Abstract
Activation of thromboxane receptors (TPr) may promote atherosclerosis by enhancing oxidative stress and the inflammation. This study examined the role of Nox1, an NADPH-oxidase subunit, in the enhancement of interleukin (IL)-1β-induced monocyte adhesion by TPr. In cultured rat aortic vascular smooth muscle cells (VSMCs), U46619, a stable thromboxane A2 mimetic, together with interleukin-1β significantly enhanced Nox1 mRNA expression, as well as adhesion of THP-1 monocytes. Activation of TPr also enhanced IL-1β-induced vascular cell adhesion molecule (VCAM)-1 expression, but inhibited inducible nitric oxide synthase (iNOS) expression. Silencing Nox1 expression by siRNA prevented the U46619 enhancement of IL-1β-induced monocyte adhesion, but had no significant effect on VCAM-1 or iNOS expression. Furthermore, monocyte adhesion was inhibited by superoxide dismutase, enhanced by a specific iNOS inhibitor, L-N6-(1-iminoethyl)-lysine, but not influenced by catalase. U46619 inhibited IL-1β-induced cyclic GMP production, and the inhibition was partially prevented by superoxide dismutase. In conclusion, activation of TPr enhances IL-1β-induced Nox1 expression in VSMCs, which is responsible for the up-regulation of monocyte adhesion. The effect of Nox1 is independent of the changes in VCAM-1 and iNOS expression, but depends upon the inactivation of nitric oxide via generation of superoxide anion.
Keywords: interleukin, monocyte adhesion, NADPH oxidase, nitric oxide, thromboxane receptor
Excess generation of reactive oxygen species (ROS), a situation known as oxidative stress, has been widely implicated in cardiovascular pathology including atherosclerosis [1]. Not only can increased ROS levels result in damage to cell components, including proteins, lipids, and DNA structures, but they also can regulate cell signaling pathways and functions [2–4]. In the vasculature, the multi-component NADPH-oxidase is one of the major sources of ROS [5, 6]. Superoxide anion generation by NADPH-oxidase is catalyzed by electron transfer to oxygen being catalyzed by two membrane bound subunits, gp91phox (also known as Nox2) and p22phox, and cytosolic activating subunits including p40phox, p47phox, and p67phox, as well as Rac-1 [7, 8]. Rodent vascular smooth muscle cells (VSMCs) do not express gp91phox, but express Nox1, a gp91phox homologue [9, 10]. Another gp91phox homologue, Nox4, is expressed throughout the vascular wall and is important for maintenance of differentiation markers such as α-actin and smooth muscle myosin heavy chain [10, 11]. Human, but not rodent, VSMCs also express Nox5, which may have a role in enhancing PDGF-induced cell proliferation [12]. Although Nox1 is implicated in regulation of VSMC dedifferentiation and proliferation, the role of Nox1 in modulation of cell inflammatory responses is unclear.
Diabetic mice treated with S18886, an antagonist of thromboxane A2/prostaglandin H2 receptors (TPr), showed attenuated tissue oxidant markers including nitrotyrosine compared with untreated diabetic mice [13, 14], suggesting involvement of TPr in regulating oxidative stress. However, the causal relationship between TPr activation and inflammation remains to be determined. In a prior study we showed that TPr activation potentiates cytokine-mediated inflammatory responses by a Jun N-terminal kinase (JNK) and activator protein (AP)-1 dependent mechanism [15]. In the present study, we investigated the role of TPr activation in IL-1β-induced Nox1 expression and monocyte adhesion in rat VSMCs. We found that activation of TPr dramatically enhanced IL-1β-induced Nox1 expression, which is responsible for the up-regulation of monocyte adhesion. The role of Nox1 in up-regulating adhesion was independent of VCAM-1 and iNOS expression, but likely dependent on the inactivation of nitric oxide by Nox1-generated superoxide.
Materials and methods
Materials
DMEM and FBS were purchased from Invitrogen. Recombinant human IL-1β (specific activity: 1.9 × 107 U/mg) was kindly provided by Dr. Aurigemma, the Biological Resources Branch Preclinical Repository, National Cancer Institute. U46619 was from Biomol Research Laboratories. SP600125 was from Calbiochem. L-N6-(1-iminoethyl)-lysine (L-NIL), Cu/Zn superoxide dismutase (SOD), catalase, and 3-isobutyl-1-methylxanthine (IBMX) were purchased from Sigma Aldrich. SQ29548 was from Cayman Chemical. Antibody against iNOS was from BD Transduction Laboratories. Antibody against VCAM-1 was from Santa Cruz Biotechnology. Antibody against ERK was from Cell Signaling. GeneEraser siRNA transfection reagent was purchased from Stratagene.
Cell culture
Rat VSMCs, isolated from rat thoracic aorta of 8-week-old male Wistar rats [16], were cultured in DMEM with 10% FBS. The cells were used between passages 6 and 12. Human THP-1 monocytic cells (ATCC) were cultured in suspension in DMEM with 10% FBS. For adhesion studies, the THP-1 monocytes were co-cultured with VSMCs as described below. The use of rats for preparation of VSMCs was approved by the Institutional Animal Care and Use Committee of Boston University Medical Center.
siRNA transfection
siRNAs targeting Nox1 were designed according to the database sequences and synthesized by Eurogentec (Seraing, Belgium). The following sequences were used: Sense Nox1 5’-GGU CGU GAU UAC CAA GGU UTT-3’ and antisense Nox1 5’-AAC CUU GGU AAU CAC GAC CTT-3’. VSMCs at 50–70% confluence were used for transfection of siRNAs. For each well transfection, 3–9 µL of 100 µmol/L siRNA-Noxl or siRNA-Control (scramble siRNA) was mixed with 1–3 µL of GeneEraser in serum free and antibiotic free DMEM, and incubated for 30 minutes [17, 18]. The medium containing siRNA and GeneEraser was then applied to the cells with a final concentration of 60 nmol/L siRNA. After 6 hours of incubation, medium with 10% FBS was added to all wells and the cells were cultured up to confluence. This usually took 2 days. Medium was then changed to DMEM with 0.1% FBS for 24 hours and the medium was refreshed before treatment for designated times with IL-1β in the absence or in the presence of U46619, as well as other additions as indicated in the results.
