Abstract
The crystal structure of anthranilate synthase (AS) from Serratia marcescens, a mesophilic bacterium, has been solved in the presence of its substrates, chorismate and glutamine, and one product, glutamate, at 1.95 Å, and with its bound feedback inhibitor, tryptophan, at 2.4 Å. In comparison with the AS structure from the hyperthermophile Sulfolobus solfataricus, the S. marcescens structure shows similar subunit structures but a markedly different oligomeric organization. One crystal form of the S. marcescens enzyme displays a bound pyruvate as well as a putative anthranilate (the nitrogen group is ambiguous) in the TrpE subunit. It also confirms the presence of a covalently bound glutamyl thioester intermediate in the TrpG subunit. The tryptophan-bound form reveals that the inhibitor binds at a site distinct from that of the substrate, chorismate. Bound tryptophan appears to prevent chorismate binding by a demonstrable conformational effect, and the structure reveals how occupancy of only one of the two feedback inhibition sites can immobilize the catalytic activity of both TrpE subunits. The presence of effectors in the structure provides a view of the locations of some of the amino acid residues in the active sites. Our findings are discussed in terms of the previously described AS structure of S. solfataricus, mutational data obtained from enteric bacteria, and the enzyme's mechanism of action.
Keywords: anthranilate synthase x-ray structure, tryptophan feedback inhibition, anthranilate biosynthesis
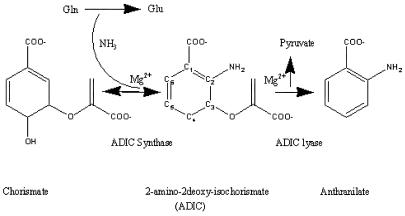
Anthranilate synthase (AS) catalyzes the initial two reactions unique to tryptophan biosynthesis (Scheme S1) (1). The enzyme from Serratia marcescens consists of two polypeptide chains that form a complex TrpE2:TrpG2 (2). The TrpE subunit binds chorismate (CA) and is the site of formation of anthranilate from CA and NH3, either aqueous, or physiologically supplied from glutamine hydrolysis by TrpG. TrpG is a member of the glutamine amidotransferase “triad” family (3, 4). In S. marcescens, Trp, a competitive inhibitor of CA, abolishes both Scheme S1 reactions when bound to TrpE (2).
Scheme 1.
The S. marcescens AS has been well characterized (3, 5–7). Known are the Mr of its subunits, the kinetic constants of its effectors, the extent of cooperativity, some parameters of the 2-amino-2-deoxy-isochorismate (ADIC) synthase and lyase reactions, and other properties. There have been two recent reports on the crystallization and structure determination of AS. One is preliminary from Salmonella typhimurium (8). The second is an analysis at 2.5 Å of AS without ligands from Sulfolobus solfataricus, an archeobacterium (9). This organism lives optimally at 90°C and pH 4.5 (10). Its TrpE displays a novel fold and has 421 residues; TrpG has 195 residues, whereas the TrpE and TrpG subunits of S. marcescens have 520 and 193 residues, respectively. The enzyme was crystallized in the presence of its ligands with a view to: (i) clarifying the mechanism of feedback inhibition by Trp; (ii) defining the binding sites of its ligands; and (iii) comparing the structures of the hyperthermophile and mesophile enzymes.
Materials and Methods
The Sequences of the Subunits of S. marcescens AS.
S. marcescens trpE was subcloned from plasmid pGM6 (11) and sequenced by dideoxy sequencing (GenBank accession no. AY027546). The sequence of trpG was published by Tso et al.(12).
Enzyme Isolation.
A 4.5-kb EcoRI/HindIII fragment containing S. marcescens trpEGDC was subcloned from pGM6 into a derivative of the expression plasmid ptacterm (13) and named pttSmEDC-3, with expression of trpEGDC now under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible tac promoter. pttSmEDC-3 was transformed into strain W3110 metB trpR ΔtrpED24 containing pMS421, a plasmid with the lacIq gene. Cultures were grown in 1× minimal medium (14) containing 0.05% acid hydrolyzed casein, 0.2% glucose, 20 μg/ml l-tryptophan, 30 μg/ml l-methionine, 50 μg/ml spectinomycin, and 100 μg/ml ampicillin, and shaken at 37°C to midlogarithmic phase (OD at 600 nm, 0.5–0.6). IPTG was then added to 1 mM. After an additional 4 hours at 37°C, cells were harvested, washed, and stored frozen. After cell disruption by sonication, the enzyme was purified as in ref. 5, sterilized by filtration, and stored at 4°C.
