Deshane JS, Tadie JM, Chaplin DD, Takashima S, Abraham E. AMP-activated protein kinase enhances the phagocytic ability of macrophages and neutrophils. FASEB J. 2011 doi: 10.1096/fj.11-190587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang S, Dale GL, Song P, Viollet B, Zou MH. AMPKalpha1 deletion shortens erythrocyte life span in mice: role of oxidative stress. J Biol Chem. 2010;285:19976–19985. doi: 10.1074/jbc.M110.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Foller M, Sopjani M, Koka S, Gu S, Mahmud H, Wang K, Floride E, Schleicher E, Schulz E, Munzel T, Lang F. Regulation of erythrocyte survival by AMP-activated protein kinase. FASEB J. 2009;23:1072–1080. doi: 10.1096/fj.08-121772. [DOI] [PubMed] [Google Scholar]
- 221.Foretz M, Guihard S, Leclerc J, Fauveau V, Couty JP, Andris F, Gaudry M, Andreelli F, Vaulont S, Viollet B. Maintenance of red blood cell integrity by AMP-activated protein kinase alpha1 catalytic subunit. FEBS Lett. 2010;584:3667–3671. doi: 10.1016/j.febslet.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 222.Randriamboavonjy V, Isaak J, Fromel T, Viollet B, Fisslthaler B, Preissner KT, Fleming I. AMPK alpha2 subunit is involved in platelet signaling, clot retraction, and thrombus stability. Blood. 2010;116:2134–2140. doi: 10.1182/blood-2010-04-279612. [DOI] [PubMed] [Google Scholar]
- 223.Fleming I, Schulz C, Fichtlscherer B, Kemp BE, Fisslthaler B, Busse R. AMP-activated protein kinase (AMPK) regulates the insulin-induced activation of the nitric oxide synthase in human platelets. Thrombosis and haemostasis. 2003;90:863–871. doi: 10.1160/TH03-04-0228. [DOI] [PubMed] [Google Scholar]
- 224.Toyoda T, Hayashi T, Miyamoto L, Yonemitsu S, Nakano M, Tanaka S, Ebihara K, Masuzaki H, Hosoda K, Inoue G, Otaka A, Sato K, Fushiki T, Nakao K. Possible involvement of the alpha1 isoform of 5′AMP-activated protein kinase in oxidative stress-stimulated glucose transport in skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287:E166–173. doi: 10.1152/ajpendo.00487.2003. [DOI] [PubMed] [Google Scholar]
- 225.Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem. 2002;277:32552–32557. doi: 10.1074/jbc.M204512200. [DOI] [PubMed] [Google Scholar]
- 226.Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WGt, Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 227.Zou MH, Hou XY, Shi CM, Kirkpatick S, Liu F, Goldman MH, Cohen RA. Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J Biol Chem. 2003;278:34003–34010. doi: 10.1074/jbc.M300215200. [DOI] [PubMed] [Google Scholar]
- 228.Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LA, Chandel NS, Sznajder JI. Alpha1-AMPactivated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol. 2009;29:3455–3464. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, Budinger GR, Chandel NS. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med. 2009;46:1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia Triggers AMPK Activation through Reactive Oxygen Species-Mediated Activation of Calcium Release-Activated Calcium Channels. Mol Cell Biol. 2011;31:3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Soo Kim S, Ha J. The regulation of AMP-activated protein kinase by H(2)O(2) Biochem Biophys Res Commun. 2001;287:92–97. doi: 10.1006/bbrc.2001.5544. [DOI] [PubMed] [Google Scholar]
- 232.Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem. 2010;285:33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Song P, Wu Y, Xu J, Xie Z, Dong Y, Zhang M, Zou MH. Reactive nitrogen species induced by hyperglycemia suppresses Akt signaling and triggers apoptosis by upregulating phosphatase PTEN (phosphatase and tensin homologue deleted on chromosome 10) in an LKB1-dependent manner. Circulation. 2007;116:1585–1595. doi: 10.1161/CIRCULATIONAHA.107.716498. [DOI] [PubMed] [Google Scholar]
- 234.Shirwany NA, Zou MH. AMPK in cardiovascular health and disease. Acta Pharmacol Sin. 2010;31:1075–1084. doi: 10.1038/aps.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ruderman NB, Cacicedo JM, Itani S, Yagihashi N, Saha AK, Ye JM, Chen K, Zou M, Carling D, Boden G, Cohen RA, Keaney J, Kraegen EW, Ido Y. Malonyl-CoA and AMP-activated protein kinase (AMPK): possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochemical Society transactions. 2003;31:202–206. doi: 10.1042/bst0310202. [DOI] [PubMed] [Google Scholar]
- 236.Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120–127. [PubMed] [Google Scholar]
- 237.Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, Wenzel P, Munzel T, Keaney JF., Jr Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118:1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008;57:3222–3230. doi: 10.2337/db08-0610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 239.McCarty MF, Barroso-Aranda J, Contreras F. AMP-activated kinase may suppress NADPH oxidase activation in vascular tissues. Med Hypotheses. 2009;72:468–470. doi: 10.1016/j.mehy.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 240.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 241.Bess E, Fisslthaler B, Fromel T, Fleming I. Nitric oxide-induced activation of the AMP-activated protein kinase alpha2 subunit attenuates IkappaB kinase activity and inflammatory responses in endothelial cells. PLoS One. 2011;6:e20848. doi: 10.1371/journal.pone.0020848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010;285:37503–37512. doi: 10.1074/jbc.M110.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ewart MA, Kennedy S. AMPK and vasculoprotection. Pharmacol Ther. 2011;131:242–253. doi: 10.1016/j.pharmthera.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 244.Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, Foretz M, Andreelli F, Ventura-Clapier R, Bertrand L. AMPK: Lessons from transgenic and knockout animals. Frontiers in bioscience: a journal and virtual library. 2009;14:19–44. doi: 10.2741/3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nagata D, Hirata Y. The role of AMP-activated protein kinase in the cardiovascular system. Hypertens Res. 2010;33:22–28. doi: 10.1038/hr.2009.187. [DOI] [PubMed] [Google Scholar]
- 246.Xu Q, Si LY. Protective effects of AMP-activated protein kinase in the cardiovascular system. Journal of cellular and molecular medicine. 2010;14:2604–2613. doi: 10.1111/j.1582-4934.2010.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sun W, Lee TS, Zhu M, Gu C, Wang Y, Zhu Y, Shyy JY. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 248.Tang L, Zhang Y, Jiang Y, Willard L, Ortiz E, Wark L, Medeiros D, Lin D. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood) 2011;236:1051–1063. doi: 10.1258/ebm.2011.010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 250.Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, Choi HC, Zou MH. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 2010;121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51:2199–2206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- 252.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]