Adenoviral constructs and infection
The adenovirus expressing JNK1-dn, a mutant with T183A and Y185F, was created using the Ad-Easy system (Qbiogene) as previously described [15, 19]. Adenovirus expressing β-galactosidase (LacZ) was used as a control. Confluent VSMCs cultured in 6-well plates were infected with the adenoviruses (4 × 1010 viral particles/mL) in DMEM with 0.1% FBS for 24 hours and the medium was refreshed before treatment for 24 hours with IL-1β in the absence or in the presence of U46619, as indicated.
Western blot analysis
The cells were washed once with ice-cold PBS and scraped into ice-cold cell lysis buffer (Cell Signaling). Protein content of the cell lysates was determined with BCA protein assay reagent (Pierce), with bovine serum albumin used as a standard. Equal amounts of cell proteins were loaded onto 10% SDS-PAGE. Western blot analysis was performed as previously described [15].
Reverse transcriptase-polymerase chain reaction (RT-PCR) and real time PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen). The cDNA was generated from 1 µg total RNA by reverse transcription. RNA expression levels were determined by real-time PCR using SYBR Green supermix (Bio-Rad Laboratories, Inc.) containing forward and reverse primers (200 nmol/L each) and sample cDNA. The gene-specific primers (synthesized by Invitrogen) for Nox1 were forward, 5’-GAA CAA CAG CAC TCA CCA ATG-3’, and reverse, 5’-TCA AGA AGG AAG CAA AGG G-3’; for VCAM-1: forward, 5’-ATG TGC TGC TGT TGG CTG TGA CTC-3’, and reverse, 5’-GGC TCA GCG TCA GTG TGG ATG TAG-3’; for iNOS: forward, 5’- CTT GGA GCG AGT TGT GGA TTG-3’, and reverse, 5’-GGT GGG AGG GGT AGT GAT GTC-3’; and for GAPDH: forward, 5’-AAC CCA TCA CCA TCT TCC AGG-3’, and reverse, 5’-GGG GCA TCA GCG GAA GG-3’. All transcripts were normalized to GAPDH that was quantified in parallel.
For RT-PCR, the synthetic gene-specific primers were: Nox1 forward, 5’-GAA CAA CAG CAC TCA CCA ATG G-3’, and reverse, 5’-GAA AAC CCC CAC CAC AGA C -3’, which amplified a 462-bp product; Nox4 forward, 5’-ATG GTG GTG GTA TTG TTC CTC-3’, and reverse, 5’-GCA TCG GTA AAG TCT CTC AGC-3’, which amplified a 408-bp product; GAPDH forward, 5’-GCC ATC AAC GAC CCC TTC AT-3’, and reverse, 5’-CGC CTG CTT CAC CAC CTT CT-3’, which amplified a 702-bp product. The PCR schedule was denaturation, annealing, and extension at 95°C, 56°C and 72°C for 40 seconds, 30 seconds, and 1 minute, respectively, for 28 cycles. PCR products were electrophoresed on 1.2% agarose gels containing ethidium bromide, and visualized by UV-induced fluorescence.
Monocyte adhesion assay
VSMCs were cultured on 6-well plates under the same conditions described above. After treatment with IL-1β in the absence or in the presence of U46619, THP-1 monocytes were added to VSMCs and co-cultured in 37°C incubator for 3 hours. The medium was then removed by aspiration and the plates were washed with PBS for 3 times to remove all non-bound THP-1 monocytes. For bound monocytes, microscopic images were recorded on a linked computer, and bound THP-1 monocytes were counted and expressed as numbers per image area.
Determination of nitrite
The release of nitric oxide from cells cultured in 24-well plate was assessed by the determination of nitrite in culture medium with Griess reagent as described previously [16].
Cellular cyclic GMP measurement
Rat VSMCs at confluence were untreated or treated for 24 hours with additions as indicated, in the medium containing the phosphodiesterase inhibitor IBMX to inhibit cyclic GMP metabolism. Cells were then lysed in 0.1 mol/L HCl. Cellular cyclic GMP was measured by ELISA following manufacturer’s manual (Cayman Chemical).
Statistical analysis
Quantitative results were expressed as means ± SD. Statistical analysis was performed using Student’s t test and 1-way analysis of variance for comparison between 2 groups and among multiple groups, respectively. P<0.05 was considered significant.