Crystallization.
Two crystal forms of AS were obtained by vapor diffusion at room temperature. The first, the T-crystal, was produced by equilibrating the protein against 10 mM Tris buffer, pH 6.5/15% glycerol (vol/vol)/2 mM DTT/10 mM EDTA and concentrating it to 25 mg/ml. The protein solution was combined with an equal volume (10 μl) of 25% polyethylene glycol 6000/100 mM citrate, pH 5.4/100 mM glutamate/34.5 mM glutamine/8 mM MgCl2/0.5 mM tryptophan. Seed crystals were added, which grew to full size (0.3 × 0.3 × 0.2 mm3) in about 3 weeks.
The second crystal form, the C-crystal, was grown identically, with 0.5 mM CA substituted for tryptophan.
Data Collection.
The T-crystal belongs to space group P212121 with cell dimensions a = 72.13 Å, b = 124.61 Å, c = 178.49 Å. Intensity data from one T-crystal were collected both in house and at the National Synchrotron Light Source (Brookhaven, NY) to a minimum Bragg spacing of 2.4 Å. Data were reduced with denzo and merged with scalepack (15) (Table 1).
Table 1.
Data collection and refinement statistics
| T-crystal | C-crystal | |
|---|---|---|
| Space group | P212121 | P21 |
| Unit cell, Å | 72.13, 124.61, 178.49 | 87.32, 68.98, 116.36 |
| β angle, ° | — | 108.52 |
| Resolution, Å | 50.0–2.4 | 50.0–1.95 |
| No. unique reflections, completeness % | 59,826 (93.8%) | 88,320 (92.0%) |
| Redundancy | 5.4 | 2.6 |
| Rsym*, % | 5.5 | 8.6 |
| No. of protein atoms | 10,852 | 10,956 |
| No. of hetero atoms | 30 | 52 |
| No. of waters | 0 | 1,335 |
| Ligands present in electron density | l-tryptophan | Glutamyl, benzoate, pyruvate, Mg2+ |
| R factor, % | 24.8 | 18.1 |
| Free R factor, % | 31.8 | 24.6 |
| rms deviation bond lengths, Å | 0.018 | 0.014 |
| rms deviation bond angles, ° | 2.08 | 1.84 |
| rms deviation dihedral angles, ° | 25.19 | 24.92 |
| rms deviation improper angles, ° | 1.35 | 1.20 |
| Mean B factor, Å2 | 60.85 | 24.19 |
ΣhklΣi|Ii − 〈I〉|/(ΣhklΣiIi)
Data for the C-crystal were collected at Beamline 7.1 of the Stanford Synchrotron Radiation Laboratory. The data were reduced and merged with mosflm (16) and scala (17). The C-crystal belongs to space group P21 with cell dimensions a = 87.32 Å, b = 68.98 Å, c = 116.36 Å, and β = 108.52°. Data were of good quality, diffracting to a minimum Bragg spacing of 1.95 Å (Table 1).
Structure Solution and Refinement.
Initial phases for the T-crystal form were generated with an automated script for molecular replacement (G.S., unpublished work) by using the unaltered heterodimeric (TrpG):(TrpE) structure of AS from S. solfataricus as a search model (9) (PDB ID code 1QDL) and the package amore (18). The script performed the following procedures automatically: analysis of the solvent content and detection of a strong nonorigin peak in the self-rotation function on the κ = 180° section indicated there were two TrpG:TrpE heterodimers in the asymmetric unit. Molecular replacement was conducted by using data between 8.0 and 4.0 Å. The rotation function with a radius of integration of 25 Å produced one clear solution 3.0 standard deviations above other peaks in the list. A translation function performed with this solution produced a peak with a correlation coefficient of 12.3% and an R factor of 51.3%. The top peak in the self-rotation function was then used to orient the second molecule relative to the first. A translation search conducted with the second molecule, holding the first fixed, correctly positioned it. After rigid-body refinement of the individual subunits, the final R factor and correlation coefficients were 49.8 and 16%, respectively. Phases and figures of merit were calculated from a polyalanine model constructed from the search model and molecular averaging carried out with sigmaa and dm within the CCP4 package (17, 19, 20). The resulting electron density map clearly showed density for many of the missing side chains and some additional loops. Noncrystallographic symmetry restrained refinement and map improvement were carried out with cns and refmac/warp (21–23), with all data between 50.0 and 2.4 Å incorporating corrections for bulk solvent and an overall anisotropic B factor, followed by rounds of automatic and manual building by using warp (23) and o (24). The refinement strategy was judged by the effects of various models on the free R-factor set (25) (5% of reflections excluded from the refinement). The final R factor and free R factor were 24.6 and 31.4%, respectively. In all, 10 residues were not included in the model because of the absence of electron density: residues 1 from TrpG and 1–3 and 43–48 in TrpE. Density was weak for a number of regions in the structure, and they were tentatively modeled.