Results
Changes induced by U46619 and IL-1β on Nox1, Nox4, VCAM-1, and iNOS expression
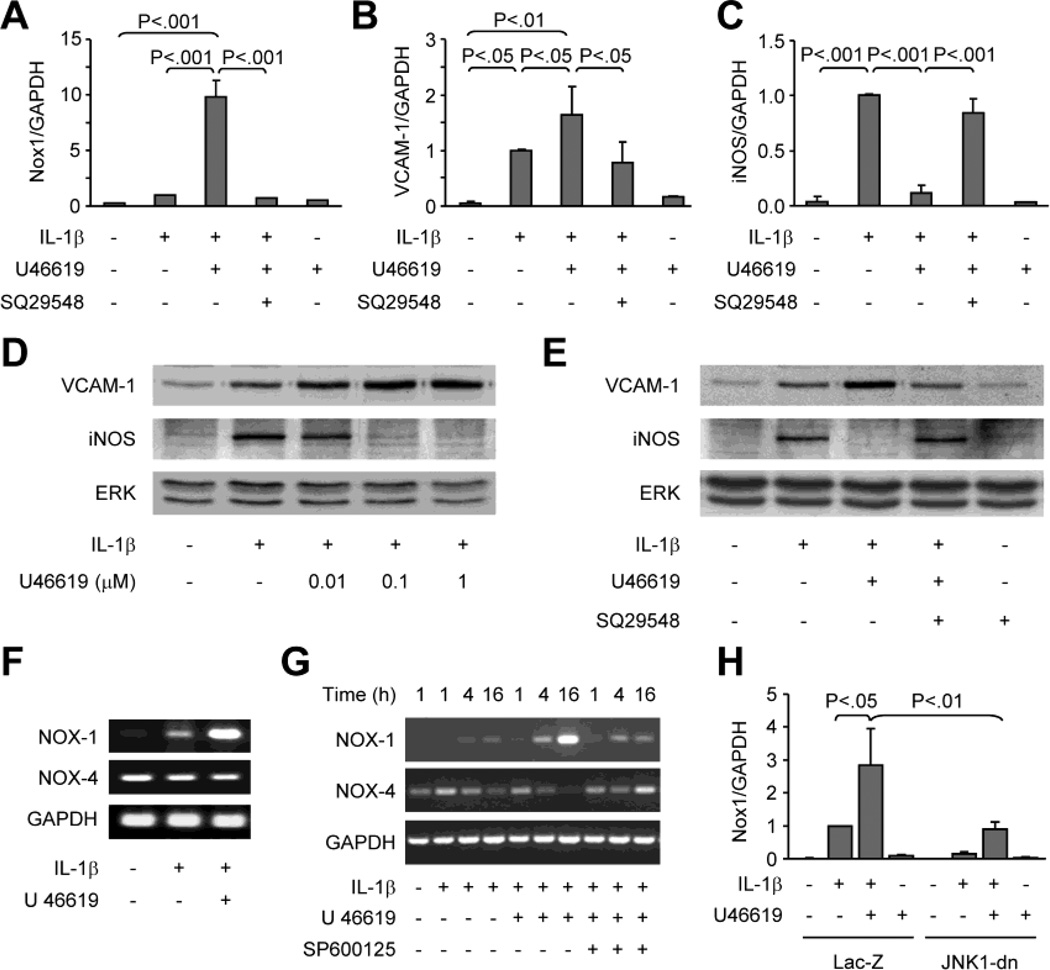
IL-1β (3 ng/mL, 24 h) induced VCAM-1 and iNOS mRNA, but had no significant effect on Nox1 mRNA (Figure 1A-C). U46619, a stable thromboxane A2 mimetic known to activate TPr, alone (0.1 µmol/L) did not induce Nox1, VCAM-1 or iNOS. However, when combined with IL-1β, U46619 significantly increased Nox1 over levels in the cells treated with IL-1β alone. TPr activation also increased VCAM-1 mRNA levels, but in contrast, prevented the increase in iNOS mRNA induced by IL-1β. The role of U46619 in differential regulation of VCAM-1 and iNOS expression was also reflected by changes in their respective protein levels, which was in a U46619 concentration-dependent manner (Figure 1D). The effects of U46619 were completely abolished by a selective antagonist of TPr, SQ29548 (1 µmol/L) (Figure 1A-C, and E). Interestingly, while Nox1 mRNA levels were up-regulated, Nox4 mRNA levels were down-regulated by IL-1β plus U46619 (Figure 1F and G). Furthermore, the induction of Nox1 transcription by IL-1β and U46619 was prevented either by pretreatment of the cells with JNK inhibitor SP600125 (Figure 1G), or by over-expression of a dominant negative mutant of JNK1 (Figure 1H), suggesting that activation of JNK signaling is required for Nox1 gene expression.
Fig. 1. U46619 differentially modulates IL-1β-induced expression of Nox1, Nox4, VCAM-1, and iNOS.
A to E, Rat VSMCs were treated for 24 hours with IL-1β, U46619, or both. TPr antagonist SQ29548 was added 1 hour prior to IL-1β and U46619. A to C, Real-time RT-PCR analysis of mRNA levels of Nox1, VCAM-1, and iNOS. D and E, Western blot analysis of VCAM-1 and iNOS protein expression. Total ERK levels are shown as a reference for equal loading. F and G, RT-PCR analysis of Nox1 and Nox4 mRNA expression in rat VSMCs treated with IL-1β or IL-1β plus U46619 for 16 h (F) or the time as indicated (G). In G, JNK inhibitor SP600125 (1 µmol/L) was added 1 h prior to IL-1β and U46619. H, Real-time RT-PCR analysis of Nox1 mRNA levels in rat VSMCs infected with adenovirus expressing Lac Z or JNK1-dn, and then treated for 24 hours with IL-1β, U46619, or both, as indicated. Bar graphs A to C represent data from 3 separate experiments, and H from 2 separate experiments. The mRNA results were normalized to GAPDH mRNA levels and expressed as fold-changes compared to IL-1β- treated cells.
Nox1 mediates the effect of U46619 on IL-1β-induced binding of THP-1 monocytes to VSMCs
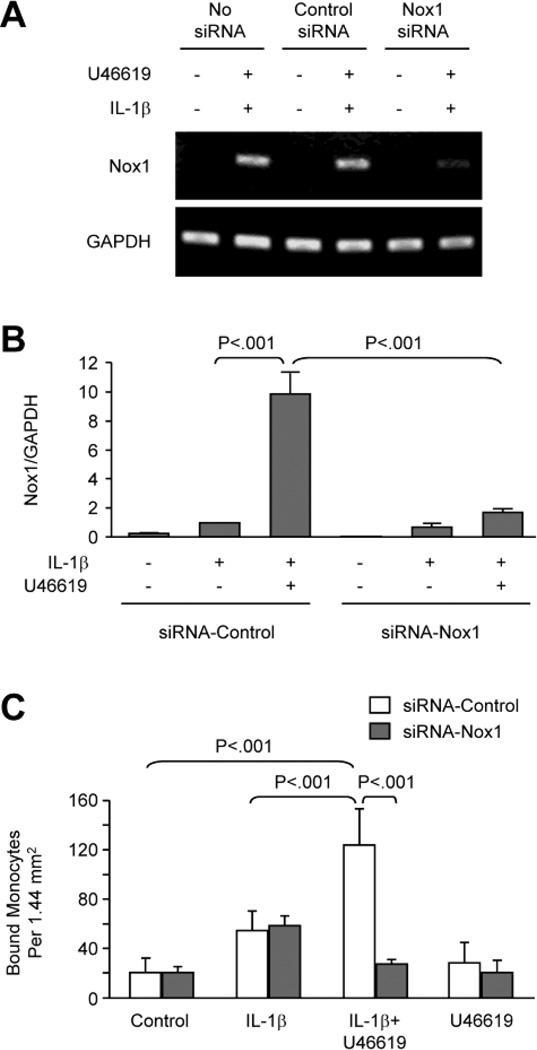
To investigate the functional role of Nox1, monocyte adhesion to VSMCs was measured and siRNA targeting was used to silence Nox1 gene expression. The strong induction of Nox1 mRNA by U46619 and IL-1β was significantly suppressed in cells transfected with Nox1 siRNA, whereas transfection with control siRNA showed no effect (Figure 2A and B). The siRNA transfection did not affect GAPDH expression, indicating that the siRNA specifically silenced the Nox1 gene, consistent with the previous report by Schröder et al [17].