Crystals of the C-crystal were solved by molecular replacement with the same script by using the partially refined heterodimer of the T-crystal and amore (14). Again, a TrpG2:TrpE2 complex was present in the asymmetric unit. Two clear peaks were visible in the rotation function, which when translated and put on a common origin resulted in a correlation coefficient of 27.4% and an R factor of 47.4%, this after rigid-body refinement, by using data between 8.0 and 4.0 Å. Refinement and rebuilding were carried out on all data between 50 and 1.95 Å with cns, warp, and refmac (21–23) (incorporating warp for map improvement and solvent addition and cns for bulk solvent and overall anisotropic B-factor correction). Again, the refinement strategy was adjudicated by monitoring the free R factor. This resulted in a model with an R factor of 18.3% and free R factor of 24.0% (Table 1). The model has good geometry (Table 1), with only one catalytically important residue (TrpG Cys-85) in a disallowed region of the Ramachandran plot.
Results and Discussion
General Structure.
The numbering of individual residues is based on the S. marcescens sequence (Fig. 1 a and b).
Figure 1.
Sequence alignments of AS subunits from S. marcescens and S. solfataricus calculated from aligned structures of the two molecules. Structure alignment was performed with o (24) and stamp (36) and the figure produced by alscript (37). Secondary structures were assigned by dssp (38). Numbering is based on the S. marcescens sequence. Conserved hydrophobic residues are shaded yellow, conserved nonhydrophobic residues are shaded green, conserved polar residues are in bold font, conserved small residues in small font, and conserved structural regions are shown boxed. Those residues underlined in a shaded blue box are solvent inaccessible in the heterodimer TrpE:TrpG interface, whereas those in brown are solvent excluded in the heterotetramer interface. β-Sheet regions are shown as light purple arrows, whereas α-helices are in blue. (a) Alignment of TrpGs: residues of the active site are shaded red, whereas those in close contact with the glutamyl residue are shaded gold. (b) Alignment of TrpE: residues in close contact to anthranilate shaded red, whereas those in close contact with pyruvate are in light blue. Residues shaded gold are in close contact with tryptophan.
TrpG.
TrpG is an α/β structure defined by the scop database (26) as a Class I glutamine amidotransferase domain. The catalytic residues Cys-85 His-170 and Glu-172 are in conformations identical to previously determined folds of this type (4, 27). The active-site Cys-85 is strained in a characteristically disallowed region of the Ramachandran plot (28). The subunit contains one cis-proline at position 127.
TrpE.
TrpE of S. marcescens has the novel fold first characterized by Knöchel et al. in S. solfataricus (9) consisting of two subdomains, I and II. It has 125 residues in addition to the thermophile, mostly confined to the N-terminal 240 residues (Fig. 1b). Subdomain I is composed of residues 1–57, 178–308, and 468–520, with one extra α-helix (α3a:202–219) relative to S. solfataricus. The remaining residues comprise subdomain II; they serve to extend the central antiparallel β-sheet from 9 to 12 strands (β4,β4a,β4b) and add two α-helices (α1a,α1b) (Figs. 1b and 2a). All insertions are at the periphery, removed from residues involved in catalysis. The interface between subdomains I and II formed from the hydrophobic surface of the 9- and 12-stranded β-sheets forms a deep crevice containing many of the residues involved in feedback inhibition by tryptophan An adjacent crevice that communicates with TrpG contains residues important for substrate transformation (Figs. 1b and 2b). One cis-proline is present at position 430.