Fig. 2. Silencing of Nox1 expression by siRNA inhibits monocyte adhesion to VSMC induced by IL-1β and U46619.
Rat VSMCs transfected with siRNA-Control or siRNA-Nox1 were treated for 24 hours as indicated. A, RT-PCR and B, Real-time RT-PCR show siRNA silencing of Nox1 expression. C, Monocyte adhesion. THP-1 monocytes were co-cultured for 3 hours with VSMCs that had been treated for 24 hours as indicated, and then unbound monocytes were washed away. Bar graphs show numbers of monocytes bound to VSMC per area and represent data from 6 separate experiments.
The functional role of TPr-mediated induction of Nox1 in IL-1β-activated VSMCs was investigated by determining the effect of Nox1 siRNA on monocyte adhesion. As shown in Figure 2C, IL-1β increased THP-1 monocyte adhesion to VSMCs, and U46619 significantly enhanced this adhesion. Although the Nox1 siRNA had no effect on the adhesion of THP-1 cells caused by IL-1β alone, it significantly decreased the THP-1 cell adhesion in VSMCs stimulated with both U46619 and IL-1β to levels not significantly different from untreated control VSMCs.
Nox1 does not mediate the U46619-induced enhancement of VCAM-1 or inhibition of iNOS expression induced by IL-1β
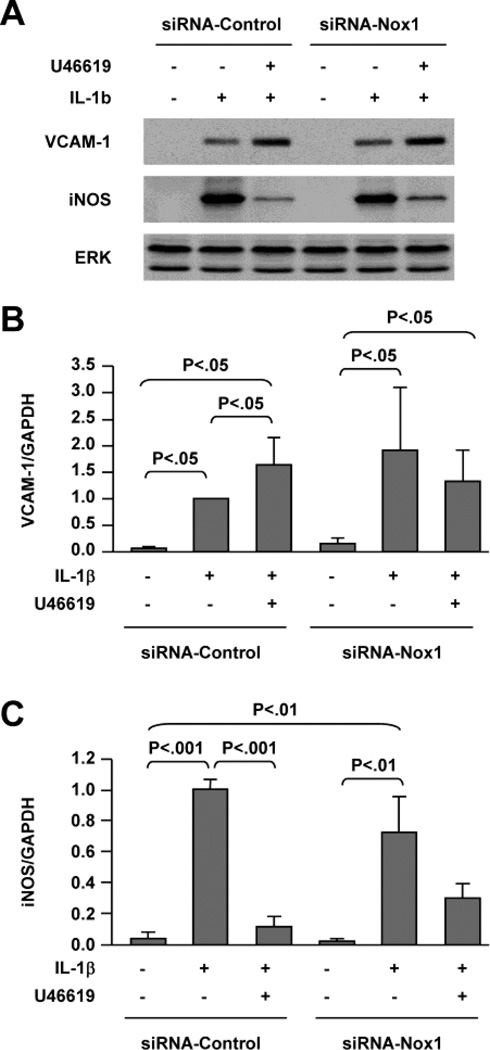
To examine whether Nox1-mediated monocyte adhesion is related to changes in expression of VCAM-1 or iNOS, protein and mRNA levels were analyzed by Western blot and real-time RT-PCR in the cells transfected with control or Nox1 siRNA. Induction of VCAM-1 expression was not significantly changed by silencing Nox1 gene expression in cells treated with IL-1β or IL-1β plus U46619 (Figures 3A and B). Likewise, iNOS expression under either condition was not significantly affected by silencing Nox1 gene expression (Figures 3A and C). These results indicate that the inhibition of monocyte adhesion to VSMCs by siRNA-Nox1 is not related primarily to regulation of VCAM-1 or iNOS expression.
Fig. 3. Silencing of Nox1 expression does not affect VCAM-1 or iNOS expression.
Rat VSMCs transfected with control or Nox1 siRNA were treated for 24 hours as indicated. A, Western blot analysis of VCAM-1 and iNOS protein expression. Total ERK levels are shown as a reference for equal loading. B and C, Real-time RT-PCR analysis of VCAM-1 and iNOS mRNA levels. Bar graphs represent 3 separate experiments. The results were normalized to GAPDH mRNA levels and expressed as fold-changes compared to IL-1β-treated cells (column 2).