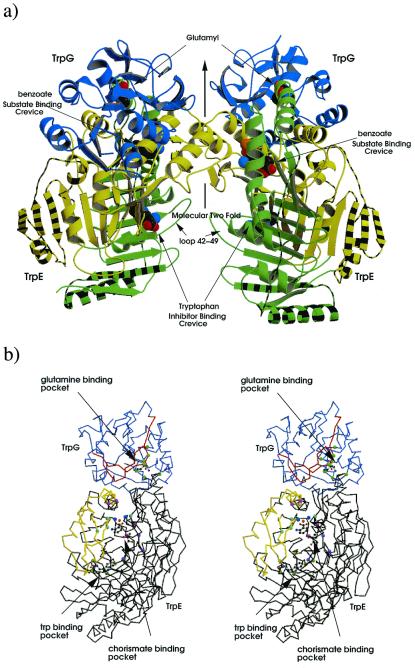
Figure 2.
Structure of the AS of S. marcescens. (a) Ribbon diagram of the AS oligomer, TrpG subunits shown in blue, TrpE subdomain I shown in green subdomain II in yellow. Striped regions correspond to additional structure in S. marcescens compared with that of S. solfataricus. Glutamyl, benzoate, pyruvate, and tryptophan are shown as cpk models. (b) Stereo diagram of the heterodimer; TrpG shown in lilac, TrpE in black; regions of TrpG that move on addition of tryptophan relative the C-crystal are shown in red, whereas those of TrpE are in yellow; residues important to the CA-binding pocket (G328, T329, H398, G485) are shown as light blue balls, residues involved in pyruvate interactions (Y449, R469, G483) are in purple, residues involved in magnesium coordination (E358,361, E495, E498) are colored light purple, magnesium ion in orange, water molecules in dark blue, Trp-binding residues (S40, P291, M293, V453, Y455) are light green, and residues involved in glutamine binding (P57, G58, G60, C85, L86, Q89, S135, S136) are in green. Benzoate, pyruvate, magnesium, and glutamyl are shown as ball-and-stick figures. Produced by bobscript and raster 3d (39–42).
The Oligomeric Structure.
In contrast to the heterodimer in the asymmetric unit of the S. solfataricus structure, both crystal forms of S. marcescens AS contain one heterotetramer in the asymmetric unit. The TrpG:TrpE interface of both bacterial enzymes is the same. The S. marcescens TrpG:TrpE protomer interface occludes 3,100 Å2 of the solvent accessible area, as calculated by the Lee and Richards algorithm (29) in cns (21). Importantly, the heterotetramer interface is completely different in the crystals of the two organisms. We find that the heterotetramer interface in both S. marcescens crystal forms is mediated by the TrpE subunits, whereas the S. solfataricus crystal was found to have a TrpG:TrpG interface. The 2-fold axis in the S. marcescens molecule forms a bridge structure connecting the active sites by using the TrpE α-helices 6 and 7 (residues 342–360) and β strand 16 (residues 382–389) (Figs. 1b, 2a, and 3d). The heterotetramer interface occludes a solvent accessible area of 2,400 Å2 on binding. The identical finding in both T and C crystals suggests that this quaternary conformation is unlikely to be a crystallization artifact. The change from the TrpG:TrpG interface to TrpE:TrpE between bacteria is perplexing. Particularly, because there is strong conservation between the two species of many of the residues in the S. marcescens heterotetramer interface (Fig. 1b). Further, should the S. solfataricus AS form a quaternary conformation identical to that of S. marcescens, the resulting TrpE:TrpE interface would occlude an area of 3,400 Å2 in contrast to the 1,000 Å2 occluded in the thermophile crystal.
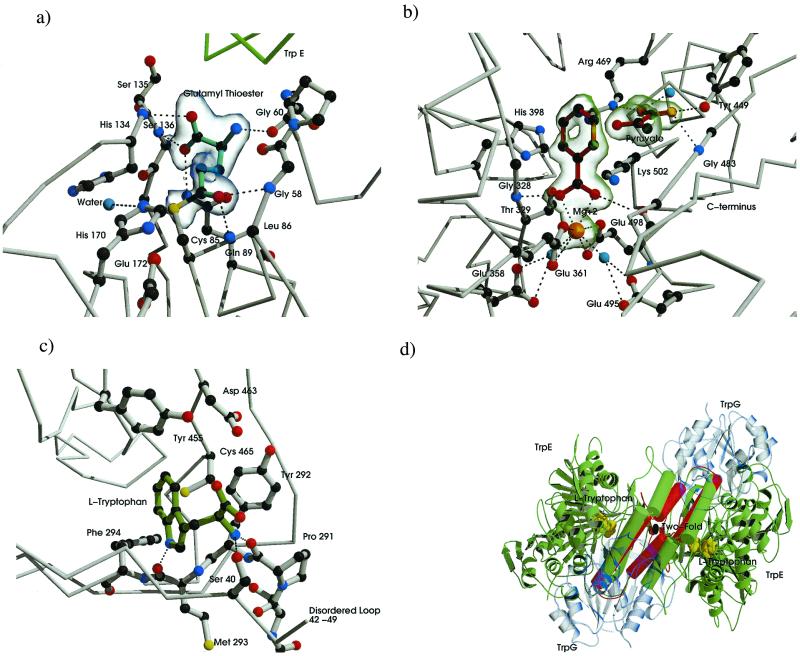
Figure 3.