Oxidants and nitric oxide bioavailability differentially regulate THP-1 monocyte adhesion induced by IL-1β and U46619
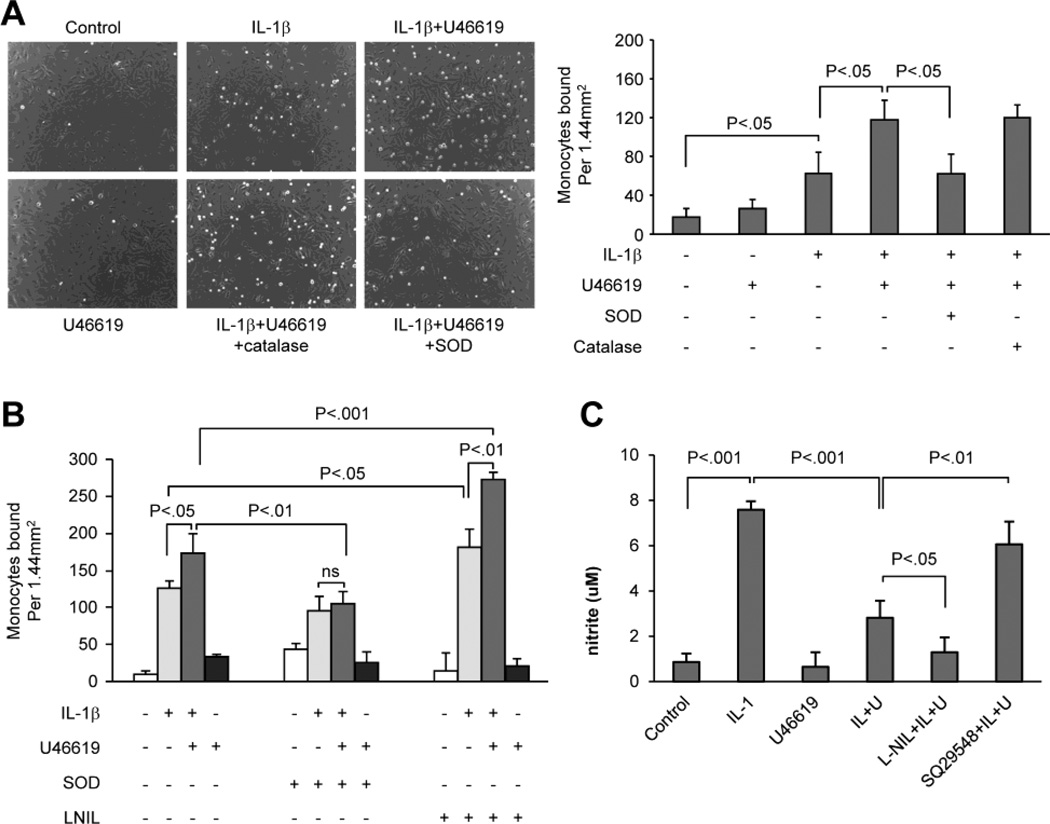
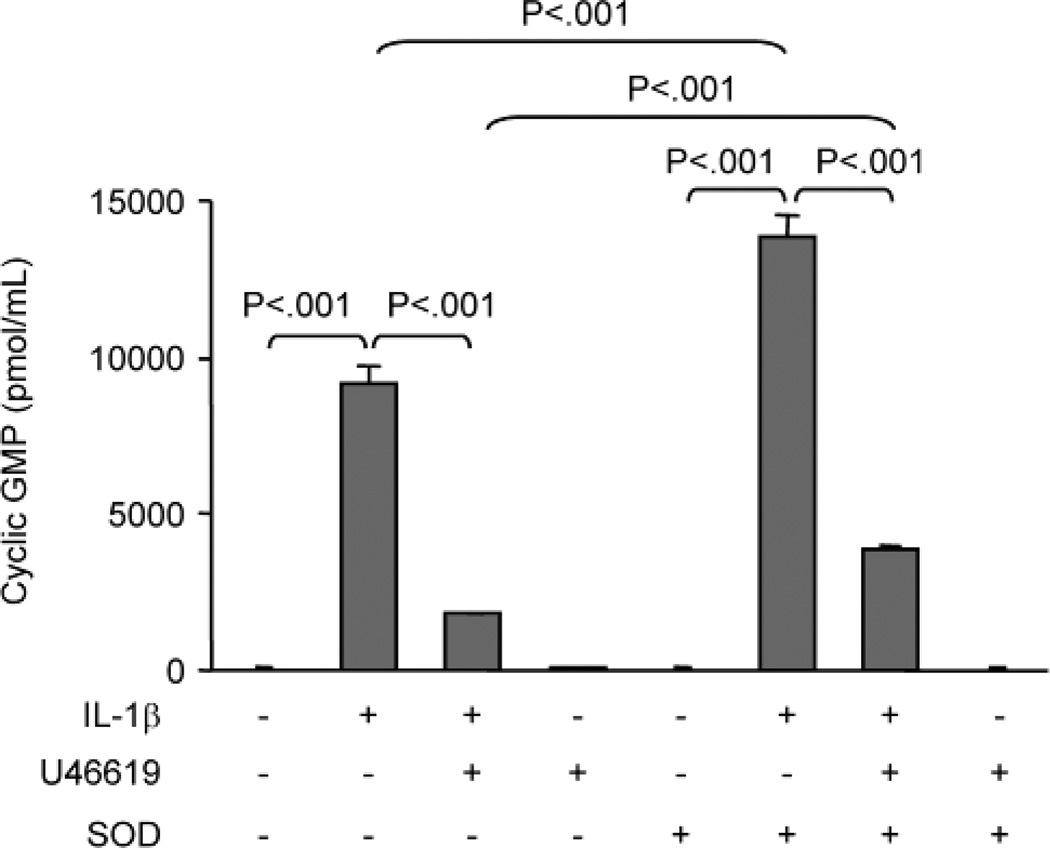
Because the increase in adhesion of monocytes to VSMCs induced by IL-1β and U46619 was associated with increased VCAM-1 and Nox1 expression as well as decreased iNOS expression, the role of oxidants generated by Nox1 and nitric oxide derived from iNOS were assessed by using oxidant scavengers and an inhibitor of iNOS. VSMCs were treated with IL-1β or U46619 or both for 24 h in the absence or presence of SOD or catalase, and then adhesion of monocytes for 3 h was assessed. As shown in Figure 4A, in cells treated with SOD, the augmentation of IL-1β-induced monocyte adhesion by U46619 was completely prevented, suggesting that Nox1-derived superoxide anion mediates the increased adhesion. Consistent with this conclusion, catalase had no effect on the enhanced monocyte binding to VSMCs induced by IL-1β plus U44619. Furthermore, adhesion of monocytes to VSMCs caused by IL-1β alone was not significantly affected by SOD (Figure 4B). In contrast, L-NIL, an iNOS inhibitor, further augmented adhesion of monocytes to VSMCs induced either by IL-1β alone or by IL-1β plus U46619 (Figure 4B). As shown in Figure 4C, U46619 decreased IL-1β-induced nitric oxide production as indicated by measurement of nitrite, which was further reduced by the presence of L-NIL but was reversed by TPr antagonist SQ29548. The fact that L-NIL enhanced monocyte binding to VSMCs induced by IL-1β alone or by IL-1β plus U46619 indicates that the bioactivity of nitric oxide produced by iNOS inhibits monocyte adhesion to VSMCs. To confirm this possibility, VSMC cyclic GMP levels were determined. Figure 5 shows that consistent with changes in iNOS mRNA and protein levels, treatment with IL-1β alone significantly increased cyclic GMP levels, and that addition of U46619 significantly reduced the IL-1β-induced cyclic GMP production. SOD significantly enhanced cyclic GMP concentrations in cells treated either with IL-1β alone or with IL-1β plus U46619. Together with the results obtained in VSMCs treated with L-NIL, these results suggest that in addition to increased VCAM-1 expression and decreased iNOS expression, superoxide anion produced by Nox1 inactivates sufficient amounts of nitric oxide produced by iNOS to increase monocyte adhesion to VSMCs.