Substrate product and Trp-binding sites of the AS molecule. Carbon atoms are dark gray, nitrogen blue, and oxygen red. Electrostatic and hydrogen-bond interactions are shown as black dotted lines. (a) Binding residues for glutamyl thioester intermediate of TrpG. Glutamyl moiety drawn with cyan bonds. A σA-weighted Fo−Fc map contoured at 3.5 SD is shown in transparent blue. (b) CA-binding pocket, anthranilate, and pyruvate are drawn with dark red bonds, magnesium ion in orange, ordered waters in cyan. A σA-weighted Fo−Fc map contoured at 3.0 SD is shown in transparent green. (c) Trp-binding pocket, tryptophan shown in green. (d) Conformational states associated with anthranilate, pyruvate, and Trp-bound forms. The molecule is viewed with the molecular 2-fold perpendicular to the page (i.e., perpendicular to the view in Fig. 2a). TrpG subunits shown in transparent blue, α helixes and loops involved in heterotetramer rearrangement shown as rods, C-crystal representation shown in green rearrangement in the T-crystal, shown in red. Tryptophan is shown as cpk model in yellow. Produced by bobscript and raster 3d (39–42).
C-Crystal.
The enzyme was crystallized with various amounts of substrates and products with the view of trapping a molecule that would define the CA-binding site. A combination of glutamine, CA, and glutamate succeeded in giving crystals that diffract to 1.95 Å.
TrpG Active Site.
TrpG generates NH3 from glutamine (3). Clear density in the active site shows a glutamyl thioester intermediate with a carbon–sulfur covalent bond (1.7 Å). This is similar to the intermediates described by Thoden et al. (30), with the notable exception that none of the catalytic residues of the S. marcescens AS have been altered by mutation. His-170 and Glu-172 adopt conformations identical to other members of the glutamine amidotransferase family (4, 30–33).
A number of residues are in close contact with the glutamyl moiety (Fig. 3a). The nitrogens of Ser-135 and Ser-136 and Gln-89 Oɛ1 are within H-bonding distances to the carboxyl group of the glutamyl moiety. Glutamyl Oɛ1 is in close contact with Gly-58 N and Leu-86 N. The glutamyl moiety is 25 Å away from the active site of the TrpE subunit. The absence of a closed tunnel between the two subunits suggests a directed passage of the ammonia down the TrpE crevice to the CA-binding site.
TrpE Active Site.
In the CA-binding site, clear electron density showed in an initial Fo−Fc map for a planar ring structure, benzoic acid, coordinated to Mg2+ by a carboxyl group, and for a cleaved pyruvate moiety separated from the ring by 3.0 Å (Fig. 3b). No density was seen at position C4 on the ring for the CA hydroxyl group or for a nitrogen at C2 (Fig. 3b; Scheme S1).
A number of residues are within H-bonding/electrostatic distance to the pyruvate (Fig. 3b). The pyruvate carboxyl group is coordinated via H bonds to an ordered water, to the hydroxyl group of Tyr-449, the N of Gly-483, and the Nɛ of Arg-469. The carboxyl moiety of benzoate is coordinated via two main chain H bonds with Gly-328 N and Gly-485 N. A Mg2+ ion is coordinated by the carboxyl moiety of the benzoate and a charged pocket provided by Glu-498 and Glu-361, with which it directly coordinates, and two water molecules held in position by Glu-358 and Glu-495 (Fig. 3b). Thr-329 Oγ and His-398 Nδ are both within 2.6 Å of the benzoate ring strongly supporting their role in catalysis as proposed by mutational studies (5). The absence of an −NH2 group on benzoate needed to create anthranilate is an enigma. The electron density in TrpG clearly shows at least in the majority of the unit cells that a nitrogen has been cleaved from the glutamine. It is possible that ADIC may never have been formed, with benzoate made directly from CA, or anthranilate may have been made in the crystal but with the nitrogen removed. Clearly, the fate of the amino group remains to be solved.
Mechanism of the Lyase Reaction, the Conversion of ADIC to Anthranilate (AA).
Some of the details of the conversion of ADIC to AA (Scheme S1) can now be surmised. The key was the finding (1) that TrpE His-398 was indispensable. The C-crystal shows His-398 to be in close proximity to C2 of the benzene ring. A standard second-order elimination can be invoked to yield the double bond between C2 and C3 with His-398 as the base abstracting the C2 proton and pyruvate as the leaving group. There is no assisting acid residue near C3. However, pyruvate is a good leaving group, and any Lewis acid, Mg2+ or H2O, should suffice. An E1 mechanism seems less likely because ADIC was trapped by the His-398 mutation with the pyruvate intact on the ring. The crystal offers no information on the removal of the hydroxyl group from CA.
T-Crystal
A Structural Basis for Competitive End-Product Inhibition by Tryptophan.
Electron density for tryptophan was clearly seen in a σA-weighted map produced by refmac (22). Tryptophan binds in a pocket distinct from the CA-binding site in the hydrophobic β-sandwich formed between β-sheets from subdomains I and II. The Trp residue H-bonds with Ser-40 Oγ, Pro-291 N, and Met-293 N; all are implicated in feedback inhibition by mutational analysis (5) (Fig. 3c).
A conformational change brought about by the binding of tryptophan to a site 18 Å from the CA-binding site is responsible for disabling the enzyme. In general, the overall structure of the C-crystal appears to be more ordered, the crystals diffracting to significantly higher resolution and possessing a significantly lower mean B factor (Table 1). The structures from the two crystal forms are very similar, 0.713 Å rms deviation on 181 aligned Cas in TrpG, and 1.02 Å rms deviation on 471 aligned Cas in TrpE. However, when the two structures are superposed, it is noted that a number of regions are displaced relative to one another. In the T-crystal, a motif formed by the secondary structure elements β15,α6,α7,β16,β17 (residues 327–363 and 387–403) is shifted at most 7.0 Å away from the CA-binding pocket relative to the C-crystal (Fig. 3d). Significantly, this movement removes Thr-329, His-398, and all of the glutamate residues responsible for Mg2+ binding from the vicinity of the active site. The rearrangement seems to be in part because of an ordering of loop 42–49 (Fig. 2a), which shows no visible density in the T-crystal (or in the S. solfataricus structure), but which is perfectly ordered in the C-crystal. When compared by superposition with the S. solfataricus AS, crystallized without ligands, the corresponding region is in an intermediate position between that of the T- or C-crystal form. This suggests that without any ligand, this motif may reside in this mean position. In the presence of CA, the correct active site is assembled by moving this region toward the active-site crevice; in the presence of Trp, the motif is forced to move away. This seesaw effect explains the competitive interaction between tryptophan and CA (Fig. 3d); further, it offers a rational explanation for competitive inhibition by molecules with different structures. That the loops involved in the active site are also those situated at the heterotetramer interface also explains the observation that one tryptophan molecule can inhibit both TrpE subunits (34). Because of the molecular 2-fold axis, a movement in one subunit must concomitantly move that of the other subunit in the same direction (Fig. 3d).
The presence of tryptophan also affects the TrpG subunit. Two β-sheets, 103–109 and 130–140 (β5 and β6), move in response to tryptophan binding, presumably communicated through α-helix 344–376 (α7) in TrpE. The sheets become largely disordered in the T-crystal and were tentatively modeled and appear to move in such a way as to disrupt the residues that are necessary for glutamine binding. This is consistent with the known lack of glutaminase activity by TrpG until CA binds (3).
Summary
AS from the mesophile S. marcescens and the hyperthermophile S. solfataricus share many structural features, including the “triad” glutamine amidotransferase fold of TrpG and the novel TrpE fold first described in the thermophile. The striking oligomeric difference between the enzymes is that the TrpG:TrpE protomers of S. marcescens associate through the TrpE subunits instead of the TrpG subunits as in the thermophile. We have no explanation for the lack of TrpE:TrpE interaction in the thermophile, given the strong amino acid homology of the enzymes, particularly in the interaction regions.
The C-crystal reveals a γ-glutamyl thioester with Cys-85 and a 1.7 Å C–S bond. The other members of the catalytic triad His-170 and Glu-172 are positioned as expected.