Fig. 4. L-NIL and SOD but not catalase differentially regulate THP-1 monocyte adhesion induced by IL-1β and enhanced by U46619.
A and B, Rat VSMCs were untreated or treated for 24 hours with IL-1β alone, IL-1β plus U46619, or U46619 alone. Catalase (A), SOD (A and B), or L-NIL (B) were added 1 hour prior to IL-1β and U46619 treatment. THP-1 monocytes were co-cultured with VSMCs for 3 hours and then unbound monocytes were washed away. The images in A were observed at 100x magnification. Bar graphs show monocytes bound to VSMC per area and represent data from 6 separate experiments. C, Nitrite production in cells untreated or treated for 24 hours with IL-1β, U46619, or IL-1β plus U46619. L-NiL or SQ29548 was added 1 hour prior to IL-1β and U46619 treatment.
Fig. 5. SOD enhances IL-1β-induced cGMP levels and partially prevents the inhibition of cGMP by U46619.
Rat VSMCs were untreated or treated for 24 hours with IL-1β alone, IL-1β plus U46619, or U46619 alone in the absence or presence of SOD in medium containing IBMX. Cells were lysed in 0.1 M HCl and cellular cGMP was measured by ELISA. Bar graphs represent data from 3 separate experiments.
Discussion
Oxidant stress is a key component in inflammation [2, 3]. Here we demonstrated that Nox1, a Nox2 NADPH-oxidase subunit isoform, participates in TPr-mediated up-regulation of monocyte adhesion to VSMC induced by IL-1β. The fact that Nox1 expression is significantly induced by the TPr agonist in the presence of IL-1β, and that silencing of Nox1 with siRNA prevents the TPr-mediated enhancement of monocyte adhesion in IL-1β-treated VSMCs establishes an important novel role of Nox1 in the modulation of the inflammatory response in VSMCs.
Our data indicate that activation of TPr by U46619 differentially regulates the expression of Nox1, VCAM-1, and iNOS induced by IL-1β. U46619 alone did not induce Nox1 expression, but dramatically enhanced that caused by IL-1β. We previously have demonstrated that U46619 activation of JNK and AP-1 is responsible for the augmentation of IL-1β-induced VCAM-1 expression by U46619 [15]. Because the Nox1 promoter contains both NF-κB and AP-1 binding sites [20], the requirement that we demonstrate here for JNK signaling in Nox1 expression induced by IL-1β and U46619 suggests that the mechanism for the enhancement in Nox1 is similar to that for VCAM-1. Interestingly, while Nox1 was induced by IL-1β and U46619, the constitutively expressed Nox4 was down-regulated. Because Nox1 and Nox4 may differently regulate VSMC proliferation and differentiation [11], the reciprocal changes in expression of Nox1 and Nox4 in response to inflammatory stimulation found in this study suggest an intriguing regulation of vascular function by Nox isoforms in pathological conditions, which is worthy of further studies.
The increase in monocyte adhesion caused by IL-1β and U46619 was associated with increased expression of both VCAM-1 and Nox1. However, in cells stimulated with both IL-1β and U46619, knockdown of Nox1 significantly decreased monocyte adhesion to VSMC but did not cause a parallel decrease in VCAM-1 expression, suggesting that although VCAM-1 mediates adhesion of monocytes to VSMC [21], other mechanisms are important. We postulated that the role of Nox1 in monocyte adhesion could be through influencing nitric oxide bioavailability, particularly in the presence of TPr agonists such as U46619 that may further reduce nitric oxide bioavailability by down regulating iNOS expression in addition to scavenging nitric oxide by Nox1-generated superoxide. This hypothesis was confirmed by using L-NIL and SOD. First, L-NIL, a specific iNOS inhibitor significantly increased monocyte adhesion, indicating that nitric oxide produced by iNOS limits monocyte adhesion to VSMCs. Secondly, monocyte binding to VSMCs was inhibited by SOD, suggesting that scavenging of nitric oxide by superoxide anion regulates monocyte adhesion. The role of superoxide in inflammatory cell adhesion is consistent with earlier observations by our laboratory and others indicating that nitric oxide and superoxide, which react with each other, differentially regulate leukocyte adhesion [22, 23]. Importantly, adhesion was far more affected by SOD in the cells treated with IL-1β plus U46619 than in the cells treated with IL-1β alone, consistent with the much greater expression of Nox1 in the combined presence of IL-1β and U46619. In fact, in the presence of SOD, U46619 caused no enhancement of monocyte adhesion, consistent with the fact that Nox1 siRNA blocked the enhancement of monocyte adhesion caused by U46619. In agreement with previous reports by our laboratory and others [23, 24], catalase showed no effect on monocyte adhesion, indicating that superoxide, but not hydrogen peroxide, mediates the role of TPr in regulating monocyte adhesion to VSMC. Although we cannot completely exclude potential effects of L-NIL and SOD on the monocytes added to the assays, control studies in which the treatments were removed prior to addition of the monocytes showed no difference in results. Addition of SOD significantly increased cyclic GMP levels in the cells treated with either IL-1β alone or IL-1β plus U46619, indicating that scavenging superoxide by SOD enhances the bioavailability of nitric oxide. Although statistically significant, the effects of SOD on cGMP levels were small, suggesting the interesting possibility that the actions of nitric oxide in suppressing monocyte adhesion were cGMP-independent.