The CA-binding site has been structurally characterized. There is unambiguous density for an aromatic benzoic acid and for pyruvate clearly separated from the ring. That benzoate occupies the CA-binding site is supported by the following evidence: the topological precision of the Mg2+ chelate pocket, the elimination of the CA hydroxyl group, and the proximity of benzoate to all of the amino acids assigned to the catalytic site by mutation (5). Of these amino acids inferred to belong to the CA-binding site by mutation, His-398 has been characterized as a putative base for the extraction of the proton at C2 of the ring. What is ambiguous is the position and existence of the ortho-amino group, which is not visible in the electron density map.
The T-crystal offers a structural interpretation of the competition between tryptophan and CA through a moiety that can oscillate between two binding sites, depending on occupancy. Further, occupancy of only one of these two sites is sufficient to abolish all enzyme activity as a consequence of the 2-fold molecular axis.
Sequence and structure comparison of the homologous enzymes does not offer insights into thermophile/mesophile differences additional to those already empirically observed with other pairs. A more substantial contribution may arise from tailoring experiments designed to eliminate the S. marcescens loops and decrease the entropy of the denatured form (35).
Acknowledgments
We thank Benjamin Boe and David Braun for excellent technical assistance, Mark Miller for advice, and Peter Schultz for continued encouragement. For help with data collection and processing, we thank the Brookhaven National Laboratory and Stanford Synchrotron Radiation Laboratory. These studies have been supported in part by National Institutes of Health (NIH) Grant GM09738 to C.Y., and NIH–National Cancer Institute National Research Service Award T32 CA09532 to C.K.
Abbreviations
- AS
anthranilate synthase
- CA
chorismate
- ADIC
2-amino-2-deoxy-isochorismate
Note Added in Proof.
We have learned that an analysis of a crystal of anthranilate synthase from Salmonella typhimurium, corresponding to our T-crystal, has been published with parallel results (43).
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY027546).
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID codes 1I7S and 1I7Q for the T- and C-crystals, respectively).
References
- 1.Morollo A A, Bauerle R. Proc Natl Acad Sci USA. 1993;90:9983–9987. doi: 10.1073/pnas.90.21.9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zalkin H, Hwang L H. J Biol Chem. 1971;246:6899–6907. [PubMed] [Google Scholar]
- 3.Zalkin H. Adv Enzymol Relat Areas Mol Biol. 1993;66:203–309. doi: 10.1002/9780470123126.ch5. [DOI] [PubMed] [Google Scholar]
- 4.Tesmer J J, Klem T J, Deras M L, Davisson V J, Smith J L. Nat Struct Biol. 1996;3:74–86. doi: 10.1038/nsb0196-74. [DOI] [PubMed] [Google Scholar]
- 5.Bauerle R, Hess J, French S. Methods Enzymol. 1987;142:366–386. doi: 10.1016/s0076-6879(87)42049-1. [DOI] [PubMed] [Google Scholar]
- 6.Robb F T, Hutchinson M A, Belser W H. J Biol Chem. 1971;246:6908–6912. [PubMed] [Google Scholar]
- 7.Yanofsky C, Miles E W, Bauerle R, Kirschner K. Encyclopedia of Molecular Biology. Vol. 4. New York: Wiley; 1999. pp. 2676–2687. [Google Scholar]
- 8.Tolbert W D, Chatterji S, Bauerle R, Kretsinger S. Acta Crystallogr. 1999;D55:305–306. doi: 10.1107/S0907444998010233. [DOI] [PubMed] [Google Scholar]
- 9.Knöchel T, Ivens A, Hester G, Gonzalez A, Bauerle R, Wilmanns M, Kirschner K, Jansonius J, N. Proc Natl Acad Sci USA. 1999;96:9479–9484. doi: 10.1073/pnas.96.17.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segerer, A. & Stetter, K. O. in Bergeyt's Manual of Systematic Bacteriology (Williams & Wilkins, Baltimore), Vol. 3, pp. 2250–2251.