Thus, involvement of TPr in enhancing adhesion of monocytes to VSMC may depend on a combination of factors including an increase in expression of adhesion molecules [15, 25], i.e. VCAM-1, decrease of nitric oxide production by iNOS, and an increase of nitric oxide scavenging by superoxide anion produced by Nox1 (Figure 6). Because the inflammatory response of VSMCs is critical to both vascular injury and repair [2, 3, 26], the balance of mediators regulating monocyte adhesion and retention within atherosclerotic lesions is likely to be important. Similar interplay of VCAM-1 expression, oxidants, and nitric oxide bioavailability and TPr in regulating monocyte adhesion to inflamed endothelial cells has been implicated in promoting atherosclerosis [14, 27] and our results here highlight the importance of the contribution of both VSMCs and endothelial cells to atherosclerotic lesions. IL-1β induces vascular expression of both VCAM-1 and iNOS and increased generation of TPr agonists is evident in many chronic inflammatory vascular disease conditions including hyperlipidemia, hypertension, and diabetes mellitus [13, 14, 27]. The fact that TPr activation enhances the inflammatory action of cytokines such as IL-1β by increasing oxidants and decreasing nitric oxide bioavailability may help to explain the therapeutic effect of blocking TPr in diabetic and atherosclerotic vascular disease.
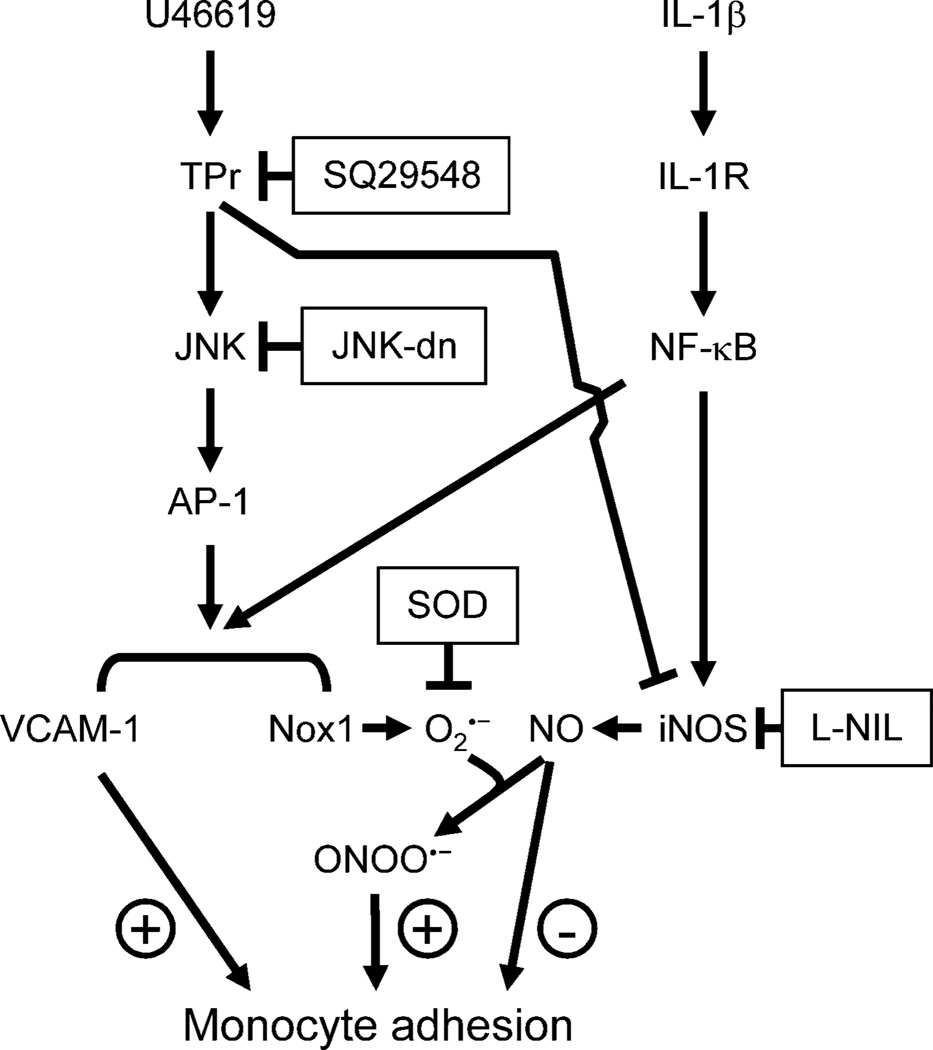
Fig. 6. A schematic representation of the involvement of Nox1 in the modulation by TPr of IL-1β-induced monocyte adhesion.
In VSMCs, IL-1β activates NF-κB leading to Nox1, VCAM-1 and iNOS expression. The TPr agonist U46619 augments both Nox1 and VCAM-1 expression through activation of JNK/AP-1 signaling, but inhibits iNOS expression induced by IL-1β. Adhesion of monocytes to VSMC is regulated by the balance of VCAM-1 expression, and nitric oxide (NO) and superoxide anion (O2.−) production. Activation of TPr results in increased expression of Nox1 that enhances superoxide generation, which inactivates nitric oxide produced by iNOS, likely forming peroxynitrite (OONO−), and contributes to enhanced monocyte adhesion.
Highlights.
Activation of TPr enhances interleukin-1-induced Nox1 expression.
Activation of TPr differentially regulates VCAM-1 and iNOS expression.
Nox1 expression enhances monocyte adhesion through inactivation of nitric oxide.
Acknowledgments
This study was supported in part by an American Heart Association award 09GRNT210017 (to B.J), National Institute of Health grants R01 HL083358 (to B.J.), R01 AG27080, R01 HL105287 (to R.A.C), and a Strategic Alliance between the Vascular Biology Section at Boston University Medical Center and the Institut de Recherche Servier.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 3.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 4.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 7.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 8.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 9.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 10.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 11.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med. 2008;45:329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu S, Jiang B, Maitland KA, Bayat H, Gu J, Nadler JL, Corda S, Lavielle G, Verbeuren TJ, Zuccollo A, Cohen RA. The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes. 2006;55:110–119. [PubMed] [Google Scholar]
- 14.Zuccollo A, Shi C, Mastroianni R, Maitland-Toolan KA, Weisbrod RM, Zang M, Xu S, Jiang B, Oliver-Krasinski JM, Cayatte AJ, Corda S, Lavielle G, Verbeuren TJ, Cohen RA. The thromboxane A2 receptor antagonist S18886 prevents enhanced atherogenesis caused by diabetes mellitus. Circulation. 2005;112:3001–3008. doi: 10.1161/CIRCULATIONAHA.105.581892. [DOI] [PubMed] [Google Scholar]
- 15.Bayat H, Xu S, Pimentel D, Cohen RA, Jiang B. Activation of thromboxane receptor upregulates interleukin (IL)-1beta-induced VCAM-1 expression through JNK signaling. Arterioscler Thromb Vasc Biol. 2008;28:127–134. doi: 10.1161/ATVBAHA.107.150250. [DOI] [PubMed] [Google Scholar]
- 16.Jiang B, Brecher P. N-Acetyl-L-cysteine potentiates interleukin-1beta induction of nitric oxide synthase : role of p44/42 mitogen-activated protein kinases. Hypertension. 2000;35:914–918. doi: 10.1161/01.hyp.35.4.914. [DOI] [PubMed] [Google Scholar]
- 17.Schroder K, Helmcke I, Palfi K, Krause KH, Busse R, Brandes RP. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1736–1743. doi: 10.1161/ATVBAHA.107.142117. [DOI] [PubMed] [Google Scholar]
- 18.Pleskova M, Beck KF, Behrens MH, Huwiler A, Fichtlscherer B, Wingerter O, Brandes RP, Mulsch A, Pfeilschifter J. Nitric oxide down-regulates the expression of the catalytic NADPH oxidase subunit Nox1 in rat renal mesangial cells. FASEB J. 2006;20:139–141. doi: 10.1096/fj.05-3791fje. [DOI] [PubMed] [Google Scholar]
- 19.Jiang B, Xu S, Hou X, Pimentel DR, Cohen RA. Angiotensin II differentially regulates interleukin-1-beta-inducible NO synthase (iNOS) and vascular cell adhesion molecule-1 (VCAM-1) expression: role of p38 MAPK. J Biol Chem. 2004;279:20363–20368. doi: 10.1074/jbc.M314172200. [DOI] [PubMed] [Google Scholar]
- 20.Kuwano Y, Kawahara T, Yamamoto H, Teshima-Kondo S, Tominaga K, Masuda K, Kishi K, Morita K, Rokutan K. Interferon-gamma activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C433–C443. doi: 10.1152/ajpcell.00135.2005. [DOI] [PubMed] [Google Scholar]
- 21.Cai Q, Lanting L, Natarajan R. Interaction of monocytes with vascular smooth muscle cells regulates monocyte survival and differentiation through distinct pathways. Arterioscler Thromb Vasc Biol. 2004;24:2263–2270. doi: 10.1161/01.ATV.0000146552.16943.5e. [DOI] [PubMed] [Google Scholar]
- 22.Kubes P, Kanwar S, Niu XF, Gaboury JP. Nitric oxide synthesis inhibition induces leukocyte adhesion via superoxide and mast cells. FASEB J. 1993;7:1293–1299. doi: 10.1096/fasebj.7.13.8405815. [DOI] [PubMed] [Google Scholar]
- 23.Cayatte AJ, Rupin A, Oliver-Krasinski J, Maitland K, Sansilvestri-Morel P, Boussard MF, Wierzbicki M, Verbeuren TJ, Cohen RA. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets nadph oxidase. Arterioscler Thromb Vasc Biol. 2001;21:1577–1584. doi: 10.1161/hq1001.096723. [DOI] [PubMed] [Google Scholar]
- 24.Lin SJ, Shyue SK, Hung YY, Chen YH, Ku HH, Chen JW, Tam KB, Chen YL. Superoxide dismutase inhibits the expression of vascular cell adhesion molecule-1 and intracellular cell adhesion molecule-1 induced by tumor necrosis factor-alpha in human endothelial cells through the JNK/p38 pathways. Arterioscler Thromb Vasc Biol. 2005;25:334–340. doi: 10.1161/01.ATV.0000152114.00114.d8. [DOI] [PubMed] [Google Scholar]
- 25.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu S, Zhi H, Hou X, Cohen RA, Jiang B. I{kappa}B{beta} Attenuates Angiotensin II-Induced Cardiovascular Inflammation and Fibrosis in Mice. Hypertension. 2011;58:310–316. doi: 10.1161/HYPERTENSIONAHA.111.172031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cayatte AJ, Du Y, Oliver-Krasinski J, Lavielle G, Verbeuren TJ, Cohen RA. The thromboxane receptor antagonist S18886 but not aspirin inhibits atherogenesis in apo E-deficient mice: evidence that eicosanoids other than thromboxane contribute to atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:1724–1728. doi: 10.1161/01.atv.20.7.1724. [DOI] [PubMed] [Google Scholar]