- 11.Miozarri G, Yanofsky C. Nature (London) 1979;277:486–489. doi: 10.1038/277486a0. [DOI] [PubMed] [Google Scholar]
- 12.Tso J Y, Hermodson M A, Zalkin H. J Biol Chem. 1980;255:1451–1457. [PubMed] [Google Scholar]
- 13.Paluh J L, Yanofsky C. Nucleic Acids Res. 1996;14:7851–7860. doi: 10.1093/nar/14.20.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel H J, Bonner D M. J Biol Chem. 1957;218:97–106. [PubMed] [Google Scholar]
- 15.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 16.Leslie A, A W. Jnt. CCP4/ESF-EACMB Newslett. Protein Crystallogr. 1992. 26. [Google Scholar]
- 17.Collaborative Computing Project Number 4. Acta Crystallogr. 1994;D50:760–763. [Google Scholar]
- 18.Navaza J. Acta Crystallogr. 1994;A50:157–163. [Google Scholar]
- 19.Read R J. Acta Crystallogr. 1986;A42:140–149. [Google Scholar]
- 20.Cowtan K. Jnt CCP4/ESF-EACMB Newslett Protein Crystallogr. 1994;31:34. [Google Scholar]
- 21.Brünger A T, Adams P D, Clore G M, DeLano W L, Gros P, Grosse-Kunstleve R W, Jiang J-S, Kuszewski J, Nilges M, Pannu N S, et al. Acta Crystallogr. 1998;D54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 22.Murshudov G N, Vagin A A, Dodson E J. Acta Crystallogr. 1997;D53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 23.Perrakis A, Sixma T K, Wilson K S, Lamzin V S. In: Recent Advances in Phasing. Wilson K S, Davies G, Ashton A W, Bailey S, editors. Daresbury Laboratories, Warrington, U.K.: SERC; 1997. pp. 82–91. [Google Scholar]
- 24.Jones T A, Zou J Y, Cowan S W, Kjeldgaard M. Acta Crystallogr. 1991;A47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 25.Brünger A T. Methods Enzymol. 1997;277:366–396. doi: 10.1016/s0076-6879(97)77021-6. [DOI] [PubMed] [Google Scholar]
- 26.Murzin A G, Brenner S E, Hubbard T, Chothia C. J Mol Biol. 1995;247:536–540. doi: 10.1006/jmbi.1995.0159. [DOI] [PubMed] [Google Scholar]
- 27.Thoden J B, Holden H M, Wesenberg G, Raushel F M, Rayment I. Biochemistry. 1997;36:6305–6316. doi: 10.1021/bi970503q. [DOI] [PubMed] [Google Scholar]
- 28.Ollis D L, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken S M, Harrel M, Remington S J, Silman I, Schrag J, et al. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- 29.Richards F M. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- 30.Thoden J B, Miran S G, Howard A J, Phillips J C, Raushel F M, Holden H M. Biochemistry. 1998;37:8825–8831. doi: 10.1021/bi9807761. [DOI] [PubMed] [Google Scholar]
- 31.Thoden J B, Huang X, Raushel F M, Holden H M. Biochemistry. 1999;38:16158–16166. doi: 10.1021/bi991741j. [DOI] [PubMed] [Google Scholar]
- 32.Raushel F M, Thoden J B, Holden H, M. Biochemistry. 1999;38:7891–7899. doi: 10.1021/bi990871p. [DOI] [PubMed] [Google Scholar]
- 33.Thoden J B, Wesenberg G, Raushel F M, Holden H M. Biochemistry. 1999;38:2347–2357. doi: 10.1021/bi982517h. [DOI] [PubMed] [Google Scholar]
- 34.Caligiuri M G, Bauerle R. J Biol Chem. 1991;266:8328–8335. [PubMed] [Google Scholar]
- 35.Matthews B W, Nicholson H, Becktel W J. Proc Natl Acad Sci USA. 1987;84:6663–6667. doi: 10.1073/pnas.84.19.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell R B, Barton G J. Proteins Struct Funct Genet. 1992;14:309–323. doi: 10.1002/prot.340140216. [DOI] [PubMed] [Google Scholar]
- 37.Barton G J. Protein Eng. 1993;6:37–40. doi: 10.1093/protein/6.1.37. [DOI] [PubMed] [Google Scholar]
- 38.Kabsch W, Sander C. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 39.Esnouf R M. J Mol Graphics. 1997;10:132–134. doi: 10.1016/S1093-3263(97)00021-1. [DOI] [PubMed] [Google Scholar]
- 40.Kraulis P J. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]
- 41.Meritt E A, Murphy M E P. Acta Crystallogr. 1994;D50:869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- 42.Esnouf R M. Acta Crystallogr. 1999;D55:938–940. doi: 10.1107/s0907444998017363. [DOI] [PubMed] [Google Scholar]
- 43.Morollo, A. A. & Eck, M. J. Nat. Struct. Biol. 8, 243–247. [DOI] [PubMed]